Abstract
GPR109A, a G-protein-coupled receptor, is activated by niacin and butyrate. Upon activation in colonocytes, GPR109A potentiates anti-inflammatory pathways, induces apoptosis, and protects against inflammation-induced colon cancer. In contrast, GPR109A activation in keratinocytes induces flushing by activation of Cox-2-dependent inflammatory signaling and, the receptor expression is upregulated in human epidermoid carcinoma. Thus, depending on the cellular context and tissue, GPR109A functions either as a tumor suppressor or a tumor promoter. However, the expression status and the functional implications of this receptor in the mammary epithelium are not known. Here we show that GPR109A is expressed in normal mammary tissue and, irrespective of the hormone receptor status, its expression is silenced in human primary breast tumor tissues, breast cancer cell lines, and in tumor tissues of three different murine mammary tumor models. Functional expression of this receptor in human breast cancer cell lines decreases cAMP production, induces apoptosis, and blocks colony formation and mammary tumor growth. Transcriptome analysis revealed that GPR109A activation inhibits genes, which are involved in cell survival and anti-apoptotic signaling, in human breast cancer cells. In addition, deletion of Gpr109a in mice increased tumor incidence and triggered early onset of mammary tumorigenesis with increased lung metastasis in MMTV-Neu mouse model of spontaneous breast cancer. These findings suggest that GPR109A is a tumor suppressor in mammary gland and that pharmacological induction of this gene in tumor tissues followed by its activation with agonists could be an effective therapeutic strategy to treat breast cancer.
Introduction
GPR109A and GPR109B are highly homologous seven-transmembrane G-protein-coupled receptors of Gi-family members (1). GPR109A was originally identified in mice in a search for genes that were differentially expressed in IFN-γ and TNF-α-stimulated macrophages (2). Subsequently, three different groups have independently demonstrated that GPR109A functions as a high-affinity receptor for the B-complex vitamin niacin while GPR109B is little affected (3–5). GPR109A is highly expressed in adipocytes and in various immune cells, including macrophages (2, 6–8). It is also expressed in spleen, colon, and retinal pigment epithelial cells (4, 9–11). Niacin, though a normal biological constituent in blood and cells, is not present at concentrations high enough to activate the receptor under physiologic conditions; however, at pharmacologic doses, circulating levels of niacin rise high enough to activate the receptor (12). In addition, butyrate is the physiologic agonist for GPR109A in colon (9) whereas β-hydroxybutyrate (β-OHB), a ketone body produced by the oxidation of fatty acids, activates the receptor at physiologic concentrations in non-colonic tissues (13). GPR109A activation in adipose tissue decreases the cellular levels of cAMP via inhibition of adenylyl cyclase in a pertussis toxin-sensitive manner (3–5). Similarly, activation of the receptor in colon cancer cells leads to apoptosis via inhibition of Bcl-2, Bcl-xL and cyclin D1 and activation of death receptor signaling pathway (9). GPR109A activation in neutrophils leads to induction of caspase-dependent apoptosis (6). Activation of this receptor in retinal pigment epithelial cells leads to inhibition of TNF-α-induced IL-6 and Ccl2 production (14). However, GPR109A expression is increased with increasing disease progression of squamous cell carcinoma and squamous cell carcinoma cell lines. Interestingly, the increased GPR109A expression observed in squamous cell carcinoma cells are non-functional, the receptor protein shows a diffuse intracellular localization and failed to elicit Gi-mediated cAMP inhibition and associated signaling (15, 16). This suggests that depends on the cellular context and tissue, GPR109A functions either as a tumor suppressor or a tumor promoter.
Butyrate and β-hydroxybutyrate are low-affinity endogenous agonists for the receptor. The EC50 is 1.6 mM for butyrate and 0.7 mM for β-hydroxybutyrate (13). The normal physiologic level of butyrate in circulation is ~ 10 μM, which is not sufficient to elicit any activation signal on GPR109A in most tissues; in contrast, even though the circulating levels of β-hydroxybutyrate under fed conditions are too low (~0.2 mM), its levels increase under fasting conditions sufficient to activate the receptor (17, 18). However, butyrate is present at high levels (~10 mM) in colonic lumen due to bacterial fermentation of dietary fiber (17). Mammary gland is another tissue where butyrate is naturally produced during lactation. Breast milk contains a significant amount of butyrate and the butyrate content of bovine milk fat is approximately 2–5% by weight (19–21). Butyrate has been shown to provide protection against several human malignancies including breast cancer (22, 23). Butyrate decreases the development of carcinogen-induced mammary tumor, suppresses the expression of ERα and progesterone receptor, and induces growth arrest in breast cancer cell lines (24–27). Thus, we hypothesize that GPR109A activation could be involved in maintaining cellular homeostasis in mammary epithelium by induction of cellular differentiation in normal cells, and inhibition of cell survival and induction of apoptosis in tumor cells. However, the expression status of GPR109A and functional implications of this receptor in mammary epithelium have not been studied. Here we show that GPR109A is expressed in normal mammary epithelium and that the receptor functions as an effective tumor suppressor in this tissue.
Materials and Methods
Cell lines
The human immortalized normal mammary epithelial cell lines: HMEC (obtained from Lonza, Walkersville, MD in June 2009), MCF10A (obtained from ATCC, Manassas, VA in September 2008) and HBL100, was kindly provided by Dr. S. Sukumar (Johns Hopkins University, Baltimore, MD) in June 2005. ER-positive human breast cancer cell lines (MCF7, T47D, ZR75.1, BT474) and the ER-negative human breast cancer cell lines (MDA-MB231, MDA-MB453, MDA-MB468 and HCC1937) were obtained from ATCC in July 2011. The HMEC and MCF10A cells were grown in MEGM complete medium. HBL100 cells was grown in McCoy5A with 10% FBS. MCF7 and BT20 cells were grown in DMEM medium with 10% FBS. T47D, ZR75.1, BT474 and HCC1937 cells were grown in RPMI1640 medium with 10% FBS. MDA-MB-231, -453, and MDA-MB-468 cells were grown in Leibovit’s L-15 medium with 10% FBS. MDA-MB415 cells were grown in Leibovit’s L-15 medium with 15% FBS and 0.01mg/ml insulin. All cell lines were authenticated twice by morphologic and isoenzyme analyses during the study period. Cell lines were routinely checked for mycoplasma contamination using the Universal mycoplasma detection kit (ATCC) and consistently found to be negative. The last mycoplasma test was performed in February 2013.
Generation of pCDH and GPR109A-pCDH stable cell lines
Human GPR109A cDNA was subcloned into pCDH-CMV-MCS-EF1-Puro vector (System Biosciences, Mountain View, CA) at EcoRI/NotI site. The resultant plasmid was sequenced to confirm the authenticity of the insert. Recombinant lentivirus was produced by co-transfection into 293FT cells with pCDH and GPR109A-pCDH constructs and three other helper vectors, pLP-1, pLP-2, and pVSVG (Invitrogen), using Lipofectamine-2000 transfection reagent. Lentiviral supernatants were harvested at 72 h post-transfection and filtered through a 0.45-μm membrane. ZR75.1 and MDA-MB231 cells were infected for 24 h with fresh lentivirus expressing either pCDH vector control or GPR109A-pCDH construct in medium containing 8 μg/ml polybrene, and cultured for an additional 48 h. The cells were selected for puromycin resistance (4 μg/ml) for 1 week, and maintained in medium containing 1 μg/ml puromycin. The level of GPR109A mRNA expression in ZR75.1 and MB231 cells expressing pCDH and GPR109A-pCDH constructs were analyzed by RT-PCR and qPCR. GPR109A protein expression was assessed by FACS with an antibody specific for human GPR109A. GPR109A function was monitored by nicotinate binding as well as by nicotinate-induced inhibition of basal and forskolin-induced cAMP levels.
RT-PCR
Expression of human GPR109A and GPR109B, and mouse Gpr109a mRNA were determined by semi-quantitative and real-time PCR analyses. Similarly, WEE1, IQGAP3, AVEN, PLK1, IRF6, IL24 and RASSF2 expressions were determined by real-time PCR. Total RNA, isolated from human normal and primary breast tumor tissues, normal and breast cancer cell lines, and mouse mammary tissues, was reverse-transcribed using the GeneAmp RNA PCR kit (Applied Biosystems). PCR was performed on Veriti thermocycler (Applied Biosystems) using specific primers (Supplementary Table S1). Real-time PCR was carried out on StepOne Plus instrument (Applied Biosystem) using the power SYBR green PCR master mix (Applied Biosystems) as per the manufacturer’s instructions.
Cell cycle analysis
Cells were fixed in 50% ethanol, treated with 0.1% sodium citrate, 1 mg/mL RNase A, and 50μg/mL propidium iodide, and subjected to fluorescence-activated cell sorting (FACS, Becton Dickinson).
ER-positive and ER-negative primary breast cancer tissues
Details about obtaining the primary breast tumor and adjacent normal tissues, RNA extraction and cDNA preparation were published in our recent manuscript (28). Human breast tissue array was obtained from US Biomax Inc., Rockville, MD and details of GPR109A immunostaining are given in Supplementary Material.
Generation of MMTV-Neu mice with Gpr109a+/+, Gpr109a+/− and Gpr109a−/− backgrounds
Gpr109a−/− mouse (34), a generous gift from Dr. Stefan Offermanns, Max-Planck-Institute for Heart and Lung Research, Germany, was bred with MMTV-Neu-Tg mice (Jackson Laboratory, Stock #002376), and the resulting Gpr109a+/−/MMTV-Neu mice, which were in mixed genetic background, were again interbred to generate Gpr109a+/+-MMTV-Neu, Gpr109a+/−-MMTV-Neu and Gpr109a−/−-MMTV-Neu mice. We used 12 mice/group and repeated the experiment 3 times, thus giving 36 mice in each group. We monitored time of tumor appearance, tumor size, the number of tumors, and time and percent of lung metastasis in each of these three groups. When the mice became morbid due to increased tumor burden and/or lung metastasis, the animals were euthanized and the tumor tissues harvested. If mice did not develop tumor until 15 months of age, we removed these mice from the experiment, and noted that these mice were tumor-free.
Details for cAMP assay, immunoblot, microarray, nicotinate binding and colony formation assays as well as mouse xenograft are given in supplementary materials section.
Institutional compliance
The Georgia Regents University (GRU) IACUC and Biosafety Committees approved the animal experiments reported in this study. Human breast cancer tissues and the surrounding normal tissues were obtained from the GRU tumor bank with approval from the Institutional Review Board and Human Assurance Committee.
Statistical analyses
Statistical analysis was conducted by statistical software SAS 9.3 at significance level 0.05. A Mixed model was used to compare tumor volume changes over time. We also used two-way ANOVA followed by Bonferroni multiple comparison test by using Graph Pad Prism software, version 5.0. A p value <0.05 was considered statistically significant.
Results
GPR109A is silenced in human primary breast tumor tissues, human breast cancer cell lines, and in mouse mammary tumor
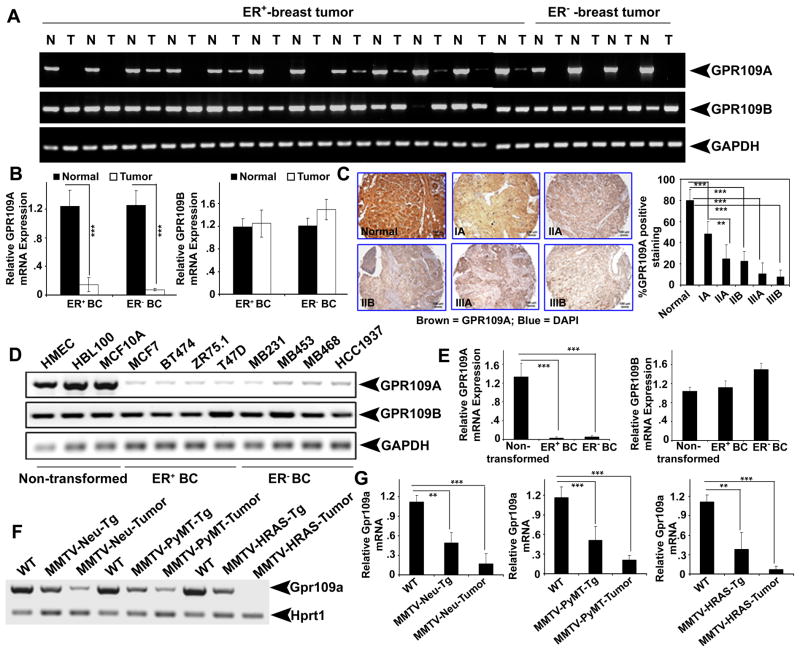
We first investigated the expression of GPR109A and GPR109B in human normal breast and in breast cancer tissues. Irrespective of estrogen receptor status, GPR109A expression was decreased in more than 70% of primary breast cancer samples compared with corresponding normal breast tissues (Fig. 1A). Real-time PCR analysis confirmed this observation (Fig. 1B). We also analyzed GPR109A protein expression using human tissue array, which has normal and breast tumors at various stages of the disease. We found that GPR109A expression was significantly reduced even in early stage of breast tumor (stage IA) and almost undetectable in advanced invasive (stage IIIB) breast tumor (Fig. 1C). The decreased GPR109A expression was also evident in several breast cancer cell lines (Fig. 1D and E). However, there was no significant change in GPR109B expression in these samples. We also examined the expression of Gpr109a in normal mammary glands at different developmental stages (virgin, pregnant, lactation and involution) and in three different murine spontaneous mammary tumor tissues obtained from MMTV-Neu-Tg, MMTV-PyMT-Tg and MMTV-HRAS-Tg mice. Gpr109a mRNA transcript was present in normal virgin mammary tissues and drastically induced during mammary gland involution, but the expression level was markedly decreased in premalignant and malignant tumor tissues from the three transgenic mice (Fig. 1F, G and unpublished data).
Figure 1.
GPR109A expression is silenced in breast cancer. A and B, expression of GPR109A and GPR109B in ER-positive (ER+) and ER-negative (ER−) breast tumor tissues and corresponding normal tissues analyzed by semi-quantitative and qPCR analyses, respectively. C. Representative images of GPR109A expression in normal and different breast tumor specimens. Images are shown at 10x magnification. D and E, GPR109A and GPR109B expressions in human immortalized normal and ER+ and ER− breast cancer cell lines. F and G, Gpr109a expression in normal, pre-malignant and tumor tissues of MMTV-Neu-Tg, MMTV-PyMT-Tg and MMTV-HRAS-Tg mice. Data are means ± SEM. ***p<0.001; ***p<0.001 by t-test.
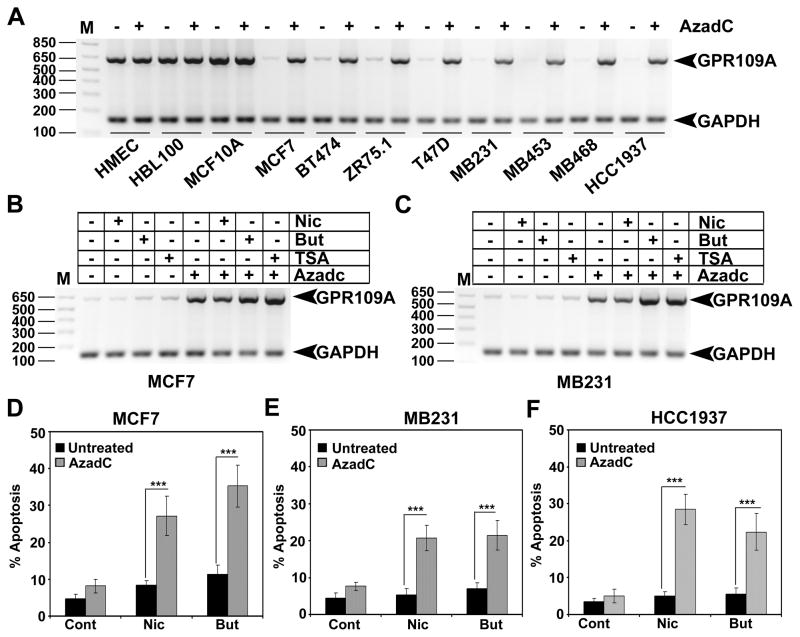
We have previously shown that GPR109A is silenced in human colon cancer cells by DNA methylation and that treatment of these cells with the DNA methyltransferase inhibitor 5′-aza-2-deoxycytidine (AzadC) re-activated its expression (9). To determine whether the decrease in GPR109A expression in human breast cancer is also due to DNA methylation, we treated immortalized normal mammary epithelial and breast cancer cell lines with AzadC, and examined the expression of GPR109A. The AzadC treatment did not affect GPR109A expression in immortalized normal cell lines. In contrast, the expression of the receptor was re-activated in breast cancer cell lines (Fig. 2A and S1A). Further, one of the GPR109A agonists, butyrate, is a well known HDAC inhibitor and studies have shown that HDAC inhibitors can restore the expression of tumor suppressors in cancer cells. We tested whether butyrate treatment alone can restore GPR109A expression in breast cancer cells. We treated two breast cancer cell lines with butyrate and TSA, a pan-HDAC inhibitor. As a positive control, we treated these cells with AzadC either alone or in combination with HDAC inhibitors. Nicotinate was used as a negative control. As shown in Fig. 2B, C and S1B, neither butyrate nor TSA treatment alone were able to restore GPR109A expression. However, co-treatment of HDAC inhibitors along with AzadC significantly increased GPR109A expression.
Figure 2.
DNA methylation inhibitor re-activates GPR109A expression in human breast cancer cells. A, GPR109A expression was analyzed in human immortalized normal mammary epithelial cell lines and human breast cancer cell lines with and without 5′-aza-2′-deoxycytidine (AzadC). B, GPR109A expression was analyzed in two human breast cancer cell lines that were treated with HDAC inhibitors (butyrate and TSA) either alone or in combination with AzadC. Nicotinate, which does not inhibit HDACs, was used as a negative control. C, Three human breast cancer cell lines were first treated with 5′-AzadC and then treated with nicotinate or butyrate. Cells were harvested and cell cycle analysis was carried out using FACS. The apoptotic cell death was quantitated in sub G0/1 phase of the cell cycle. Values are expressed as means ± SEM. ***p<0.001 by t-test.
Ectopic expression of GPR109A induces apoptosis in human breast cancer cells
To determine the functional importance of GPR109A in human mammary epithelial cells, we exposed MCF10A, MCF7 and MB231 cells to GPR109A agonists niacin and butyrate. The agonists did not have any effect in both immortalized normal and breast cancer cell lines (Fig. S1D). We then transiently expressed GPR109A and control vector in these cells and the effects of niacin and butyrate were evaluated. Ectopic expression of GPR109A in human breast cancer cell lines induced apoptosis in response to niacin and butyrate treatment (Fig. S1C–D). In contrast, these ligands did not affect normal MCF10A cells or control vector-transfected breast cancer cell lines, suggesting that activation of GPR109A induces apoptosis specifically in breast cancer cells.
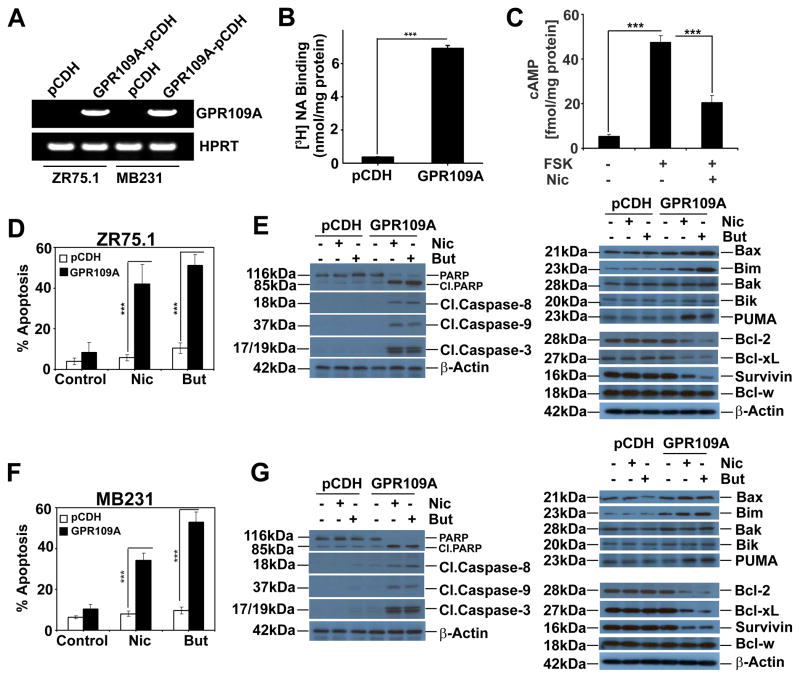
To study the functional implications of GPR109A in breast cancer in more detail, we developed lentiviral-vector mediated stable expression of GPR109A in ER-positive (ZR75.1) and Triple-negative (MB231) breast cancer cell lines. The expression of GPR109A mRNA and protein were confirmed in these stable cells (Fig. 3A and S2A–B). The expression was further confirmed by a ligand-binding assay using the nicotinate. The binding of nicotinate was ~7-fold higher in GPR109A-expressing cells than in vector controls (Fig. 3B). The binding was specific and saturable as evident from the competitive inhibition of [3H]-nicotinate binding by unlabeled nicotinate (Fig. S2C). To confirm that the expressed GPR109A was functional, we measured forskolin-stimulated cAMP levels in the presence and absence of nicotinate in GPR109A-expressing MB231 cells. As shown in Fig. 3C, the GPR109A agonist nicotinate efficiently decreased the forskolin-induced cAMP levels. We then exposed these cells to nicotinate and butyrate and performed cell cycle analysis. As shown in Fig. 3D and F, nicotinate and butyrate induced apoptosis in GPR109A-expressing cells but not in control cells. Protein expression analysis confirmed that GPR109A activation leads to activation of caspases (9, 8 and 3), cleavage of PARP and activation of pro-apoptotic proteins (Bax, Bim and Puma) with inhibition of anti-apoptotic proteins (Bcl-2, Bcl-xL and Survivin) in both ZR75.1 (Fig. 3E) and MB231 cells (Fig. 3G).
Figure 3.
Stable expression of GPR109A in human breast cancer cell lines induces agonist-dependent apoptosis. A, ectopic expression of GPR109A in two human breast cancer cell lines. B GPR109A protein function in MB231-GPR109A cells was assessed by nicotinate binding assay. C, cAMP levels in MB231-GPR109A cells in the presence and absence of forskolin (10 μM) and nicotinate (1 mM). D and F, percent of apoptosis in control and GPR109A-expressing stable cell lines in the presence and absence of nicotinate and butyrate in ZR75.1 and MB231 cells. Data are means ± SEM. ***p<0.001 by t-test. E and G, expression of pro-apoptotic and anti-apoptotic proteins in control and GPR109A-expressing stable cell lines in the presence and absence of nicotinate or butyrate in ZR75.1 and MB231 cells.
GPR109A re-activation induces ligand-dependent apoptosis in breast cancer cells
To test whether GPR109A re-activation by AzadC could induce apoptosis upon activation with agonists, we treated three breast cancer cell lines with AzadC and then exposed them to nicotinate or butyrate. Exposure to nicotinate and butyrate induced apoptosis in AzadC-treated breast cancer cells but not in control cells (Fig. 2D–F), showing that re-activation of endogenous GPR109A induces apoptosis in breast cancer cells in the presence of GPR109A agonists.
GPR109A expression inhibits cell survival and anti-apoptotic genes in human breast cancer cells
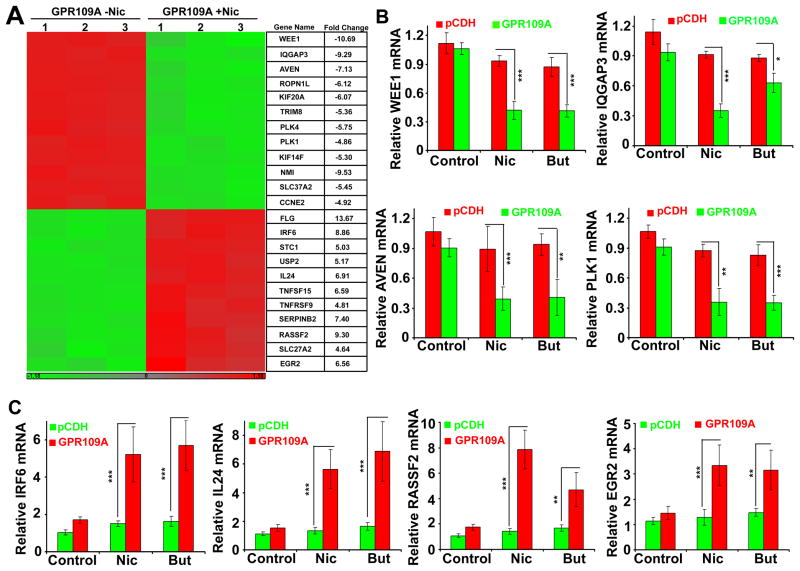
To determine the molecular mechanism of GPR109A-induced apoptosis in human breast cancer, we performed microarray analysis using MB231-pCDH and MB231-GPR109A cells with and without niacin. Following the treatment of cells with niacin, total RNA was isolated and the quality was analyzed using Agilent 2100 Bioanalyzer (Fig. S3A). Based on differentially expressed gene transcript, we generated a heat map for all four groups. As shown in Fig. S3B, there were no significant changes between MB231-pCDH untreated and niacin-treated samples. Similarly, there were very few changes between MB231-pCDH untreated and MB231-GPR109A untreated samples (Supplementary Table S2). However, we found that 1219 genes that were differentially expressed (≥ 2-fold) between MB231-GPR109A untreated and niacin-treated samples. As shown in Fig. 4A, we generated a heat map for 12 upregulated and 12 downregulated genes that are involved in cell cycle regulation and apoptosis. We also selected the top 100 highly up- and down-regulated genes and shown in Supplementary Tables S3 and S4. Further, we selected a few up- and down-regulated genes and validated the changes in the expression by qPCR analysis using gene-specific primers (Supplementary Table S1). We found that WEE1, IQGAP3, AVEN and PLK1, which are involved in cell cycle progression, were significantly downregulated in MB231-GPR109A cells treated with niacin and butyrate. Similarly, we also found that IRF6, IL24, RASSF2 and EGR2, which are involved in tumor suppression, were significantly upregulated in MB231-GPR109A cells treated with niacin and butyrate.
Figure 4.
GPR109A activation in breast cancer cells inhibits anti-apoptotic genes and induces pro-apoptotic genes. A, heat map generated for 12 genes that were up- and down-regulated in MB231-GPR109A cells in the presence and absence of nicotinate. B and C, qPCR confirmation of the changes in the expression of pro-survival and pro-apoptotic genes in control and GPR109A-expressing cells in the presence and absence of nicotinate or butyrate. Data are means ± SEM. *p<0.05; **p<0.01; ***p<0.001 by t-test.
GPR109A activation differentially regulates genes involved in cell cycle and DNA replication pathways in human breast cancer
We created a functional analysis map using the Ingenuity Pathway Analysis (IPA) program and found that genes which are involved in cell cycle, DNA replication, cell death and survival were differentially expressed between untreated and niacin-treated samples (Fig. S4A and C). Similarly, genes that are involved in solid tumor, epithelial neoplasia, breast cancer and tumor rejection were also differentially expressed between these two groups (Fig. S4B). All these data suggest that GPR109A activation specifically inhibits genes that are involved in cell cycle progression and anti-apoptosis in breast cancer cells.
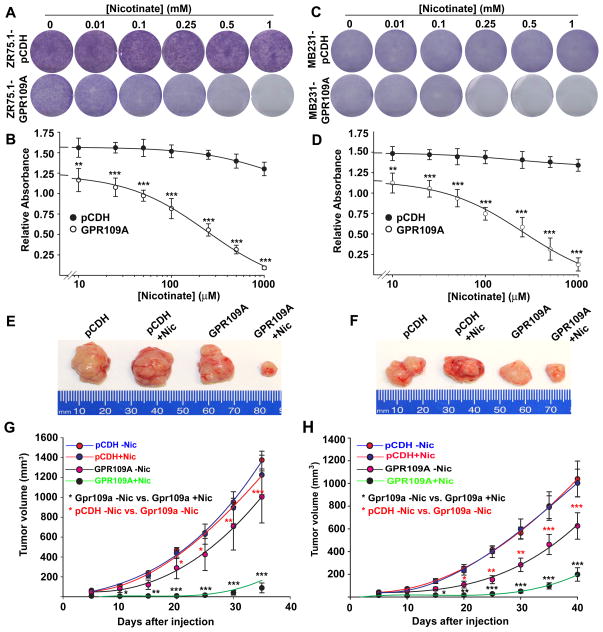
GPR109A expression blocks colony formation and tumor development in mouse xenografts
To confirm the above observations, we monitored the ability of GPR109A in inhibition of colony formation in these stable cells in the presence and absence of niacin. GPR109A expression alone inhibited colony formation significantly and a further dose-dependent inhibition of the colony formation in both ZR75.1-GPR109A and MB231-GPR109A cells when treated with niacin (Fig. 5A–D). However, niacin treatment did not affect the colony formation in control vector-transfected cells. This phenomenon was also confirmed in vivo in a mouse xenograft model. Mouse xenografts with control cells grew in a time-dependent manner and niacin, when administered in drinking water (10 mM), was unable to inhibit the growth of these tumor cells (Fig. 5E–H). In contrast, the tumor growth was significantly reduced in ZR75.1-GPR109A and MB231-GPR109A cells even in the absence of niacin treatment. But niacin treatment reduced tumor formation much more robustly in both cell lines (Fig. 5E–H). These results suggest that GPR109A activation in human breast cancer cells inhibits tumor growth.
Figure 5.
GPR109A activation in breast cancer cells inhibits cell survival and mammary tumor growth. A–D, control and GPR109A-expressing ZR75.1 and MB231cells were subjected to colony-formation assay in the presence or absence of nicotinate at various concentrations for 2 weeks and the resulting colonies were stained with Giemsa dye (A and C) and the bound Giemsa dye were dissolved and quantified by spectrophotometer analysis (B and D). E–H, two days before tumor induction, female athymic Balb/c nude mice (6 mice per group) were treated with and without nicotiante (10 mM in drinking water). Two days after nicotinate treatment, ZR75.1-pCDH and ZR75.1-GPR109A as well as MB231-pCDH and MB231-GPR109A cells (1×107 cells in 100 μl PBS) were injected subcutaneously in the mammary fat pad. Treatment with or without nicotinate continued throughout the experiment. Tumor volume was measured once in every 5 days for 30 days after cell injections (G and H). We compared tumor volume of pCDH-cells and GPR109A-expressing cells with and without nicotinate treatment and the statistical significance was calculated. At end of the experimental period, tumors were dissected and photographed. Representative images are shown (E and F). Data are means ± SEM. **p<0.01; ***p<0.001 by t-test.
As seen in Fig. 5A–H, re-expression of GPR109A alone significantly inhibited colony formation and mouse tumor growth. However, the transcriptome analysis between control vector and GPR109A-expressing MB231 cells did not show notable changes in the gene expression (Fig. S3B). To understand the discrepancy between this 2D cell culture and 3D colony and tumor growth, we performed a dropout experiment using the nicotinate (NA) binding assay. First, we analyzed the NA binding assay using the binding buffer (50 mM Tris-HCl, pH7.4 and 2 mM MgCl2) and L15 media and found that NA binding was significantly reduced in the presence of L15 media (Fig. S5A), indicating that a component present either in the cell culture medium or in the serum could be an agonist for GPR109A. To investigate this further, we repeated the binding assay using binding buffer and culture media with and without serum. Media alone, without serum, dramatically reduced the NA binding and addition of serum had only a moderate effect (Fig. S5B). This suggests the presence of a GPR109A agonist in the culture medium. We analyzed the formulation of both L15 and RPMI media and found that both media contain 1 mg/L niacinamide (NAM), as the source of niacin. Though NAM is not an agonist for GPR109A, it could be converted into niacin by hydrolysis and activate GPR109A when the cells are cultured for several day as in the colony formation assay. However, the transcriptome analysis was carried out in cells that were exposed for only 24 h in the cultured media. This could explain the discrepancy between the data obtained from the 2D cell culture and 3D colony and tumor growth experiments.
Deletion of Gpr109a in mice increases spontaneous mammary tumor development and provokes early onset mammary tumorigenesis
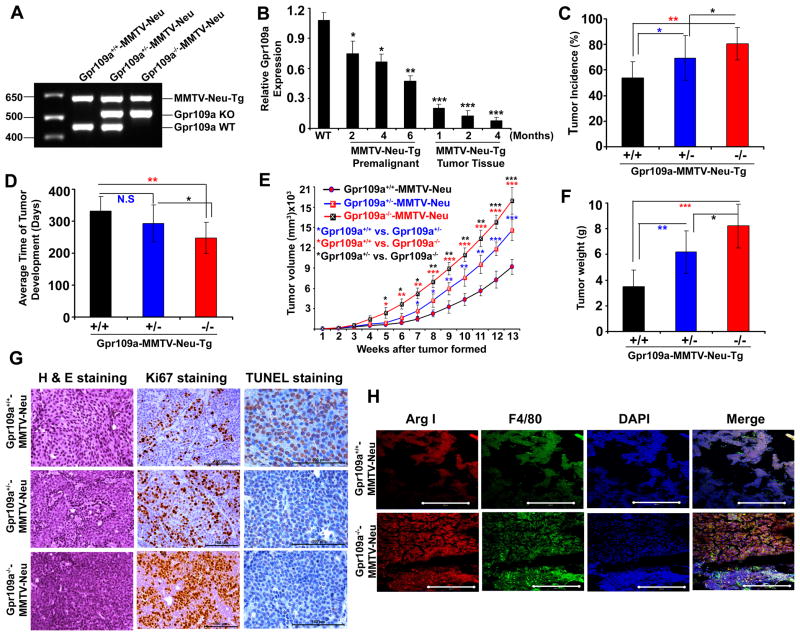
To investigate the tumor-suppressor function of GPR109A further, we crossed Gpr109a-null mice with MMTV-Neu transgenic mice and generated Gpr109a+/+-MMTV-Neu, Gpr109a+/−-MMTV-Neu and Gpr109a−/−-MMTV-Neu mice (Fig. 6A). To test whether loss of Gpr109a in mammary tumor development is an early event or occurs during later stages of tumor development, we analyzed Gpr109a expression in the mammary tissue and mammary tumor harvested from MMTV-Neu-Tg mice at different time points. As shown in Fig. 6B, MMTV-Neu transgene expression itself resulted in a significant reduction in Gpr109a expression. The expression of the receptor decreased further in premalignant mammary tissue and was almost undetectable in tumor tissues. We next monitored the tumor incidence, time of tumor appearance, tumor size and histological changes in all three Gpr109a-MMTV-Tg genotypes. As shown in Fig. 6C, 53% of Gpr109a+/+-MMTV-Neu, 69% Gpr109a+/−-MMTV-Neu and 80% of Gpr109a−/−-MMTV-Neu mice developed mammary tumors. Further, there was a significant decrease in the number of days needed for the onset of tumor formation in Gpr109a−/−-MMTV-Neu mice (Fig. 6D). We also measured the tumor volume and tumor tissue weight in all three genotypes. As shown in Fig. 6E and F, significantly increased tumor volume with corresponding increase in tumor weight were observed in Gpr109a−/−-MMTV-Neu mice than in wild type or heterozygous genotype mice. Interestingly, tumors developed in Gpr109a+/−- and Gpr109a−/− -MMTV-Neu mice were highly proliferative, more aggressive, and invasive (Fig. 6G). Ki67 and TUNEL staining showed a dramatically increased cell proliferation with correspondingly reduced apoptosis in tumors derived from Gpr109a+/−- and Gpr109a−/−-MMTV-Neu mice than those from wild type mice (Fig. 6G). All these results show that Gpr109a inactivation is associated with early onset of mammary tumor formation with aggressive tumor phenotype.
Figure 6.
Deletion of Gpr109a is associated with early onset of mammary tumor formation. A, Representative PCR image of genotype analysis of wild type and Gpr109a-knockout mice in MMTV-Neu-Tg background. B, Gpr109a expression was analyzed in wild type and MMTV-Neu-Tg mouse at different time of pre-malignant (2, 4 and 6 months) and different stages of tumor development (1, 2 and 4 months after the formation of the tumors. n=3 mice in each time point. Tumor incidence (C), time of tumor formation (D), tumor size (E), tumor weight (F), representative H & E histological sections (40x), Ki67 (40x) and TUNEL immunostaining (63x) (G) of Gpr109a+/+-, Gpr109a+/−- and Gpr109a−/− -MMTV-Neu-Tg mice. Data are means ± SEM (36 mice in each Gpr109a+/+-MMTV-Neu, Gpr109a+/−-MMTV-Neu and Gpr109a−/−-MMTV-Neu group). *p<0.05; **p<0.01; ***p<0.001 by t-test. H, Representative immunofluorescence (IF) images of tumor tissue sections from Gpr109a+/+- and Gpr109a−/− -MMTV-Neu-Tg mice probed for Arginase I (Arg I), F4/80 and DAPI. Images are shown at 40x magnification.
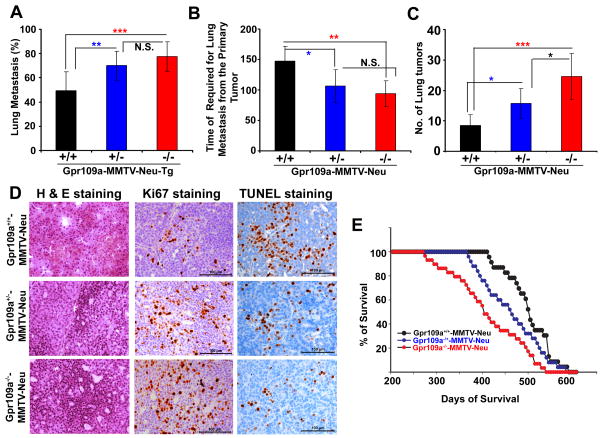
Deletion of Gpr109a in mice is associated with increased lung metastasis and reduced survival rate
Tumors developed in MMTV-Neu mice usually metastasize to the lung, and we found that about 48% of Gpr109a+/+-MMTV-Neu, 69% of Gpr109a+/−-MMTV-Neu and 76% of Gpr109a−/−-MMTV-Neu mice showed lung metastasis with an average of 147, 106 and 93 days, respectively, after the initial tumor formation at the primary site (Fig. 7A and B). Further, the number of tumor nodules in the lung also increased corresponding to the aggressiveness of the metastatic process (Fig. 7C). The histological sections from lung metastasis of Gpr109a−/−-MMTV-Neu mice showed evidence of more aggressive phenotype than the corresponding sections from Gpr109a+/+-MMTV-Neu mice (Fig. 7D). This was corroborated with Ki67 and TUNEL staining (Fig. 7D). The average survival of Gpr109a−/−-MMTV-Neu mice was significantly decreased (379 days) compared to Gpr109a+/−-MMTV-Neu mice (405 days) and Gpr109a+/+ -MMTV-Neu mice (495 days) (Fig. 7E). These observations suggest that GPR109A plays a crucial role in cellular homeostasis in the mammary epithelium and that inactivation of this gene is associated with early onset of mammary tumorigenesis with accelerated lung metastasis.
Figure 7.
Gpr109a deletion increased lung metastasis and reduced survival. The average percent of lung metastasis (A), average time required for lung metastasis from the onset of primary tumor (B), and average number of metastatic nodules (C) were monitored in Gpr109a+/+-, Gpr109a+/−- and Gpr109a−/−-MMTV-Neu-Tg mice. To determine the morphological changes, we also monitored the H & E histological sections, Ki67 and TUNEL staining in these three groups of mice (D). Images are shown at 40x magnification. The mean survival time for these mice was also monitored (E). Data are means ± SEM (36 mice each in Gpr109a+/+-, Gpr109a+/− and Gpr109a−/− -MMTV-Neu mice). **p<0.01; ***p<0.001 by t-test.
It is interesting to note that in spite of Gpr109a expression being almost undetectable even in primary tumors, Gpr109-knockdown is associated with increased lung metastasis. Though the molecular mechanism underlying this interesting observation is not understood, one possibility is that Gpr109a expressed in immune cells, especially in macrophages, could play a role in the regulation of tumor growth and progression. Further, macrophages serve as a source for many pro-angiogenic factors, including VEGF and IL-6, which contribute further to tumor growth (29). Tumor associated macrophages (M2) have also been shown to infiltrate a number of tumors and their number correlates with poor prognosis in human malignancies including breast cancer (30). Thus, to investigate this possibility, we analyzed macrophage expression in tumor tissue sections of Gpr109a+/+-MMTV-Neu-Tg and Gpr109a−/− -MMTV-Tg mice using Arginase I (Arg I) and F4/80 staining. As shown in Fig. 6H, Gpr109a−/−-MMTV-Tg mice showed relatively more macrophage infiltration (co-localization of Arg I and F4/80) than the respective control suggesting that Gpr109a plays a crucial role in regulation of macrophage infiltration thereby controlling tumor growth and metastasis.
Discussion
GPR109A is a newly discovered GPCR for niacin and butyrate. Niacin has long been known for its unique beneficial effect on lipid profiles in humans and it is one of the most effective drugs for lowering triglycerides with raising HDL levels (31). The role of niacin on lipid-modification and anti-atherosclerotic effects has been well documented. However, there is no conclusive evidence to support the role of niacin in prevention and/or suppression of human malignancies. In breast cancer, especially in postmenopausal breast cancer, diet and obesity are positively associated to the risk. Obesity is plausibly related to unfavorable lipid profiles, which have been linked to breast cancer. Several epidemiological studies have investigated the lipid profiles in the context of breast cancer and demonstrated possible associations between cholesterol and lipoprotein levels and breast cancer risk (32, 33). Previous studies have shown that a significantly higher total- and LDL-cholesterol, triglycerides and free fatty acids with decreased HDL-cholesterol in breast cancer cases than in the respective controls (34, 35). Similarly, studies have also shown that a low serum HDL level is an independent predictor of increased postmenopausal breast cancer risk, mainly because the low level of HDL is associated with increased levels of cancer-promoting hormones such as insulin, and IGF-I (36, 37). Thus, the lipid-lowering potential of niacin could have a great impact in suppression of breast cancer.
Similarly, butyrate is a naturally occurring fatty acid in the large intestine where it plays a critical role in inducing differentiation in colonic epithelial cells and maintaining their function through its ability to serve as a source of metabolic energy and to inhibit HDACs. Breast milk constitutes the primary source of butyrate in the mammary epithelium and butyrate plays an important role in induction of cell differentiation, growth arrest and apoptosis. Recently butyrate has been discovered as an agonist for GPR109A and butyrate-induced GPR109A signaling induces apoptosis in colon cancer cells (9). Further, the tumor-suppressor role of butyrate has been well documented and butyrate has been suggested as a candidate for protection against several human malignancies, including breast cancer. This suggests that niacin and butyrate have therapeutic potential for the treatment of human malignancies including breast cancer.
The present study describes a novel mode of action of niacin and butyrate in the mammary epithelium, where these two ligands activate GPR109A. The gene expression analysis in different tissues showed that mouse mammary gland expresses Gpr109a at levels comparable to those in spleen and intestine. We also found an abundant expression of GPR109A in human and mouse normal mammary tissues and in human immortalized normal mammary epithelial cell lines. However, GPR109A expression is silenced in human breast tumor tissues and cell lines and in three different mouse mammary tumor models. This suggests that GPR109A may function as a tumor suppressor in the mammary epithelium. Previous studies have also shown that GPR109A expression is silenced in human colon tumor tissues and cell lines and loss of function of GPR109A in human squamous cell carcinoma cell lines (9, 19). However, in keratinocytes GPR109A functions as a pro-survival and GPR109A activation leads to induction of Cox-2 expression (38). This tissue specific differential function of GPR109A is not known. However, in colon and mammary epithelium, GPR109A activation leads to induction of tumor suppressor and pro-apoptotic genes while in keratinocytes it activates pro-angiogenic signaling. Thus, depending on the cellular context, GPR109A functions as either a pro-survival or a tumor suppressor.
Our studies also show that DNA methylation-associated epigenetic mechanism is involved in GPR109A inactivation in breast cancer, and that treatment with DNMTs inhibitors re-activates GPR109A expression and induces apoptosis in the presence of its agonists. This observation is very intriguing because it suggests that use of niacin alone may not show significant efficacy in the treatment of breast cancer. This is true in three previous studies in which the association between niacin intake and disease progression was investigated in breast cancer patients, and no significant association was observed (39–41). This may be because GPR109A is silenced in breast cancer, and niacin has no target once the tumor is formed. However, the present studies show that it may be beneficial to use niacin along with DNMT inhibitors because the latter re-activate the niacin receptor in tumor tissues.
In our study, we found that GPR109A activation in human breast cancer cells inhibits several anti-apoptotic and pro-survival genes and induces several tumor suppressor and pro-apoptotic genes suggesting that GPR109A-induced apoptosis involves maintenance of low pro-survival and high pro-apoptotic gene expression in human breast cancer cells. Further, GPR109A activation in breast cancer cells blocks colony formation and mammary tumor growth in mice. Interestingly, deletion of Gpr109a in the MMTV-Neu transgenic mouse model of mammary tumor increases tumor incidence, provokes early onset of tumorigenesis and promotes lung metastasis. These data provide strong evidence that GPR109A functions as a tumor suppressor in vivo. Overall our study suggests that either physiological means to preserve the functional GPR109A or pharmacological means to re-activate this receptor could have potential in the prevention and treatment of breast cancer.
Supplementary Material
Acknowledgments
We thank Dr. Stefan Offermanns, Max-Planck-Institute, Germany, for the generous gift of Gpr109a-knockout mouse. We also thank the Georgia Regents University (GRU) Integrated Genomic Core for the microarray analysis, Flow Cytometry Core for FACS analysis, and the Histology and Pathology Core for histological sections. This work was supported by grants from the National Institute of Health (NIH) (R01 CA131402), Department of Defense (DOD) (BC074289) and a GRU Intramural Pilot Study grant.
Footnotes
Author contribution: M. T. designed experiments and analyzed data; S. E., R. P., S. R. and M. T. performed most of the experiments; S. A. performed GPR109A binding assays; P. M. M. performed immunohistochemical staining; R. N. P. performed pathway analysis; L. H. performed microarray analysis; S. E. and N. S. performed FACS analysis; S. E. and P. D. P. generated GPR109A-pCDH lenti-viral constructs and developed stable cell lines; L. L. performed statistical analysis; V. G. and M. T. wrote the manuscript.
All authors declare no conflict of interest.
References
- 1.Offermanns S. The nicotinic acid receptor GPR109A (HM74A or PUMA-G) as a new therapeutic target. Trends Pharmacol Sci. 2006;27:384–90. doi: 10.1016/j.tips.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Schaub A, Futterer A, Pfeffer K. PUMA-G, an IFN-gamma-inducible gene in macrophages is a novel member of the seven transmembrane spanning receptor super family. Eur J Immunol. 2001;31:3714–25. doi: 10.1002/1521-4141(200112)31:12<3714::aid-immu3714>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, Pfeffer K, Offermanns S. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med. 2003;9:352–55. doi: 10.1038/nm824. [DOI] [PubMed] [Google Scholar]
- 4.Wise A, Foord SM, Fraser NJ, Barness AA, Elshourbagy N, Eilert M, et al. Molecular identification of high and low affinity receptors for nicotinic acid. J Biol Chem. 2003;278:9869–74. doi: 10.1074/jbc.M210695200. [DOI] [PubMed] [Google Scholar]
- 5.Soga T, Kamohara M, Takasaki J, Matsumoto S, Ohishi T, Hiyama H, et al. Molecular identification of nicotinic acid receptor. Biochem Biophys Res Commun. 2003;303:364–69. doi: 10.1016/s0006-291x(03)00342-5. [DOI] [PubMed] [Google Scholar]
- 6.Kostylina G, Simon D, Fey MF, Yousefi S, Simon HU. Neutrophil apoptosis mediated by nicotinic acid receptors (GPR109A) Cell Death Differ. 2008;15:134–42. doi: 10.1038/sj.cdd.4402238. [DOI] [PubMed] [Google Scholar]
- 7.Benyo Z, Gille A, Kero J, Csiky M, Suchankiva MC, Nusing RM, et al. GPR109A (PUMA-G/HM74A) mediates nicotinic acid induced flushing. J Clin Invest. 2005;115:3634–40. doi: 10.1172/JCI23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maciejewski-Lenoir D, Richman JG, Hakak Y, Gaidarov I, Behan DP, Connolly DT. Langerhans cells release prostaglandin D2 in response to nicotinic acid. J Invest Dermatol. 2006;126:2637–46. doi: 10.1038/sj.jid.5700586. [DOI] [PubMed] [Google Scholar]
- 9.Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009;69:2826–32. doi: 10.1158/0008-5472.CAN-08-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cresci GA, Thangaraju M, Mellinger JD, Liu K, Ganapathy V. Colonic gene expression in conventional and germ-free mice with a focus on the butyrate receptor GPR109A and the butyrate transporter SLC5A8. J Gastrointest Surg. 2010;14:449–61. doi: 10.1007/s11605-009-1045-x. [DOI] [PubMed] [Google Scholar]
- 11.Martin PM, Ananth S, Cresci G, Roon P, Smith S, Ganapathy V. Expression and localization of GPR109A (PUMA-G/HM74A) mRNA and protein in mammalian retinal pigment epithelium. Mol Vis. 2009;15:362–72. [PMC free article] [PubMed] [Google Scholar]
- 12.Jung JK, Johnson BR, Duong T, Decaire M, Uy J, Gharbaoui T, et al. Analogues of acifran: agonists of the high and low affinity niacin receptors, GPR109a and GPR109b. J Med Chem. 2007;50:1445–48. doi: 10.1021/jm070022x. [DOI] [PubMed] [Google Scholar]
- 13.Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, et al. (D)-beta- Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem. 2005;280:26649–52. doi: 10.1074/jbc.C500213200. [DOI] [PubMed] [Google Scholar]
- 14.Gambhir D, Ananth S, Veeranan-Karmegam R, Elangovan S, Hester S, Jennings E, et al. GPR109A as an anti-inflammatory receptor in retinal pigment epithelial cells and its relevance to diabetic retinopathy. Invest Ophthalmol Vis Sci. 2012;53:2208–17. doi: 10.1167/iovs.11-8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bermudez Y, Benavente CA, Meyer RG, Coyle WR, Jacobson MK, Jacobson EL. Nicotinic acid receptor abnormalities in human skin cancer: implications for a role in epidermal differentiation. PLoS One. 2011;6:e20487. doi: 10.1371/journal.pone.0020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou L, Tang Y, Cryan EV, Demarest KT. Human epidermoid A431 cells express functional nicotinic acid receptor HM74a. Mol Cell Biochem. 2007;294:243–48. doi: 10.1007/s11010-006-9150-6. [DOI] [PubMed] [Google Scholar]
- 17.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–19. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 18.Owen OE, Felig P, Morgan AP, Wahren J, Cahill GF., Jr Liver and kidney metabolism during prolonged starvation. J Clin Invest 1969. 1969;48:574–83. doi: 10.1172/JCI106016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glass RL, Jenness R, Lohse LW. Comparative biochemical studies of milks. V. The triglyceride composition of milk fats. Comp Biochem Physiol. 1969;28:783–86. doi: 10.1016/0010-406x(69)92113-6. [DOI] [PubMed] [Google Scholar]
- 20.Smith LM, Hardjo S. Fatty acid composition of monkey milk lipids. Lipids. 1974;9:674–78. doi: 10.1007/BF02532174. [DOI] [PubMed] [Google Scholar]
- 21.Ochoa-Zarzosa A, Villarreal-Fernandez E, Cano-Camacho H, Lopez-Meza JE. Sodium butyrate inhibits Staphylococcus aureus internalization in bovine mammary epithelial cells and induces the expression of antimicrobial peptide genes. Microb Pathog. 2009;47:1–7. doi: 10.1016/j.micpath.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Sowa Y, Sakai T. Butyrate as a model for “gene-regulating chemoprevention and chemotherapy. Biofactors. 2000;12:283–87. doi: 10.1002/biof.5520120142. [DOI] [PubMed] [Google Scholar]
- 23.Heerdt BG, Houston MA, Anthony GM, Augenlicht LH. Initiation of growth arrest and apoptosis of MCF-7 mammary carcinoma cells by tributyrin, a triglyceride analogue of the short-chain fatty acid butyrate, is associated with mitochondrial activity. Cancer Res. 1999;59:1584–91. [PubMed] [Google Scholar]
- 24.Belobrajdic DP, McIntosh GH. Dietary butyrate inhibits NMU induced mammary cancer in rats. Nutr Cancer. 2000;36:217–23. doi: 10.1207/S15327914NC3602_11. [DOI] [PubMed] [Google Scholar]
- 25.DeFazio A, Chiew YE, Donoghue C, Lee CSL, Sutherland RL. Effect of sodium butyrate on estrogen receptor and epidermal growth factor receptor gene expression in human breast cancer cell lines. J Biol Chem. 1992;267:18008–12. [PubMed] [Google Scholar]
- 26.Horwitz KB, Mockus MB, Lessey BA. Variant T47D human breast cancer cells with high progesterone-receptor levels despite estrogen and antiestrogen resistance. Cell. 1982;28:633–42. doi: 10.1016/0092-8674(82)90218-5. [DOI] [PubMed] [Google Scholar]
- 27.Graham KA, Buick RN. Sodium butyrate induces differentiation in breast cancer cell lines expressing the estrogen receptor. J Cell Physiol. 1988;136:63–71. doi: 10.1002/jcp.1041360108. [DOI] [PubMed] [Google Scholar]
- 28.Elangovan S, Ramachandran S, Venkatesan N, Ananth S, Gnana-Prakasam JP, Martin PM, et al. SIRT1 is essential for oncogenic signaling by estrogen/estrogen receptor α in breast cancer. Cancer Res. 2011;71:6654–64. doi: 10.1158/0008-5472.CAN-11-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin EY, Li JF, Gnatovskiy L, Deng, Grzesik DA, Qian H, Xue XN, Pollard JW. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–46. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 30.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–65. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 31.Pike NB. Flushing out the role of GPR109A (HM74A) in the clinical efficacy of nicotinic acid. J Clin Invest. 2005;115:3400–03. doi: 10.1172/JCI27160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ray G, Husain SA. Role of lipids, lipoproteins and vitamins in women with breast cancer. Clin Biochem. 2001;34:71–6. doi: 10.1016/s0009-9120(00)00200-9. [DOI] [PubMed] [Google Scholar]
- 33.Hoyer AP, Engholm G. Serum lipids and breast cancer risk: a cohort study of 5207 Danish women. Cancer Causes Control. 1992;3:403–8. doi: 10.1007/BF00051352. [DOI] [PubMed] [Google Scholar]
- 34.Gaard M, Tretli S, Urdal P. Risk of breast cancer in relation to blood lipids: a prospective study of 31,209 Norwegian women. Cancer Causes Control. 1994;5:501–9. doi: 10.1007/BF01831377. [DOI] [PubMed] [Google Scholar]
- 35.Thangaraju M, Kumar K, Gandhirajan R, Sachdanandam P. Effect of tamoxifen on plasma lipids and lipoproteins in postmenopausal women with breast cancer. Cancer. 1994;73:659–63. doi: 10.1002/1097-0142(19940201)73:3<659::aid-cncr2820730325>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 36.Furberg AS, Veierod MB, Wilsgaard T, Bernstein L, Thune I. Serum high-density lipoprotein cholesterol, metabolic profile, and breast cancer risk. J Natl Cancer Inst. 2004;96:1152–60. doi: 10.1093/jnci/djh216. [DOI] [PubMed] [Google Scholar]
- 37.Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393–96. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 38.Hanson J, Gille A, Zwykiel S, Lukasova M, Clausen BE, Ahmed K, Tunaru S, Wirth A, Offermanns S. Nicotinic acid- and monomethyl fumarate-induced flushing involves GPR109A expressed by keratinocytes and COX-2-dependent prostanoid formation in mice. J Clin Invest. 2010;120:2910–19. doi: 10.1172/JCI42273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levi F, Pasche C, Lucchini F, La Vecchia C. Dietary intake of selected micronutrients and breast-cancer risk. Int J Cancer. 2001;91:260–63. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1041>3.3.co;2-r. [DOI] [PubMed] [Google Scholar]
- 40.Kabat GC, Miller AB, Jain M, Rohan TE. Dietary intake of selected B vitamins in relation to risk of major cancers in women. Br J Cancer. 2008;99:816–21. doi: 10.1038/sj.bjc.6604540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shrubsole MJ, Shu XO, Li HL, Cai H, Yang G, Gao YT, Gao J, Zheng W. Dietary B vitamin and methionine intakes and breast cancer risk among Chinese women. Am J Epidemiol. 2011;173:1171–82. doi: 10.1093/aje/kwq491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.