Abstract
Depression is a debilitating mental disease affecting a large population worldwide, the pathophysiological mechanisms of which remain incompletely understood. Prenatal infection and associated activation of the maternal immune system (MIA) are prominently related to an increased risk for the development of several psychiatric disorders including schizophrenia and autism in the offsprings. However, the role of MIA in the etiology of depression and its neurobiological basis are insufficiently investigated. Here we induced MIA in mice by challenge with polyinosinic:polycytidylic phosphate salt—a synthetic analog of double-stranded RNA, which enhances maternal levels of the cytokine interleukin-6 (IL-6)—and demonstrate a depression-like behavioral phenotype in adult offsprings. Adult offsprings additionally show deficits in cognition and hippocampal long-term potentiation (LTP) accompanied by disturbed proliferation of newborn cells in the dentate gyrus and compromised neuronal maturation and survival. The behavioral, neurogenic and functional deficiencies observed are associated with reduced hippocampal expression of vascular endothelial growth factor (VEGF)A-VEGFR2. IL-6-STAT3-dependent aberrant VEGFA-VEGFR2 signaling is proposed as neurobiological mechanism mediating the effects of MIA on the developing fetal brain and ensuing consequences in adulthood.
Keywords: depression, hippocampal neurogenesis, maternal immune activation, VEGF
Introduction
Early life events are reported to have a profound effect on the physiological and psychological development of an organism. Evidence in literature suggests that traumatic events during early stages of life, including the pre- and perinatal period are associated with an enhanced risk for psychiatric morbidities later in life.1, 2, 3 Among the multiple potential early life adversities, several lines of evidence suggest infections during pregnancy as a powerful factor contributing to the development of mental illnesses in adulthood.4, 5, 6
Major depressive disorder is a highly complex mental illness affecting a large percentage of the population worldwide,7 the underlying neurobiological mechanisms of which remain poorly understood. Although several brain structures are known to be involved in the neural circuitry of depression, the role of the hippocampus has gained considerable attention over the past 20 years.8,9 Rodent models of depression demonstrate a reduction in adult hippocampal neurogenesis,10 dendrite reorganization11 and structural rearrangement of the hippocampus.9,12 Moreover, the ‘neurogenesis hypothesis of depression' states that the pathophysiology of depression is related to alterations in adult hippocampal neurogenesis, which can be bidirectionally modulated by exposure to stress and antidepressant treatment.13,14
The search for the underlying molecular mechanisms have provided evidence for an involvement of altered neurotrophic and growth factor in the neurogenic deficiencies associated with depression.15 Here, vascular endothelial growth factors (VEGF) have recently become a focus of investigation based upon a series of studies indicating a neuroprotective effect of VEGF and its contribution to adult hippocampal neurogenesis.16 Conversely, pro-depressogenic factors, such as exposure to various type of stress, including immune stress, are known to affect expression of VEGF and other neurotrophic factors, thereby contributing to the development of depression.17
Although prenatal social stress has been tightly linked to the suppression of hippocampal neurogenesis18,19 and subsequent behavioral20 and cognitive21 dysfunctions related to depression in adulthood, a possible involvement of immune stress resulting from maternal infections during gestation has not been systematically investigated in this context. Excellent mouse models of maternal immune activation (MIA) have been previously described, where an induction of a specific set of cytokines in the maternal and fetal compartments22 leads to endocrine changes in the placenta23 and subsequent alterations in fetal brain development.24,25 For this study, we have employed this mouse model to address the role of MIA in the subsequent development of depression-like behavior and to examine its morphological, functional and molecular correlates in the hippocampus of adult offsprings.
Materials and methods
Animals
C57BL/6 N mice used for the study were purchased from Charles River, Sulzfeld, Germany. All behavioral studies, neurogenesis analysis, gene expression analysis and electrophysiological studies were carried out on adult male offsprings from maternal immune activation with polyinosinic:polycytidylic phosphate salt at embryonic day 12.5 (PIC) and age-matched control animals. Animals were housed under standard conditions and all animal procedures were approved by the Austrian Ethics committee for animal testing (GZ 66.009/268-II/3b/2011).
Breeding
Female mice (10–14 weeks) were subjected to previously described timed mating procedures.26 Female mice were housed in groups of five animals per cage for 7 days. The bedding of female cages was replaced with used bedding from male animals for a 48h period. At the end of this time, each female was introduced to a single housed male 1 h before the onset of the dark period of the light cycle. The development of vaginal plugs was ascertained the following morning within 1 h of the onset of the light phase of the light cycle, and confirmation of a vaginal plug at this time was denoted at embryonic day 0.5 (E0.5).27
Behavioral studies
Animals were habituated to the experimental room for 30 min before the start of behavioral experiments. Mice underwent a battery of behavioral tests prior to depression evaluation.
Sucrose preference test
Sucrose preference testing followed a previously described protocol.28 Sucrose preference was calculated as a percentage of liquid consumption during the 3 h testing period.
Forced swim test
Forced swim test was modified from previous studies.28 Mouse behavior patterns were tracked by VIDEOTRACK (PORSOLT) software provided (Viewpoint, Champagne au mont d'Or, France). The test had a total duration of 6 min and the last 4 min of the test were used for the analysis of immobility.
Morris water maze (MWM)
MWM followed a set up previously described.29 The spatial acquisition phase consisted of three trials per day (120 s each) over the course of 3 days. Using distal cues, mice had to find a submerged platform (in the target quadrant) in the opaque testing pool. The testing pool was made opaque by nontoxic white paint (Viewpoint). Mice were tracked from above the experimental setup by a camera coupled to computational tracking software (Videotrack [02-WATERMAZE-NSHD-LBW] Viewpoint). Probe trial consisted of one session (60 s) without the submerged platform. Time spent in the target quadrant as compared with the time spent in the other three quadrants was evaluated for the first 15 s of the probe trial.
Open field
Locomotor activity was monitored by a computational tracking system (Activity Monitor, MedAssociates, St Albans, VT, USA). Total distance covered in the 60min testing time was recorded using a protocol previously described.29
Rota rod
Rota rod (USB Rota Rod ‘SOF-ENV-57X', Medassociates) test was performed in triplicate for each mouse at 5 min sessions in order to evaluate motor coordination following a published procedure.29
Maternal immune activation
Polyinosinic:polycytidylic phosphate salt (Poly(I:C) (Sigma, Vienna, Austria) and vehicle control (0.9% NaCl) were administered to pregnant dams at embryonic day 12.5. The 20-mg kg−1 dose of Poly(I:C) was calculated based on the weight of Poly(I:C) in the mixture itself.23 The volume of injections was held at 10 ml kg−1. All injections were administered intraperitoneally.
Cytokine response analysis
Plasma collection
Mice were injected with Poly(I:C) 20 mg kg−1 (PIC stimulated) or NaCl (Control) and were deeply anesthetized after 3 h using a ketamine/xylazine cocktail (100 mg per 10 mg intraperitoneally; Ketasol, Graeub Veterinary Products, Bern, Switzerland/Rompun, Bayer, Bayer Animal Health, Leverkusen, Germany). Transcardial blood collection was performed using a 20-G needle, and the blood was transferred to autoclaved Eppendorf tubes containing heparin solution (working stock 1000 U ml−1; 7.5 U heparin per 200 ml blood) over ice. Blood samples were then centrifuged for 5 min at 14 000 r.p.m. at 4 °C. Plasma volume was recorded and isolated plasma was transferred to a fresh Eppendorf tube to be stored at -80 °C until further analysis.
Enzyme-linked immunosorbent assay analysis
Mouse interleukin (IL)-6 ELISA Ready-SET-Go! kit (eBioscience, Vienna, Austria) was used for enzyme-linked immunosorbent assay. All procedures followed the manufacturer's suggestions. IL-6 concentrations in the plasma were calculated based on standard curve analysis using the standards provided in the kit.
Neurogenesis analysis
Two injection paradigms were employed for neurogenesis analysis in the proliferation and survival paradigm.30 Upon deep anesthesia by a ketamine/xylazine cocktail (100 mg per 10 mg intraperitoneally; Ketasol, Graeub Veterinary products/ Rompun, Bayer), transcardial in situ perfusions were performed using 4% paraformaldehyde solution made in 0.1 M phosphate-buffered saline. Modified 5-bromodeoxyuridine (BrdU) immunofluorescence chemistry protocols were employed31 and free-floating sections were cut on a cryostat and collected in cryoprotective solution (30% glycerol, 30% ethylene glycol in 0.1 M phosphate-buffered saline; pH 7.4). Every 10th free-floating section of the entire rostro-caudal span of the hippocampus was used for evaluation (n=5–6 animals per group). Double labeling protocols for astrocytes and mature granular neurons were followed as previously described.32
Immunofluorescence
Doublecortin (DCX) immunofluorescence chemistry protocols were used as previously published.33 Double staining of BrdU/neuronal marker (BrdU+/NeuN+)31,32,34 and BrdU/glial fibrillary acidic protein (BrdU+/GFAP+)31,32,35 analysis was carried out according to published studies.
Quantification of BrdU+, BrdU+/DCX+, BrdU+/GFAP+ and BrdU+/NeuN+ immunofluorescence
For the proliferation paradigm, quantification included all BrdU-labeled cells in the subgranular zone of the dentate gyrus (DG). All quantification of BrdU+ was performed blind to experimental conditions. For the survival paradigm, all double-positive cells within 50 μm of the DG were additionally quantified in order to account for cell migration. Fluorescence microscopy pictures were taken on Carl-Zeiss Axiovert-Apotome System (Oberkochen, Germany) coupled to Axiovision software version 4.8.
Electrophysiological recordings
Long-term potentiation (LTP) and short-term presynaptic-dependent facilitation were evaluated on hippocampal slices. All procedures for the hippocampal slice preparation and electrophysiological recordings followed previously published protocols with minor modifications.29 Analysis of data was done by an AxoClamp-2B amplifier (Bridge mode) and a Digidata-1440 interface and the pClamp-10 Program software (Axon Instruments, Foster City, CA, USA; Molecular Devices Germany, Biberach an der Riss, Germany).
Gene expression analysis
Brain dissection
Subjects were killed by neck dislocation and brains were rapidly dissected over ice. Isolated hippocampal tissues were stored in RNA later (Ambion, Vienna, Austria) at -20 °C until further processing.
Real-time PCR (quantitative reverse transcriptase-PCR)
Hippocampal RNA was isolated using miRNeasy kit (Qiagen, Hilden, Germnay) according to the manufacturer's instructions. Nine hundred nanograms of total RNA was used for complementary DNA synthesis following the manufacturer's instructions provided with Moloney Murine Leukemia Virus reverse-transcriptase first-strand complementary DNA synthesis kit, G1 (Biozym, Vienna, Austria). Complementary DNA reaction mix (1:5 dilution) was used for PCR amplification using the Fast SYBR Green Mastermix (Applied Biosystems, Foster City, CA, USA) on a StepOnePlus realtime PCR system (Applied Biosystems, serial number 271000455). Target genes were normalized to ß-actin. All primer sequences are listed in Supplementary Materials (Supplementary Table 1).
Statistical analysis
For analysis of the acquisition phase of the MWM and LTP analysis, mixed-model repeated-measures analysis of variances were used with acquisition days and percentage of slope change, respectively, as the repeated measure (within-subject factor) and treatment as between-subject factor. For behavioral tests, neurogenesis analysis and quantitative reverse transcriptase-PCR data, Student's t-tests were carried out. Results were considered significant when P-values were <0.05. All statistical analyses were performed using SPSS software, for windows, Version 19 (IBM corporation, Chicago, IL, USA).
Results
MIA induces depression-like behavior in adult offsprings
To test the hypothesis that MIA induces depression later in life, we used a previously described MIA mouse model.24,25 To ascertain immune activation resulting from Poly(I:C) treatment in the present study, IL-6 levels were assessed in the serum of the dam. A significant, more than 1000-fold over control levels increase in circulating IL-6 was measured 3 h after Poly(I:C) challenge (Supplementary Figure 1).
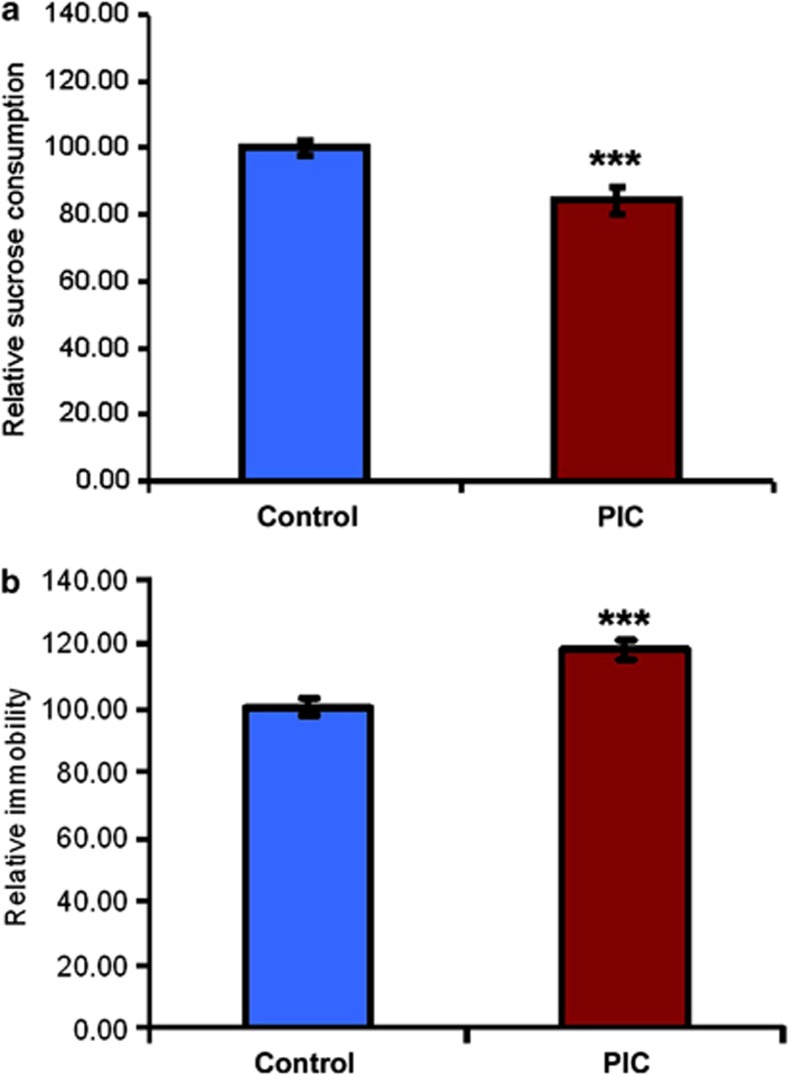
Male adult offsprings from pregnant dams injected with Poly(I:C) at embryonic day 12.5 (PIC) and control animals were subjected to two standard paradigms assessing depression-like behavior in mice, the sucrose preference test for evaluation of anhedonic behavior and the forced swim test, measuring behavioral despair. A highly significant reduction of sucrose preference in the sucrose preference test was observed in PIC compared with control mice (P<0.001; Figure 1a). The forced swim test analysis revealed significantly heightened levels of behavioral despair-related immobility in PIC male offsprings (P<0.001; Figure 1b).
Figure 1.
Maternal immune activation (MIA) at embryonic day 12.5 (PIC) increases depression-like behavior in adulthood. (a) Sucrose preference test to measure anhedonic behavior. (b) Behavioral despair analysis by the forced swim test. Controls (n=34) and PIC (n=20) mice. Data represent mean±s.e.m. of PIC relative to control mice. ***P<0.001.
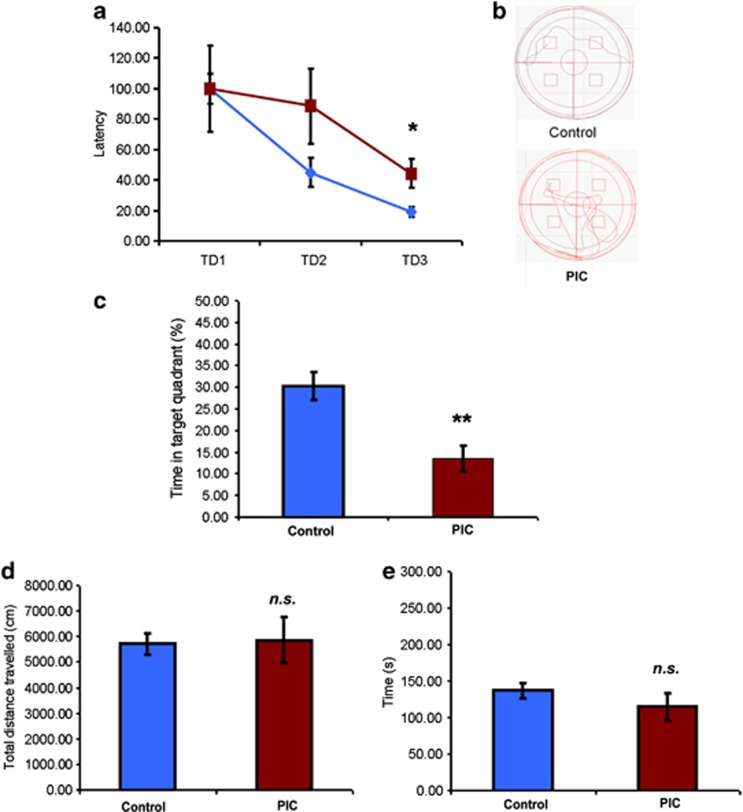
MIA induces cognitive deficits and impairs LTP and paired pulse facilitation in hippocampal slices but does not alter general behavioral function
In order to examine whether depression-like behavior in PIC mice was paralleled by cognitive deficits, as frequently observed in depressed patients, hippocampal-dependent learning and memory was tested using the MWM task and ex vivo correlates were examined using electrophysiological analysis of hippocampal slices. During the training phase, the latency over the training days 1, 2 and 3 (TD1, TD2 and TD3, respectively) to find the hidden platform was longer in PIC mice (significant treatment × time point interaction F(2,48)=4.05, P<0.05) with a significant effect on TD3 (P<0.05), indicative of deficient spatial learning capabilities (Figures 2a and b). During the probe trial, assessing hippocampal-dependent memory, PIC mice spent significantly less time (P<0.01) in the target quadrant, which had contained the platform during the training phase (Figure 2c). To control for possible unspecific effects of MIA on performance in the MWM, the open field test, examining exploratory and locomotor activity as well as the rota rod, for the evaluation of motor coordination were carried out. No significant differences in total distance traveled in the open field (Figure 2d) or latency on the rota rod (Figure 2e) were observed between groups.
Figure 2.
Maternal immune activation (MIA) induces cognitive deficits but does not alter general behavioral function. Cognitive evaluation of adult PIC mice using the Morris water maze (MWM) task. (a) Latencies in the acquisition phase of MWM of PIC (red) relative to controls (blue). (b) Sample tracking pathways during the last training day (TD3). Target platform in top left quadrant. (c) Percentage of time spent in the target quadrant during the first 15 s of the probe trial. (d) Locomotor activity evaluated by the total distance traveled in the open field. (e) Motor coordination evaluation using the rota rod. n=10–34 per group. Data represent mean±s.e.m. *P<0.05, **P<0.01, n.s. not significant.
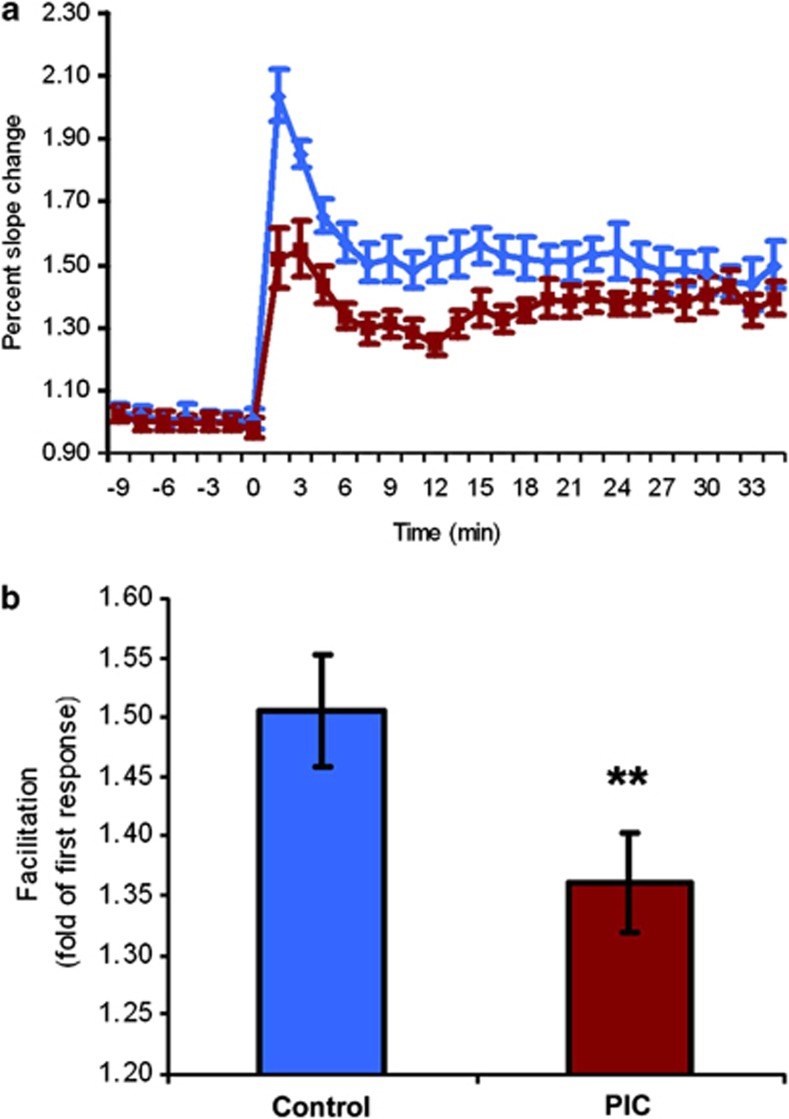
In order to corroborate that the findings obtained in the MWM indeed relate to altered hippocampal function, LTP and short-term presynaptic-dependent facilitation were evaluated in hippocampal slices of PIC and control mice. A mixed-model repeated-measure analysis of variance revealed deficient LTP in the hippocampal slices of PIC mice (significant main effect of treatment F(2,32)=17.90, P<0.001, significant treatment × repeat interaction F(2,32)=4.56, P<0.001; Figure 3a), indicative of altered postsynaptic activity. In addition, aberrant presynaptic functionality was suggested by significantly reduced paired pulse facilitation in hippocampal slices of PIC mice (P<0.01; Figure 3b).
Figure 3.
Maternal immune activation (MIA) impairs long-term potentiation (LTP) and paired pulse facilitation (ppF) in hippocampal slices. (a) Temporal course of percent slope change of field excitatory postsynaptic potentials (fEPSP) in hippocampal slices differed significantly between PIC (red) and controls (blue) as evaluated by mixed-model repeated-measures analysis of variance (ANOVA). (b) ppF as fold change from first response. n=16 per group. Data represent mean±s.e.m. **P<0.01.
MIA compromises adult hippocampal neurogenesis and affects differentiation of neuronal cells in the hippocampal DG
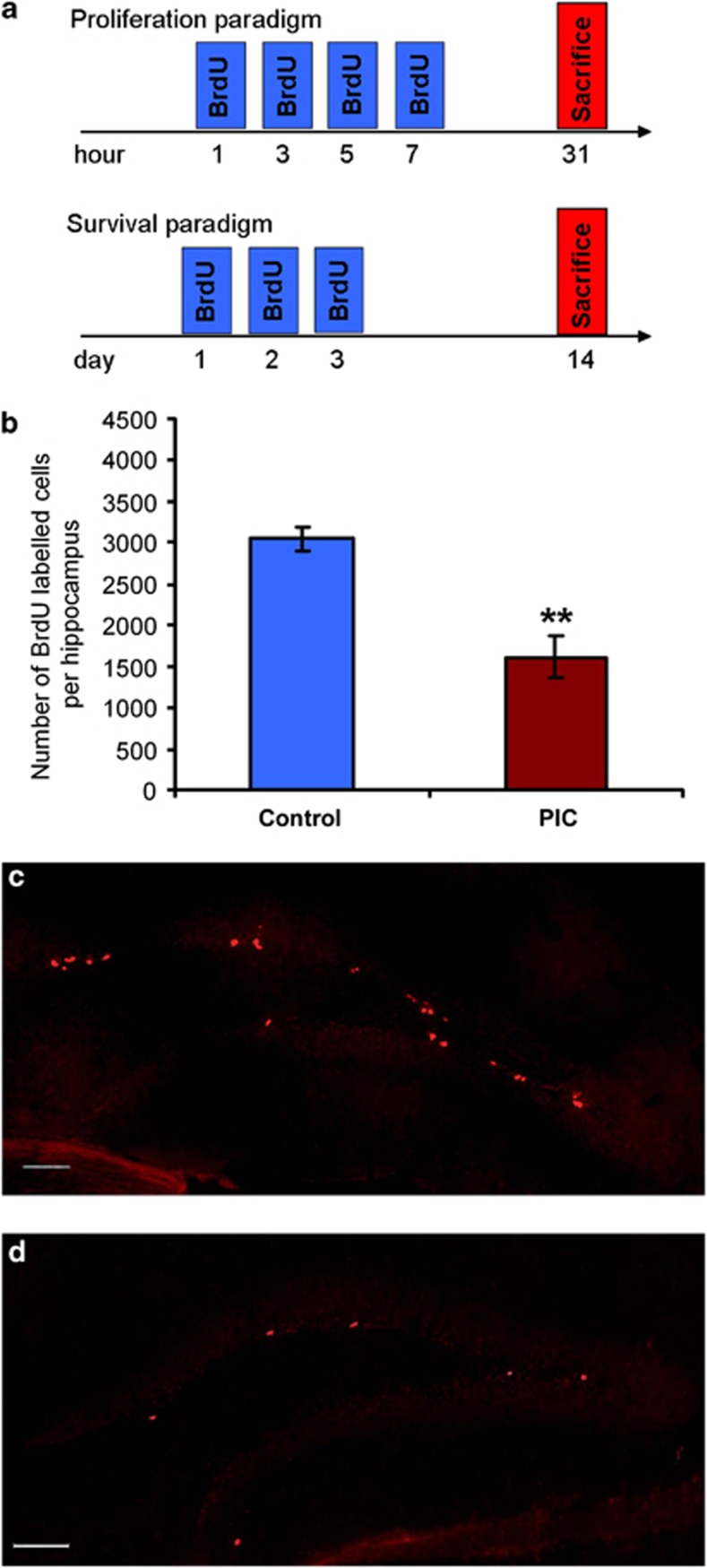
As a next step, we set out to examine potential neurobiological correlates of the observed depression-like behavioral phenotype at the cellular level. To this end, we focused on the analysis of cell proliferation in the subgranular zone of the hippocampal DG, highly implicated in the pathophysiology of depression and response to antidepressant treatment.10,36 Adult hippocampal neurogenesis was evaluated using two standard paradigms assessing proliferation and survival of newly born cells using BrdU labeling (Figure 4a). A significant reduction of BrdU-labeled cells 24 h after injection (BrdU+) was measured in the hippocampus of PIC mice, indicating deficient hippocampal cell proliferation (Figures 4b–d).
Figure 4.
Maternal immune activation (MIA) decreases proliferation of newborn cells in the dentate gyrus (DG) of the hippocampus. (a) Schematic representation of 5-bromodeoxyuridine (BrdU) injection protocols for proliferation and survival paradigms. (b) Approximated number of BrdU-labeled cells per hippocampus in the proliferation paradigm. Sample fluorescence microscope images (20 × ) of BrdU-labeled cells (red) show differences in proliferation between (c) control and (d) PIC mice. n=5–6 animals per group. Scale bars denote 100 μm. Data represent mean±s.e.m. **P<0.01.
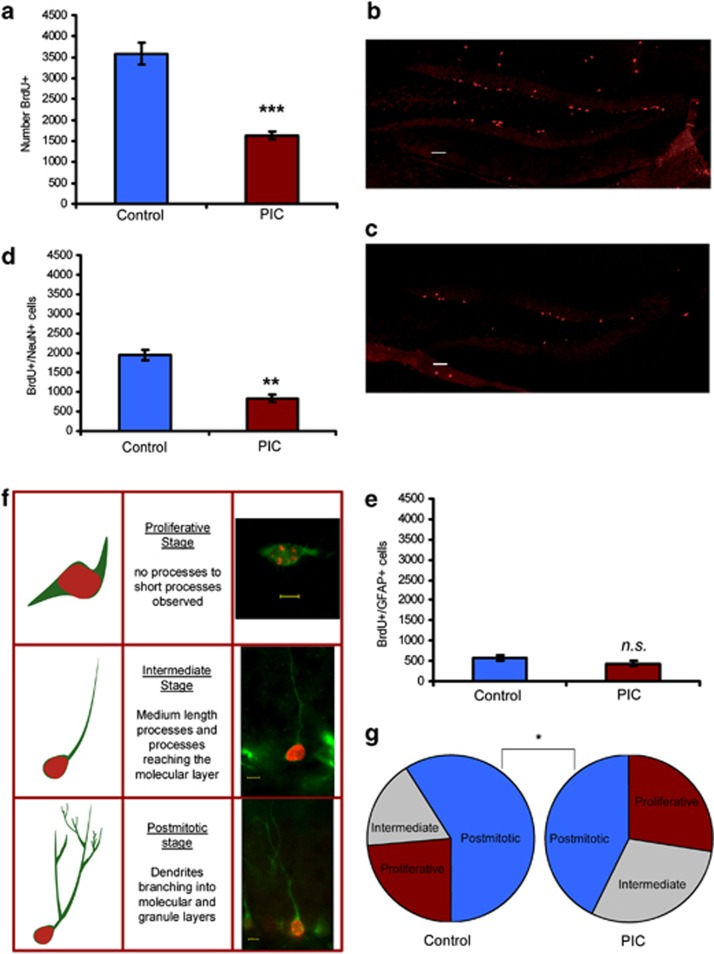
Quantification of BrdU+ cells 2 weeks after the first BrdU injection in the ‘survival paradigm' revealed a significant decrease of surviving BrdU+ cells in the hippocampus of PIC mice (P<0.001; Figures 5a–c). Next, we aimed to determine whether the observed findings in the survival paradigm could be explained by an effect of MIA on the process of differentiation into neuronal cells or an impact on survival of mature neurons. To this end, double labeling experiments with the mature granular neuronal marker, NeuN (BrdU+/NeuN+) and the astrocytic marker GFAP (BrdU+/GFAP+) were carried out. Quantification of double-labeled cells revealed a significant reduction of surviving mature neurons, indicated by a significantly lower number of BrdU+/NeuN+labeled cells in the PIC hippocampus (P<0.01) compared with control hippocampus (Figure 5d), but no differences in the number of BrdU+/GFAP+ cells (Figure 5e). In order to understand at which developmental stage MIA affected the differentiating neurons, we examined the relative distributions of newly formed cells within three major stages of development. A characterization scheme by morphological criteria (Figure 5f) during the DCX period, using BrdU-DCX double-labeling (BrdU+/DCX+) was employed.37 A significant and selective reduction (P<0.05) of BrdU+/DCX+ cells in the postmitotic stage was observed (Figure 5g).
Figure 5.
Maternal immune activation (MIA) reduces survival of new born cells and affects differentiation morphology of neuronal cells in the dentate gyrus (DG) of the hippocampus. Total number of 5-bromodeoxyuridine (BrdU)-labeled cells in the DG. (a) Quantification of surviving BrdU-positive (BrdU+) cells show significantly lower number of surviving cells in PIC mice. Sample images (20 × ) depict a significant difference in the number of surviving BrdU-labeled cells between (b) control and (c) PIC animals. (d) Schematic and sample pictures (63 × ) illustrating differentiation analysis by morphological criteria using doublecortin (DCX) staining. (e) Percentages of BrdU+ cells in different stages of developmental morphology during the DCX period. (f) PIC animals show significantly lower number of BrdU+/NeuN+ cells. (g) No differences observed in BrdU+/glial fibrillary acidic protein (GFAP)+ cells. n=5-6 animals per group. Gray scale bars denote 50 μm, yellow scale bars denote 5 μm. Data represent mean±s.e.m. *P<0.05, **P<0.01, ***P<0.001, n.s. not significant.
MIA leads to changes in neurotrophic factor expression patterns in the hippocampus of adult offsprings
To examine whether the observed behavioral and functional deficiencies resulting from MIA may be related to insufficient trophic support, we examined the expression of VEGFs and their respective receptors in hippocampal tissue of PIC and control mice. Quantitative reverse transcriptase-PCR analysis revealed a significant decrease in mRNA levels of VEGFA (P<0.05; Supplementary Figure 2a) and its receptor VEGFR2 (P<0.01; Supplementary Figure 2b) in PIC hippocampal tissue, whereas no significant changes in the expression of VEGFB, VEGFR1, VEGFC, VEGFR3 or VEGFD were observed (Supplementary Figures 2c and g).
Discussion
Maternal immune activation (MIA) during gestation is known to cause important, irreversible changes in the fetal brain38, 39, 40, 41 that may subsequently lead to behavioral alterations related to various psychopathologies in adulthood.22,24,42,43 Although there has been a consistent association between MIA and an enhanced risk of schizophrenia in the adult offsprings (reviewed in Brown and Susser44), this association is less clear with regard to the offspring's chances for developing affective disorders45 as those few specific epidemiological studies tackling the topic have found contrasting results.46,47 Why is this so? To address this question, it has to be considered that an individual's genetic makeup may determine not only their vulnerability to the impact of adverse (early) life events (including maternal infection), but may also define the specific psychopathology to which each individual is rendered susceptible as a result of this early environmental impact. Thus, it may be speculated that the relative paucity of epidemiological studies analyzing the role of MIA in mood disorder development as opposed to schizophrenia relates to a more than 80% association with heritability for schizophrenia cases as opposed to ~40% for major depression48,49 and, that, therefore, genetic contributions to the pathogenesis of schizophrenia are greater than to depression. Consequently, larger study pools with defined genetic and environmental risk factors are required to comprehensively examine the etiology of depression in epidemiological analysis.50 Accordingly, the complexity of interaction between genetic and environmental factors contributing to the pathogenesis of depressive disorders renders the need for reductionist animal models even more apparent. We herein present such a model by specifically elucidating the contribution of a MIA as one single etiological component and describing its impact on behavioral, cellular and molecular characteristics commonly associated with depression.
Using this model, we provide evidence for a role of MIA as an environmental risk factor contributing to emotional disturbances related to depression later in life, as augmented anhedonic states together with an increase in behavioral despair are observed in PIC offsprings. The specific relevance of the observed alterations in emotional behaviors to depressive disorders is supported by our findings on deficient hippocampal-dependent cognitive abilities in PIC offsprings, as evidenced by the impaired performance in the MWM. Compromised cognitive capacities are an ubiquitous characteristic in human patients diagnosed with major depressive disorder and have been suggested to be largely resulting from improper hippocampal functionality.51 Hippocampal dysfunction as neurobiological correlate of the observed cognitive deficit is further supported by results of the ex vivo electrophysiological studies, reporting deficient LTP and dysfunctional presynaptic activity in hippocampal slices of PIC offsprings. Interestingly, paralleling our observations on the effects of early immune stress, early psychosocial stress has been reported to lead to impaired performance in the MWM and attenuated hippocampus-dependent LTP in rodents.52
In order to further elucidate the neurobiological mechanisms potentially underlying the observed behavioral phenotype in PIC offsprings, we proceeded to evaluate the level of neurogenesis in the hippocampal DG owing to its well-documented relevance for both depression-related behavior and cognitive function.10,53, 54, 55, 56 To comprehensively characterize the potential effects of MIA on adult hippocampal neurogenesis, proliferation and survival of newly generated cells of the DG—alterations of both of which have been described in several animal models of depression30,53,57—were analyzed. Our findings of significantly reduced basal rates of neuronal precursor cell proliferation in adult PIC animals are in line with previous reports describing the effects of other models of gestational infection on adult hippocampal neurogenesis.58,59 Moreover, as an association between altered hippocampal cell proliferation and impairments in learning and memory is suggested,56,60,61 impaired hippocampal neurogenesis may also relate to the observed cognitive deficiencies in PIC offsprings. The significant reduction of BrdU+ 2 weeks after BrdU administration could reflect the deficits in neuronal precursor cell proliferation over time or may relate to aberrant neural progenitor cell differentiation and/or survival of mature neurons. Although no significant differences in the number of total surviving BrdU+/GFAP+ cells were found, we observed a significantly reduced number of surviving BrdU+/NeuN+mature granular neurons in PIC offsprings, suggesting a specific sensitivity of neuronal cells to effects of MIA. These results still do not ascertain whether MIA affects rather neuronal differentiation or the survival of mature neurons. To address this question, we evaluated the morphology of immature neurons, and focused on the period of DCX expression during which the majority of regulatory processes are thought to occur.37 Quantification of three different stages of neuronal differentiation shows no difference on the premitotic and intermediate stages but demonstrated that PIC animals show a selective reduction of postmitotic immature neuronal cells. These data suggest that neurons in the postmitotic stages are specifically vulnerable to the detrimental impact of MIA. Moreover, as postmitotic neurons are also NeuN+, the reduction of neurons in postmitotic stage suggests that the reduced number of BrdU+/NeuN+ cells mainly reflects an effect of MIA on neuronal differentiation.
With regard to the mechanism potentially mediating the effect of MIA on behavior, hippocampal function and neurogenesis, it can be hypothesized that enhanced maternal production of IL-6, which can transfer through the placental barrier hereby affecting fetal brain development,24 represents a key molecular intermediary. Indeed, blockade of IL-6, which has been tightly linked to depression in both experimental animals and human patients,28,62,63 has been proven to preclude the behavioral effects in PIC offsprings.24,64
IL-6 has also been related to impaired neuronal differentiation in vitro,60 suggesting that it could have a role in the observed disturbances of neuronal maturation in PIC mice. Intriguingly, a similar deficiency in neuronal differentiation with a specific reduction of stage-3 neurons has been recently reported in a different model for adult depression-like behavior resulting from prenatal disturbances.65
Searching for the molecular correlates of depression-like behavior and deficient neuronal proliferation and differentiation, we went on to examine whether MIA may further induce alterations in neurotrophic support in the offsprings brains, which may be associated with the observed behavioral and cellular phenotypes. Although brain-derived neurotrophic factor is regarded as one of the key molecules implicated in the pathogenesis of depression and response to antidepressant treatment,66,67 VEGF has lately emerged as an important mediator at the interface between the vascular and the nervous system orchestrating the cooperative link between angiogenesis and neural precursor cell proliferation.68 Furthermore, the VEGF signaling pathway has been recently implicated in the pathophysiology of several stress-related disorders, including major depression.61,65,69, 70, 71
Although the design of the present study does not allow to establish a direct causal relationship between the observed behavioral, neurogenic and electrophysiological alterations in PIC mice, significantly reduced expression of hippocampal VEGFA and VEGFR2 may provide the molecular link between these phenomena as blockade of VEGFA has also been demonstrated to abrogate in vivo hippocampal LTP72 and impair learning and memory.16,72 Thus, as VEGF is a target of the STAT3 (signal transducer and activator of transcription) signaling pathway,73 classically induced by IL-6, we hypothesize that the effects of MIA on the developing fetal brain and ensuing consequences in adulthood may result from IL-6-STAT3-dependet aberrant VEGFA–VEGFR2 signaling. This hypothesis remains to be tested in future experiments.
Acknowledgments
DDP is supported by the Austrian Science Fund (FWF): P22424 and member of the special research network (SFB) 35. DK received a fellowship from ‘Verein zur Foerderung der Forschung auf dem Gebiet der Neonatologie und paediatrischen Intensivmedizin'.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Brown AS, Vinogradov S, Kremen WS, Poole JH, Deicken RF, Penner JD, et al. Prenatal exposure to maternal infection and executive dysfunction in adult schizophrenia. Am J Psychiatry. 2009;166:683–690. doi: 10.1176/appi.ajp.2008.08010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babulas V, Factor-Litvak P, Goetz R, Schaefer CA, Brown AS. Prenatal exposure to maternal genital and reproductive infections and adult schizophrenia. Am J Psychiatry. 2006;163:927–929. doi: 10.1176/ajp.2006.163.5.927. [DOI] [PubMed] [Google Scholar]
- Buka SL, Cannon TD, Torrey EF, Yolken RH. Collaborative study group on the perinatal origins of severe psychiatric disorders. Maternal exposure to herpes simplex virus and risk of psychosis among adult offspring. Biol Psychiatry. 2008;63:809–815. doi: 10.1016/j.biopsych.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Kolevzon A, Gross R, Reichenberg A. Prenatal and perinatal risk factors for autism: a review and integration of findings. Arch Pediatr Adolesc Med. 2007;161:326–333. doi: 10.1001/archpedi.161.4.326. [DOI] [PubMed] [Google Scholar]
- Mednick SA, Huttunen MO, Machon RA. Prenatal influenza infections and adult schizophrenia. Schizophr Bull. 1994;20:263–267. doi: 10.1093/schbul/20.2.263. [DOI] [PubMed] [Google Scholar]
- Bitanihirwe BK, Peleg-Raibstein D, Mouttet F, Feldon J, Meyer U. Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology. 2010;35:2462–2478. doi: 10.1038/npp.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- Posener JA, Wang L, Price JL, Gado MH, Provine MA, Miller MI, et al. Mapping of the hippocampus in depression. Am J Psychiatry. 2003;160:83–89. doi: 10.1176/appi.ajp.160.1.83. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Abazyana B, Nomura J, Kannana G, Ishizukai K, Tamashirob KL, Nuciforaa F, et al. Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol Psychiatry. 2010;68:1172–1181. doi: 10.1016/j.biopsych.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, et al. Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry. 2011;16:1177–1188. doi: 10.1038/mp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duric V, Duman RS. Depression and treatment response: dynamic interplay of signaling pathways and altered neural processes. Cell Mol Life Sci. 2013;70:39–53. doi: 10.1007/s00018-012-1020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi G, Brovedani The hippocampus, neurotrophic factors and depression: possible implications for the pharmacotherapy of depression. CNS. 2011;25:913–931. doi: 10.2165/11595900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Kiuchi T, Lee H, Mikami T. Regular exercise cures depression-like behavior via VEGF-Flk-1 signaling in chronically stressed mice. Neuroscience. 2012;207:208–217. doi: 10.1016/j.neuroscience.2012.01.023. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, LeMoal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. PNAS. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka A, Fujioka T, Ishida Y, Maekawa T, Nakamura S. Differential effects of prenatal stress on the morphological maturation of hippocampal neurons. Neuroscience. 2006;141:907–915. doi: 10.1016/j.neuroscience.2006.04.046. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 2001;65:427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- Xu J, Yang B, Yan C, Hu H, Cai S, Liu J, et al. Effects of duration and timing of prenatal stress on hippocampal myelination and synaptophysin expression. Brain Res. 2013;8993:00892–00895. doi: 10.1016/j.brainres.2013.06.025. [DOI] [PubMed] [Google Scholar]
- Garay PA, Hsiao EY, Patterson PH, McAllister AK. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun. 2013;31:54–68. doi: 10.1016/j.bbi.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun. 2012;25:604–615. doi: 10.1016/j.bbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Smith SE, Malkova N, Tse D, Su Y, Patterson PH. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav Immun. 2009;23:116–123. doi: 10.1016/j.bbi.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Simard M, Cote M, Provost PR, Tremblay Y. Expression of genes related to the hypothalamic-pituitary-adrenal axis in murine fetal lungs in late gestation. Reprod Biol Endocrinol. 2010;8:1477–7827. doi: 10.1186/1477-7827-8-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje FJ, Cabatic M, Divisch I, Kim EJ, Herkner KR, Binder BR, et al. Constant darkness induces IL-6-dependent depression-like behavior through the NF-{kappa}B signaling pathway. J Neurosci. 2011;31:9075–9083. doi: 10.1523/JNEUROSCI.1537-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Monje FJ, Li L, Höger H, Pollak DD, Lubec G. Alzheimer's disease risk factor lymphocyte-specific protein tyrosine kinase regulates long-term synaptic strengthening, spatial learning and memory. Cell Mol Life Sci. 2012;70:743–759. doi: 10.1007/s00018-012-1168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak DD, Monje FJ, Zuckerman L, Denny CA, Drew MR, Kandel ER. An animal model of a behavioral intervention for depression. Neuron. 2008;60:149–161. doi: 10.1016/j.neuron.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah A, Schmuckermair C, Sartori SB, Gaburro S, Kandasamy M, Irschick R, et al. Anxiety- rather than depression-like behavior is associated with adult neurogenesis in a female mouse model of higher trait anxiety- and comorbid depression-like behavior. Transl Psychiatry. 2012;1038:94. doi: 10.1038/tp.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, et al. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- Viikki M, Anttila S, Kampman O, Illi A, Huuhka M, Setälä-Soikkeli E, et al. Vascular endothelial growth factor (VEGF) polymorphism is associated with treatment resistant depression. Neurosci Lett. 2010;477:105–108. doi: 10.1016/j.neulet.2010.04.039. [DOI] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26:12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci. 1992;12:3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson ND, Owens MJ, Nemeroff CB. Depression, antidepressants, and neurogenesis: a critical reappraisal. Neuropsychopharmacology. 2011;36:2589–2602. doi: 10.1038/npp.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plümpe T, Ehninger D, Steiner B, Klempin F, Jessberger S, Brandt M, et al. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci. 2006;7:77. doi: 10.1186/1471-2202-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayle DA, Beloosesky R, Desai M, Amidi F, Nuñez SE, Ross MG. Maternal LPS induces cytokines in the amniotic fluid and corticotropin releasing hormone in the fetal rat brain. Am J Physiol. 2004;286:1024–1029. doi: 10.1152/ajpregu.00664.2003. [DOI] [PubMed] [Google Scholar]
- Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun. 2010;24:881–897. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Garbett KA, Hsiao EY, Kalman S, Patterson PH, Mirnics K. Effects of maternal immune activation on gene expression patterns in the fetal brain. Transl Psychiatry. 2012;2012:24. doi: 10.1038/tp.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc Natl Acad Sci USA. 2012;109:12776–12781. doi: 10.1073/pnas.1202556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Cheung C, Wei R, Hui ES, Feldon J, Meyer U, et al. Prenatal immune challenge is an environmental risk factor for brain and behavior change relevant to schizophrenia: evidence from MRI in a mouse model. PLoS One. 2009;4:e6354. doi: 10.1371/journal.pone.0006354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Susser ES. In utero infection and adult schizophrenia. Ment Retard Dev Disabil Res Rev. 2002;8:51–57. doi: 10.1002/mrdd.10004. [DOI] [PubMed] [Google Scholar]
- Markham JA, Koenig JI. Prenatal stress: role in psychotic and depressive diseases. Psychopharmacology. 2011;214:89–106. doi: 10.1007/s00213-010-2035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang D, Syed S, Fine P, Jones PB. No association between prenatal viral infection and depression in later life—a long-term cohort study of 6152 subjects. Can J Psychiatry. 2009;54:565–570. doi: 10.1177/070674370905400809. [DOI] [PubMed] [Google Scholar]
- Parboosing R, Bao Y, Shen L, Schaefer CA, Brown AS. Gestational influenza and bipolar disorder in adult offspring. JAMA Psychiatry. 2013;70:677–685. doi: 10.1001/jamapsychiatry.2013.896. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Wray NR, Pergadia ML, Blackwood DH, Penninx BW, Gordon SD, Nyholt DR, et al. Genome-wide association study of major depressive disorder: new results, meta-analysis, and lessons learned. Mol Psychiatry. 2012;17:36–48. doi: 10.1038/mp.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993;13:3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Huang S, Cao J, Chen T, Zhu P, Zhu R, et al. The timing of maternal separation affects morris water maze performance and long-term potentiation in male rats. Dev Psychobiol. 2013;25:21130. doi: 10.1002/dev.21130. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar VK, Hattiangady B, Kuruba R, Shuai B, Shetty AK. Predictable chronic mild stress improves mood, hippocampal neurogenesis and memory. Mol Psychiatry. 2011;16:171–183. doi: 10.1038/mp.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp JR, Beasley CL, Galea LA. Increased hippocampal neurogenesis and p21 expression in depression: dependent on antidepressants, sex, age, and antipsychotic exposure. Neuropsychopharmacology. 2013;2013:132. doi: 10.1038/npp.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Wang H, Gondre-Lewis MC. Prenatal nicotine and maternal deprivation stress de-regulate the development of CA1, CA3, and dentate gyrus neurons in hippocampus of infant rats. PLoS One. 2013;8:e65517. doi: 10.1371/journal.pone.0065517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui K, Ashdown H, Luheshi GN, Boksa P. Effects of prenatal immune activation on hippocampal neurogenesis in the rat. Schizophr Res. 2009;113:288–297. doi: 10.1016/j.schres.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Forrest CM, Khalil OS, Pisar M, Smith RA, Darlington LG, Stone TW. Prenatal activation of Toll-like receptors-3 by administration of the viral mimetic poly(I:C) changes synaptic proteins, N-methyl-D-aspartate receptors and neurogenesis markers in offspring. Mol Brain. 2012;5:1756–6606. doi: 10.1186/1756-6606-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomi M, Aoki T, Takagi Y, Nishimura M, Ohsugi Y, Mihara M, et al. Single and local blockade of interleukin-6 signaling promotes neuronal differentiation from transplanted embryonic stem cell-derived neural precursor cells. J Neurosci Res. 2011;89:1388–1399. doi: 10.1002/jnr.22667. [DOI] [PubMed] [Google Scholar]
- Lee BH, Kim YK. Increased plasma VEGF levels in major depressive or manic episodes in patients with mood disorders. J Affect Disord. 2012;136:181–184. doi: 10.1016/j.jad.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Anisman H, Merali Z, Poulter MO, Hayley S. Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des. 2005;11:963–972. doi: 10.2174/1381612053381701. [DOI] [PubMed] [Google Scholar]
- Sukoff Rizzo SJ, Neal SJ, Hughes ZA, Beyna M, Rosenzweig-Lipson S, Moss SJ, et al. Evidence for sustained elevation of IL-6 in the CNS as a key contributor of depressive-like phenotypes. Transl Psychiatry. 2012;1038:120. doi: 10.1038/tp.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson AM, Jennische E, Hansson HA, Holmang A. Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABA(A) dysregulation and impaired spatial learning. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1345–R1356. doi: 10.1152/ajpregu.00268.2005. [DOI] [PubMed] [Google Scholar]
- Kahl KG, Bens S, Ziegler K, Rudolf S, Kordon A, Dibbelt L, et al. Angiogenic factors in patients with current major depressive disorder comorbid with borderline personality disorder. Psychoneuroendocrinology. 2009;34:353–357. doi: 10.1016/j.psyneuen.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Castren E, Rantamaki T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev Neurobiol. 2010;70:289–297. doi: 10.1002/dneu.20758. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Isung J, Aeinehband S, Mobarrez F, Mårtensson B, Nordström P, Asberg M, et al. Low vascular endothelial growth factor and interleukin-8 in cerebrospinal fluid of suicide attempters. Transl Psychiatry. 2012;2:e196. doi: 10.1038/tp.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi M, Hashimoto R, Hisaoka K, Tsuchioka M, Kunugi H. Plasma levels of vascular endothelial growth factor and fibroblast growth factor 2 in patients with major depressive disorders. J Neural Transm. 2010;117:1119–1122. doi: 10.1007/s00702-010-0452-1. [DOI] [PubMed] [Google Scholar]
- Iga J, Ueno S, Yamauchi K, Numata S, Tayoshi-Shibuya S, Kinouchi S, et al. Gene expression and association analysis of vascular endothelial growth factor in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:658–663. doi: 10.1016/j.pnpbp.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Licht T, Goshen I, Avital A, Kreisel T, Zubedat S, Eavri R, et al. Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci USA. 2011;108:5081–5086. doi: 10.1073/pnas.1007640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Weinberger NM, Roozendaal B, McGaugh JL. Is the amygdala a locus of ‘conditioned fear'? Some questions and caveats. Neuron. 1999;23:227–228. doi: 10.1016/s0896-6273(00)80774-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.