Abstract
Although the involvement of genetic abnormalities in autism spectrum disorders (ASD) is well-accepted, recent studies point to an equal contribution by environmental factors, particularly environmental toxicants. However, these toxicant-related studies in ASD have not been systematically reviewed to date. Therefore, we compiled publications investigating potential associations between environmental toxicants and ASD and arranged these publications into the following three categories: (a) studies examining estimated toxicant exposures in the environment during the preconceptional, gestational and early childhood periods; (b) studies investigating biomarkers of toxicants; and (c) studies examining potential genetic susceptibilities to toxicants. A literature search of nine electronic scientific databases through November 2013 was performed. In the first category examining ASD risk and estimated toxicant exposures in the environment, the majority of studies (34/37; 92%) reported an association. Most of these studies were retrospective case–control, ecological or prospective cohort studies, although a few had weaker study designs (for example, case reports or series). Toxicants implicated in ASD included pesticides, phthalates, polychlorinated biphenyls (PCBs), solvents, toxic waste sites, air pollutants and heavy metals, with the strongest evidence found for air pollutants and pesticides. Gestational exposure to methylmercury (through fish exposure, one study) and childhood exposure to pollutants in water supplies (two studies) were not found to be associated with ASD risk. In the second category of studies investigating biomarkers of toxicants and ASD, a large number was dedicated to examining heavy metals. Such studies demonstrated mixed findings, with only 19 of 40 (47%) case–control studies reporting higher concentrations of heavy metals in blood, urine, hair, brain or teeth of children with ASD compared with controls. Other biomarker studies reported that solvent, phthalate and pesticide levels were associated with ASD, whereas PCB studies were mixed. Seven studies reported a relationship between autism severity and heavy metal biomarkers, suggesting evidence of a dose–effect relationship. Overall, the evidence linking biomarkers of toxicants with ASD (the second category) was weaker compared with the evidence associating estimated exposures to toxicants in the environment and ASD risk (the first category) because many of the biomarker studies contained small sample sizes and the relationships between biomarkers and ASD were inconsistent across studies. Regarding the third category of studies investigating potential genetic susceptibilities to toxicants, 10 unique studies examined polymorphisms in genes associated with increased susceptibilities to toxicants, with 8 studies reporting that such polymorphisms were more common in ASD individuals (or their mothers, 1 study) compared with controls (one study examined multiple polymorphisms). Genes implicated in these studies included paraoxonase (PON1, three of five studies), glutathione S-transferase (GSTM1 and GSTP1, three of four studies), δ-aminolevulinic acid dehydratase (one study), SLC11A3 (one study) and the metal regulatory transcription factor 1 (one of two studies). Notably, many of the reviewed studies had significant limitations, including lack of replication, limited sample sizes, retrospective design, recall and publication biases, inadequate matching of cases and controls, and the use of nonstandard tools to diagnose ASD. The findings of this review suggest that the etiology of ASD may involve, at least in a subset of children, complex interactions between genetic factors and certain environmental toxicants that may act synergistically or in parallel during critical periods of neurodevelopment, in a manner that increases the likelihood of developing ASD. Because of the limitations of many of the reviewed studies, additional high-quality epidemiological studies concerning environmental toxicants and ASD are warranted to confirm and clarify many of these findings.
Keywords: autism, environmental medicine, gene–environment interaction, heavy metals, polymorphisms, toxicants
Introduction
Autism spectrum disorders (ASD) are a heterogenous group of neurodevelopmental disorders that are behaviorally defined and characterized by impairments in communication and social interaction along with restrictive and repetitive behaviors.1 ASD includes autistic disorder, Asperger syndrome and pervasive developmental disorder-not otherwise specified. ASD affects an estimated 1 out of 88 individuals in the United States2 with four times more males than females being affected.3
The etiology of ASD is unclear at this time. Although several genetic syndromes, such as Fragile X and Rett syndrome, have been associated with ASD, empirical studies have estimated that single gene and chromosomal defects only account for a minority of ASD cases.4 Recently, evidence has accumulated implicating a role that environmental factors have in ASD. For example, one recent study of 192 twin pairs reported that environmental factors were estimated to account for 55% of the risk of developing autistic disorder compared with 37% for genetic factors; a similar risk pattern was also observed for developing the broader diagnosis of ASD.5
Although many of the cognitive and behavioral features of ASD are thought to arise from dysfunction of the central nervous system, evidence from many fields of medicine has documented multiple non-central nervous system physiological abnormalities associated with ASD,6 suggesting that, in some individuals, ASD arises from systemic, rather than organ-specific abnormalities. Specifically, in recent decades, research and clinical studies have implicated physiological and metabolic systems that transcend specific organ dysfunction, such as immune dysregulation, inflammation, impaired detoxification, redox regulation/oxidative stress and energy generation/mitochondrial systems.6,7 In this context, ASD may arise from, or at least involve, systemic physiological abnormalities rather than being a purely central nervous system disorder,8 at least in a subset of individuals with ASD.
Exposures to environmental toxicants such as mercury, lead, arsenic, polychlorinated biphenyls (PCBs) and toluene are known causes of neurodevelopmental disorders.9 Approximately 85 000 chemicals have been manufactured in the United States, and although only about 2800 are used in high volumes (more than one million pounds produced per year), little information exists about the developmental toxicity for most of these, including many that are in common use today.10 Because of limitations inherent to toxicant studies in assessing subtle changes in neurobehavioral outcomes and accurately measuring toxicant exposures, the risk of developing a neurodevelopmental disorder after exposure to a particular toxicant probably tends to be underestimated rather than overestimated.11 Furthermore, individual variability in genetic susceptibility can influence responses to environmental toxicants and contribute to increased disease vulnerabilities.12 For example, several studies have reported that some individuals with ASD express polymorphisms in genes involved in the detoxification of environmental pollutants. These genes have been termed ‘environmental response genes'13 and more than 100 such genes may contribute to ASD risk.14 Single nucleotide polymorphisms (SNPs) in environmental response genes are believed to increase susceptibilities to the adverse effects of environmental toxicants.15
Until recently, the study of potential environmental toxicant contributions to the development of ASD has been generally ‘neglected'.16 However, several large studies examining the role of environmental factors in ASD are currently underway. One recent review reported that 190 articles (including review articles and animal studies) published since 1971 have examined environmental toxicants in ASD with 170 (89%) implicating an association with ASD.6 This current review explores potential associations between ASD and environmental toxicants, including environmental exposures to toxicants, biomarkers of toxicants and genetic polymorphisms that might be associated with impaired detoxification. Although prior reviews have examined the evidence for an association between ASD and toxicants, this review systematically examines and differentiates studies examining estimated exposures to environmental toxicants from those measuring biomarkers of toxicants, while also examining the evidence for exposure risk during specific developmental time periods. In addition, this review examines the role of environmental response genes in relation to specific environmental toxicants found to be implicated in ASD in order to determine whether the notion of shared environmental and genetic risk factors can be supported for environmental toxicant exposures. Through this analysis, we demonstrate that evidence exists to support the notion that environmental toxicant exposures across multiple developmental periods can increase the risk of developing ASD and that studies support shared environmental and genetic etiological risk factors contributing to the development of ASD.
Materials and methods
Search strategy and selection criteria
We systematically reviewed and collated studies into the following three categories: (a) published studies concerning potential associations between estimated exposures to toxicants in the environment and the risk of ASD; (b) studies regarding biomarkers of environmental toxicants and ASD; and (c) studies examining potential genetic susceptibilities to environmental toxicants. Five studies in the first category (a) utilized biomarkers of toxicant exposure17, 18, 19, 20, 21 to create dichotomous toxicant exposure groups and then prospectively investigated whether these exposure groups were associated with ASD development later in life. As these studies were not primarily concerned with the relationship between these biomarkers and ASD, these studies were placed into category (a) instead of (b). To identify publications in the first two categories—(a) and (b)—a search of Pubmed, Scopus, EMBASE, Google Scholar, CINAHL, ERIC, AMED, PsychInfo and Web of Science databases through November 2013 was conducted to identify pertinent articles using the search terms ‘autism', ‘autistic', ‘ASD', ‘Asperger', ‘pervasive developmental disorder' and ‘PDD' in all combinations with the terms ‘toxicant', ‘toxin', ‘metal', ‘mercury', ‘lead', ‘chemical', ‘pesticide', ‘PCB', ‘phthalate', ‘solvent', ‘pollutant', ‘pollution', ‘xenobiotic' and ‘detoxification.' The references cited in identified publications were also searched to locate additional studies. Review articles, hypothesis papers and letters to the editor that did not present unique or new data were excluded from the analysis. Publications of animal models were also excluded. Studies concerning potential toxicant exposures related to medications (for example, mercury or aluminum in medicinal preparations, including vaccines and dental amalgams), food additives, cocaine, alcohol, smoking, allergens, maternal stressors and infectious agents (for example, viruses, yeast and bacteria) were excluded. Figure 1 lists the PRISMA flowchart for publications examining estimated environmental toxicant exposures and/or biomarkers of toxicants in ASD identified from this search. A total of 118 publications were identified with 84 publications (71%) implicating toxicants in ASD, 7 studies (6%) reporting on treatments for toxicants in ASD and 27 publications (23%) reporting no significant association between environmental toxicants and ASD.
Figure 1.
PRISMA flow chart of publications examining estimated environmental toxicant exposures and toxicant biomarkers in autism spectrum disorder (ASD).
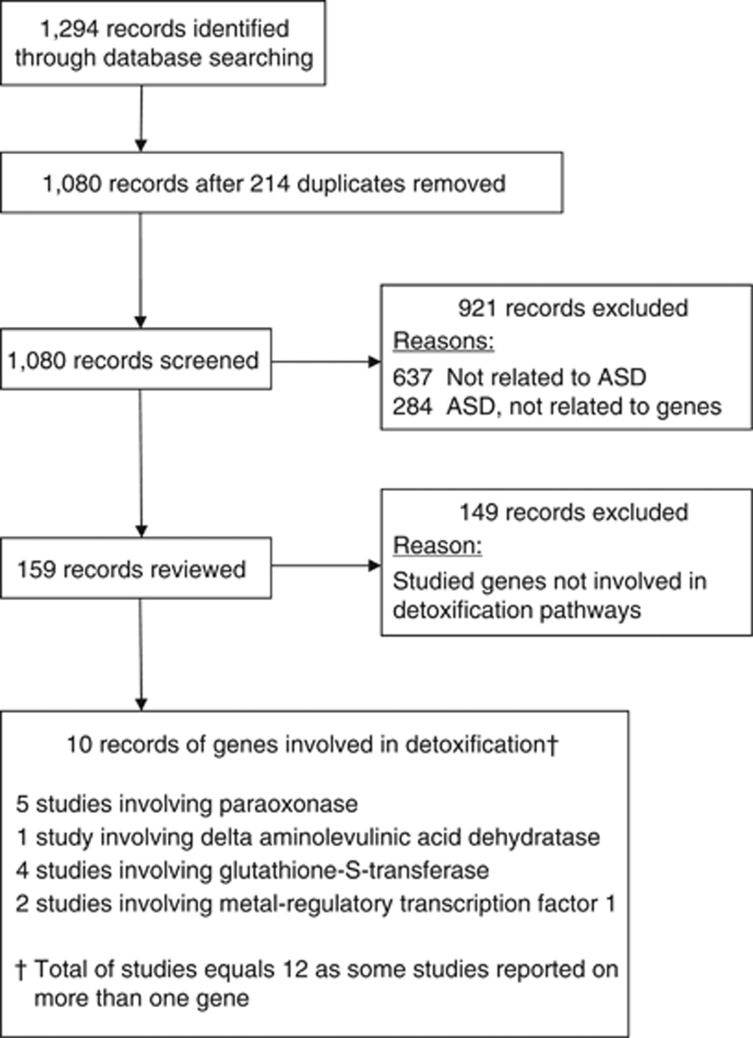
To identify publications in category (c), a second search using the same databases was performed to identify genes involved in detoxification that have been implicated in ASD by using the search terms ‘autism', ‘autistic', ‘ASD', ‘Asperger', ‘pervasive developmental disorder' and ‘PDD' in all combinations with a list of genes from two web-based environmentally related genomic databases: the National Institute of Environmental Health Sciences Environmental Genome Project (Phase 1 finished genes, http://egp.gs.washington.edu/finished_genes.html) and SeattleSNPs (http://pga.gs.washington.edu/finished_genes.html). The lists of genes from these databases used in the search are present in Supplementary Material Table S1. This search revealed several genes known to be involved in the detoxification of xenobiotics and also implicated in ASD, including PON, glutathione S-transferase, δ-aminolevulinic acid dehydratase (ALAD2), divalent metal ion transporter SLC11A3 and the metal regulatory transcription factor. Figure 2 lists the PRISMA flowchart for the 10 publications reporting genes involved in toxicant elimination and ASD identified from this search.
Figure 2.
PRISMA flow chart of publications examining genes involved in toxicant elimination in autism spectrum disorder (ASD).
Studies were grouped into the following three sections in this review: (a) epidemiological and other studies exploring potential associations between estimated toxicant exposures in the environment and ASD risk; (b) studies measuring biomarkers of toxicants and potential associations with ASD; and (c) studies examining polymorphisms in genes involved in detoxification and potential associations with ASD.
Results
Potential associations between ASD and environmental toxicant exposures
Some studies examined estimated environmental toxicant exposures in parents of children with ASD during the preconceptional and gestational periods, whereas others examined estimated exposures during childhood in children who developed ASD. Therefore, these three developmental time periods are discussed separately. For the gestational and childhood exposure sections, the reviewed studies examined estimated exposures to specific categories of environmental toxicants; therefore, each category of environmental toxicants is also discussed separately. A majority of the studies reviewed in this section were retrospective case–control studies or prospective cohort studies, although several had a weaker design (for example, case reports or series). Limitations of studies and further research needs are also listed.
Preconceptional exposures
Three retrospective case–control studies examined estimated toxicant exposure during the preconceptional period in parents of children with ASD, with each reporting an association with ASD. The first study by Coleman,22 published in 1976, contained 78 children with ASD and 78 typically developing (TD) children who were age-/sex-matched friends or neighbors and reported that the parents of the ASD children were significantly more likely to work in an occupation involving chemical exposures during the preconception period (26% of families) compared with parents of TD children (1% of families). As recruited participants knew the goal of the study, Coleman was concerned about recruitment bias in her sample. In order to control for this bias, Felicetti23 selected parents of 20 ASD children and 20 non-autistic children with intellectual disability who attended the same school for the developmentally disabled. Twenty TD children who were friends or neighbors of the ASD cases were randomly selected and used as controls. Parents of children with ASD demonstrated a significantly higher frequency of estimated occupational exposure to chemicals during the preconception period (21% exposed; approximately two-thirds of those exposed were chemists) compared with parents of non-autistic children with intellectual disability (3% exposed) and TD children (10% exposed).23 Finally, the last study examined estimated parental occupational exposure from preconception through the early life of the child in 93 parents of ASD children and 81 parents of TD children as assessed by industrial hygienists as well as parental recall. Parents of children with ASD were more likely to have occupational workplace exposures to lacquer (odds ratio (OR)=7.3; 95% confidence interval (CI), 1.6–33.5), varnish (OR=4.7; 95% CI, 1.0–22.0), xylene (OR=2.7; 95% CI, 1.1–6.7), solvents (OR=3.1; 95% CI, 1.3–7.7) and asphalt (OR=6.9; 95% CI, 1.5–32.4) during the 3 months preceding pregnancy through birth or weaning (if breast feeding) compared with parents of TD children.24 Notably, these three studies reported a potential association between toxicant exposures in the preconceptional period and autism risk; however, given the limited number of these studies, the limitations of the retrospective study design and the relatively small sample sizes, further studies are needed to investigate this apparent association.
Gestational exposures
Pesticides
One retrospective case–control and three prospective cohort studies examined ASD risk and gestational exposure to pesticides with each study reporting an association with ASD. The retrospective case–control study identified 465 children with ASD through the California Department of Developmental Services and 6975 TD children and examined estimates of pesticide exposure, as obtained from the California Department of Pesticide Regulation. This study analyzed the effects of combinations of three separate pesticide exposure factors during pregnancy: type of pesticide, timing of exposure and residential distance from pesticide application. Estimated prenatal exposure to organochlorine pesticides (specifically dicofol and endosulfan) during the 8 weeks immediately following the time of cranial neural tube closure was associated with an increased risk of ASD (OR=6.1; 95% CI, 2.4–15.3) in children of mothers who lived within 500 m of fields that had the highest quartile of estimated pesticide exposure compared with children whose mothers lived more than 1750 m from exposure, and therefore had the lowest exposure levels.25 Notably, another study used the same data set as well as Bayesian modeling to define the critical periods before, during and after pregnancy when proximity to organochlorine pesticides would be most likely to result in ASD. This model identified two peaks of developmental vulnerability, one that extended from 38 days before fertilization to 163 days following fertilization and a second postnatal peak ranging from 346 to 529 days post fertilization.26
The first prospective cohort study followed 254 inner-city newborn infants who were prenatally exposed to the organophosphate (OP) insecticide chlorpyrifos. Children with higher estimated exposure levels (as determined by an umbilical cord plasma chlorpyrifos concentration greater than 6.17 pg g−1) were significantly more likely to develop symptoms of PDD by 36 months of age as measured by answers provided by mothers on the 99-item Child Behavior Checklist compared with children with lower estimated exposure levels (OR=5.39; 95% CI, 1.21–24.11).20 The second cohort study followed 531 newborn infants from Latino farm-worker families in California who were exposed to OP pesticides during pregnancy, as estimated by measuring urinary biomarkers of OP pesticides (dialkylphosphate (DAP) metabolites) collected from their mothers during pregnancy. A significantly increased risk of PDD symptoms at 2 years of age, as measured by answers provided by mothers on the Child Behavior Checklist, was found for each 10-fold increase in DAP metabolites (OR=2.3; 95% CI, 1.0–5.2).18 Finally, the last cohort study of 75 children with autism and 75 TD controls matched on sex, birth year, urbanization and maternal age measured several toxicant metabolites during pregnancy, including pesticides, PCBs and other organic pollutants. This study reported a trend in elevated risk of ASD for children at 7 years of age or older who had the highest 10th percentile of estimated exposure in total PCBs (OR=1.91; 95% CI, 0.57–6.39) and in dichlorodiphenyldichloroethylene (OR=1.79; 95% CI, 0.52–6.21).17 Notably, these four studies ranged from 75 to 531 children with three studies being prospective in nature. Collectively, these studies point to a relatively strong association between pesticide exposure during gestation and ASD, with some studies reporting a two- to fivefold increased OR.
Air pollution
Six retrospective case–control studies examined ASD risk and estimated exposure to air pollution during gestation, with each reporting an association. These studies selected children diagnosed with ASD and used birth records or parental interviews to determine their residence during gestation (although one study used an additional questionnaire). The first study was population based and contained 304 children with ASD and 259 TD controls, and reported that maternal residences during the third trimester (OR=2.22; 95% CI, 1.16–4.42) and at the time of delivery (OR=1.86; 95% CI, 1.04–3.45) were more likely to be located near a freeway in the ASD group compared with the TD controls. The investigators suggested that closer residence to a freeway was a surrogate for higher exposure to air pollution.27 The second study compared estimated perinatal exposure to 35 air pollutants between 383 children with ASD and 2829 children who had speech and language impairment. Exposures to ambient concentrations of metal, particulate and volatile organic air compounds were assessed in relationship to the child's birth residence. Hazardous air pollutants associated with an elevated risk of ASD included quinoline (OR=1.4; 95% CI, 1.0–2.2) and styrene (OR=1.8; 95% CI, 1.0–3.1).28 The third study enrolled 7603 children with autism matched to 10 controls per autism case. This study reported a 12–15% estimated increase in the risk of autism for each increase in the interquartile range of ozone (OR=1.12; 95% CI, 1.06–1.19) and particulate matter <2.5 μm (PM2.5) in aerodynamic diameter (OR=1.15; 95% CI, 1.06–1.24) while controlling for the effect of each pollutant on the other pollutants.29 The fourth study was population based and contained 279 children with ASD and 245 controls, and reported that residences with the highest quartile of traffic-related air pollution were associated with ASD during gestation (OR=1.98; 95% CI, 1.20–3.31), including estimated exposures to PM2.5 (OR=2.08; 95% CI, 1.93–2.25), particulate matter <10 μm (PM10) in aerodynamic diameter (OR=2.17; 95% CI, 1.49–3.16) and nitrogen dioxide (OR=1.81; 95% CI, 1.37–3.09).30 A fifth study of 325 children with ASD and 22 101 controls reported that perinatal exposure to the highest versus lowest quintile of air pollutants was significantly associated with an increased risk of ASD, including pooled metals (OR=1.5; 95% CI, 1.3–1.7), mercury (OR=2.0; 95% CI, 1.2–3.3), lead (OR=1.6; 95% CI, 1.1–2.3), nickel (OR=1.7; 95% CI, 1.1–2.5), manganese (OR=1.5; 95% CI, 1.1–2.2), diesel particulate (OR=2.0; 95% CI, 1.0–4.0) and methylene chloride (OR=1.8; 95% CI, 1.2–2.7). Notably, a stronger association was observed in boys compared with girls for most pollutants, suggesting a sex-specific interaction.31 Finally, the last case–control study was population based and examined air pollution exposure (including traffic-related air pollution, PM2.5, PM10, nitrogen dioxide and ozone) during the prenatal period in 252 children with ASD and 156 TD controls as well as a genetic variant in the MET receptor tyrosine kinase (MET) gene. Children who had both a MET rs1858830 CC genotype and higher exposures to certain air pollutants (in the top exposure quartile) had a greater risk of ASD compared with those with lower exposures and the CG/GG genotypes. The air pollutants found to have a significant association with ASD included traffic-related air pollution (adjusted OR=2.9; 95% CI, 1.0–10.6), PM10 (adjusted OR=3.2; 95% CI, 1.3–9.1) and nitrogen dioxide (adjusted OR=3.6; 95% CI, 1.3–12.7), whereas PM2.5 and ozone did not demonstrate this association.32 Collectively, these six case–control studies ranged from 252 to 7603 children with ASD and their results point to an association between ASD and air pollution, but these findings are limited by the retrospective nature of these studies.
Other toxicants
Two prospective and four retrospective studies examined ASD risk and other gestational environmental toxicant exposures with five out of six reporting an association. The prospective studies quantitatively estimated toxicant concentrations while the retrospective studies used questionnaires. The first prospective study measured urinary metabolites of phthalates and bisphenol A during the third trimester of 137 pregnancies, and reported that children with the highest estimated exposure to phthalates, but not bisphenol A, had a trend toward greater social deficits (OR=1.53; 95% CI, 0.25–2.9) at 7–9 years of age as measured by maternal ratings on the Social Responsiveness Scale (a quantitative scale for measuring the severity of social impairment related to ASD) compared with children with less estimated exposure.19 The second prospective study of 1784 children and young adults from the Republic of Seychelles examined prenatal exposure to methylmercury (predominantly through fish consumption) as measured in maternal hair samples collected around the time of birth and found no significant association between methylmercury exposure and ASD, as measured by the Social Communication Questionnaire administered to parents and the Social Responsiveness Scale administered to teachers at 10.7 years of age.21 As previously mentioned, one case–control study retrospectively estimated parental occupational exposure from preconception through early life of the child and reported that parents with ASD children were more likely to have occupational workplace exposure during gestation to lacquer, varnish, xylene, solvents and asphalt.24 In the second retrospective case–control study, maternal knowledge about environmental toxicants as well as estimated exposures to toxicants during the brain growth spurt (BGS)—a period of time extending from the third trimester of pregnancy through the first 2 years of life—were examined in 106 mothers of children with ASD and 324 mothers of TD children. Mothers of children with ASD were found to be significantly less knowledgeable about environmental toxicants and had higher estimated exposures during the BGS to toxicants including polybrominated diphenyl ethers, PCBs, bisphenol A and polychlorinated dibenzo-p-dioxin related to canned foods, waste incinerators, old electronics, plastics, microwavable food and textiles.33 A third retrospective case–control study of 284 children with ASD and 682 partially matched TD children of similar age from regions in the San Francisco Bay area reported that mothers of children with ASD were twice as likely (14.4 versus 7.2%) during gestation to work in an occupation with exposure to toxicants such as exhaust and combustion products (OR=12.0; 95% CI, 1.4–104.6) and disinfectants (OR=4.0; 95% CI, 1.4–12.0); paternal occupational exposure was not found to be associated with autism.34 Finally, the fourth retrospective case–control study from Spain examined 70 children with ASD and 136 controls, and reported that parental occupational exposures to solvents (including paints, varnishes, lacquers, adhesives, glues, degreasing chemicals, cleaning supplies, dyes, polymers, plastics, textiles and printing inks) were associated with an increased risk of ASD when the mother (OR=2.88; 95% CI, 1.28–6.17) or the father (OR=2.81; 95% CI, 1.01–7.86) worked with solvents.35 Collectively, these studies ranged from 70 to 1784 children and provided limited evidence for an association between exposures to other toxicants during gestation and ASD. One of the prospective studies reported a trend toward an association between phthalates and ASD symptoms. However, the largest prospective study reported no significant association between methylmercury and ASD.
Childhood exposures
Pesticides
Three studies examined estimated pesticide exposures during childhood and ASD with each reporting an association with ASD. As previously discussed, one prospective cohort study measured biomarkers of OP pesticides (DAP metabolites) in 531 children from Latino farm-worker families in California to determine estimated exposure levels to OP pesticides during gestation and early postnatal life. A significantly increased risk of PDD symptoms as measured by answers provided by mothers on the Child Behavior Checklist was found for each 10-fold increase in DAP metabolites measured in the child at 24 months of age (OR=1.7; 95% CI, 1.0–2.9). The investigators noted, however, that the association between postnatal OP pesticide exposure and PDD symptoms should be interpreted with caution as greater postnatal exposure was also associated with better scores on the Bayley Mental Developmental Index.18 One cross-sectional retrospective study of 1532 children from farm families exposed to pesticides reported that two children with parentally reported ASD had fathers directly exposed to phosphine, a fungicide.36 Finally, using computer-based modeling of toxicant–protein interactions and data from the Online Mendelian Inheritance in Man database and the Comparative Toxicogenomics Database, one study reported that the dichlorodiphenyltrichloroethane metabolite o,p′-dichlorodiphenyltrichloroethane was linked to ASD.37 Collectively, these studies provide limited evidence for an association between pesticide exposure in childhood and ASD. One study was prospective but the authors warned the results should be interpreted with caution, and the other two studies were limited by either a small sample size of ASD children36 or because the study was based on a computer model.37 Therefore, the evidence linking pesticide exposure in ASD does not appear as strong during childhood as during the gestational period particularly because there are fewer studies examining this factor during childhood; therefore, additional studies are warranted.
Toxic waste sites
Two studies retrospectively examined an association between ASD prevalence and the residential distance to US Environmental Protection Agency Superfund sites with both reporting an association with ASD. The first study was a case series of 495 children with ASD followed in a neurology clinic at the UMDNJ-New Jersey Medical School, which reported that the prevalence of ASD in child-specific zip codes of New Jersey was significantly associated with the density of toxic landfill sites within that zip code (P=0.019). These investigators also demonstrated that the estimated prevalence of ASD in each state (excluding Oregon) significantly correlated with the number of Superfund sites in that state (P=0.015).38 A cross-sectional ecological study analyzed the prevalence of ASD in 334 school districts in Minnesota (obtained from the Minnesota Department of Education for the 2007–2008 school year). School districts with higher rates of ASD were significantly more likely to be located within a 20-mile radius of a Superfund site (P=0.0001) compared with those farther away.39 These studies are limited by a cross-sectional design that prevents firm conclusions on causation, but provide evidence for an association between ASD and toxic waste sites; further studies are warranted to examine this in more detail.
Air pollution
Three retrospective case–control studies examined the effects of air pollution in children with ASD compared with controls with each reporting an association with ASD. The first study of 284 children with ASD and 657 partially matched TD children of similar age found that regions in the San Francisco Bay area with the highest quartile compared with the lowest quartile of atmospheric mercury concentration, as estimated using data from the US Environmental Protection Agency, demonstrated a significantly higher ASD prevalence (OR=1.92; 95% CI, 1.36–2.71). Prevalent cases of ASD were identified by data from the California autism surveillance system ~2 years after the child's birth. The prevalence of ASD was also significantly associated with the highest versus lowest quartile of atmospheric concentrations for cadmium (OR=1.54; 95% CI, 1.08–2.20), nickel (OR=1.46; 95% CI, 1.04–2.06), trichloroethylene (OR=1.47; 95% CI, 1.03–2.08), vinyl chloride (OR=1.75; 95% CI, 1.25–2.43) and diesel particulate matter (OR=1.44; 95% CI, 1.03–2.02).40 As previously discussed, the second study was population based and contained 279 children with ASD and 245 controls and reported that residences with the highest quartile of traffic-related air pollution were associated with ASD during the first year of life (OR=3.1; 95% CI, 1.76–5.57), including estimated exposures to PM2.5 (OR=2.12; 95% CI, 1.45–3.10), PM10 (OR=2.14; 95% CI, 1.46–3.12) and nitrogen dioxide (OR=2.06; 95% CI, 1.37–3.09).30 Finally, a population-based study of 49 073 children from Taiwan reported that exposure to air pollution in the preceding 1–4 years was associated with an increased risk of ASD, including a 59% higher risk per 10 p.p.b. increase in ozone (95% CI, 1.42–1.78), 37% higher risk per 100 p.p.b. increase in carbon monoxide (95% CI, 1.31–1.44), 343% higher risk per 10 p.p.b. increase in nitrogen dioxide (95% CI, 3.33–5.90) and a 18% higher risk per 1 p.p.b. increase in sulfur dioxide (95% CI, 1.09–1.28).41 Notably, the first two studies were relatively large, ranging from 279 to 284 children with ASD, whereas the last study was extremely large at over 49 thousand children. Collectively, these studies furnish stronger evidence that air pollution is associated with ASD risk, especially when viewed in light of the gestational data previously reviewed associating ASD with air pollution.
Water pollutants
Two ecological studies examined the effects of water pollutants in children with ASD with neither reporting an association with ASD. The first study reported that the prevalence of ASD during 1996–2000 in Nevada was not significantly related to perchlorate levels in the drinking water during 1997–2001.42 In the second study from 2000, the Agency for Toxic Substances and Disease Registry examined autism prevalence and the presence of water chlorination byproducts (specifically chloroform, bromoform and tetrachloroethylene) in the Brick Township, New Jersey and determined that it was not likely these chemicals contributed to the prevalence of ASD based on correlations between the concentration of estimated exposure and/or the timing of exposure.43 These studies are limited by a cross-sectional design that prevents firm conclusions on causation, but they do not provide evidence for an association between ASD and water pollutants.
Heavy metals
Eight ecological studies examined potential associations between estimated heavy metal exposures in the environment and ASD prevalence with all eight reporting some type of an association. In the first study from 2006, the amount of mercury released into the environment in 254 counties in Texas, as estimated using data from the US Environmental Protection Agency Toxic Release Inventory from 1998, was compared with the prevalence of autistic disorder for 2002, as obtained from the Texas Education Agency. An increased relative risk of 1.614 (95% CI, 1.487–1.752) in autistic disorder prevalence was calculated for every 1000 pounds of mercury released.44 Two other studies reanalyzed data from this latter study and/or examined similar data from the same Texas counties. One study corrected for a potential overprediction of autism risk owing to the possibility that counties with low autistic disorder numerical counts might delay the release of these results. However, the adjusted analysis using a Bayesian approach nonetheless showed a significant relative risk of 1.42 (95% CI, 1.09–1.78).45 The second study demonstrated that the results of the study by Palmer et al.44 could not be replicated using estimated environmental mercury exposure data from different years and derived from different databases for the same Texas counties, or when the diagnostic data 5 years following the exposure data was considered (for example, assuming gestational or early life exposure). However, a significant association was observed for nickel air emission data and autism (relative risk=1.71; 95% CI, 1.12–2.60), which was a novel finding compared with the study by Palmer et al.44 The investigators suggested that either the relationship between autism and mercury emissions as reported by Palmer et al.44 was inconsistent or that the reported association was spurious.46
Another ecological study in Texas reported that the residential distances to industrial or power plant (P<0.05 for both) sources of mercury (estimated from the US Environmental Protection Agency Toxic Release Inventory) were independently correlated with autistic disorder prevalence such that prevalence increased exponentially with increasing proximity to mercury sources.47 In another study from Texas and California, the prevalence of autism was significantly greater (P=0.01 for Texas; P=0.04 for California) in geographical areas that had the highest concentrations of ambient mercury. In addition, a significant correlation was observed between the mercury concentration in ambient air and the autism prevalence by state.48 ASD prevalence was significantly correlated with mercury and lead environmental atmospheric concentrations in another study using Combinatorial Fusion Analysis and Association Rule Mining.49 The prevalence of ASD for 59 parishes in Louisiana, as obtained from the Louisiana Department of Education, significantly correlated (P<0.001) with the mercury concentrations of 7652 fish samples measured throughout the state by the Louisiana Department of Environmental Quality in another study.50 Finally, the eighth ecological study reported that fish advisories related to mercury were significantly correlated with autism prevalence for all 50 states (r=0.48, P<0.001).51 Collectively, these eight ecological studies are limited by a cross-sectional design that prevents firm conclusions on causation, but they provide evidence for an association between ASD and heavy metal exposures in the environment.
Two other studies reported a potential association between estimated heavy metal exposures and ASD. The first was a case report that described the development of autistic features in an 11-month old child ~4 weeks after exposure to mercury from a broken thermometer in the home.52 The second was a retrospective case–control study of 256 mothers of children with ASD and 752 control mothers, which reported a higher prevalence of maternally reported childhood lead exposure (8.6% compared with 2.4%, P<0.001) in the children with ASD; however, only two cases of lead exposure could be confirmed with chart abstraction data.53 These two studies are limited by small sample sizes of the participants (case report) or the number of confirmed exposures (two cases) and thus do not add significant support for an association between heavy metals and ASD.
In-house flooring material
One cohort study examined ASD risk and retrospectively estimated environmental toxicant exposures in children. This Swedish study administered two questionnaires to parents of 4779 children living in one Swedish county. The first questionnaire (in 2000) assessed exposures to certain environmental factors when the children were between 1 and 6 years of age. The second questionnaire (in 2005) identified children who had developed ASD over the 5-year interval. This study reported that polyvinyl chloride flooring material (a source of airborne phthalates), in comparison with wood flooring, located in the parent's room (OR=2.51; 95% CI, 1.38–4.57) or the child's room (OR=1.96; 95% CI, 1.07–3.61) was associated with an increased risk of ASD.54
Summary
Only three studies examined estimated preconceptional exposures to toxicants in parents, with each reporting a positive association with ASD in offspring; however, all of these studies were retrospective. A total of 16 studies inspected estimated gestational exposures to toxicants and ASD with all but one (94%) reporting a positive association. The toxicant exposures during gestation most commonly associated with ASD included pesticides, solvents, PCBs and air pollutants. Three of the four studies examining estimated pesticide exposures during gestation were prospective, whereas the remainder of the studies examining toxicant exposures during gestation were retrospective, except for two that examined endocrine disruptors19 and methylmercury.21 Twenty-one studies examined estimated childhood exposures to toxicants and ASD with 19 (90%) reporting a positive association. The toxicants most implicated included pesticides, toxic waste sites, phthalates, air pollutants and heavy metals. The only prospective study of these 21 examined pesticides.18
Three studies spanned two developmental time periods.18,24,30 Collectively, 37 unique studies examined estimated exposures to environmental toxicants in relation to ASD, with 34 (92%) reporting some type of an association. The three studies that reported no significant association between ASD and toxicants were concerning water pollutants42,43 and methylmercury.21 Most of the reviewed studies were retrospective case–control studies or prospective cohort studies, although a few had weaker study designs (for example, case reports or series). The toxicants that appeared to have the strongest association with ASD were pesticides and air pollutants.
Out of the 37 studies, only 5 (14%) studies were prospective. All 5 of these studies were strengthened by the fact that they also measured biomarkers estimating actual toxicant exposures.17, 18, 19, 20, 21 Fourteen of the studies (39%) suggested evidence of a dose–effect relationship—that is, ASD risk was associated with higher estimated toxicant exposure levels as gauged by measuring biomarkers,17, 18, 19, 20 a closer proximity to estimated toxicant exposures25,27,30,38,39,47,48 or questionnaires.24,33,34 One study reported that a genetic variant in MET was associated with a greater risk of ASD in children exposed to higher levels of air pollutants, suggesting that genetic factors may have a role in increasing susceptibility to toxicants in some ASD children.
Most of the studies suffered from limitations. Many of the studies (32/37, 87%) were retrospective and none of these retrospective studies measured biomarkers to estimate toxicant exposures. Some studies relied on parental recall or questionnaires/surveys. Most studies lacked objective confirmation of ASD cases and/or did not measure toxicant exposures on an individual level. Some studies used estimates of ASD prevalence instead of measuring actual prevalence. Many of the studies only examined a select set of toxicants and did not control for other potential toxicant classes. Some of the studies had inadequate matching of cases and controls. Despite these limitations, the majority of the reviewed studies implicated multiple toxicants in ASD risk. Additional studies are warranted to confirm and clarify these findings and to better control for these limitations.
Studies of toxicant biomarkers and ASD
Although the previous section reviewed estimated exposures to toxicants in the environment and ASD risk, this section reviews studies investigating biomarkers of toxicants. These biomarkers were obtained from blood, urine, hair, brain or teeth of children with ASD. Biomarkers can be helpful to gauge acute toxicant exposures as well as the bioaccumulation of toxicants. However, because concentrations of blood and urinary biomarkers for various toxicants are affected by multiple factors, they may serve to indicate the presence rather than the precise quantity of stored compounds within the body and may also act, to some degree, as a quantitative indicator of recent or ongoing exposure. Primarily, biomarkers for heavy metals, solvents, pesticides, PCBs, phthalates and polybrominated diphenyl ethers have been studied in relation to ASD. These studies are reviewed below and are categorized by toxicant type and by the category of tissue/body fluid. For measurements of heavy metals in the blood, studies used measurements in whole blood, plasma, serum or red blood cells (RBC); however, not all studies noted which type of blood sample was used. Limitations of the reviewed studies and the need for further research are also listed.
Heavy metals
A significant amount of research has concentrated on heavy metal toxicants in relation to ASD. A number of studies have examined specific heavy metals, particularly mercury, lead cadmium, aluminum and arsenic, whereas other studies have attempted to estimate the body burden of heavy metals. Table 1 lists the 40 case–control studies reporting measurements of blood, hair, brain, teeth and/or urinary heavy metals in children with ASD compared with control children.
Table 1. Case–control studies reporting blood, hair, urinary, tooth or brain concentrations of heavy metals in children with ASD compared with controls.
| Study, year, location | No. ASD | No. controls | Blood Hg | Hair Hg | Urine Hg | Tooth Hg | Brain Hg | Blood Pb | Hair Pb | Urine Pb | Tooth Pb | Blood Cd | Hair Cd | Urine Cd | Blood Al | Hair Al | Urine Al | Blood other | Hair other | Urine other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdullah et al., 2012,88 United States |
22 |
62 |
|
|
|
↔ |
|
|
|
|
↔ |
|
|
|
|
|
|
|
|
|
| Adams et al., 2006,78 United States |
51 |
40 |
|
↔ |
|
|
|
|
↔ |
|
|
|
↔ |
|
|
↓ |
|
|
↔ |
|
| Adams et al., 2007,86 United States |
15 |
11 |
|
|
|
↑ |
|
|
|
|
↔ |
|
|
|
|
|
|
|
|
|
| Adams et al., 2008,77 United States |
78 |
31 |
|
↔ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Adams et al., 2013,59 United States |
55 |
44 |
↔ |
|
|
|
|
↑ |
|
↑ |
|
↓ |
|
↔ |
|
|
↔ |
↔ |
|
↑ |
| Al-Ayadhi., 2005,70 Saudi Arabia |
77a |
80 |
|
↑ |
|
|
|
|
↑ |
|
|
|
↑ |
|
|
↔ |
|
|
↑ |
|
| Al-Farsi et al., 2012,107 Oman |
27 |
27 |
|
|
|
|
|
|
↑ |
|
|
|
↑ |
|
|
↑ |
|
|
↑ |
|
| Albizzati et al., 2012,58 Italy |
17 |
20 |
↔ |
↔ |
↔ |
|
|
↔ |
↔ |
↔ |
|
|
↔ |
↔ |
↔ |
↔ |
↔ |
|
|
|
| Blaurock-Busch et al., 2011,63 Saudi Arabia |
25 |
25 |
|
↔ |
↑ |
|
|
|
↑ |
↑ |
|
|
↑ |
↔ |
|
↔ |
↑ |
|
↑ |
↔ |
| Blaurock-Busch et al., 2012,74 Saudi Arabia |
44 |
146 |
|
↑ |
|
|
|
|
↑ |
|
|
|
↑ |
|
|
↑ |
|
|
↑ |
|
| Bradstreet et al., 2003,65 United States |
221 |
18 |
|
|
↑b |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cohen et al., 1976,100 United States |
18 |
16 |
|
|
|
|
|
↑ |
|
|
|
|
|
|
|
|
|
|
|
|
| Cohen et al., 1982,103 United States |
33 |
16 |
|
|
|
|
|
↔ |
|
|
|
|
|
|
|
|
|
|
|
|
| De Palma et al., 2012,81 Italy |
44 |
61 |
|
↔ |
|
|
|
|
↔ |
|
|
|
↔ |
|
|
↔ |
|
|
↔ |
|
| El-Ansary et al., 2010,101 Saudi Arabia |
14 |
12 |
|
|
|
|
|
↑ |
|
|
|
|
|
|
|
|
|
|
|
|
| El-Ansary et al., 2011,102 Saudi Arabia |
25 |
16 |
|
|
|
|
|
↑ |
|
|
|
|
|
|
|
|
|
|
|
|
| El-Baz et al., 2010,71 Egypt |
32 |
15 |
|
↑ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Elsheshtawy et al., 2011,72 Egypt | 32 | 32 | ↑ | ↑ | ||||||||||||||||
| Fido and Al-Saad., 2005,69 Kuwait |
40 |
40 |
|
↑ |
|
|
|
|
↑ |
|
|
|
↔ |
|
|
↔ |
|
|
↑ |
|
| Gentile et al., 1983,108 United States |
47 |
37 |
|
|
|
|
|
|
↔ |
|
|
|
|
|
|
↔ |
|
|
↔ |
|
| Geier et al., 2010,57 United States |
83 |
89 |
↑ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Hertz-Picciotto et al., 2010,62 United States |
332 |
166 |
↔ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Holmes et al., 2003,84 United States |
94 |
45 |
|
↓ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Ip et al., 2004,55 Hong Kong |
82 |
55 |
↑c |
↔ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Kern et al., 2007,82 United States |
45 |
45 |
|
↔ |
|
|
|
|
↓ |
|
|
|
↓ |
|
|
|
|
|
↓ |
|
| Lakshmi Priya and Geetha, 2011,73 Indiad |
45 |
50 |
|
↑ |
|
|
|
|
↑ |
|
|
|
|
|
|
|
|
|
|
|
| Majewska et al., 2010,75 Poland |
91 |
75 |
|
** |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Obrenovich et al., 2011,83 United States |
26 |
39 |
|
↓ |
|
|
|
|
↔ |
|
|
|
|
|
|
|
|
|
↑ |
|
| Rahbar et al., 2012,110 Jamaica |
65 |
65 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
↓ |
|
|
| Rahbar et al., 2013,60 Jamaica |
65 |
65 |
↔ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Sajdel-Sulkowska et al., 2008,89 United States |
6 |
9 |
|
|
|
|
↔ |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Shearer et al.,109 1982, United States |
12 |
12 |
|
|
|
|
|
|
↔ |
|
|
|
↓ |
|
|
|
|
|
|
|
| Soden et al., 2007,66 United States |
15 |
4 |
|
|
↔b |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stamova et al., 2011,61 United States |
33 |
51 |
↔ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Tian et al., 2011,104 United States |
37 |
15 |
|
|
|
|
|
↔ |
|
|
|
|
|
|
|
|
|
|
|
|
| Vergani et al., 2011,105 Italy | 28 | 32 | ↔ | ↑ | ↔ | ↑ | ||||||||||||||
| Wecker et al., 1985,79 United States |
12 |
22 |
|
↔ |
|
|
|
|
↔ |
|
|
|
↔ |
|
|
|
|
|
↔ |
|
| Williams et al., 2008,80 United States |
15 |
16 |
|
↔ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Wright et al., 2012,64 United Kingdom |
56 |
197 |
|
|
↔ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Yorbik et al., 2010,106 Turkey | 30 | 20 | ↓ | ↓ |
Abbreviations: ASD, autism spectrum disorder; Al, aluminum; Cd, cadmium; Hg, mercury; Pb, lead.
↑, significantly higher in ASD compared with controls; ↓, significantly lower in ASD compared with control; ↔, no significant difference between ASD and controls; **, younger children were lower and older children higher.
Included eight patients with ADD.
After dimercaptosuccinic acid.
Reanalyzed by Desoto and Hitlan,56 and a significant association was found.
Also used nail samples.
Mercury-related biomarkers
Mercury was examined in 29 case–control studies of ASD and TD children (Table 1), with 12 studies (41%) reporting at least one elevation. Of these 12 latter studies, only 3 (25%) were performed in the United States. Concerning the 17 studies reporting similar or lower mercury levels in the ASD group compared with controls, 13 (76%) were performed in the United States.
Blood
The first case–control study to examine blood mercury levels in ASD compared mean whole blood and hair mercury concentrations in 82 children with ASD aged 4–11 years from Hong Kong and 55 age-matched TD children who had similar estimated environmental mercury exposures (determined by parental questionnaire). This study originally reported no significant differences between groups (P=0.15 for whole blood; P=0.79 for hair).55 However, a reanalysis of this study was performed after other investigators noted typographical and statistical errors in the published analytical data. After correcting these errors, this reanalysis reported that the mean whole-blood mercury concentration in the ASD group was significantly higher than in the control group (P=0.017).56 Another retrospective case–control study reported that the mean RBC mercury level was 1.9-fold higher (P<0.0001) in 83 children with ASD (mean age 7.3 years, s.d. 3.7) compared with 89 unmatched control children (mean age 11.4 years, s.d. 2.2).57 These two studies are limited by relatively small sample sizes and have not been replicated by other studies (reviewed in next paragraph).
In contrast, five case–control studies totaling 502 children with ASD and 346 TD children reported no significant difference in mean whole blood58, 59, 60, 61, 62 or RBC59 mercury levels between the two groups, although one of these studies (reviewed below) reported differing regulated genes with increasing blood mercury levels in the ASD group compared with controls,61 and another study reported that both whole blood and RBC mercury were related to standardized questionnaires of ASD severity.59 Collectively, these five studies have a larger sample size compared with the two previous studies (that reported a higher blood mercury level in the ASD group compared with controls) and therefore could carry more weight. However, it is clear that not every study measured mercury from the same type of blood sample and the fact that one study found a relationship between autism severity and mercury levels, despite not finding a significant group difference,59 suggests that characteristics of the sample population (that is, less versus more severe autism) could skew the mean difference between groups. Therefore, further studies are needed to clarify whether a relationship exists between blood mercury and autism.
Urine
One case–control study from Egypt reported a significantly higher mean urinary mercury level in 25 children with ASD aged 3–9 years of age compared with 25 age- and gender-matched controls; hair mercury levels were similar.63 Another case–control study reported similar urinary mercury levels between 56 ASD children and several unmatched control groups including 42 siblings of ASD children, 121 children without ASD in a mainstream school and 34 children in a special educational school.64 These two studies are limited by smaller sample sizes and the lack of replication between the two studies.
A higher urinary excretion of mercury after administration of oral dimercaptosuccinic acid (DMSA) was reported in one retrospective study of 221 children with ASD compared with 18 unmatched controls without ASD who were referred to a clinic for evaluation of possible mercury exposure.65 A smaller case–control study found similar urinary mercury concentrations after DMSA administration between 15 ASD children and 4 TD controls.66 Finally, in an uncontrolled study of 44 children with ASD from Egypt, the administration of oral DMSA led to a significantly increased urinary excretion of mercury, lead and cadmium as well as improvements in autistic behavior as measured by the Childhood Autism Rating Scale (CARS).67 Collectively, two of these three studies reported increased urinary excretion of heavy metals after administration of a chelator65,67 which suggests a higher metal burden in the children with ASD.68 However, these studies suffered from limitations as there was no placebo control and the status of the patient was not blinded to the treating physician. Therefore, further studies are warranted to investigate these findings in more detail.
Hair
Six case–control studies (all performed outside the United States) reported a higher mean hair mercury concentration in children with ASD compared with TD children; these studies were performed in Kuwait on 40 children with ASD and 40 age- and gender-matched controls;69 in Saudi Arabia on 77 children with ASD (although attention deficit disorder was considered an ASD in eight subjects) and 80 age- and gender-matched controls;70 in Egypt on 32, 2–13-year old, children with ASD and 15 age- and gender-matched controls;71 in Egypt on 32 children with ASD and 32 age- and gender-matched controls;72 in India on 45 children with ASD aged 4–12 and 50 age- and gender-matched controls;73 and in Saudi Arabia on 44 children with ASD aged 3–9 years and 146 age-matched controls.74 Besides these six studies, an additional case–control study of 91 children with ASD and 75 age- and sex-matched TD controls from Poland reported an unusual relationship between hair mercury concentrations and age: the mean hair mercury level was significantly lower in younger children with ASD (ages 3–4 years) compared with the respective age-matched control group, but significantly higher in older children with ASD (ages 7–9 years) compared with the respective age-matched control group.75 Finally, one recent uncontrolled study from Japan reported that 56 out of 1967 ASD children (2.8%) had an elevated level of scalp hair mercury compared with a normative reference range.76 Collectively, these studies support an association between ASD and elevated levels of heavy metals in hair samples; however, because all of these studies occurred outside of the United States, these findings might have limited applicability in the United States. These studies are also limited by relatively small sample sizes.
In contrast, a number of other case–control studies were unable to find a significant association between hair mercury and ASD. Nine studies reported similar mean concentrations of hair mercury in a total of 369 children with ASD compared with 315 TD children;55,58,63,77, 78, 79, 80, 81, 82 in one of these studies, urinary and blood mercury levels were also similar.58 As previously mentioned, two of these latter studies reported higher mercury in the blood55,56 or urine63 in the ASD group. In addition, two other studies reported a lower mean hair mercury level in a total of 120 children with ASD compared with 84 TD controls.83,84 A recent meta-analysis of seven studies reported a similar mean hair mercury level in a total of 343 ASD children and 317 TD children.81 These studies provide additional evidence that hair mercury levels are not associated with ASD, at least in the United States, especially since all but three of the studies55,58,63 occurred in the United States.
Three studies reported an intriguing relationship between ASD severity and hair mercury concentrations. The first study reported that a lower mean hair mercury in children with ASD was associated with more severe language impairments.84 Another study of 78 children with ASD and 31 control children reported that, compared with children who had higher hair mercury levels, children with lower levels of hair mercury were 2.5-fold more likely to have ASD.77 Finally, one case–control study reported a significantly lower mean hair mercury level in children with ASD at 3–4 years of age but a significantly higher mean mercury level in other children with ASD at 7–9 years of age compared with their respective age-matched controls.75 Collectively, these investigators suggested that these findings are evidence of impaired mercury excretion in younger children with ASD as less hair mercury (believed to be a marker of excretion) was associated with a higher ASD severity or risk of ASD.75,77,84 However, it should be noted that, in the normal population, the relationship between estimated total mercury burden and hair levels of mercury is variable and may be linked to polymorphisms in detoxification genes.85 Further studies are needed to clarify these complicated findings.
Teeth
One case–control study of 15 children with ASD and 11 TD children found a 2.1-fold higher concentration of mercury (P<0.05) in deciduous teeth in the ASD group but similar lead and zinc concentrations.86 Notably, the measurement of heavy metal concentrations in deciduous teeth may be a biomarker of cumulative exposures during gestation.87 However, another case–control study reported similar mercury concentrations in the deciduous teeth of 22 children with ASD compared with 20 children with disruptive behavior and 42 TD children matched on the child's gender and race, and parents' education and marital status.88 These two studies are limited by small sample sizes and the lack of replication between the two studies.
Brain
One post-mortem case–control study compared six ASD individuals with nine TD individuals and reported a nonsignificant elevation (68%) in cerebellar mercury concentration in the ASD group. However, cerebellar 3-nitrotyrosine (a putative biomarker of oxidative stress) was found to be significantly elevated in the ASD group (P=0.045) and was significantly correlated with mercury concentrations (r=0.796, P=0.0001). The investigators suggested that the tissue mercury burden could partially contribute to the increased oxidative stress observed in the cerebellum of the ASD subjects.89 This study is limited by a small sample size and the lack of replication by other studies; further studies examining toxicants in brain tissue of individuals with ASD are warranted.
Genetics
One case series examined the prevalence of ASD in 1380 grandchildren of 522 patients who had a history of infantile acrodynia (Pink disease) and reported that the prevalence of ASD in the grandchildren was 1 in 22 (incidence ratio=7.02; 95% CI, 4.28–10.84), suggesting that mercury sensitivity might be a heritable risk factor for ASD.90 Another study reported that children with ASD might have genetic differences in the ability to metabolize mercury. In this case–control study, significant differences in the relationship between the expression of 189 genes and mercury levels were found in 33 boys with ASD compared with 51 TD boys matched on age, despite no significant difference in mean blood mercury levels, suggesting that children with ASD might metabolize mercury differently than TD children.61 On the basis of these studies, additional studies are warranted to determine whether children with ASD have genetic differences in the ability to metabolize toxicants compared with TD children.
Lead-related biomarkers
Lead was examined in 25 case–control studies of ASD and TD children (Table 1), with 11 studies (44%) reporting at least one elevation. Of these 11 latter studies, 2 (18%) were performed in the United States. Concerning the 14 studies reporting similar or lower lead levels in the ASD group, 10 (71%) were performed in the United States.
Blood
Seven case reports/series described lead toxicity in individuals with ASD as measured by an elevated blood lead level;91, 92, 93, 94, 95, 96, 97 one study used whole-blood samples94 while the remaining studies presumably measured whole-blood lead but did not specifically note this. In addition, a retrospective, uncontrolled case series from 1980 reported that 15 out of 77 children with ASD (19%) had a blood lead concentration (not noted whether the sample was whole blood, plasma, serum or RBC) above 35 μg dl−1 and that the blood lead concentration was inversely correlated with intellectual functioning.98 Finally, a case series from Canada found that none of 48 ASD children selected from a convenience sample had a lead level (not noted whether the sample was whole blood, plasma, serum or RBC) above 0.48 μmol l−1 (the Centers for Disease Control and Prevention (CDC) threshold for intervention); however, nine children (19%) with a level above 0.1 μmol l−1 exhibited significantly more pica or oral-related behaviors.99 These studies are limited by small sample sizes (some are case reports), the retrospective nature and the lack of a control group.
Two of these studies reported that the presentation of lead toxicity can appear unusual in individuals with ASD: one case report described a 4-year old autistic boy who had a flu-like syndrome with an elevated blood lead level of 216 μg dl−1,96 and another case report depicted an individual with ASD who developed weight loss, abdominal pain, diarrhea and vomiting who had a blood lead level of 147 μg dl−1.94
Eight case–control studies compared blood lead concentrations in ASD individuals compared with controls, with four studies reporting a higher mean blood lead concentration in the ASD group. In the first study published in 1976, the whole-blood lead concentration was significantly higher in 18 children with autism compared with 16 unmatched non-autistic psychotic ‘atypical' children and 10 TD siblings despite the fact that none of the children had any known episodes of acute lead exposure. In 11 of the 18 children with autism (61%), blood lead concentrations were more than two s.d. above the mean for the sibling group.100 The second study of 55 children with ASD and 44 TD children reported significantly higher mean urinary and RBC lead levels (but not whole-blood lead) in the ASD group.59 The third study from Saudi Arabia reported a significantly higher mean RBC lead concentration (2.6-fold higher) in 14 ASD children compared with 12 age-matched controls. In the ASD group, the blood lead concentration significantly correlated with markers of mitochondrial dysfunction (P=0.028) and oxidative stress (P=0.045).101 Finally, the last case–control study, also from Saudi Arabia, reported a significantly higher RBC lead concentration in 25 children with ASD compared with 16 age-matched controls.102 These studies are limited by relatively small sample sizes.
However, four case–control studies did not confirm a higher blood lead concentration in the ASD group. In a study from 1982, no significant difference in the mean blood lead concentration (not noted whether sample was whole blood, plasma, serum or RBC) was found in 33 ASD children, 34 control children with Tourette syndrome and 16 TD children (all children were enrolled in the same school), although a lead concentration above 26 μg dl−1 was found in 14% of the ASD group but none of the TD group.103 Another case–control study reported a similar mean whole-blood lead concentration in 37 ASD children compared with 15 TD children of similar age.104 Similar whole-blood levels were found in one study of 17 children with ASD and 20 TD controls,58 and similar plasma levels were reported in another study of 28 children with autistic disorder and 32 TD controls.105 Similar to the previous four studies, these studies are also limited by smaller sample sizes and also by the fact that not every study measured lead from the same type of blood sample; therefore, larger case–control studies are warranted.
One study from a lead treatment program in Boston suggested that children with ASD might be more easily exposed to lead compared with non-ASD children. This study used a retrospective chart review to identify 17 ASD children treated for lead poisoning and compared them with 30 randomly selected non-ASD children with lead poisoning. Notably, the children with ASD were significantly older at the diagnosis of lead toxicity (46 versus 30 months of age, P=0.03) had elevated blood lead concentrations for a longer period of time during treatment with a chelator (39 versus 14 months, P=0.013) and were more likely to be re-exposed to lead during the study period (75 versus 23% re-exposed, P=0.001) despite close monitoring, environmental inspections and adequate lead cleanup procedures or alternative housing.95
Urine
A significantly higher mean urinary lead level was found in one study of 25 children with ASD compared with 25 controls from Saudi Arabia.63 However, a lower mean urinary lead concentration was reported in 30 ASD children compared with 20 controls in another study from Turkey.106 Finally, a similar urinary lead level was reported in a study from Italy of 17 children with autism and 20 controls.58 These three urinary studies are limited by small sample sizes and the lack of replication between studies.
Hair
Studies examining hair lead concentrations in children with ASD have demonstrated mixed results. Seven case–control studies performed in Kuwait,69 Saudi Arabia,63,70,74 Egypt,72 Oman,107 and India73 have reported a higher mean hair lead concentration in a total of 290 children with ASD compared with 400 TD children. In two of these studies, higher hair and nail lead levels were significantly correlated with more severe ASD symptoms as measured by CARS,73 and higher hair lead levels were significantly and negatively correlated with Intelligence Quotient (IQ) in the ASD group.72 One recent uncontrolled study from Japan reported that 94 out of 1967 ASD children (4.8%) had an elevated level of scalp hair lead compared with a normative reference range.76 Similar to the hair mercury studies, these hair lead studies are limited by relatively small sample sizes, and as all of them took place outside the United States, their findings may be limited in applicability in the United States.
In contrast, seven case–control studies examining hair lead concentrations in a total of 209 children with ASD reported similar concentrations compared with 231 TD children;58,78,79,81,83,108,109 as previously mentioned, one of these studies reported similar urinary levels.58 In addition, one study reported a lower mean concentration of hair lead in 45 children with ASD compared with 45 TD controls.82 Notably, a recent meta-analysis of five studies81 reported a significantly higher mean hair lead concentration in 167 children with ASD compared with 217 controls (P=0.021); however, these findings were driven predominantly by the results from one study. These studies were limited by smaller sample sizes, but provide further evidence that hair lead is not associated with ASD, at least in the United States, as all but one of these studies58 occurred in the United States.
Teeth
Similar lead concentrations were reported in deciduous teeth between a total of 37 children with ASD and 73 TD controls in two studies.86,88 These two studies were limited by small sample sizes, but did replicate each other.
Genetics
One case–control study reported that children with ASD might have genetic differences in the ability to metabolize lead. In this study, significant differences in the relationship between the expression of 162 genes and lead levels were found in 37 children with ASD compared with 15 TD children matched on age, despite no significant difference in mean blood lead levels, suggesting that children with ASD might metabolize lead differently than TD children.104 This study was limited by small sample sizes and therefore additional studies are needed to investigate potential genetic differences in the ability to metabolize lead in individuals with ASD.
Cadmium
Cadmium was examined in 14 case–control studies of ASD and TD children (Table 1), with five studies (36%) reporting at least one elevation. Of these latter five studies, none were performed in the United States. Concerning the nine studies reporting similar or lower cadmium levels in the ASD group, five (56%) were performed in the United States.
Blood
Only two studies examined blood cadmium levels. One study reported a significantly higher plasma cadmium level in 28 children with autistic disorder compared with 32 TD children.105 The other study reported a significantly lower mean whole blood cadmium level in 55 children with ASD compared with 44 TD controls.59 These two studies are limited by small sample sizes and the lack of replication between studies.
Urine
Three studies reported similar urinary cadmium levels in a total of 97 children with ASD and 89 TD controls,58,59,63 whereas another study reported a lower mean urinary cadmium concentration in 30 children with ASD compared with 20 controls.106 These studies are also limited by small sample sizes but do report similar findings.
Hair
Five studies reported similar hair cadmium levels in a total of 164 children with ASD compared with 183 TD controls.58,69,78,79,81 Two studies reported a lower mean hair cadmium level in a total of 57 children with ASD and 57 TD controls.82,109 Only four studies reported a higher hair cadmium hair level in a total of 173 children with ASD compared with 278 TD children.63,70,74,107 One recent uncontrolled study from Japan reported that 168 out of 1967 ASD children (8.5%) had an elevated level of scalp hair cadmium compared with a normative reference range.76 A recent meta-analysis of four studies totaling 152 children with ASD and 167 TD controls found no significant association between hair cadmium concentrations and ASD.81 Similar to the studies on hair mercury and lead, these studies are limited by small samples sizes, lack of replication between studies and by whether or not these findings are applicable in the United States.
Aluminum
Eleven case–control studies examined aluminum levels in ASD (Table 1), with three (27%) reporting at least one elevation. Of these three latter studies, none were performed in the United States. Concerning the eight studies reporting similar or lower aluminum in the ASD group, three (38%) were performed in the United States.
Blood
Two studies totaling 45 children with ASD and 52 TD children reported similar whole blood58 or plasma aluminum levels105 between the two groups. These studies are limited by small sample sizes and by measurements taken from different types of blood samples, but do replicate each other.
Urine
One study reported higher urinary aluminum in 25 children with ASD compared with 25 controls,63 wherease two studies reported similar urinary aluminum in 72 children with ASD compared with 64 TD controls.58,59 These studies are also limited by small sample sizes and the lack of replication between studies.
Hair
Six studies totaling 250 children with ASD and 263 controls reported similar hair aluminum levels.58,63,69,70,81,108 Another study reported lower hair aluminum in 51 children with ASD compared with 40 controls,78 whereas two studies reported a higher mean hair aluminum level in a total of 71 ASD children compared with 173 controls.74,107 Finally, one recent uncontrolled study from Japan reported that 339 out of 1967 ASD children (17.2%) had an elevated level of scalp hair aluminum compared with a normative reference range.76 Similar to the previous hair studies on other heavy metals, these studies are limited by small sample sizes and might not be applicable in the United States, as many took place outside the United States.
Other heavy metals
Fourteen case–control studies examined other heavy metals, with eight studies (57%) reporting at least one elevation (Table 1).
Arsenic
Eight case–control studies examined arsenic levels in ASD children, with five studies (63%) reporting at least one elevation. Four studies reported a higher mean hair arsenic level in a total of 172 children with ASD compared with 290 TD controls,63,70,74,83 whereas one study reported lower hair arsenic in 45 ASD children compared with 45 TD controls.82 One study of 55 children with ASD and 44 TD controls reported similar whole blood, RBC and urinary arsenic levels.59 Another study from Jamaica reported significantly lower whole-blood arsenic in 65 children with ASD compared with 65 age- and sex-matched controls, with ASD status not significantly linked to blood arsenic concentration.110 A significantly higher mean plasma arsenic level in 28 children with autistic disorder compared with 32 TD children was reported in another study from Italy.105 Finally, one recent uncontrolled study from Japan reported that 52 out of 1967 ASD children (2.6%) had an elevated concentration of scalp hair arsenic compared with a normative reference range.76 These studies on arsenic are limited by relatively small sample sizes and the lack of replication between studies.
Nickel
Two studies reported a higher hair nickel in a total of 71 children with ASD compared with 173 TD controls.74,107 Another study of 55 children with ASD and 44 TD controls reported similar urinary nickel levels.59 These nickel studies are limited by small sample sizes and the lack of replication between studies.
Uranium
One study reported elevated hair uranium in 40 children with ASD compared with 40 TD controls.69 Another study of 55 children with ASD and 44 TD controls reported similar urinary uranium levels.59 The two studies are also limited by small sample sizes and the lack of replication between studies.
Tin
One study reported a significantly higher mean urinary tin in 55 children with ASD compared with 44 TD controls.59
Biomarkers of heavy metals and ASD severity
Seven studies reported a possible relationship between autism severity and biomarkers of heavy metals. One uncontrolled treatment study reported that the severity of autism was significantly associated with the toxic metal body burden as estimated by the excretion of heavy metals into the urine after the administration of oral DMSA in 63 children with ASD.111 Another study of 55 children with autism and 44 controls reported that the whole blood and RBC levels of toxic metals significantly correlated with autism severity.59 As previously discussed, two other studies reported that higher scores on the CARS (indicating more severe autism symptoms) were significantly related to higher hair and nail mercury and lead levels73 and higher hair mercury concentration.72 One study reported that higher hair lead levels were significantly correlated with lower IQ.72 Higher blood lead concentrations were correlated with lower intellectual functioning in children with ASD in another study.98 One study reported that higher hair levels of lead were correlated with verbal communication problems and more ASD symptoms as measured by CARS, and that higher mercury levels were correlated with greater problems in object use and auditory response.74 Finally, an uncontrolled study of 18 children found that elevated hair mercury levels correlated with higher ASD severity as measured by CARS.112 Collectively, these studies report a possible relationship between autism severity and heavy metal biomarkers, suggesting evidence of a dose–effect relationship. These studies also demonstrate the limitations of simply examining group differences as at least one study found a relationship between autism severity and mercury levels despite not finding a significant group difference,59 suggesting that characteristics of the sample population (that is, less versus more severe autism) could skew the mean difference between groups when autism severity is not taken into account.
Twelve studies reported improvements in biomarkers of toxicants113,114 or in clinical symptoms67,91,92,100,115, 116, 117, 118, 119, 120 in children with ASD using treatments incorporating detoxification methods. No significant adverse effects were reported in these studies. However, none of these studies contained a control group or were placebo controlled. Additional studies examining detoxification methods in children with ASD are warranted to confirm the effects of these treatments.121
Urinary porphyrin studies
A number of studies have reported that urinary porphyrin concentrations may be biomarkers of estimated heavy metal exposure or burden in children with ASD. Porphyrins are molecular precursors of heme; heavy metals inhibit enzymes in the heme porphyrin pathway and result in specific porphyrin excretion patterns in the urine.122 For example, mercury exposure has been reported to cause an increase in the urinary excretion of precoproporphyrin, coproporphyrin, and pentacarboxyporphyrin.122,123 In animal studies, urinary porphyrin concentrations have been found to significantly correlate (r~0.9) with the renal content and body burden of mercury.124 In addition, in the context of occupational mercury exposure, urinary porphyrin concentrations have been correlated with neurobehavioral deficits.125
Four uncontrolled case series126, 127, 128, 129 and seven case–control studies114,130, 131, 132, 133, 134, 135 have reported abnormal urinary porphyrin concentrations in children with ASD. One study reported that urinary porphyrin levels were strong predictors of ASD.132 Five studies reported that higher urinary porphyrin levels were correlated with more severe ASD symptoms.114,127, 128, 129, 130 Two studies reported that porphyrin levels significantly correlated with either plasma oxidized glutathione (GSH) concentrations127 or other oxidative stress markers in children with ASD.135 Two studies reported that porphyrin levels demonstrated significant reductions following treatment with 2,3-dimercaptopropane-1-sulfonate or DMSA.114,131 Most of the investigators in these studies suggested that the urinary porphyrin abnormalities were markers of increased heavy metal burden in children with ASD. However, in one study, porphyrin levels were not significantly correlated with urinary mercury levels or estimates of previous mercury exposure in children with ASD.134
Collectively, these studies observed higher mean urinary porphyrin concentrations in children with ASD compared with controls with a significant correlation in some studies between the degree of porphyrin elevation and ASD severity or physiological abnormalities. Therefore, these studies suggest that the processes that change porphyrin excretion may be associated with processes related to underlying ASD pathophysiology, and that the specific changes in the urinary porphyrin excretion patterns observed could be biomarkers of such processes. Although porphyrin concentrations may be related to heavy metal body burden,124 other physiological processes such as oxidative stress or mitochondrial dysfunction might also contribute to changes in porphyrin excretion.136 Additional controlled studies investigating urinary porphyrin levels in comparison with heavy metal exposures and other biomarkers are warranted to assess whether or not these findings are accurate reflections of increased heavy metal burden in individuals with ASD.
Solvents, pesticides and PCBs
One case series of 18 children with autism reported blood concentrations of solvents that were above the upper limit of normal values established for adults in 16 of the children (89%), including triethylbenzene (44%), xylene (39%), trimethylbenzene (33%), ethylbenzene (33%), 2-methylpentane (33%) and 3-methylpentane (33%).137 A second case series measured blood concentrations of organochlorine pesticides, PCBs and solvents in 38 children with autism and reported levels above the upper limit of normal values established for adults in 34 of the children (89%), including 3-methylpentane (61%), n-hexane (45%), toluene (34%), 2-methylpentane (34%), tetrachloroethylene (26%), mirex (16%) and trimethylbenzene (16%). When examined by xenobiotic class, elevated concentrations of solvents were found in 33 children (87%), pesticides in 9 children (24%) and PCBs in 4 children (11%). The children were also given caffeine and acetaminophen to estimate liver detoxification capacity; abnormalities in phase II detoxification were reported, including impairments in glycine conjugation in 31% of children, GSH conjugation in 22%, sulphation in 19% and glucuronidation in 19%. The investigators hypothesized that increased exposures to xenobiotics in conjunction with altered liver detoxification might increase the risk of developing ASD.138 These two studies were limited by the lack of a control group, the retrospective nature, the use of adult laboratory reference ranges as controls and relatively small sample sizes.
Two case–control studies measured PCB concentrations in ASD. The first study reported that 17 children with ASD and 7 healthy siblings had similar concentrations of PCBs in umbilical cord samples.139 The second study examined post-mortem brain samples and measured 7 polybrominated diphenyl ether and 7 PCB congeners; only one congener, PCB95, was associated with a genetic form of ASD (32 children with 15q11–13 duplications/deletions and other genetic syndromes) but this finding was not observed in 32 individuals with idiopathic ASD or 43 controls.140 These two studies do not support a strong association between biomarkers of PCBs and ASD, although they are limited by small sample sizes.
Phthalates
One study of 48 children with ASD and 45 control children reported that urinary concentrations of two phthalates (5-OH-MEHP and 5-oxo-MEHP) were significantly increased in the ASD group compared with the control group.141 Another study reported that 50 children with ASD had decreased glucuronidation of diethylhexyl phthalate as measured by urinary metabolites compared with 53 age-matched TD controls despite similar phthalate exposure levels.142 Notably, glucuronidation is a significant pathway involved in the metabolism of xenobiotics and lower glucuronidation might lead to a decreased detoxification capacity for phthalates. These two studies are limited by relatively small sample sizes, but do provide some overlap of findings.
Polybrominated diphenyl ethers
One case–control study examined the effects of the xenobiotic 2,2′,4,4′-tetrabrominated biphenyl on the immune response of lipopolysaccharide-stimulated peripheral blood mononuclear cells in 19 children with ASD compared with 18 age-matched TD children, and reported that cells from the ASD group demonstrated significantly higher in vitro interleukin-1β (P=0.033) and interleukin-8 (P<0.04) production after 2,2′,4,4′-tetrabrominated biphenyl exposure compared with controls. The investigators suggested that the ASD group had ‘altered sensitivity' to 2,2′,4,4′-tetrabrominated biphenyl consistent with increased immune activation.143 This study is limited by small sample sizes and the lack of replication by other studies, and therefore additional studies are needed to investigate these findings.
Summary
Forty case–control studies compared heavy metal concentrations from various tissues/body fluids in a total of 2089 children with ASD and 1821 TD children, with 21 (53%) of these studies (totaling 1109 children with ASD and 999 TD children) reporting that heavy metal levels were similar or lower in children with ASD (Table 1). Of these 21 studies, 15 (71%) studies took place in the United States. Of the 19 studies reporting a positive association, 6 (32%) took place inside the United States. This suggests that heavy metal exposures in children with ASD may be less common in the United Staes compared with the remainder of the world, and that more work is needed to counteract the adverse effects of toxicants in countries outside the United States.
The most studied heavy metals were mercury (29 case–control studies) and lead (25 case–control studies). The percentage of studies showing an elevated metal level in at least one tissue/sample ranged from 27% (aluminum) to 63% (arsenic), with studies for mercury (41%), lead (44%) and other metals (57%) in between.
One study found significant associations between lead concentrations and markers of mitochondrial dysfunction and oxidative stress.101 Seven studies correlated increased heavy metal levels with either impaired intellectual functioning or increased ASD severity/behaviors, suggesting evidence of a dose–effect relationship.
Two uncontrolled studies reported elevated levels of solvents, pesticides and PCBs compared with the upper limit of normal values established for adults. Two studies reported similar or lower PCB levels in children with ASD compared with controls. One case–control study reported higher urinary concentrations of two different phthalates in the ASD group compared with controls.
Some studies reported differences that could have a genetic basis in children with ASD compared with controls concerning the ability to metabolize toxicants. For example, two studies suggested that children with ASD appear to metabolize mercury61 and lead104 differently than TD children. One study found ‘altered sensitivity' to 2,2′,4,4′-tetrabrominated biphenyl in children with ASD compared with controls.143 Another study found abnormalities in phase II detoxification in an uncontrolled study of children with ASD, suggesting altered liver detoxification.138 Finally, two studies reported evidence of impaired glucuronidation in children with ASD.138,142 These findings raise the possibility that some children with ASD might not metabolize toxicants as efficiently as TD children and therefore might experience adverse effects of toxicants at lower concentrations compared with TD children. Additional studies are warranted to determine whether the reported differences in the ability to metabolize lead and mercury and other toxicants contribute to more severe problems in children with ASD compared with TD children.
Most of the studies reviewed suffered from limitations that were listed with the corresponding studies. Some of these limitations included relatively small sample sizes, the lack of replication of some findings, questionable applicability of the findings to the United States (as some studies took place outside the United States) and the lack of a control group in some studies. Overall, the evidence linking biomarkers of toxicants to ASD does not appear as strong as the evidence linking estimated exposures to toxicants in the environment and ASD risk (as reviewed in the first section). This suggests that current biomarkers may be limited in their ability to identify an association between environmental toxicants and ASD; that this association is more easily identified when examining ASD as a group, rather than on an individual level; or that unaccounted for variations in genetic factors could result in different thresholds for susceptibility to certain toxicants.
Another significant issue in biomonitoring toxicant levels is that biomarkers in hair, blood and urine do not necessarily reflect retained levels within specific tissues. Toxicants are potentially mobile and their presence may be abundant in tissues with no evidence of toxicants in blood or urine testing.144 Toxicant tissue levels within specific tissues can also be dynamic depending on various physiological determinants within the body such as caloric state, exercise, fever and so on.145 Furthermore, even within identical tissues in different locations within the same person, toxicant levels can vary considerably.146 Accordingly, snapshot testing of hair, urine or blood for toxicant levels are notoriously unreliable and may underestimate the toxicant burden within the body. Emerging testing methods incorporating tissue mobilization of toxicants with techniques such as caloric restriction may better reflect the level of retained toxicants.147 Consequently, the lack of a strong association between levels of toxicant biomarkers and ASD in studies published to date may reflect limitations with toxicant biomonitoring rather than the absence of a definitive link. It is also possible that future research might identify better biomarkers of toxicants in ASD.148 Given these findings, additional studies on biomarkers of environmental toxicants in individuals with ASD are warranted.
Gene–toxicant interactions in ASD
This section discusses studies (Figure 2) that have examined SNPs in environmental response genes that are involved in the detoxification of environmental pollutants in ASD individuals (Supplementary Table S1).
Paraoxonase abnormalities
Paraoxonase (PON) is an enzyme that hydrolyzes and inactivates a number of OP pesticides. Four studies have reported decreased PON1 activity in individuals with ASD or autistic symptoms.149, 150, 151, 152
Polymorphisms in PON1 have been examined in five studies of ASD, with three studies (60%) reporting an association. One case–control study of 312 children with autism and 676 first-degree relatives investigated three common SNPs in PON1 (C-108T, Q192R and L55M) and reported that the L55/R192 haplotype, which confers less PON1 activity in vitro, was associated with a significantly increased risk of autism in Caucasian-American families living in North America (P=0.015) but not in Italian families living in Italy; the investigators suggested higher exposure levels to pesticides in North America accounted for this difference in risk.153 Another study of 353 2-year-old children and their mothers reported that children with the PON1-108T allele were more likely to be reported by their mothers as having symptoms of PDD as measured by the Child Behavior Checklist; interactions between PON1 polymorphisms and urinary DAP metabolites were not found to be significant.149 Finally, one study of 174 patients with ASD, 175 first-degree relatives and 144 controls reported that ASD was associated with SNPs in PON1, which can alter protein amounts (rs705379: C108T) and substrate specificity (rs662: Q192R).152 This study verified the functional significance of these SNPs by showing a significant decrease in PON1 arylesterase activity in the ASD group compared with the two control groups.
However, two studies were unable to verify an association between polymorphisms in PON1 and ASD. No significant association was found between PON1 genotype and ASD (P=0.12) in 196 families with at least two affected family members with ASD.154 A Romanian study of 50 children with ASD and 30 TD children found no significant differences in Q192R and L55M PON1 SNPs between the two groups, despite documenting decreased plasma PON1 arylesterase (P<0.001) and PON (P<0.05) activities in the ASD group compared with controls.151
Although four studies have reported lower PON1 activity in individuals with ASD or autistic symptoms,149, 150, 151, 152 the genetic studies reviewed above only provide mixed support for an association between genetic polymorphisms in the PON1 gene and ASD. Clearly, further studies will be needed to investigate this potential association and determine whether other factors besides genetic polymorphisms could be responsible for the changes in enzyme activity observed in the ASD group. Interestingly, one study suggested that the differences in the relationship between genetic polymorphisms and ASD diagnosis could be dependent on the level of exposure to specific toxicants,153 demonstrating the complexity of the interactions between genetic susceptibilities, toxicant exposures and ASD risk.
Glutathione and glutathione S-transferase abnormalities
Glutathione S-transferases (GST) catalyze the detoxification of heavy metals and xenobiotic compounds by catalyzing the conjugation of GSH to compounds including xenobiotics. One case–control study of 20 children with ASD demonstrated a significantly lower activity of GST compared with 20 controls.155
Four case–controlled studies have examined GST polymorphisms in ASD individuals or their mothers that could potentially affect enzyme activity, with three (75%) reporting an association. One study of 54 ASD case–parent trios and 172 controls reported a significantly higher frequency of GSTM1-null in the ASD group (OR=2.02; 95% CI, 1.03–4.04).156 Another study examining the GSTM1 gene found a marginal increase in GSTM1-null frequency in 80 children with ASD compared with 73 TD children (OR=1.37; 95% CI, 0.98–1.96).157 Interestingly, mothers with a polymorphism in the GSTP1 gene were 2.7-fold (95% CI, 1.39–5.13) more likely to have a child with autism compared with control mothers in another study.158 However, one study of 196 families with at least two affected family members with ASD found no significant association between the GSTP1 gene and ASD.154
Although few, these studies support the notion that polymorphisms in the GSTM1 or GSTP1 genes could be associated with ASD. Although none of these studies provided specific verification of enzyme dysfunction associated with a specific polymorphism, polymorphisms in GST genes, particularly GSTM1, have been associated with increased susceptibility in non-ASD populations to mercury toxicity,159,160 ethyl mercury sensitization161 and xenobiotic toxicity, including PCBs162 and polycyclic aromatic hydrocarbons.163 In addition, as polymorphisms in genes responsible for the production of GSH, the key substrate of GST, have been associated with ASD,157,164,165 and deficiencies in GSH have also been associated with ASD compared with controls,157,166, 167, 168 examination of both functional and genetic changes in the GSH pathway in future studies may help clarify important interactions between genes, GSH metabolism and toxicant elimination in ASD.
Other genetic abnormalities
In one case–control study, a polymorphism in ALAD2, but not in coproporphyrin oxidase, was found to be more common in 450 children with ASD compared with 251 TD children (OR=1.66; 95% CI, 1.06–2.62). Interestingly, the ALAD2 polymorphism was also associated with significantly lowered mean plasma total GSH (P=0.007) in the ASD group.169 Studies in non-ASD individuals have demonstrated that polymorphisms in ALAD2 are associated with increased susceptibility to lead toxicity170 and cognitive impairments from lead exposure.171
One case–control study of 196 families with at least two ASD children found an association between ASD and SNPs in the divalent metal ion transporter SLC11A3 and the metal regulatory transcription factor 1 (MTF1), but not in other genes involved in the detoxification of xenobiotics, including ABCC1 and SLC11A2.154 Both SLC11A3 and MTF1 are important in heavy metal metabolism: SLC11A3 is involved in the intracellular transport of heavy metals, including iron, lead, nickel and cadmium; MTF1 is important in the activation of metallothionein after exposure to heavy metals, which may consequently help limit metal toxicity. Finally, a smaller case–control study of 24 children with ASD and 24 controls found no significant association between polymorphisms in four genes implicated in mercury transportation (MT1a, DMT1, LAT1 and MTF1) and ASD.172
Summary
Ten unique studies of genes involved in toxicant elimination were reviewed in this section; one of the studies examined multiple genes (PON1, GSTP1, SLC11A3 and MTF1).154 Collectively, eight of these studies reported that children with ASD (or their mothers) were significantly more likely to have genetic variations in enzymes important in the detoxification of xenobiotics, including PON (three of five studies), GST (three of four studies), ALAD (one study), SLC11A3 (one study) and MTF (one of two studies). These findings might lead to increased susceptibility to the adverse effects of toxicants in children with ASD who have these SNPs compared with controls. However, the genes from only two groups of enzymes (that is, PON1 and GST) have undergone multiple studies, with slightly mixed results, and many of the studies reviewed have small sample sizes. In addition, genes encoding for enzymes important in xenobiotic elimination that have been demonstrated to have reduced activity in ASD, such as phenolsulphotransferase173,174 have not been studied to date in ASD. In addition, many studies that reported genetic abnormalities in enzymes did not measure enzymatic activity. For example, although four studies reported lower PON1 activity and one study reported lower GST activity in children with ASD compared with controls, most of these studies did not examine whether genetic abnormalities contributed to this finding. It is important to recognize that genetic polymorphisms are only one potential reason for changes in enzyme activity. Indeed, enzyme activity in vivo is modulated by such factors as gene expression, substrate and cofactor availability and metabolic modulators. Thus, the investigation of metabolism on a systems level may provide a clearer picture of the factors and interactions involved in potential differences in detoxification between ASD and TD individuals. In addition, as genetic susceptibility may only demonstrate a significant association with ASD in the context of a specific toxicant exposure, samples derived from diverse environmental exposures may demonstrate different relationships.
Discussion
Studies of potential associations between ASD and estimated environmental exposures to toxicants
A majority (34/37, 92%) of the studies examining a potential association between ASD and estimated environmental toxicant exposures reported a significant relationship. In fact, only three studies did not find a significant association.21,42,43 Fourteen studies suggested a dose–effect relationship—that is, higher estimated levels of gestational and/or early postnatal toxicant exposures significantly correlated with either an increased risk of developing ASD or a higher ASD prevalence. The four prospective studies reporting an association between ASD and environmental exposures to toxicants incorporated biomarkers estimating actual environmental toxicant exposure levels.17, 18, 19, 20 Collectively, these results suggest that occupational and environmental exposures in parents and children to known neurodevelopmental toxicants may be related to the development of ASD. Indeed, most of the toxicants reviewed have known neurological sequelae when exposure is significant. However, these studies do not prove a causative relationship but indicate that further investigation is warranted. The studies discussed in this review provide an indication of the types of toxicants that may be of interest in future studies and provide insight into the sources of such exposures. In addition, one study reported that mothers of children with ASD were less knowledgeable than mothers of TD children about environmental toxicants and had higher estimated exposures to toxicants during pregnancy.33 This suggests that increasing the knowledge concerning sources of toxicant exposure and the potential adverse effects of toxicants might lead to preventative strategies to help decrease the risk of ASD in some children.
One key remaining question is the mechanism by which these exposures could result in neurodevelopmental perturbations that could cause or contribute to the development of ASD. Measuring individual biomarkers of actual toxicant exposures at specific times may be useful to understand the types and quantities of toxicants that are necessary for increasing the risk of ASD. However, the use of biomarkers has limitations (discussed below). In addition, epidemiological studies provide insights into particular substances and times in neurodevelopment (that is, gestation) when biomarker measurements cannot be easily obtained and provide for the study of large populations that cannot be practically studied in detail on an individual level.
Studies examining biomarkers of toxicants and ASD
Forty case–control studies examined concentrations of heavy metals in blood, urine, hair, brain and/or teeth of children with ASD compared with TD children. The results of these studies were mixed, with almost half (19 studies) reporting at least one elevated level in ASD compared with controls. Approximately 40% of the mercury and lead studies reported a positive association with ASD, whereas about one-third or less showed a positive association with cadmium and aluminum. Many (68%) of the studies reporting a positive association with ASD were performed outside the United States, and some of these studies were older in nature when exposure levels in the environment may have been higher. These inconsistent findings concerning toxicant biomarkers and ASD suggest the relationship between ASD and toxicants is complex, currently available biomarkers may not be sufficient to identify an association between toxicants and ASD, and studies with more rigorous experimental designs are needed.
Most of the studies measuring biomarkers of toxicants examined blood, urine or hair. Only a few studies examined biomarkers in brain or teeth, which are areas that may better reflect bioaccumulated toxicant concentrations as they represent tissue where toxicants are deposited. Indeed, only two studies examined brain levels of toxicants.89,140 As the brain is the major organ system affected in ASD, additional studies examining toxicant concentrations in the brain are warranted. However, the identification of toxicants at the tissue level (for example, fat or organs) may be difficult as levels can vary across tissues, even in the same individual.146
Several studies suggested that children with ASD might metabolize toxicants differently than TD children based on genetic differences (discussed below). This finding raises the possibility that children with ASD may experience toxicity to pollutants at a lower concentration compared with TD children. For example, a child with a PON1 SNP might experience toxicity to a pesticide at a lower exposure level compared with a child without this SNP. Additional studies are warranted to examine whether the reported differences in the ability to metabolize lead and mercury and other toxicants cause more problems in children with ASD compared with TD children.
Links between biomarkers of toxicants and the severity of ASD symptoms
Seven studies reported that biomarkers of environmental toxicants were associated with ASD severity. For example, two studies reported that the severity of ASD was associated with the estimated heavy metal body burden.59,111 Five other studies correlated heavy metal levels with more severe autistic behaviors or intellectual problems.72, 73, 74,98,112 Five studies reported that urinary porphyrin concentrations correlated with the severity of ASD symptoms.114,127, 128, 129, 130 Collectively, these studies support the notion that higher exposures to environmental toxicants are associated with more severe ASD symptomatology. As biomarkers represent an important method of identifying environmental toxicant exposures and subsequent adverse effects on an individual level, additional studies are warranted to investigate this possibility.
Associations between toxicants and physiological abnormalities in ASD
In several studies, biomarkers of environmental toxicants were associated with physiological abnormalities in some individuals with ASD. For example, one study from Saudi Arabia reported that the blood lead concentration significantly correlated with markers of mitochondrial dysfunction and oxidative stress in children with ASD.101 In another study, a brain oxidative stress marker (3-nitrotyrosine) was significantly correlated with brain mercury concentrations in some individuals with ASD.89 Two studies reported that urinary porphyrin concentrations significantly correlated with oxidized GSH levels127 or other oxidative stress markers.135 Notably, many toxicants can produce abnormal physiology similar to that reported in some children with ASD, including depleting GSH levels, increasing oxidative stress, impairing cellular signaling, causing immune dysregulation and impairing mitochondrial function.6,175,176
Of note, the male-to-female ratio reported in ASD (4:1) may partially explain the apparent link between environmental toxicants and physiological abnormalities reported in ASD. For example, when compared with females, males are generally more susceptible to the toxic effects of heavy metals,177 pesticides,178 and PCBs.179 This heightened susceptibility may be due to a range of factors; some examples are higher oxidative stress180 and lower GSH levels181 generally observed in males compared with females. Animal studies also suggest males excrete mercury less readily than females,182,183 and that testosterone may increase the toxicity of mercury,184 whereas estrogen is protective.185 Notably, one of the reviewed studies reported that ASD was more significantly associated with pollutants in boys compared with girls.31 A recent systematic review suggested gender-related differences in the susceptibility to toxicants, with males generally more susceptible.186 Collectively, these male-related hormonal factors may amplify the adverse effects of environmental toxicants and contribute to the higher male prevalence observed in ASD. Further studies investigating this possibility are warranted.
Notably, children with ASD who have pre-existing physiological abnormalities, such as oxidative stress or mitochondrial dysfunction, might have heightened susceptibility to the adverse effects of toxicants. For example, some investigators have suggested that children with ASD who have oxidative stress, lowered GSH and/or impaired conjugation of GSH to toxicants might possess insufficient metabolic reserve to efficiently detoxify environmental pollutants.157,187 Several case–control studies have reported that a number of children with ASD have lower GSH levels compared with TD children.157,166, 167, 168 A higher frequency of genetic polymorphisms that could impair one-carbon metabolism and indirectly the synthesis of GSH was also observed in the ASD group in one of these studies, including homozygous variants in transcobalamin II (G776C) and the reduced folate carrier (G80A).157 Lower GSH concentrations could contribute to slower elimination of heavy metals and xenobiotics.187 For example, animal studies have demonstrated that the secretion of mercury into bile is diminished by GSH depletion and that intravenous injections of GSH increase mercury biliary secretion.182,188 Taken together, these findings suggest that disturbances in either GSH production or GSH conjugation to toxicants could increase susceptibilities to the adverse effects of toxicants and/or increase toxicant body burden in individuals with ASD. Additional controlled studies are warranted to investigate these possibilities.
Studies of gene and toxicant interactions in ASD
Several polymorphisms in genes that could adversely affect the ability to efficiently eliminate environmental toxicants were reported in some children with ASD. However, only two genes (PON1 and GST) were examined in multiple studies and many of the studies had limited sample sizes. In addition, only a few studies confirmed the functional consequences of the polymorphism. In fact, four studies reported decreased PON1 activity and one study reported decreased GST activity in children with ASD compared with controls. It is possible that these findings could be due to SNPs in PON1 and GST, respectively, but further studies are needed to clarify this possibility.
Two studies examined a collection of genes and suggested that children with ASD may not metabolize heavy metals as efficiently as TD children owing to differences in genetics.61,104 Most importantly, as discussed below, the interactions between genes and environment are complex, suggesting that a multifactorial, systems level approach of examining detoxification metabolism is needed in future studies. Notably, the reviewed studies examining changes in genes involved in toxicant elimination could lead to preventative or treatment strategies. For example, one study reported the mothers with a polymorphism in GSTP1 were more likely to have a child with ASD compared with mothers without such a change.158 This suggests that screening mothers for this polymorphism could lead to strategies to prevent ASD. Similarly, the identification of polymorphisms in genes involved in toxicant elimination may help to identify children who are at a higher risk of adverse events upon exposure to toxicants and/or who are at higher risk of developing ASD, and therefore may also lead to preventative strategies.
Several studies described findings that could represent synergistic interactions between genes and toxicants. For example, while several studies associated estimated pesticide exposures with an increased ASD risk, other studies found that polymorphisms in PON1, a gene critical for detoxification of pesticides, were also associated with an elevated ASD risk. However, only one of the reviewed studies simultaneously examined both estimated pesticide exposures and PON1 polymorphisms in the same children,149 although synergistic effects between these two factors might be significant contributors to these reported associations. The lack of measuring both toxicant exposures and polymorphisms in most studies could explain the inconsistent findings in some studies. For example, the difference in the association between PON1 SNPs and ASD observed between Italy and North America in one study could be due to differences in exposures to household pesticides between the two countries.153
None of the studies examining SNPs in genes involved in toxicant elimination in children with ASD examined potential interactions between different genes. For example, an individual who has both GST and PON1 abnormalities might be more susceptible to the adverse effects of toxicants compared with an individual with an abnormality in only one of these genes. Given the complex and polygenic nature of ASD, epistatic interactions among multiple SNPs (even those commonly observed in the general population, such as SNPs in GST) may act synergistically to amplify the adverse effects of environmental toxicants, thereby increasing the risk of developing ASD in children who are most susceptible.187 These synergistic interactions may occur by either impairing detoxification or by adversely affecting biochemical pathways simultaneously damaged by toxicants.189
The effects of polymorphisms in genes involved in detoxification pathways may be hard to predict. For example, one reviewed study examining PON1 demonstrated a significant gene–gene interaction (P<0.025) between RELN and PON1 variants.153 As reelin (the enzyme encoded by RELN) activity has been shown to be inhibited by OP pesticides,190 it is possible that the indirect consequence of impaired elimination of OP pesticides due to PON1 polymorphisms may disproportionally affect individuals with RELN polymorphisms that predispose them to decreased reelin expression. As reelin is essential for neurodevelopment and reelin abnormalities have been associated with ASD,191,192 such gene–gene interactions could be significant. Therefore, these two reported genetic variants in RELN and PON1 in conjunction with OP pesticide exposure during critical periods of neurodevelopment, could cause reelin levels to drop below the threshold necessary for proper neuronal migration, thereby increasing the risk of developing ASD.193
Polymorphisms in genes not involved in detoxification pathways could also interact with toxicants to amplify a common pathophysiology. For example, one study suggested that exposure to certain air pollutants, some of which have been linked to decreased MET protein expression, when combined with a polymorphism known to decrease MET gene expression (thereby decreasing protein expression), combine to increase the risk of developing ASD, presumably by synergistically decreasing MET protein production as decreased MET expression has been linked to an increased risk of developing ASD.32 In this same manner, other synergistic interactions between environmental toxicants and genetic predispositions could be related to increasing the risk of developing ASD. For example, several lines of evidence point to an increased excitatory-inhibitory ratio in the ASD brain due to either increased glutamatergic (excitatory) or reduced GABAergic (inhibitory) signaling.194,195 Certain environmental toxicants can adversely affect glutamatergic and/or GABAergic pathways, thereby altering this excitatory-inhibitory balance of the brain.196,197 Mercury198 and PCBs199 can amplify glutamate signaling, whereas certain pesticides can impair GABAergic signaling.176,189 Given that autism has been associated with variations in genes responsible for glutamate receptors,200,201 GABA receptors,202,203 GABAergic interneurons204 and interactions between glutamatergic and GABAergic neurons,205,206 all of which can also increase the excitatory-inhibitory ratio of the brain, it is very possible that a heritable pre-existing imbalance in the ratio of neuronal excitation to inhibition found in some children with ASD could be amplified by the adverse effects of environmental toxicants that simultaneously and additionally perturb this balance.
Collectively, the studies reviewed in this paper support the possibility that gene–environment interactions in enzymes important for detoxification are associated with ASD. Notably, SNPs in genes that may impair the elimination of toxicants might not become functionally relevant in individuals with ASD until toxicant exposure levels reach a critical threshold and normal defense mechanisms have been overwhelmed.207 Differences in genes essential for detoxification could reduce this threshold in a subgroup of ASD children, making them more susceptible to the adverse effects of environmental toxicants. Without identifying this subgroup in advance, even large cohort studies could potentially miss these types of susceptibilities to toxicant exposures.208 Additional controlled and adequately powered studies that incorporate potential gene–environment interactions as well as polymorphisms in genes involved in the detoxification of environmental pollutants are warranted. If positive, such studies would support the notion that some children with ASD may be more susceptible to the adverse effects of pollutants compared with TD children and that similar exposure levels to toxicants may cause adverse effects in some children with ASD that may not occur as readily in TD children. If these potential susceptibility factors (for example, variants in gene expression, different activity levels of enzymes related to detoxification and so on) are not taken into account, then studies examining only toxicant exposures might miss subtle adverse effects in the ASD group.
Identification of toxicant exposure in ASD
Several studies suggested that toxicant exposures in children with ASD may be difficult to identify. For example, one retrospective chart review reported that, compared with controls, children with ASD were significantly older at the diagnosis of lead toxicity, had elevated blood lead concentrations for a longer period of time during treatment and were more likely to be re-exposed to lead during the study period despite close monitoring, environmental inspections and adequate lead cleanup procedures or alternative housing.95 Two case reports suggested that the presentation of lead toxicity may be unusual in some individuals with ASD, and can present as a flu-like syndrome96 or as weight loss, abdominal pain, diarrhea and vomiting.94 Therefore, a high index of suspicion for toxicant exposures may be needed in children with ASD, as they may be more easily exposed to toxicants compared with non-ASD children. This may be because children with ASD generally have a longer oral-motor stage than TD children and are more likely to exhibit pica behavior.91,100 Because of these findings, periodic screening for lead exposure in children with ASD has been recommended.209 Although the mean blood lead concentration in US children has declined over the last 30 years, a number of children still have lead levels high enough to impair cognitive functioning.210 Recent evidence implicates even very low lead exposure (blood lead level less than 5 μg dl−1) in neurodevelopmental deficits.211 In addition, because low levels of lead exposure have been shown to have a role in large studies of children with ADHD,212 similar large studies are warranted in children with ASD to determine whether even low levels of lead exposure increase the risk of ASD or increase ASD symptoms. As some children with ASD exhibit SNPs in ALAD169 and also may metabolize lead differently than TD individuals,104 incorporating genetic testing into studies of lead exposure may help identify children most at risk of developing toxicity from lead. Additional controlled studies examining lead concentrations in children with ASD are warranted, particularly studies that examine differences in lead metabolism in children with ASD, and also whether or not the cognitive effects of lead are different in children with ASD compared with TD children.
Limitations of reviewed studies
Many of the reviewed studies were case–control but did not always match participants on age or gender and, even when they did, often had an uneven number of case and controls without a rational for matching. Some of the studies were limited by possible recall bias, especially studies that relied on parental recall. For example, one study reported a higher prevalence of maternally reported childhood lead exposure (8.6%) in children with ASD; however, only two cases of lead exposure could be confirmed with chart abstraction data.53 This finding could be consistent with poor parental recall and/or poor data collection. Many of the reviewed studies also had small sample sizes or were uncontrolled, and several studies relied on questionnaires. Others relied on laboratory reference ranges (sometimes using values established for adults) for comparisons instead of using actual measurements in control individuals as a reference. Therefore, these laboratory ranges might not have been representative of the ASD population studied. Finally, some studies measured heavy metals from different types of blood samples (whole blood, plasma, serum or RBC) or did not note which type of sample was used, making it difficult to account for differences in measurements between studies. For example, the RBC concentration of a heavy metal may be a measurement of long-term exposure (several months) compared with the whole-blood level.59
Some studies reported that ASD symptoms were associated with toxicant exposures, but did not objectively confirm ASD diagnosis using standard criteria. For example, many autism research studies use one or two gold-standard instruments, either the standardized interactive examination known as the Autism Diagnostic Observation Schedule or a standardized historical interview known as the Autism Diagnostic Interview-Revised. These instruments were used in very few of the reviewed studies, and some studies provided an estimation of autism prevalence within a zip code, state or school district, leaving the exact instruments for ASD diagnosis possibly different across the populations sampled. Other studies used estimated ASD prevalence data rather than measuring actual prevalence. In addition, as the diagnosis of autism is less reliable in younger children, and children diagnosed with autism can sometimes improve and lose their diagnosis,213 differences in ages across samples could also be a source of variability. Particularly good studies used state autism surveillance systems that standardized the autism diagnostic workup. In addition, some studies used questionnaires such as the SRS, which has a particularly high correlation with the Autism Diagnostic Observation Schedule.
Some of the studies estimated toxicant levels or used a proxy for toxicant exposures, but did not directly measure toxicant levels in individuals. Some studies had insufficient accounting for other sources of toxicant exposure or were unable to confirm toxicant exposures on an individual level. The ecological studies were generally limited by a cross-sectional design that prevented firm conclusions on causation, or by an assumption that environmental toxicant concentrations were a marker for actual toxicant exposure in the children studied. Findings from the hair studies were limited by the potential unreliability of laboratory methods for hair metal analysis.214
Many of the studies only assessed estimated toxicant exposures during specific periods of time, such as during gestation or early childhood, or did not constrain the exposure time precisely. As there are several critical time periods during development, the precise timing when exposure to a toxicant is most disruptive is unclear. As a case in point, one interesting study used Bayesian modeling to define the critical periods before, during and after pregnancy when proximity to organochlorine pesticides would be most likely to result in ASD, and found two peaks of developmental vulnerability.26 This demonstrates the complexity of the phenomenon being studied and suggests that better integration of biological information with respect to periods of peak vulnerability could help refine the statistical models used to study the neurodevelopmental effects of toxicant exposure.
Many of the reviewed studies revealed a positive association between environmental toxicants and ASD. These findings could be partly due to publication bias (studies finding a negative association might not have been published). The studies examining biomarkers of toxicant exposure were inconsistent, especially with respect to heavy metals and PCBs, and some studies lacked replication of findings. Some studies indicated that several biomarkers were significantly higher on average in ASD, or at least in some individuals with ASD, compared with controls; this supports the need for further careful investigation to confirm these findings. Although almost all of the reviewed studies (92%) found a significant association between estimated environmental toxicant exposures and ASD risk, the inconsistent findings concerning biomarkers suggest that currently available biomarkers may not be sufficient to identify such an association. In addition, the associations reported between environmental toxicants and ASD could be incidental and not causal. As many of the studies in this review had significant weaknesses, further investigations and controlled studies are needed.
Conclusions
The reviewed studies support an association between environmental toxicants and ASD. This may be due, in part, to higher toxicant exposures unique to individuals with ASD (that is, pica behavior) and/or increased susceptibilities to the adverse effects of toxicants. However, many of the reviewed studies contain significant weaknesses and reveal a need for more high-quality epidemiological studies concerning environmental toxicants and ASD. Many of the studies were retrospective or based on population estimates and did not confirm the ASD diagnosis. Notably, many studies did not account for the fact that exposures can occur at particular developmentally sensitive periods as many studies measured biomarkers and/or exposure at times other than these developmentally sensitive periods.
Despite these limitations, the reviewed studies support the notion that shared environmental and genetic factors could converge to result in neurotoxic mechanisms that may lead to the development of ASD. Potential susceptibilities to toxicants implicated in some individuals with ASD—including altered detoxification, genetic factors, lower GSH levels, oxidative stress, altered neuronal development and synaptic function, and hormonal factors—could act synergistically and amplify the adverse effects of toxicants during critical periods of neurodevelopment, particularly during the prenatal and early postnatal periods. Additional studies are needed to investigate the possibility that individuals who develop ASD may be more susceptible to the adverse effects of environmental toxicants compared with TD individuals, as well as the mechanisms that may mediate this susceptibility.
Unlike previous review articles on toxicants and ASD, this review examined both environmental and genetic mechanisms for toxicants contributing to ASD and synthesized this information. The findings of this review suggest that the etiology of ASD involves complex interactions between genetic factors and environmental toxicants that may act synergistically or in parallel during critical periods of neurodevelopment, conferring enhanced susceptibilities to the adverse effects of environmental toxicants in a manner that increases the likelihood of developing ASD. This review also offers many targets for additional research that may be helpful in investigating this possibility. The incorporation of potential gene–environment interactions and genetic polymorphisms affecting the detoxification of environmental pollutants should be considered in the search for candidate ASD genes, especially as some of these genetic factors have not been adequately studied to date in ASD.14
Acknowledgments
This study was supported by a grant from the Autism Research Institute, San Diego, CA.
DAR and SJG utilize detoxification methods in their clinical practices. The remaining author declares no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- APA Diagnostic and Statistical Manual of Mental Disorders4th edn. American Psychiatric Association: Washington, DC, USA; 1994 [Google Scholar]
- Baio J. Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61:1–19. [PubMed] [Google Scholar]
- Rice C. Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, 14 sites, United States, 2002. MMWR. 2007;56:12–28. [PubMed] [Google Scholar]
- Schaefer GB, Mendelsohn NJ. Clinical genetics evaluation in identifying the etiology of autism spectrum disorders: 2013 guideline revisions. Genet Med. 2013;15:399–407. doi: 10.1038/gim.2013.32. [DOI] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68:1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol DA, Frye RE. A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol Psychiatry. 2012;17:389–401. doi: 10.1038/mp.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X, Brimacombe M, Chaaban J, Zimmerman-Bier B, Wagner GC. Autism spectrum disorders: concurrent clinical disorders. J Child Neurol. 2008;23:6–13. doi: 10.1177/0883073807307102. [DOI] [PubMed] [Google Scholar]
- Herbert MR. Autism: a brain disorder or a disorder that affects the brain. Clin Neuropsychiatry. 2005;2:354–379. [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Goldman LR, Koduru S. Chemicals in the environment and developmental toxicity to children: a public health and policy perspective. Environ Health Perspect. 2000;108:443–448. doi: 10.1289/ehp.00108s3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Perez M. Development neurotoxicity: implications of methylmercury research. Int J Environ Health. 2008;2:417–428. [Google Scholar]
- Faustman EM, Silbernagel SM, Fenske RA, Burbacher TM, Ponce RA. Mechanisms underlying Children's susceptibility to environmental toxicants. Environ Health Perspect. 2000;108:13–21. doi: 10.1289/ehp.00108s113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler CP, Croen LA, Grether JK. Van de Water J. Identifying environmental contributions to autism: provocative clues and false leads. Ment Retard Dev Disabil Res Rev. 2004;10:292–302. doi: 10.1002/mrdd.20043. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Russo JP, Yang S, Roohi J, Blaxill M, Kahler SG, et al. Autism and environmental genomics. Neurotoxicology. 2006;27:671–684. doi: 10.1016/j.neuro.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Livingston RJ, von Niederhausern A, Jegga AG, Crawford DC, Carlson CS, Rieder MJ, et al. Pattern of sequence variation across 213 environmental response genes. Genome Res. 2004;14:1821–1831. doi: 10.1101/gr.2730004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler CP. The ‘environment' for autism research: signs of improvement. Environ Health Perspect. 2008;116:A416–A417. doi: 10.1289/ehp.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheslack-Postava K, Rantakokko PV, Hinkka-Yli-Salomaki S, Surcel HM, McKeague IW, Kiviranta HA, et al. Maternal serum persistent organic pollutants in the Finnish Prenatal Study of Autism: a pilot study. Neurotoxicol Teratol. 2013;38:1–5. doi: 10.1016/j.ntt.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miodovnik A, Engel SM, Zhu C, Ye X, Soorya LV, Silva MJ, et al. Endocrine disruptors and childhood social impairment. Neurotoxicology. 2011;32:261–267. doi: 10.1016/j.neuro.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, et al. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:e1845–e1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijngaarden E, Davidson PW, Smith TH, Evans K, Yost K, Love T, et al. Autism spectrum disorder phenotypes and prenatal exposure to methylmercury. Epidemiology. 2013;24:651–659. doi: 10.1097/EDE.0b013e31829d2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. The Autistic Syndromes. North-Holland Publishing: New York, NY, USA; 1976. [Google Scholar]
- Felicetti T. Parents of autistic children: some notes on a chemical connection. Milieu Ther. 1981;1:13–16. [Google Scholar]
- McCanlies EC, Fekedulegn D, Mnatsakanova A, Burchfiel CM, Sanderson WT, Charles LE, et al. Parental occupational exposures and autism spectrum disorder. J Autism Dev Disord. 2012;42:2323–2334. doi: 10.1007/s10803-012-1468-1. [DOI] [PubMed] [Google Scholar]
- Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environ Health Perspect. 2007;115:1482–1489. doi: 10.1289/ehp.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EM, English PB. Bayesian modeling of time-dependent vulnerability to environmental hazards: an example using autism and pesticide data. Stat Med. 2013;32:2308–2319. doi: 10.1002/sim.5600. [DOI] [PubMed] [Google Scholar]
- Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R. Residential proximity to freeways and autism in the CHARGE study. Environ Health Perspect. 2011;119:873–877. doi: 10.1289/ehp.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkbrenner AE, Daniels JL, Chen JC, Poole C, Emch M, Morrissey J. Perinatal exposure to hazardous air pollutants and autism spectrum disorders at age 8. Epidemiology. 2010;21:631–641. doi: 10.1097/EDE.0b013e3181e65d76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra TA, Wilhelm M, Olsen J, Cockburn M, Ritz B. Ambient air pollution and autism in Los Angeles County, California. Environ Health Perspect. 2012;121:380–386. doi: 10.1289/ehp.1205827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Lurmann F, Penfold B, Hertz-Picciotta I, McConnell R. Traffic-related air pollution, particulate matter, and autism. Arch Gen Psychiatry. 2012. pp. 1–7. [DOI] [PMC free article] [PubMed]
- Roberts AL, Lyall K, Hart JE, Laden F, Just AC, Bobb JF, et al. Perinatal air pollutant exposures and autism spectrum disorder in the children of nurses' health study II participants. Environ Health Perspect. 2013;121:978–984. doi: 10.1289/ehp.1206187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Kerin T, Lurmann F, Hertz-Picciotto I, McConnell R, Campbell DB. Autism spectrum disorder: interaction of air pollution with the MET receptor tyrosine kinase gene. Epidemiology. 2013;25:44–47. doi: 10.1097/EDE.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, Han DH, Lyoo HS, Min KJ, Kim KH, Renshaw P. Exposure to environmental toxins in mothers of children with autism spectrum disorder. Psychiatry Investig. 2010;7:122–127. doi: 10.4306/pi.2010.7.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham GC, Sumner A, Li SX, Anderson M, Katz E, Croen LA, et al. Use of birth certificates to examine maternal occupational exposures and autism spectrum disorders in offspring. Autism Res. 2013;6:57–63. doi: 10.1002/aur.1275. [DOI] [PubMed] [Google Scholar]
- Pino-Lopez M, Romero-Ayuso DM. Parental occupational exposures and autism spectrum disorder in children. Rev Esp Salud Publica. 2013;87:73–85. doi: 10.4321/S1135-57272013000100008. [DOI] [PubMed] [Google Scholar]
- Garry VF, Harkins ME, Erickson LL, Long-Simpson LK, Holland SE, Burroughs BL. Birth defects, season of conception, and sex of children born to pesticide applicators living in the Red River Valley of Minnesota, USA. Environ Health Perspect. 2002;110:441–449. doi: 10.1289/ehp.02110s3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audouze K, Grandjean P. Application of computational systems biology to explore environmental toxicity hazards. Environ Health Perspect. 2011;119:1754–1759. doi: 10.1289/ehp.1103533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X, Brimacombe M, Malek JH, Jani N, Wagner GC. Autism spectrum disorders and identified toxic land fills: co-occurrence across States. Environ Health Insights. 2008;2:55–59. doi: 10.4137/EHI.S830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desoto MC. Ockman's razor and autism: the case for developmental neurotoxins contributing to a disease of neurodevelopment. Neurotoxicology. 2009;30:331–337. doi: 10.1016/j.neuro.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Windham GC, Zhang L, Gunier R, Croen LA, Grether JK. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the San Francisco bay area. Environ Health Perspect. 2006;114:1438–1444. doi: 10.1289/ehp.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CR, Lin YT, Hwang BF. Air pollution and newly diagnostic autism spectrum disorders: a population-based cohort study in Taiwan. PLoS One. 2013;8:e75510. doi: 10.1371/journal.pone.0075510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Crothers C, Lai S, Lamm S. Pediatric neurobehavioral diseases in Nevada counties with respect to perchlorate in drinking water: an ecological inquiry. Birth Defects Res A Clin Mol Teratol. 2003;67:886–892. doi: 10.1002/bdra.10089. [DOI] [PubMed] [Google Scholar]
- ATSDR 2000. Division of Health Assessment and Consultation. Public Health Assessment, Brick Township Investigation, Appendix A—Contaminants of Concern. Available at http://www.atsdr.cdc.gov/hac/pha/pha.asp?docid=380&pg=0 (accessed 2 November 2013).
- Palmer RF, Blanchard S, Stein Z, Mandell D, Miller C. Environmental mercury release, special education rates, and autism disorder: an ecological study of Texas. Health Place. 2006;12:203–209. doi: 10.1016/j.healthplace.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Bartell SM, Lewandowski TA. Administrative censoring in ecological analyses of autism and a Bayesian solution. J Environ Public Health. 2011;2011:202783. doi: 10.1155/2011/202783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski TA, Bartell SM, Yager JW, Levin L. An evaluation of surrogate chemical exposure measures and autism prevalence in Texas. J Toxicol Environ Health A. 2009;72:1592–1603. doi: 10.1080/15287390903232483. [DOI] [PubMed] [Google Scholar]
- Palmer RF, Blanchard S, Wood R. Proximity to point sources of environmental mercury release as a predictor of autism prevalence. Health Place. 2009;15:18–24. doi: 10.1016/j.healthplace.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Blanchard KS, Palmer RF, Stein Z. The value of ecologic studies: mercury concentration in ambient air and the risk of autism. Rev Environ Health. 2011;26:111–118. doi: 10.1515/reveh.2011.015. [DOI] [PubMed] [Google Scholar]
- Schweikert C, Li Y, Dayya D, Yens D, Torrents M, Hsu DF. Analysis of autism prevalence and neurotoxins using combinatorial fusion and association rule mining. Ninth IEEE International Conference on Bioinformatics and Bioengineering. 2009. pp. 400–404.
- Rury J.Links between environmental mercury, special education, and autism in Louisiana 2008 thesis, Louisiana State University: Baton Rouge, LA, USA, 2006 [Google Scholar]
- DeSoto MC, Hitlan RT. Fish consumption advisories and the surprising relationship to prevalence rate of developmental disability as reported by public schools. J Environ Prot. 2012;3:1579–1589. [Google Scholar]
- Chrysochoou C, Rutishauser C, Rauber-Luthy C, Neuhaus T, Boltshauser E, Superti-Furga A. An 11-month-old boy with psychomotor regression and auto-aggressive behaviour. Eur J Pediatr. 2003;162:559–561. doi: 10.1007/s00431-003-1239-2. [DOI] [PubMed] [Google Scholar]
- Price CS, Thompson WW, Goodson B, Weintraub ES, Croen LA, Hinrichsen VL, et al. Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism. Pediatrics. 2010;126:656–664. doi: 10.1542/peds.2010-0309. [DOI] [PubMed] [Google Scholar]
- Larsson M, Weiss B, Janson S, Sundell J, Bornehag CG. Associations between indoor environmental factors and parental-reported autistic spectrum disorders in children 6-8 years of age. Neurotoxicology. 2009;30:822–831. doi: 10.1016/j.neuro.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip P, Wong V, Ho M, Lee J, Wong W. Mercury exposure in children with autistic spectrum disorder: case-control study. J Child Neurol. 2004;19:431–434. doi: 10.1177/088307380401900606. [DOI] [PubMed] [Google Scholar]
- Desoto MC, Hitlan RT. Blood levels of mercury are related to diagnosis of autism: a reanalysis of an important data set. J Child Neurol. 2007;22:1308–1311. doi: 10.1177/0883073807307111. [DOI] [PubMed] [Google Scholar]
- Geier DA, Audhya T, Kern JK, Geier MR. Blood mercury levels in autism spectrum disorder: is there a threshold level. Acta Neurobiol Exp (Wars) 2010;70:177–186. doi: 10.55782/ane-2010-1789. [DOI] [PubMed] [Google Scholar]
- Albizzati A, More L, Di Candia D, Saccani M, Lenti C. Normal concentrations of heavy metals in autistic spectrum disorders. Minerva Pediatr. 2012;64:27–31. [PubMed] [Google Scholar]
- Adams JB, Audhya T, McDonough-Means S, Rubin RA, Quig D, Geis E, et al. Toxicological status of children with autism vs neurotypical children and the association with autism severity. Biol Trace Elem Res. 2013;151:171–180. doi: 10.1007/s12011-012-9551-1. [DOI] [PubMed] [Google Scholar]
- Rahbar MH, Samms-Vaughan M, Loveland KA, Ardjomand-Hessabi M, Chen Z, Bressler J, et al. Seafood consumption and blood mercury concentrations in Jamaican children with and without autism spectrum disorders. Neurotox Res. 2013;23:22–38. doi: 10.1007/s12640-012-9321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamova B, Green PG, Tian Y, Hertz-Picciotto I, Pessah IN, Hansen R, et al. Correlations between gene expression and mercury levels in blood of boys with and without autism. Neurotox Res. 2011;19:31–48. doi: 10.1007/s12640-009-9137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Green PG, Delwiche L, Hansen R, Walker C, Pessah IN. Blood mercury concentrations in CHARGE Study children with and without autism. Environ Health Perspect. 2010;118:161–166. doi: 10.1289/ehp.0900736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaurock-Busch E, Amin OR, Rabah T. Heavy metals and trace elements in hair and urine of a sample of Arab children with autistic spectrum disorder. Maedica (Buchar) 2011;6:247–257. [PMC free article] [PubMed] [Google Scholar]
- Wright B, Pearce H, Allgar V, Miles J, Whitton C, Leon I, et al. A comparison of urinary mercury between children with autism spectrum disorders and control children. PLoS One. 2012;7:e29547. doi: 10.1371/journal.pone.0029547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradstreet JJ, Geier DA, Kartzinel JJ, Adams JB, Geier MR. A case-control study of mercury burden in children with autistic spectrum disorders. J Amer Physicians Surg. 2003;8:76–79. [Google Scholar]
- Soden SE, Lowry JA, Garrison CB, Wasserman GS. 24-hour provoked urine excretion test for heavy metals in children with autism and typically developing controls, a pilot study. Clin Toxicol (Phila) 2007;45:476–481. doi: 10.1080/15563650701338195. [DOI] [PubMed] [Google Scholar]
- Blaucok-Busch E, Amin OR, Dessoki HH, Rabah T. Efficacy of DMSA therapy in a sample of Arab children with autistic spectrum disorder. Maedica (Buchar) 2012;7:214–221. [PMC free article] [PubMed] [Google Scholar]
- Aposhian HV, Maiorino RM, Gonzalez-Ramirez D, Zuniga-Charles M, Xu Z, Hurlbut KM, et al. Mobilization of heavy metals by newer, therapeutically useful chelating agents. Toxicology. 1995;97:23–38. doi: 10.1016/0300-483x(95)02965-b. [DOI] [PubMed] [Google Scholar]
- Fido A, Al-Saad S. Toxic trace elements in the hair of children with autism. Autism. 2005;9:290–298. doi: 10.1177/1362361305053255. [DOI] [PubMed] [Google Scholar]
- Al-Ayadhi LY. Heavy metals and trace elements in hair samples of autistic children in central Saudi Arabia. Neurosciences (Riyadh) 2005;10:213–218. [PubMed] [Google Scholar]
- El-Baz F, Elhossiny RM, Elsayed AB, Gaber GM. Hair mercury measurement in Egyptian autistic children. Egypt J Med Hum Genet. 2010;11:135–141. [Google Scholar]
- Elsheshtawy E, Tobar S, Sherra K, Atallah S, Elkasaby R. Study of some biomarkers in hair of children with autism. Middle East Curr Psychiatry. 2011;18:6–10. [Google Scholar]
- Lakshmi Priya MD, Geetha A. Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biol Trace Elem Res. 2011;142:148–158. doi: 10.1007/s12011-010-8766-2. [DOI] [PubMed] [Google Scholar]
- Blaurock-Busch E, Amin OR, Dessoki HH, Rabah T. Toxic metals and essential elements in hair and severity of symptoms among children with autism. Maedica (Buchar) 2012;7:38–48. [PMC free article] [PubMed] [Google Scholar]
- Majewska MD, Urbanowicz E, Rok-Bujko P, Namyslowska I, Mierzejewski P. Age-dependent lower or higher levels of hair mercury in autistic children than in healthy controls. Acta Neurobiol Exp (Wars) 2010;70:196–208. doi: 10.55782/ane-2010-1791. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Yasuda Y, Tsutsui T. Estimation of autistic children by metallomics analysis. Sci Rep. 2013;3:1199. doi: 10.1038/srep01199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JB, Romdalvik J, Levine KE, Hu L-W. Mercury in first-cut baby hair of children with autism versus typically-developing children. Toxicol Environ Chem. 2008. pp. 1–14.
- Adams JB, Holloway CE, George F, Quig D. Analyses of toxic metals and essential minerals in the hair of Arizona children with autism and associated conditions, and their mothers. Biol Trace Elem Res. 2006;110:193–209. doi: 10.1385/BTER:110:3:193. [DOI] [PubMed] [Google Scholar]
- Wecker L, Miller SB, Cochran SR, Dugger DL, Johnson WD.Trace element concentrations in hair from autistic children J Ment Defic Res 198529Pt 115–22. [DOI] [PubMed] [Google Scholar]
- Williams PG, Hersh JH, Allard A, Sears LL. A controlled study of mercury levels in hair samples of children with autism as compared to their typically developing siblings. Res Autism Spect Disord. 2008;2:170–175. [Google Scholar]
- De Palma G, Catalani S, Franco A, Brighenti M, Apostoli P. Lack of correlation between metallic elements analyzed in hair by ICP-MS and autism. J Autism Dev Disord. 2012;42:342–353. doi: 10.1007/s10803-011-1245-6. [DOI] [PubMed] [Google Scholar]
- Kern JK, Grannemann BD, Trivedi MH, Adams JB.Sulfhydryl-reactive metals in autism J Toxicol Environ Health A 200770715–721.17365626 [Google Scholar]
- Obrenovich ME, Shamberger RJ, Lonsdale D. Altered heavy metals and transketolase found in autistic spectrum disorder. Biol Trace Elem Res. 2011;144:475–486. doi: 10.1007/s12011-011-9146-2. [DOI] [PubMed] [Google Scholar]
- Holmes AS, Blaxill MF, Haley BE. Reduced levels of mercury in first baby haircuts of autistic children. Int J Toxicol. 2003;22:277–285. doi: 10.1080/10915810305120. [DOI] [PubMed] [Google Scholar]
- Canuel R, de Grosbois SB, Atikesse L, Lucotte M, Arp P, Ritchie C, et al. New evidence on variations of human body burden of methylmercury from fish consumption. Environ Health Perspect. 2006;114:302–306. doi: 10.1289/ehp.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JB, Romdalvik J, Ramanujam VM, Legator MS. Mercury, lead, and zinc in baby teeth of children with autism versus controls. J Toxicol Environ Health A. 2007;70:1046–1051. doi: 10.1080/15287390601172080. [DOI] [PubMed] [Google Scholar]
- Rinderknecht AL, Kleinman MT, Ericson JE. Pb enamel biomarker: deposition of pre- and postnatal Pb isotope injection in reconstructed time points along rat enamel transect. Environ Res. 2005;99:169–176. doi: 10.1016/j.envres.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Abdullah MM, Ly AR, Goldberg WA, Clarke-Stewart KA, Dudgeon JV, Mull CG, et al. Heavy metal in children's tooth enamel: related to autism and disruptive behaviors. J Autism Dev Disord. 2012;42:929–936. doi: 10.1007/s10803-011-1318-6. [DOI] [PubMed] [Google Scholar]
- Sajdel-Sulkowska EM, Lipinski B, Windom H, Audhya T, McGinnis WR. Oxidative stress in autism: elevated cerebellar 3-nitrotyrosine levels. Am J Biochem Biotech. 2008;4:73–84. [Google Scholar]
- Shandley K, Austin DW. Ancestry of pink disease (infantile acrodynia) identified as a risk factor for autism spectrum disorders. J Toxicol Environ Health A. 2011;74:1185–1194. doi: 10.1080/15287394.2011.590097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accardo P, Whitman B, Caul J, Rolfe U. Autism and plumbism. A possible association. Clin Pediatr (Phila) 1988;27:41–44. doi: 10.1177/000992288802700108. [DOI] [PubMed] [Google Scholar]
- Eppright TD, Sanfacon JA, Horwitz EA. Attention deficit hyperactivity disorder, infantile autism, and elevated blood-lead: a possible relationship. Mo Med. 1996;93:136–138. [PubMed] [Google Scholar]
- Lidsky TI, Schneider JS. Autism and autistic symptoms associated with childhood lead poisoning. J Appl Res. 2005;5:80–87. [Google Scholar]
- Newton KE, Leonard M, Delves HT, Bowen-Jones D. A problem with her lead weight. Ann Clin Biochem. 2005;42:145–148. doi: 10.1258/0004563053492793. [DOI] [PubMed] [Google Scholar]
- Shannon M, Graef JW. Lead intoxication in children with pervasive developmental disorders. J Toxicol Clin Toxicol. 1996;34:177–181. doi: 10.3109/15563659609013767. [DOI] [PubMed] [Google Scholar]
- George M, Heeney MM, Woolf AD. Encephalopathy from lead poisoning masquerading as a flu-like syndrome in an autistic child. Pediatr Emerg Care. 2010;26:370–373. doi: 10.1097/PEC.0b013e3181db2237. [DOI] [PubMed] [Google Scholar]
- Hallaway N. From autistic to lead toxic. Can Nurse. 1993;89:53–54. [PubMed] [Google Scholar]
- Campbell M, Petti TA, Green WH, Cohen IL, Genieser NB, David R. Some physical parameters of young autistic children. J Am Acad Child Psychiatry. 1980;19:193–212. doi: 10.1016/s0002-7138(09)60697-x. [DOI] [PubMed] [Google Scholar]
- Clark B, Vandermeer B, Simonetti A, Buka I. Is lead a concern in Canadian autistic children. Paediatr Child Health. 2010;15:17–22. doi: 10.1093/pch/15.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DJ, Johnson WT, Caparulo BK. Pica and elevated blood lead level in autistic and atypical children. Am J Dis Child. 1976;130:47–48. doi: 10.1001/archpedi.1976.02120020049007. [DOI] [PubMed] [Google Scholar]
- El-Ansary A, Al-Daihan S, Al-Dbass A, Al-Ayadhi L. Measurement of selected ions related to oxidative stress and energy metabolism in Saudi autistic children. Clin Biochem. 2010;43:63–70. doi: 10.1016/j.clinbiochem.2009.09.008. [DOI] [PubMed] [Google Scholar]
- El-Ansary AK, Bacha AB, Ayahdi LY. Relationship between chronic lead toxicity and plasma neurotransmitters in autistic patients from Saudi Arabia. Clin Biochem. 2011;44:1116–1120. doi: 10.1016/j.clinbiochem.2011.06.982. [DOI] [PubMed] [Google Scholar]
- Cohen DJ, Paul R, Anderson GM, Harcherik DF. Blood lead in autistic children. Lancet. 1982;2:94–95. doi: 10.1016/s0140-6736(82)91707-x. [DOI] [PubMed] [Google Scholar]
- Tian Y, Green PG, Stamova B, Hertz-Picciotto I, Pessah IN, Hansen R, et al. Correlations of gene expression with blood lead levels in children with autism compared to typically developing controls. Neurotox Res. 2011;19:1–13. doi: 10.1007/s12640-009-9126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergani L, Cristina L, Paola R, Luisa AM, Shyti G, Edvige V, et al. Metals, metallothioneins and oxidative stress in blood of autistic children. Res Autism Spect Disord. 2011;5:286–293. [Google Scholar]
- Yorbik O, Kurt I, Hasimi A, Ozturk O. Chromium, cadmium, and lead levels in urine of children with autism and typically developing controls. Biol Trace Elem Res. 2010;135:10–15. doi: 10.1007/s12011-009-8494-7. [DOI] [PubMed] [Google Scholar]
- Al-Farsi YM, Waly MI, Al-Sharbati MM, Al-Shafaee MA, Al-Farsi OA, Al-Khaduri MM, et al. Levels of heavy metals and essential minerals in hair samples of children with autism in Oman: a case-control study. Biol Trace Elem Res. 2012;151:181–186. doi: 10.1007/s12011-012-9553-z. [DOI] [PubMed] [Google Scholar]
- Gentile PS, Trentalange MJ, Zamichek W, Coleman M. Brief report: trace elements in the hair of autistic and control children. J Autism Dev Disord. 1983;13:205–206. doi: 10.1007/BF01531820. [DOI] [PubMed] [Google Scholar]
- Shearer TR, Larson K, Neuschwander J, Gedney B. Minerals in the hair and nutrient intake of autistic children. J Autism Dev Disord. 1982;12:25–34. doi: 10.1007/BF01531671. [DOI] [PubMed] [Google Scholar]
- Rahbar MH, Samms-Vaughan M, Ardjomand-Hessabi M, Loveland KA, Dickerson AS, Chen Z, et al. The role of drinking water sources, consumption of vegetables and seafood in relation to blood arsenic concentrations of Jamaican children with and without autism spectrum disorders. Sci Total Environ. 2012;433:362–370. doi: 10.1016/j.scitotenv.2012.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JB, Baral M, Geis E, Mitchell J, Ingram J, Hensley A, et al. The severity of autism is associated with toxic metal body burden and red blood cell glutathione levels. J Toxicol. 2009;2009:1–7. doi: 10.1155/2009/532640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier DA, Kern JK, King PG, Sykes LK, Geier MR. Hair toxic metal concentrations and autism spectrum disorder severity in young children. Int J Environ Res Public Health. 2012;9:4486–4497. doi: 10.3390/ijerph9124486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JB, Baral M, Geis E, Mitchell J, Ingram J, Hensley A, et al. Safety and efficacy of oral DMSA therapy for children with autism spectrum disorders: part A—medical results. BMC Clin Pharmacol. 2009;9:16. doi: 10.1186/1472-6904-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataf R, Skorupka C, Amet L, Lam A, Springbett A, Lathe R. Porphyrinuria in childhood autistic disorder: implications for environmental toxicity. Toxicol Appl Pharmacol. 2006;214:99–108. doi: 10.1016/j.taap.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Goin-Kochel RP, Mackintosh VH, Myers BJ. Parental reports on the efficacy of treatments and therapies for their children with autism spectrum disorders. Res Autism Spect Disord. 2009;3:528–537. [Google Scholar]
- Patel K, Curtis LT. A comprehensive approach to treating autism and attention-deficit hyperactivity disorder: a prepilot study. J Altern Complement Med. 2007;13:1091–1097. doi: 10.1089/acm.2007.0611. [DOI] [PubMed] [Google Scholar]
- Adams JB, Baral M, Geis E, Mitchell J, Ingram J, Hensley A, et al. Safety and efficacy of oral DMSA therapy for children with autism spectrum disorders: part B—behavioral results. BMC Clin Pharmacol. 2009;9:17. doi: 10.1186/1472-6904-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier DA, Geier MR. A clinical trial of combined anti-androgen and anti-heavy metal therapy in autistic disorders. Neuro Endocrinol Lett. 2006;27:833–838. [PubMed] [Google Scholar]
- Kidd PM. Autism, an extreme challenge to integrative medicine. Part II: Medical management. Alternat Med Rev. 2002;7:472–499. [PubMed] [Google Scholar]
- Lonsdale D, Shamberger RJ, Audhya T. Treatment of autism spectrum children with thiamine tetrahydrofurfuryl disulfide: a pilot study. Neuro Endocrinol Lett. 2002;23:303–308. [PubMed] [Google Scholar]
- Lofthouse N, Hendren R, Hurt E, Arnold LE, Butter E. A review of complementary and alternative treatments for autism spectrum disorders. Autism Res Treat. 2012;2012:870391. doi: 10.1155/2012/870391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JS. Porphyrin Metabolism as Indicator of Metal Exposure and Toxicity. vol. 115. Springer-Verlag: Berlin; 1995. [Google Scholar]
- Heyer NJ, Bittner AC, Jr, Echeverria D, Woods JS. A cascade analysis of the interaction of mercury and coproporphyrinogen oxidase (CPOX) polymorphism on the heme biosynthetic pathway and porphyrin production. Toxicol Lett. 2006;161:159–166. doi: 10.1016/j.toxlet.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Pingree SD, Simmonds PL, Rummel KT, Woods JS. Quantitative evaluation of urinary porphyrins as a measure of kidney mercury content and mercury body burden during prolonged methylmercury exposure in rats. Toxicol Sci. 2001;61:234–240. doi: 10.1093/toxsci/61.2.234. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Ramirez D, Maiorino RM, Zuniga-Charles M, Xu Z, Hurlbut KM, Junco-Munoz P, et al. Sodium 2,3-dimercaptopropane-1-sulfonate challenge test for mercury in humans: II. Urinary mercury, porphyrins and neurobehavioral changes of dental workers in Monterrey, Mexico. J Pharmacol Exp Ther. 1995;272:264–274. [PubMed] [Google Scholar]
- Austin DW, Shandley K. An investigation of porphyrinuria in Australian children with autism. J Toxicol Environ Health A. 2008;71:1349–1351. doi: 10.1080/15287390802271723. [DOI] [PubMed] [Google Scholar]
- Geier DA, Kern JK, Garver CR, Adams JB, Audhya T, Nataf R, et al. Biomarkers of environmental toxicity and susceptibility in autism. J Neurol Sci. 2009;280:101–108. doi: 10.1016/j.jns.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Geier DA, Kern JK, Geier MR. A prospective blinded evaluation of urinary porphyrins verses the clinical severity of autism spectrum disorders. J Toxicol Environ Health A. 2009;72:1585–1591. doi: 10.1080/15287390903232475. [DOI] [PubMed] [Google Scholar]
- Kern JK, Geier DA, Adams JB, Geier MR. A biomarker of mercury body-burden correlated with diagnostic domain specific clinical symptoms of autism spectrum disorder. Biometals. 2010;23:1043–1051. doi: 10.1007/s10534-010-9349-6. [DOI] [PubMed] [Google Scholar]
- Geier DA, Geier MR. A prospective assessment of porphyrins in autistic disorders: a potential marker for heavy metal exposure. Neurotox Res. 2006;10:57–64. doi: 10.1007/BF03033334. [DOI] [PubMed] [Google Scholar]
- Geier DA, Geier MR. A prospective study of mercury toxicity biomarkers in autistic spectrum disorders. J Toxicol Environ Health A. 2007;70:1723–1730. doi: 10.1080/15287390701457712. [DOI] [PubMed] [Google Scholar]
- Heyer NJ, Echeverria D, Woods JS. Disordered porphyrin metabolism: a potential biological marker for autism risk assessment. Autism Res. 2012;5:84–92. doi: 10.1002/aur.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern JK, Geier DA, Adams JB, Mehta JA, Grannemann BD, Geier MR. Toxicity biomarkers in autism spectrum disorder: a blinded study of urinary porphyrins. Pediatr Int. 2011;53:147–153. doi: 10.1111/j.1442-200X.2010.03196.x. [DOI] [PubMed] [Google Scholar]
- Woods JS, Armel SE, Fulton DI, Allen J, Wessels K, Simmonds PL, et al. Urinary porphyrin excretion in neurotypical and autistic children. Environ Health Perspect. 2010;118:1450–1457. doi: 10.1289/ehp.0901713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn SI, Jin SH, Kim SH, Lim S. Porphyrinuria in Korean children with autism: correlation with oxidative stress. J Toxicol Environ Health A. 2010;73:701–710. doi: 10.1080/15287391003614000. [DOI] [PubMed] [Google Scholar]
- Frye RE, Rossignol DA. Mitochondrial physiology and autism spectrum disorder. OA Autism. 2013;1:5. [Google Scholar]
- Edelson SB, Cantor DS. Autism: xenobiotic influences. Toxicol Ind Health. 1998;14:553–563. doi: 10.1177/074823379801400406. [DOI] [PubMed] [Google Scholar]
- Edelson SB, Cantor DS. The neurotoxic etiology of the autistic spectrum disorders: a replication study. Toxicol Ind Health. 2000;16:239–247. [Google Scholar]
- Otake T, Yoshinaga J, Seki Y, Matsumura T, Watanabe K, Ishijima M, et al. Retrospective in utero exposure assessment of PCBs using preserved umbilical cords and its application to case-control comparison. Environ Health Prev Med. 2006;11:65–68. doi: 10.1007/BF02898144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MM, Woods R, Chi LH, Schmidt RJ, Pessah IN, Kostyniak PJ, et al. Levels of select PCB and PBDE congeners in human postmortem brain reveal possible environmental involvement in 15q11-q13 duplication autism spectrum disorder. Environ Mol Mutagen. 2012;53:589–598. doi: 10.1002/em.21722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa C, Nuti F, Hayek J, De Felice C, Chelli M, Rovero P, et al. Di-(2-ethylhexyl) phthalate and autism spectrum disorders. ASN Neuro. 2012;4:223–229. doi: 10.1042/AN20120015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein TP, Schluter MD, Steer RA, Ming X. Autism and phthalate metabolite glucuronidation. J Autism Dev Disord. 2013;43:2677–2685. doi: 10.1007/s10803-013-1822-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Schauer J, Pessah IN, Van de Water J. Preliminary evidence of the in vitro effects of BDE-47 on innate immune responses in children with autism spectrum disorders. J Neuroimmunol. 2009;208:130–135. doi: 10.1016/j.jneuroim.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibeque-Engle SL, Tessari JD, Winn DT, Keefe TJ, Nett TM, Zheng T. Comparison of organochlorine pesticide and polychlorinated biphenyl residues in human breast adipose tissue and serum. J Toxicol Environ Health. 1997;52:285–293. doi: 10.1080/00984109708984065. [DOI] [PubMed] [Google Scholar]
- Jandacek RJ, Anderson N, Liu M, Zheng S, Yang Q, Tso P. Effects of yo-yo diet, caloric restriction, and olestra on tissue distribution of hexachlorobenzene. Am J Physiol Gastrointest Liver Physiol. 2005;288:G292–G299. doi: 10.1152/ajpgi.00285.2004. [DOI] [PubMed] [Google Scholar]
- Yu GW, Laseter J, Mylander C. Persistent organic pollutants in serum and several different fat compartments in humans. J Environ Public Health. 2011;2011:417980. doi: 10.1155/2011/417980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusiecki JA, Matthews A, Sturgeon S, Sinha R, Pellizzari E, Zheng T, et al. A correlation study of organochlorine levels in serum, breast adipose tissue, and gluteal adipose tissue among breast cancer cases in India. Cancer Epidemiol Biomarkers Prev. 2005;14:1113–1124. doi: 10.1158/1055-9965.EPI-04-0356. [DOI] [PubMed] [Google Scholar]
- Ruggeri B, Sarkans U, Schumann G, Persico AM. Biomarkers in autism spectrum disorder: the old and the new. Psychopharmacology (Berl) 2013;151:181–186. doi: 10.1007/s00213-013-3290-7. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Huen K, Marks A, Harley KG, Bradman A, Barr DB, et al. PON1 and neurodevelopment in children from the CHAMACOS study exposed to organophosphate pesticides in utero. Environ Health Perspect. 2010;118:1775–1781. doi: 10.1289/ehp.1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca SP, Nemes B, Vlase L, Gagyi CE, Dronca E, Miu AC, et al. High levels of homocysteine and low serum paraoxonase 1 arylesterase activity in children with autism. Life Sci. 2006;78:2244–2248. doi: 10.1016/j.lfs.2005.09.040. [DOI] [PubMed] [Google Scholar]
- Pasca SP, Dronca E, Nemes B, Kaucsar T, Endreffy E, Iftene F, et al. Paraoxonase 1 activities and polymorphisms in autism spectrum disorders. J Cell Mol Med. 2010;14:600–607. doi: 10.1111/j.1582-4934.2008.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaita L, Manzi B, Sacco R, Lintas C, Altieri L, Lombardi F, et al. Decreased serum arylesterase activity in autism spectrum disorders. Psychiatry Res. 2010;180:105–113. doi: 10.1016/j.psychres.2010.04.010. [DOI] [PubMed] [Google Scholar]
- D'Amelio M, Ricci I, Sacco R, Liu X, D'Agruma L, Muscarella LA, et al. Paraoxonase gene variants are associated with autism in North America, but not in Italy: possible regional specificity in gene-environment interactions. Mol Psychiatry. 2005;10:1006–1016. doi: 10.1038/sj.mp.4001714. [DOI] [PubMed] [Google Scholar]
- Serajee FJ, Nabi R, Zhong H, Huq M. Polymorphisms in xenobiotic metabolism genes and autism. J Child Neurol. 2004;19:413–417. doi: 10.1177/088307380401900603. [DOI] [PubMed] [Google Scholar]
- Al-Yafee YA, Al-Ayadhi LY, Haq SH, El-Ansary AK. Novel metabolic biomarkers related to sulfur-dependent detoxification pathways in autistic patients of Saudi Arabia. BMC Neurol. 2011;11:139. doi: 10.1186/1471-2377-11-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyske S, Williams TA, Mars AE, Stenroos ES, Ming SX, Wang R, et al. Analysis of case-parent trios at a locus with a deletion allele: association of GSTM1 with autism. BMC Genet. 2006;7:8. doi: 10.1186/1471-2156-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SJ, Melnyk S, Jernigan S, Cleves MA, Halsted CH, Wong DH, et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:947–956. doi: 10.1002/ajmg.b.30366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TA, Mars AE, Buyske SG, Stenroos ES, Wang R, Factura-Santiago MF, et al. Risk of autistic disorder in affected offspring of mothers with a glutathione S-transferase P1 haplotype. Arch Pediatr Adolesc Med. 2007;161:356–361. doi: 10.1001/archpedi.161.4.356. [DOI] [PubMed] [Google Scholar]
- Gundacker C, Komarnicki G, Jagiello P, Gencikova A, Dahmen N, Wittmann KJ, et al. Glutathione-S-transferase polymorphism, metallothionein expression, and mercury levels among students in Austria. Sci Total Environ. 2007;385:37–47. doi: 10.1016/j.scitotenv.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Klautau-Guimarães MN, D'Ascenção R, Caldart FA, Grisolia CK, Souza JR, Barbosa AC, et al. Analysis of genetic susceptibility to mercury contamination evaluated through molecular biomarkers in at-risk Amazon Amerindian populations. Genet Mol Biol. 2005;28:827–832. [Google Scholar]
- Westphal GA, Schnuch A, Schulz TG, Reich K, Aberer W, Brasch J, et al. Homozygous gene deletions of the glutathione S-transferases M1 and T1 are associated with thimerosal sensitization. Int Arch Occup Environ Health. 2000;73:384–388. doi: 10.1007/s004200000159. [DOI] [PubMed] [Google Scholar]
- Tsai PC, Huang W, Lee YC, Chan SH, Guo YL. Genetic polymorphisms in CYP1A1 and GSTM1 predispose humans to PCBs/PCDFs-induced skin lesions. Chemosphere. 2006;63:1410–1418. doi: 10.1016/j.chemosphere.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Hung RJ, Boffetta P, Brennan P, Malaveille C, Hautefeuille A, Donato F, et al. GST, NAT, SULT1A1, CYP1B1 genetic polymorphisms, interactions with environmental exposures and bladder cancer risk in a high-risk population. Int J Cancer. 2004;110:598–604. doi: 10.1002/ijc.20157. [DOI] [PubMed] [Google Scholar]
- Frustaci A, Neri M, Cesario A, Adams JB, Domenici E, Dalla Bernardina B, et al. Oxidative stress-related biomarkers in autism: systematic review and meta-analyses. Free Radic Biol Med. 2012;52:2128–2141. doi: 10.1016/j.freeradbiomed.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Schmidt RJ, Hansen RL, Hartiala J, Allayee H, Schmidt LC, Tancredi DJ, et al. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology. 2011;22:476–485. doi: 10.1097/EDE.0b013e31821d0e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, et al. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr. 2004;80:1611–1617. doi: 10.1093/ajcn/80.6.1611. [DOI] [PubMed] [Google Scholar]
- James SJ, Rose S, Melnyk S, Jernigan S, Blossom S, Pavliv O, et al. Cellular and mitochondrial glutathione redox imbalance in lymphoblastoid cells derived from children with autism. FASEB J. 2009;23:2374–2383. doi: 10.1096/fj.08-128926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnyk S, Fuchs GJ, Schulz E, Lopez M, Kahler SG, Fussell JJ, et al. Metabolic imbalance associated with methylation dysregulation and oxidative damage in children with autism. J Autism Dev Disord. 2012;42:367–377. doi: 10.1007/s10803-011-1260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S, Melnyk S, Savenka A, Hubanks A, Jernigan S, Cleves MA, et al. The frequency of polymorphisms affecting lead and mercury toxicity among children with autism. Am J Biochem Biotech. 2008;4:85–94. [Google Scholar]
- Montenegro MF, Barbosa F, Jr, Sandrim VC, Gerlach RF, Tanus-Santos JE. A polymorphism in the delta-aminolevulinic acid dehydratase gene modifies plasma/whole blood lead ratio. Arch Toxicol. 2006;80:394–398. doi: 10.1007/s00204-005-0056-y. [DOI] [PubMed] [Google Scholar]
- Weuve J, Kelsey KT, Schwartz J, Bellinger D, Wright RO, Rajan P, et al. Delta-aminolevulinic acid dehydratase polymorphism and the relation between low level lead exposure and the Mini-Mental Status Examination in older men: the Normative Aging Study. Occup Environ Med. 2006;63:746–753. doi: 10.1136/oem.2006.027417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens SE, Summar ML, Ryckman KK, Haines JL, Reiss S, Summar SR, et al. Lack of association between autism and four heavy metal regulatory genes. Neurotoxicology. 2011;32:769–775. doi: 10.1016/j.neuro.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly BA, Waring RH. Enzyme and sulphur oxidation deficiencies in autistic children with known food/chemical intolerances. J Orthomol Med. 1993;8:198–200. [Google Scholar]
- Waring RH, Ngong JM, Klovrza L, Green S, Sharp H. Biochemical parameters in autistic children. Dev Brain Dysfunct. 1997;10:40–43. [Google Scholar]
- Li Z, Dong T, Proschel C, Noble M. Chemically diverse toxicants converge on Fyn and c-Cbl to disrupt precursor cell function. PLoS Biol. 2007;5:e35. doi: 10.1371/journal.pbio.0050035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton JF, Hertz-Picciotto I, Pessah IN. Tipping the balance of autism risk: potential mechanisms linking pesticides and autism. Environ Health Perspect. 2012;120:944–951. doi: 10.1289/ehp.1104553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter M, Akesson A, Liden C, Ceccatelli S, Berglund M. Gender differences in the disposition and toxicity of metals. Environ Res. 2007;104:85–95. doi: 10.1016/j.envres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Mocarelli P, Gerthoux PM, Ferrari E, Patterson DG, Jr, Kieszak SM, Brambilla P, et al. Paternal concentrations of dioxin and sex ratio of offspring. Lancet. 2000;355:1858–1863. doi: 10.1016/S0140-6736(00)02290-X. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Jusko TA, Willman EJ, Baker RJ, Keller JA, Teplin SW, et al. A cohort study of in utero polychlorinated biphenyl (PCB) exposures in relation to secondary sex ratio. Environ Health. 2008;7:37. doi: 10.1186/1476-069X-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borras C, Gambini J, Vina J. Mitochondrial oxidant generation is involved in determining why females live longer than males. Front Biosci. 2007;12:1008–1013. doi: 10.2741/2120. [DOI] [PubMed] [Google Scholar]
- Lavoie JC, Chessex P. Gender and maturation affect glutathione status in human neonatal tissues. Free Radic Biol Med. 1997;23:648–657. doi: 10.1016/s0891-5849(97)00011-7. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Clarkson TW. Dependence of biliary secretion of inorganic mercury on the biliary transport of glutathione. Biochem Pharmacol. 1984;33:1093–1098. doi: 10.1016/0006-2952(84)90519-7. [DOI] [PubMed] [Google Scholar]
- Thomas DJ, Fisher HL, Sumler MR, Mushak P, Hall LL. Sexual differences in the excretion of organic and inorganic mercury by methyl mercury-treated rats. Environ Res. 1987;43:203–216. doi: 10.1016/s0013-9351(87)80072-5. [DOI] [PubMed] [Google Scholar]
- Muraoka Y, Itoh F. Sex difference of mercuric chloride-induced renal tubular necrosis in rats—from the aspect of sex differences in renal mercury concentration and sulfhydryl levels. J Toxicol Sci. 1980;5:203–214. doi: 10.2131/jts.5.203. [DOI] [PubMed] [Google Scholar]
- Oliveira FR, Ferreira JR, dos Santos CM, Macedo LE, de Oliveira RB, Rodrigues JA, et al. Estradiol reduces cumulative mercury and associated disturbances in the hypothalamus-pituitary axis of ovariectomized rats. Ecotoxicol Environ Saf. 2006;63:488–493. doi: 10.1016/j.ecoenv.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Llop S, Lopez-Espinosa MJ, Rebagliato M, Ballester F. Gender differences in the neurotoxicity of metals in children. Toxicology. 2013;311:3–12. doi: 10.1016/j.tox.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Deth R, Muratore C, Benzecry J, Power-Charnitsky VA, Waly M. How environmental and genetic factors combine to cause autism: a redox/methylation hypothesis. Neurotoxicology. 2008;29:190–201. doi: 10.1016/j.neuro.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Clarkson TW. Biliary transport of glutathione and methylmercury. Am J Physiol. 1983;244:G435–G441. doi: 10.1152/ajpgi.1983.244.4.G435. [DOI] [PubMed] [Google Scholar]
- Pessah IN, Seegal RF, Lein PJ, LaSalle J, Yee BK, Van De Water J, et al. Immunologic and neurodevelopmental susceptibilities of autism. Neurotoxicology. 2008;29:532–545. doi: 10.1016/j.neuro.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrocchi CC, Wannenes F, Persico AM, Ciafre SA, D'Arcangelo G, Farace MG, et al. Reelin is a serine protease of the extracellular matrix. J Biol Chem. 2002;277:303–309. doi: 10.1074/jbc.M106996200. [DOI] [PubMed] [Google Scholar]
- Serajee FJ, Zhong H, Mahbubul Huq AH. Association of Reelin gene polymorphisms with autism. Genomics. 2006;87:75–83. doi: 10.1016/j.ygeno.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Stary JM, Halt AR, Realmuto GR. Dysregulation of Reelin and Bcl-2 proteins in autistic cerebellum. J Autism Dev Disord. 2001;31:529–535. doi: 10.1023/a:1013234708757. [DOI] [PubMed] [Google Scholar]
- Persico AM, Bourgeron T. Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends Neurosci. 2006;29:349–358. doi: 10.1016/j.tins.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell AE, Jeon OH, Zimmerman AW, Blue ME, Pevsner J. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology. 2001;57:1618–1628. doi: 10.1212/wnl.57.9.1618. [DOI] [PubMed] [Google Scholar]
- Kenet T, Froemke RC, Schreiner CE, Pessah IN, Merzenich MM. Perinatal exposure to a noncoplanar polychlorinated biphenyl alters tonotopy, receptive fields, and plasticity in rat primary auditory cortex. Proc Natl Acad Sci USA. 2007;104:7646–7651. doi: 10.1073/pnas.0701944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Farkas S, Kortbeek S, Zhang FX, Chen L, Zamponi GW, et al. Mercury-induced toxicity of rat cortical neurons is mediated through N-Methyl-D-Aspartate receptors. Mol Brain. 2012;5:30. doi: 10.1186/1756-6606-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez BI, Martinez ML, Montante M, Dufour L, Garcia E, Jimenez-Capdeville ME. Methylmercury increases glutamate extracellular levels in frontal cortex of awake rats. Neurotoxicol Teratol. 2002;24:767–771. doi: 10.1016/s0892-0362(02)00270-2. [DOI] [PubMed] [Google Scholar]
- Gafni J, Wong PW, Pessah IN. Non-coplanar 2,2′,3,5′,6-pentachlorobiphenyl (PCB 95) amplifies ionotropic glutamate receptor signaling in embryonic cerebellar granule neurons by a mechanism involving ryanodine receptors. Toxicol Sci. 2004;77:72–82. doi: 10.1093/toxsci/kfh004. [DOI] [PubMed] [Google Scholar]
- Jamain S, Betancur C, Quach H, Philippe A, Fellous M, Giros B, et al. Linkage and association of the glutamate receptor 6 gene with autism. Mol Psychiatry. 2002;7:302–310. doi: 10.1038/sj.mp.4000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serajee FJ, Zhong H, Nabi R, Huq AH. The metabotropic glutamate receptor 8 gene at 7q31: partial duplication and possible association with autism. J Med Genet. 2003;40:e42. doi: 10.1136/jmg.40.4.e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JD, Silverman JM, Smith CJ, Greenberg DA, Kilifarski M, Reichert J, et al. Association between a GABRB3 polymorphism and autism. Mol Psychiatry. 2002;7:311–316. doi: 10.1038/sj.mp.4001011. [DOI] [PubMed] [Google Scholar]
- Vincent JB, Horike SI, Choufani S, Paterson AD, Roberts W, Szatmari P, et al. An inversion inv(4)(p12-p15.3) in autistic siblings implicates the 4p GABA receptor gene cluster. J Med Genet. 2006;43:429–434. doi: 10.1136/jmg.2005.039693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DB, Sutcliffe JS, Ebert PJ, Militerni R, Bravaccio C, Trillo S, et al. A genetic variant that disrupts MET transcription is associated with autism. Proc Natl Acad Sci USA. 2006;103:16834–16839. doi: 10.1073/pnas.0605296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintas C, Persico AM. Autistic phenotypes and genetic testing: state-of-the-art for the clinical geneticist. J Med Genet. 2009;46:1–8. doi: 10.1136/jmg.2008.060871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P. Individual susceptibility in occupational and environmental toxicology. Toxicol Lett. 1995;77:105–108. doi: 10.1016/0378-4274(95)03278-9. [DOI] [PubMed] [Google Scholar]
- Weiss B, Bellinger DC. Social ecology of children's vulnerability to environmental pollutants. Environ Health Perspect. 2006;114:1479–1485. doi: 10.1289/ehp.9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipek PA, Accardo PJ, Baranek GT, Cook EH, Jr, Dawson G, Gordon B, et al. The screening and diagnosis of autistic spectrum disorders. J Autism Dev Disord. 1999;29:439–484. doi: 10.1023/a:1021943802493. [DOI] [PubMed] [Google Scholar]
- Jones RL, Homa DM, Meyer PA, Brody DJ, Caldwell KL, Pirkle JL, et al. Trends in blood lead levels and blood lead testing among US children aged 1 to 5 years, 1988–2004. Pediatrics. 2009;123:e376–e385. doi: 10.1542/peds.2007-3608. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. Very low lead exposures and children's neurodevelopment. Curr Opin Pediatr. 2008;20:172–177. doi: 10.1097/MOP.0b013e3282f4f97b. [DOI] [PubMed] [Google Scholar]
- Braun JM, Kahn RS, Froehlich T, Auinger P, Lanphear BP. Exposures to environmental toxicants and attention deficit hyperactivity disorder in US children. Environ Health Perspect. 2006;114:1904–1909. doi: 10.1289/ehp.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helt M, Kelley E, Kinsbourne M, Pandey J, Boorstein H, Herbert M, et al. Can children with autism recover? If so, how. Neuropsychol Rev. 2008;18:339–366. doi: 10.1007/s11065-008-9075-9. [DOI] [PubMed] [Google Scholar]
- Seidel S, Kreutzer R, Smith D, McNeel S, Gilliss D. Assessment of commercial laboratories performing hair mineral analysis. JAMA. 2001;285:67–72. doi: 10.1001/jama.285.1.67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.