Abstract
Schizophrenia and bipolar disorder share a number of common features, both symptomatically and biologically. Abnormalities in the neuroimmune and the stress-signaling pathways have been previously identified in brains of individuals with both diseases. However, the possible relationship between abnormalities in stress and neuroimmune signaling within the cortex of people with psychotic illness has not been defined. To test the hypothesis that combined alterations in brain stress responsiveness and neuroimmune/inflammatory status are characteristic of some individuals suffering from major mental illness, we examined gene expression in the Stanley Array Cohort of 35 controls, 35 individuals with schizophrenia and 34 individuals with bipolar disorder. We used levels of 8 inflammatory-related transcripts, of which SERPINA3 was significantly elevated in individuals with schizophrenia (F(2,88)=4.137, P<0.05), and 12 glucocorticoid receptor signaling (stress) pathway transcripts previously examined, to identify two clusters of individuals: a high inflammation/stress group (n=32) and a low (n=68) inflammation/stress group. The high inflammation/stress group has a significantly greater number of individuals with schizophrenia (n=15), and a trend toward having more bipolar disorder individuals (n=11), when compared with controls (n=6). Using these subgroups, we tested which microarray-assessed transcriptional changes may be associated with high inflammatory/stress groups using ingenuity analysis and found that an extended network of gene expression changes involving immune, growth factors, inhibitory signaling and cell death factors also distinguished these groups. Our work demonstrates that some of the heterogeneity in schizophrenia and bipolar disorder may be partially explained by inflammation/stress interactions, and that this biological subtype cuts across Diagnostic and Statistical Manual of Mental Disorders (DSM)-defined categories.
Keywords: bipolar disorder, glucocorticoid receptor, heterogeneity, inflammation, personalized medicine, schizophrenia
Introduction
Schizophrenia and bipolar disorder share a number of common features such as psychosis, negative symptoms and antipsychotic treatment. This commonality also extends to cellular and molecular neuropathology1, 2 and abnormalities in both the immune system and the stress response system have been proposed as mechanisms contributing to pathogenesis in both disorders. (for review see Drexhage et al.3 and Kupka et al.4)
Within the immune system, some of the key mediators are cytokines, which are small signaling molecules that can have a variety of downstream effects on both innate and adaptive immune systems. In schizophrenia, there have been a number of studies that have found increased levels of cytokines, including interleukin (IL)-1β, IL-6, IL-8 and tumor necrosis factor (TNF), in the serum of both first episode psychosis and chronic schizophrenia.5, 6, 7, 8 Similarly, in bipolar disorder, elevation of serum IL-6 and TNF have been shown across manic, euthymic and depressive states with IL-2, IL-4 and IL-8 being particularly elevated in mania.9, 10 The classic early literature suggested that there may be little direct impact of these cytokines on the brain owing to the presence of the blood brain barrier, and the brain's ‘immunoprivileged' status.11 However, there are a variety of mechanisms for immune signaling to occur across the blood brain barrier, as well as evidence that the blood brain barrier may become more porous in a disease state.12, 13 We recently found direct evidence for elevated cytokine mRNA levels in the post-mortem brains of people with schizophrenia in the Sydney Tissue Resource Center cohort that correlates with an increase in microglial density.14 However, a number of other studies have not observed any evidence of abnormal microglial density or morphological change with respect to diagnosis.15, 16, 17 In bipolar disorder patients, the IL-1 system-related mRNAs (IL1B and IL1R) have been reported to be elevated in the brain and there is a single study that observed an increase in microglial activation in the post-mortem tissue from frontal cortex.18
Although the contradictory findings with respect to microglia in both disorders indicates that there may be considerable biological heterogeneity present in these resident immune cells of the brain, it is nevertheless possible that the cellular source of the elevated markers of immune function in some individuals is microglia that may consequently result in (neuro)inflammation.14, 18, 19 The elevated inflammatory markers may be because of a genetic predisposition in some individuals with psychosis that favors increased production of cytokines as genetic linkage studies have found polymorphisms in the IL-1β gene, and in the major histocompatibility complex genes associated with schizophrenia and bipolar disorder.20, 21, 22, 23, 24 Environmental factors such as maternal infection leading to developmental or later immune abnormalities may also have a role in the pathogenesis of schizophrenia and possibly bipolar disorder.25, 26, 27 Thus, the immune system has frequently been implicated in the pathogenesis of both schizophrenia and bipolar disorder.
The stress signaling system, in particular glucocorticoids, are known to be upregulated in stress and chronic elevations result in a decrease in glucocorticoid receptor (GR) mRNA. Dysregulation of GR and other glucocorticoid signaling molecules has been observed in the brains of both schizophrenia and bipolar disorder patients.1, 28, 29, 30, 31 Although stress signaling is frequently considered independently from the immune system changes in the brain, GR and the immune/inflammatory system are intertwined, with glucocorticoid (stress) hormones being some of the most potent suppressors of the immune function.32 This suggests that abnormalities of the immune system and glucocorticoid signaling pathway, and interactions between these two systems, may contribute to the common pathophysiology found in major mental illnesses. However, it is not known whether the alterations in the stress and inflammatory systems found in psychiatric disease state co-occur, or are found in distinct subsets of individuals. Further, it is not known whether the changes in these two systems are reciprocal or whether they represent a side effect of chronic changes related to the diseases or to their treatments.33
In humans, the endogenous glucocorticoid, cortisol, binds to both the mineralocorticoid receptor (during periods of basal cortisol secretion) and the GR (during periods of elevated cortisol, such as acute or chronic stress). Cortisol-bound GR has a number of immediate and sustained actions, which include functioning as a transcription factor to modulate the expression of a variety of genes including repressing transcription of many genes involved in immune/inflammatory signaling including IL1B, IL6 and IL8.34, 35, 36, 37, 38 Interestingly, high levels of these ILs, and other cytokines, trigger increased secretion of cortisol,39, 40, 41 which in turn cyclically represses immune gene expression. In theory, a disruption in any part of this pathway could lead to runaway inflammation in the periphery or in the central nervous system and could lead to consequent cellular damage. It is possible that dysregulation of cytokine mRNA expression in psychiatric illness is not independent of GR mRNA abnormalities, but that both co-occur in the same individuals, thereby increasing the risk of disruption of neuronal and immune homeostasis in both schizophrenia and bipolar disorder.
The goals for this study were (1) to examine the extent to which changes in key mediators of the stress and inflammatory system responses co-occur in subsets of individuals with schizophrenia and bipolar disorder; (2) to determine what percentage of individuals with distinct clinical diagnoses of schizophrenia or bipolar disorder have shared molecular pathology of the inflammatory and stress signaling systems in the prefrontal cortex; and finally, (3) to examine what other molecular changes characterize these subsets of individuals.
Materials and methods
Tissue collection
Total RNA extracted from cortical gray matter that included all six layers was carefully dissected from the middle frontal gyrus, at the level anterior to the genu of the corpus callosum not including the frontal pole. White matter was carefully removed from each block of tissue with a scalpel or razor blade while frozen before RNA extraction. The total RNA was provided by the Stanley Medical Research Institute (Array Cohort) from 35 individuals with schizophrenia, 34 with bipolar disorder and 35 controls. Demographic details of this cohort are shown in Table 1.
Table 1. Demographic details of the schizophrenia, bipolar disorder and control cases in the Stanley Array Cohort.
| Control group (n=35) | Bipolar disorder group (n=34) | Schizophrenia group (n=35) | |
|---|---|---|---|
| Diagnostic subtype | — | BP1=27, BP2=4, BPNOS=2, schizoaffective=1 | SCZ(disorganized)=1, SCZ(paranoid)=8, SCZ(undifferentiated)=26 |
| Age (years) | 44.2 (31–60) | 45.4 (19–64) | 42.6 (19–59) |
| Gender | 9F, 26M | 18F, 16M | 9F, 26M |
| Hemisphere | 16L, 19R | 19L, 15R | 17L, 18R |
| pH | 6.61±0.27 | 6.43±0.30 | 6.48±0.24 |
| PMI (hours) | 29.4±12.9 | 37.9±18.6 | 31.4±15.5 |
| RIN | 7.23±0.87 | 7.34±0.88 | 7.36±0.61 |
| Manner of death | Natural=35 | Natural=19, suicide=15 | Natural=28, suicide=7 |
| Age of onset (years) | — | 25.3±9.2 | 21.3±6.1 |
| Duration of illness (years) | — | 20.2±9.6 | 21.3±10.2 |
| Lifetime antipsychotics (fluphenazine equiv., mg) | — | 10 212±22 871 | 85 004±100 335 |
| Antidepressant use | Yes=0, no=35 | Yes=19, no=15 | Yes=9, no=26 |
| Type of antidepressanta | — | SSRI=9 (fluoxetine=5), SNRI=4, SARI=5, TCA=6, other=1 | SSRI=4 (fluoxetine=2), SNRI=0, SARI=2, TCA=2, other=2 |
| Smoking around time of death | Yes=9, no=9, unknown=17 | Yes=15, no=6, unknown=13 | Yes=23, no=4, unknown=8 |
Abbreviations: BP1, bipolar disorder type 1; BP2, bipolar disorder type 2; BPNOS, bipolar disorder not otherwise specified; F, female; L, left; M, male; PMI, post-mortem interval; R, right; RIN, RNA integrity number; SARI, serotonin antagonist and reuptake inhibitor; SCZ, schizophrenia; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin–norepinephrine reuptake inhibitor; TCA, tricyclic antidepressant.
Some individuals took multiple antidepressant medications. Data quoted are mean (range)±s.d.
Quantitative PCR analysis
Preparation of complementary DNA and quantitative PCR (qPCR) experiments were performed as described previously.42 In brief, total RNA was extracted from 300 mg of gray matter using the Trizol method (Life Technologies, Carlsbad, CA USA) before having its quality determined on an Agilent Bioanalyzer 2100 (Agilent Technologies, Palo Alto, CA, USA). The complementary DNA was synthesized from this total RNA using Superscript First-Strand Synthesis Kit (Life Technologies). The following probes were used for the inflammatory genes: SERPINA3 (Hs003153674_m1), IL6 (Hs00174131_m1), IL6ST (Hs01006741_m1), IL8 (HS00174103_m1), IL1B (Hs01555410_m1), PTGS2 (Hs00153133_m1), IL18 (Hs01038788_m1), TNF (Hs99999043_m1), IL1RL1 (Hs01073300_m1), with four genes: ACTB (Hs99999903_m1), GAPDH (Hs99999905_m1), TBP (Hs00427621_m1) and UBC (Hs00824723_m1) used as housekeeper genes. None of the housekeeping genes significantly varied between diagnostic conditions. Cycling conditions and reaction amounts were as previously described.14 Previously published normalized mRNA expression data from the same cohort were used for statistical comparisons from three GRs (GR Pan, GR1B and GR1C) and eight other cofactors and chaperones of GR (FKBP5, BAG1, HSPA1A, FKBP4, DNAJB1, HSP90AA1, HSPB1 and PTGES3).43
Statistical analysis and clustering
Statistical tests of qPCR data were performed using SPSS statistics (version 21, OSX, IBM, Armonk, NY, USA). Data were non-normally distributed and a log10 transformation was applied to all qPCR measurements after normalization to the geometric mean of housekeeping gene expression. An approximate normal distribution was achieved before a Grubbs test was used to identify population/experimental outliers, (average number =1.9, range: 0–3 individuals per measurement). Stepwise regressions using demographic variables (body mass index (BMI), age, pH, post-mortem interval, brain weight and refrigerator interval) were used to identify covariates for analysis of covariance of experimentally measured mRNA values with Fisher's least significant difference post hoc tests on significant results. Significantly identified demographic variables are indicated in the individual experimental results. The α level for all statistical tests was 0.05.
The composition of the three groups, inflammatory, stress and a combined grouping of inflammatory/stress were tested using a recursive two-step cluster analysis on the entire schizophrenia/bipolar disorder/control cohort with missing values replaced by an expectation maximization algorithm. The overall model quality (Silhouette measure) was required to be >0.4, with predictors of the least importance removed until all predictors had significant contributions to the model (>0.25 on a scale of 0–1.0). Demographic factors that were significant in any regression model were included in the initial clustering lists to minimize bias. The diagnostic significance of the clustering was measured by splitting the comparison into the two diagnostic groups, respectively, compared with controls using both a one-tailed Fisher's exact test as well as a χ2-analysis. This was to minimize the influence of similar distributions between the schizophrenia and bipolar disorder groups.
If not included in the clustering variables, post hoc tests were performed to examine demographic differences in the clusters by analysis of variance with both cluster group and diagnosis included as factors. Factors examined included BMI, age, pH, post-mortem interval, brain weight and freezer storage interval. The impact of possible peripheral inflammatory response based on prior medical history/cause of death, smoking status and psychotic status was examined through the use of the χ2-statistic. Medical records were examined for any conditions that may result in peripheral cytokine elevations, including but not limited to obesity (BMI>30 kg m−2), diabetes, hypertension, hepatitis and atherosclerosis.
Microarray meta-analysis
Microarray Analysis Suite 5.0 (Affymetrix, Santa Clara, CA, USA) -normalized data from the Stanley microarray collection was downloaded from four studies using the Array collection group from dorsolateral prefrontal cortex (DLPFC) of individuals with schizophrenia, bipolar disorder and healthy controls (http://www.stanleygenomics.org, AltarA, Bahn, Dobrin and Kato44). Three of the four studies were run on Affymetrix HGU133P arrays while the fourth was run on an expanded HGU133P 2.0 series of chips. This resulted in three experiments with 22 283 probe sets and one experiment with 54 675 probe sets. Each replicated probe set was averaged using geometric mean in order to identify an unbiased mRNA expression for each set using custom R code. This was analyzed for differential expression using a series of false discovery rate-corrected t-tests on six groups comprising diagnosis (schizophrenia, bipolar disorder and control) and high inflammation/stress and low inflammation/stress as identified in the cluster analysis using CLC genomics workbench (5.51, CLC, Aarhus, The Netherlands).
Pathway analysis
Genes that were significantly changed between the high inflammation/stress in both schizophrenia as well as bipolar disorder and low inflammation/stress controls (false discovery rate P<0.05) were isolated and ingenuity pathway analysis (http://www.ingenuity.com) was used to identify other genes that may be part of an extended altered inflammatory–stress pathway dysregulation. Ingenuity pathway core analysis was performed using experimentally observed data from all animal types and central nervous system cell lines with biological pathways, networks and regulating factors explored in detail.
Results
qPCR analysis
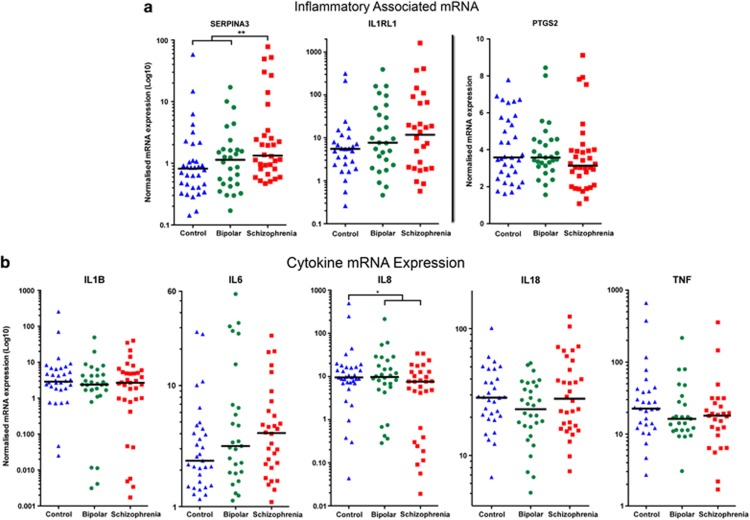
SERPINA3 mRNA was significantly changed according to diagnosis (analysis of covariance, pH, brain weight, F(2,88)=4.137, P<0.05), and we found that SERPINA3 mRNA was specifically increased in schizophrenia compared with both controls and bipolar (schizophrenia-control and schizophrenia-bipolar disorder: 184%, P<0.05, SCZ-BP: 293%, P<0.01, with % change calculated from means, Figure 1a). IL-8 mRNA showed a significant diagnostic effect (analysis of covariance, BMI and age, F(2,89)=3.897, P<0.05), but surprisingly, with a decreased expression in individuals with schizophrenia compared with controls and bipolar disorder (SCZ-CON: −73%, P<0.01, SCZ-BP: −58%, P<0.05, Figure 1b). IL-1β, IL-18, TNF and PTGS2 mRNAs showed no significant diagnostic effects overall and no consistent pattern of expression according to diagnosis. IL1RL1 and IL-6 mRNAs were not significantly changed but showed a pattern of increased expression in both psychosis groups with respect to median values (Figures 1a and b). As found in other cohorts, substantial individual differences in expression level were identified suggesting that there may be significant heterogeneity within each diagnostic category.14
Figure 1.
Quantitative PCR (qPCR) measured mRNA expression for inflammatory-associated genes (a) and cytokine-associated genes (b). Controls (blue) and individuals with bipolar disorder (green) and schizophrenia (red) are plotted with median values represented by the black line. All values with the exception of PTGS2 are plotted on a log-transformed scale because of their distribution.
Use of clustering to identify subgroups
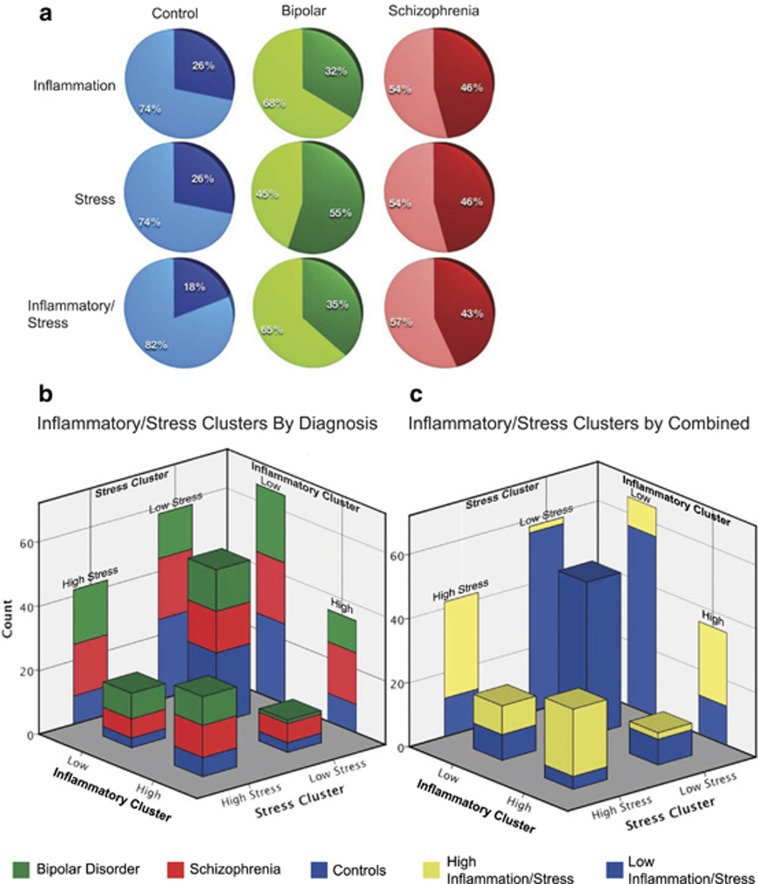
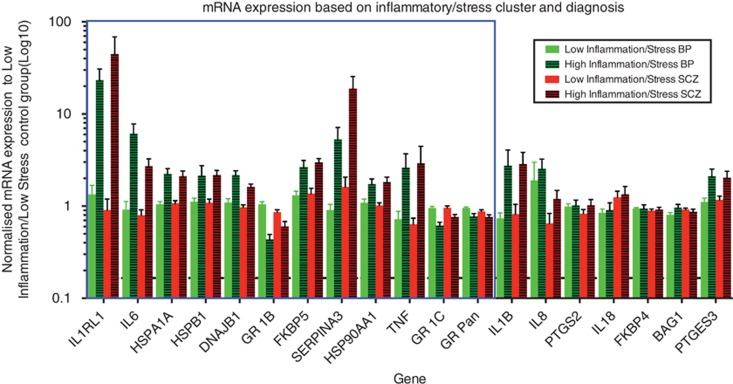
We performed three recursive two-step clustering analyses using our 8 inflammatory-related genes (SERPINA3, IL1B, IL1RL1, IL6, IL8, IL18, TNF and PTGS2) as well as previously published data on 11 mRNAs from the GR stress signaling pathway.29 The first clustering analysis using the inflammatory-related genes yielded two groups, which we entitled high (n=35) and low (n=65) based on the expression pattern of the majority of the defining genes/demographic factors, IL6, SERPINA3, IL1RL1, pH, TNF, IL1B and IL8. This model had an optimum silhouette value of 0.4. Schizophrenia showed a trend toward a non-random distribution (χ2=2.764, P<0.01, Fisher's P=0.08), although bipolar disorder did not (Figures 2a and b, x axis; Supplementary Table 1). The second clustering used only the GR and associated signaling molecules giving two groups, a high stress (n=42) and a low stress (n=58) defined by HSPA1A, HSPB1, DNAJB1, GR1B, HSP90AA1, FKBP5, GR1C and pH. The optimum silhouette value was once again 0.4 and in this cluster schizophrenia showed a trend toward a non-random distribution between the stress groups (χ2=2.764, P<0.01, Fisher's P=0.08) with bipolar disorder being significantly non-random (χ2=5.437, P<0.05, Fisher's P<0.05, Figures 2a and b, z axis; Supplementary Table 1). The overlap between these groups with regard to diagnosis shows a distinctly greater proportion of psychosis in the respective high inflammation and stress groups compared with low (Figure 2b). Third, owing to the inflammatory response and stress signaling interrelationship, we performed clustering using all the genes and found the model included only IL1RL1, IL6, HSPA1A, HSPB1, DNAJB1, GR1B, FKBP5, SERPINA3, HSP90AA1, TNF, GR1C, pH, GR Pan, (12 out of the 19 genes and 4 demographic factors we started with) in order of decreasing importance (Figure 3). Two clusters were identified with an optimum silhouette value of 0.4, a high inflammation/stress (n=32) and an opposing, low inflammation/stress group (n=68) (Figures 2a and c; Supplementary Table 1). The high inflammation/stress cluster showed a significantly different diagnostic distribution with respect to schizophrenia (χ2=5.177, P<0.05, Fisher's P<0.05) and a trend for bipolar (χ2=2.671, P<0.1, Fisher's P=0.09) with more individuals in the high group than expected (Figure 2a). The high inflammation/stress group also appears to include more individuals with high stress and low inflammation than the comparative low stress and high inflammation group (Figure 2c). None of the three clusters showed a significantly different distribution according to axis I diagnosis including schizophrenia subtypes.
Figure 2.
A series of recursive two-step cluster analyses yields different breakdowns in each diagnosis category. Three clustering operations were performed on only the inflammatory-related genes ((a), top row), stress-related genes ((a), middle row) and then both sets of genes combined ((a), bottom row). Each diagnosis is split between those in the high group ((a), darker color) and the low group ((a), lighter color). Controls (blue), bipolar disorder (green) and schizophrenia (red) are shown in the first, second and third columns, respectively. To examine the overlap of the inflammation ((b), x axis and right rear wall) and stress clusters ((b), z axis and left rear wall) with respect to diagnosis (blue, green and red color scheme), they were plotted in the form of a three-dimensional bar graph. Using the same values as b, the third, combined stress and inflammation cluster is represented by a separate color code, with blue representing low inflammation/stress and yellow high inflammation/stress (c). This indicates that the dichotomous group variables of inflammation and stress are a good, but not perfect, predictor of membership in the combined inflammation/stress group defined by clustered by the 19 continuous mRNA values.
Figure 3.
A recursive two-step clustering incorporating stress as well as inflammatory gene-related results in two groups that we titled high inflammation/stress and low inflammation/stress with relation to their inflammatory and stress signaling components. Individuals with bipolar disorder (green) and schizophrenia (red) are plotted relative to the mean expression of the low inflammation/stress control group (black-dashed line at one) on a log scale with s.e.m. represented by error bars. The blue rectangle represents the genes incorporated into the combined inflammatory/stress clustering model. Genes are ordered relative to their contribution to the model decreasing from left to right.
Demographic differences between clusters
We were primarily interested in the interaction between inflammation and stress, so further analysis focused on the two groups defined by this combined cluster, which did not have a significant difference in BMI, post-mortem interval or freezer storage interval. Individuals did not differ in age between diagnostic groups, or between inflammatory/stress groups but there was a significant interaction (F(2,94)=3.241, P<0.05) stemming from the high inflammation/stress bipolar disorder group being 9.07 years older than the high inflammation/stress controls. Although pH was included in the clustering, it is important to observe that it did not differ among diagnosis, although the low inflammation/stress group controls had a slightly higher pH as compared with bipolar disorder (ΔpH: 0.137, P=0.05) and schizophrenia (ΔpH:0.199, P<0.01). The low inflammatory/stress group also had greater brain weight (5.43%, F(1,94)=7.421, P<0.01). Gender, smoking, history of alcohol use and history of drug use status did not significantly differ between the inflammatory/stress groups. Excluding individuals with no available information concerning peripheral inflammation, we find that there is a trend toward more individuals in the high inflammation/stress group having some evidence of peripheral inflammatory processes (no (high/low): 7/24, yes: 24/32, χ2=3.577, P<0.1). When examining demographic variables present just in the bipolar disorder and schizophrenia groups, we found no significant differences in antipsychotic dose, duration of disease or age of onset between the inflammation/stress groups or interactions with diagnosis. However, the low inflammatory group were more likely to have committed suicide (n=18/3, χ2=3.833, P<0.05).
Microarray analysis of differences between inflammatory/stress groups
The total list of 54 675 probes was imported to CLC genomics workbench for analysis. We used a pair of two-tailed t-tests comparing high inflammation/stress subgroup of individuals with schizophrenia and bipolar disorder to low inflammation/stress subgroup of controls and found 57 probe sets significantly changed between the groups after false discovery rate correction (Supplementary Table 2) that were carried forward into the pathway analysis.
Pathway analysis
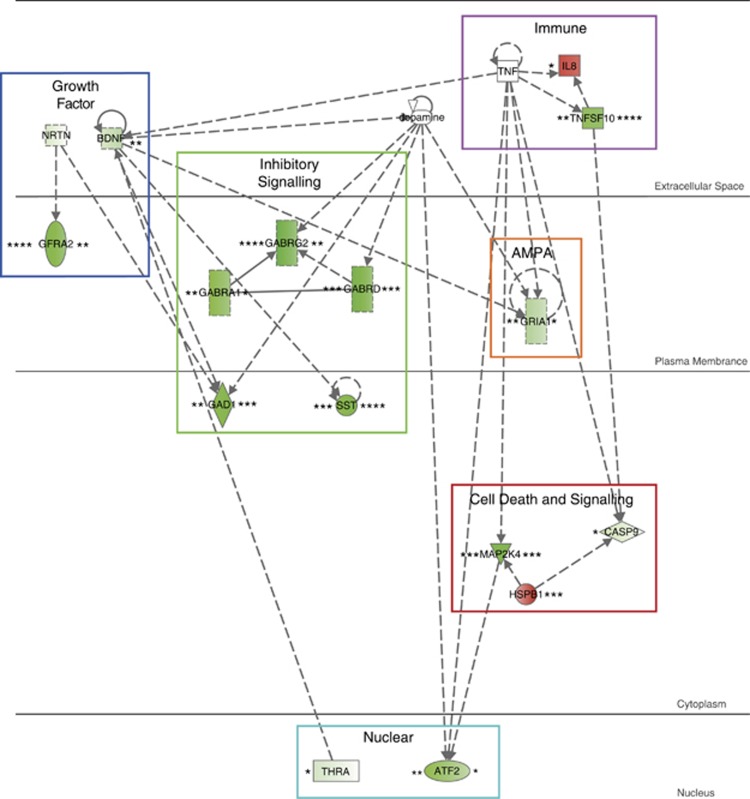
The ingenuity pathway analysis yielded five gene networks that were significantly enriched in the list of 57 probe sets (Supplementary Table 3). The top network was entitled: ‘Neurological Disease, Cell-to-Cell Signaling and Interaction, Nervous System Development and Function'. This pathway showed significant decreased expression in both high inflammation/stress bipolar disorder as well as schizophrenia compared with low inflammation/stress controls (Figure 4). Genes involved in this pathway can be roughly grouped by relation to six different functions: growth factor, immune, inhibitory signaling, cell death and signaling, AMPA receptor and nuclear factors. The majority of the individual genes show negative fold changes in individuals with bipolar/schizophrenia and high inflammation/stress. In addition, ingenuity calculated that a number of genes, including brain-derived neurotrophic factor (BDNF), TGF1 and TNF, were implicated as key upstream regulators of the overall altered gene networks (Supplementary Table 4).
Figure 4.
The top ingenuity identified network that was enriched for the 57 genes that were significantly differentially expressed, after false discovery rate (FDR) correction, between high inflammation/stress individuals with bipolar disorder and schizophrenia as compared with low inflammation/stress controls. The initial microarray data were taken from a geometric mean of four separate experiments performed on the same Stanley Array Collection that we used in this current study. On the left side of each gene is the significance and fold change for bipolar disorder (when compared with low inflammation/stress controls). On the right side of each gene is the significance and fold change for schizophrenia. Fold changes are represented by intensity of color with green showing a decreased expression in the disease state compared with controls, whereas red indicates an increased expression in the disease state high inflammation/stress group as compared with the low inflammation/stress controls. Significance of these fold changes as assayed by t-tests are the following: *P<0.05, **P<0.001, ***P<0.0001, ****P<0.00001.
Discussion
We have determined that clustering by inflammatory genes as well as clustering by stress genes results in groups enriched in individuals with a diagnosis of psychosis. Furthermore, it would seem that inflammation has a greater association with schizophrenia while stress signaling has a greater association with bipolar disorder. However, when the gene lists are combined together there are a greater than expected number of individuals with schizophrenia and a trend toward a greater number of bipolar disorder individuals categorized as in the high inflammation/stress group. Our study builds on the mounting evidence suggesting that a subset of individuals with psychotic illness have increased expression of immune-related genes in the brain,14 cerebrospinal fluid,45 and blood.46, 47 By showing that the immune changes are often concomitant with alterations in the stress signaling system, we have expanded the understanding of possible molecular mechanisms involved in the pathophysiology and of the therapies targeting these systems.
We examined the cohort demographics for evidence that the inflammatory/stress cluster could be a reflection of some external factors other than psychiatric disease state. We found decreased pH in the high inflammatory/stress group, which may be a response to the increased metabolic demands of an inflammatory immune response; however, this lowered pH could be a sign of tissue damage and in itself may exacerbate inflammation.48 This damage may be reflected in our observation of decreased brain weight in the high inflammatory/stress group, which remains significantly different even after co-varying for age and sex (data not shown). This brain mass decrease may be related to the observed volumetric and brain thickness decreases found in schizophrenia and bipolar disorder, but the link between inflammation and brain volume requires further exploration.49 It is also possible that the decrease in brain mass is related to the stress of the disease states as individuals undergoing chronic stress in adolescence and adulthood have decreased brain volumes.50, 51 Some of the observed brain inflammatory changes may be related to peripheral inflammation, which were identified in individuals found in both high and low inflammatory/stress groups. Interactions between peripheral IL-1 activation and the central nervous system have been demonstrated in animal models.52 Although obesity could be a possible cause of peripheral inflammation in some humans, it also may be directly related to psychiatric disease or to treatment.53 This is a key area that requires further research to understand the interactions.
The most robust mRNA finding from this study, and one that has been consistently replicated in other studies, was the elevation in SERPINA3 mRNA present in the DLPFC of individuals with schizophrenia. We found an almost 200% increase in the expression of SERPINA3 in schizophrenia patients compared to controls (note results on log scale) in the Stanley Array Cohort, which is now the fourth separate post-mortem cohort studied where this elevation is found.14, 54, 55 This suggests that upregulation of SERPINA3 mRNA is a highly reproducible finding in the frontal cortex in schizophrenia but its more specific involvement in schizophrenia as compared with bipolar disorder remains unexplored. SERPINA3 is known to code for a protein with an anti-chymotripsin action (SERPINA3 is also known as alpha 1-antichymotripsin), although this may not be the only protease that SERPINA3 inhibits.56 The precise cellular source of the SERPINA3 mRNA elevations in the brains of individuals with schizophrenia is unknown, but macrophages, reactive astrocytes and activated microglia have been shown to express the SERPINA3 protein in pathological conditions.57 In Alzheimer's disease, SERPINA3 forms complexes with amyloid beta protein and also alters the expression of astrocytic and inflammatory genes.58, 59 Transcription of SERPINA3 is associated with inflammatory activation and the protein itself has multiple roles including prevention of excessive tissue damage from the secretion of chymotrypsin originating in phagocytes (microglia are the equivalent central nervous system phagocytes) as well as an unidentified DNA-binding role thought to modulate chymotrypsin-like chromatin enzymes.60, 61 Recently, it has been shown that glucocorticoids, working synergistically with inflammatory molecules (specifically TNF) increase the transcription of SERPINA3.62 Although the levels of GR are lower in individuals with schizophrenia, which may be a consequence of increased stress, the GR signaling system may remain functional, and in the face of elevated TNF this may result in the elevations in SERPINA3 present in our cohorts as well as others. The evidence for TNF being elevated in schizophrenia and bipolar disorder is mixed, but nevertheless a number of studies have demonstrated TNF increases in the brain and blood.63, 64, 65 The coactivation and upregulation of SERPINA3 suggests a possible compensatory role in the disease in an attempt to attenuate a chronic inflammatory response. It is perplexing that the elevation in SERPINA3 only becomes apparent in bipolar disorder after clustering by other inflammatory/stress factors, implying that although inflammation is common between the two diseases, specific elements of the response are more prevalent or exaggerated in schizophrenia. Further research is required to elucidate the role of SERPINA3 in the brain, and particularly in schizophrenia.
We were unable to replicate the overall group increases found in the cytokines IL-6 and IL-8 mRNAs previously identified in the schizophrenia patients from our Sydney TRC cohort.14 However, the subgroup of individuals with high inflammation/stress in the current study did display elevated IL1RL1, IL-6, TNF and IL-1β mRNA compared with the majority of controls. We were also unable to replicate a previous finding of overall increased IL-1β in individuals with bipolar disorder.18 It does seem that the generalized activation of the immune/inflammatory system is a common factor between all of these studies; however, the precise factors and the percentage of individuals demonstrating these changes at the time of death appear to be variable. Alternatively, as we suspect that the number of individuals with psychosis who have active inflammation may be only 40–50% of the total, nonrandom sampling may be responsible for variable research outcomes.
Our data suggest an interrelationship between stress signaling and immune function in the frontal cortex of a portion of individuals, primarily those with bipolar disorder and schizophrenia. Although data on cortisol levels of individuals in this cohort are not currently available, it is plausible that elevated cortisol levels in individuals with schizophrenia and bipolar disorder may drive the observed changes in both stress and inflammatory gene mRNAs. Elevated glucocorticoid levels, which have been demonstrated in individuals with schizophrenia and bipolar disorder,66, 67 have been shown to decrease GR mRNA expression and protein abundance in the rodent and primate frontal cortex and hippocampus.68, 69, 70, 71 Glucocorticoids also suppress immune function and immune gene expression,34, 35, 36, 37, 72 resulting in inflammatory resurgence after chronic administration.73 In schizophrenia and bipolar disorder it is possible that chronic hypercortisolemia, in a portion of patients with greater negative symptoms,74 may cause a chronic decrease in the expression and function of GR protein leading to attenuation of the natural inhibition of the immune system by stress.
Our idea of stress-related gene expression changes being more primary originates from the greater contribution of stress-related genes to the combined inflammatory/stress clustering. Our pathway analysis expands upon the limited number of genes involved in our mRNA findings and provides an opportunity for integration of our results with other schizophrenia and bipolar disorder findings. The high inflammation/stress group shows a downregulation of a number of gene groups in the diagnosis of schizophrenia and bipolar disorder. Key among these are the growth factor-related genes (NRTN (neurturin), BDNF and GDNF family receptor α2 (GFRA2)) and the inhibitory and excitatory signaling (GABAA receptor subunit γ2, α1 and δ, glutamic acid decarboxylase 1, somatostatin and glutamate receptor ionotropic AMPA 1, respectively). NRTN works through the GDNF receptor pathway, including GFRA2, and has been shown to be neuroprotective in the cortex of animal models.75 BDNF mRNA has been previously found decreased in the DLPFC of schizophrenia and BDNF can inhibit major histocompatibility complex-II inducibility in microglia.76, 77, 78 We, as well as others, have previously shown that major histocompatibility complex II-labeled microglia are increased in individuals with schizophrenia, and this may provide a possible link to decreased BDNF.14 The health of inhibitory interneurons and their signaling has been the focus of extensive research efforts in both schizophrenia and bipolar disorder with BDNF thought to be an important survival factor for inhibitory interneurons. Knockout of BDNF in mice resulted in a decrease density of cortical interneurons of the somatostatin subtype.79 Decreased amounts of BDNF have further been shown to correlate with decreased expression of glutamic acid decarboxylase 1 (GAD67) in subjects with schizophrenia.2, 80 Decreased amounts of somatostatin and glutamic acid decarboxylase 1 mRNA in particular, have been widely replicated in schizophrenia, with glutamic acid decarboxylase 1 also found decreased in bipolar disorder.81, 82, 83, 84 These mRNA decreases are exacerbated in the high inflammation/stress group of both diseases in our current study as well as previously in the high inflammation group of the TRC schizophrenia cohort.14 The involvement of the GABAA receptor subunits with stress and inflammation, particularly the significant decreases in the almost ubiquitous γ2 and α1 encoding subunits, together with the extrasynapic δ subunit, may explain some of the contradictory findings reported in both diseases.83, 85, 86 A number of immune-related genes are also found in this pathway including TNF, IL8 and TNFSF10 all of which as well as a number of other inflammatory-related genes have been implicated in schizophrenia and bipolar disorder.14, 87, 88, 89, 90 IL-8 was significantly elevated only in bipolar, similar to our qPCR data, when using the microarray generated data. However, microarrays, owing to their sensitivity issues are not optimal for measuring cytokine mRNA.91 TNFSF10 is also known as TNF-related apoptosis-inducing ligand and works through a caspase cascade including caspase 9 to cause apoptosis.92 The decrease in TNF-related apoptosis-inducing ligand is in direct contrast to our previously observed TNFSF13 (APRIL) increases in both disease states implying abnormal apoptotic-related process may be present.88 Cytokines, including IL-1β and TNF also work through mitogen-activated protein kinases to phosphorylate ATF2, leading to increased c-Jun and anti-apoptotic activity.92 A decrease in the latter part of this pathway may indicate a reduced ability to counter the pro-apoptotic activity of elevated cytokines found in the high inflammation/stress group, although the presence of apoptosis in these diseases is disputed.
It is noteworthy that we observed a significant number of controls (18% of the group) that also displayed the elevated markers of both inflammation and stress in the brain. This may suggest that some individuals have a biological resiliency to the negative effects of chronic inflammation, the mechanisms of which have not yet been identified, or that we are observing a mix of individuals in both chronic and acute inflammatory states. Individuals identified in the high inflammation/stress control group may be in the midst of an acute inflammatory response to a stressor that may have resolved without significant or ongoing neuronal damage.31 In schizophrenia, the often observed peripheral cytokine elevations over a variety of ages would lead us to believe that the higher than normal inflammatory response may be more chronic, rather than acute, possibly leading to cumulative damage.93
In summary, we have established that our previously observed post-mortem changes in cytokine expression are not consistently detectable across all cohorts.14 However, a generalized pattern of immune activation/inflammatory processes seems to be occurring in under half of the individuals diagnosed with schizophrenia and bipolar disorder. This immune activation may also co-occur with previously observed GR signaling abnormalities found in both diseases.28 In combination, the inflammatory/stress dysfunction may integrate previously independent observations about the pathophysiology of the disorders and provide additional targets for novel, individually or biological subgroup-targeted treatment interventions.
Acknowledgments
This work was supported by the Stanley Medical Research Institute as well as the Schizophrenia Research Institute (utilizing infrastructure funding from the NSW Ministry of Health and the Macquarie Group Foundation), the University of New South Wales, and Neuroscience Research Australia. CSW is a recipient of a National Health and Medical Research Council (Australia) Senior Research Fellowship (#1021970). CSW is supported by Schizophrenia Research Institute (utilizing infrastructure funding from the NSW Ministry of Health and the Macquarie Group Foundation), the University of New South Wales, and Neuroscience Research Australia.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Webster MJ, Knable MB, O'Grady J, Orthmann J, Weickert CS. Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Mol Psychiatry. 2002;7:924. doi: 10.1038/sj.mp.4001139. [DOI] [PubMed] [Google Scholar]
- Ray MT, Weickert CS, Wyatt E, Webster MJ. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J Psychiatry Neurosci. 2011;36:195. doi: 10.1503/jpn.100048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexhage RC, Knijff EM, Padmos RC, Heul-Nieuwenhuijzen Lvd, Beumer W, Versnel MA, et al. The mononuclear phagocyte system and its cytokine inflammatory networks in schizophrenia and bipolar disorder. Expert Rev Neurother. 2010;10:59–76. doi: 10.1586/ern.09.144. [DOI] [PubMed] [Google Scholar]
- Kupka RW, Breunis MN, Knijff E, Ruwhof C, Nolen WA, Drexhage HA. Immune activation, steroid resistancy and bipolar disorder. Bipolar Disord. 2002;4:73–74. doi: 10.1034/j.1399-5618.4.s1.29.x. [DOI] [PubMed] [Google Scholar]
- Erbağci AB, Herken H, Köylüoglu O, Yilmaz N, Tarakçioglu M. Serum IL-1beta, sIL-2 R, IL-6, IL-8 and TNF-alpha in schizophrenic patients, relation with symptomatology and responsiveness to risperidone treatment. Mediators Inflamm. 2001;10:109–115. doi: 10.1080/09629350123895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz M, Ceresér KM, Goi PD, Fries GR, Teixeira AL, Fernandes BS, et al. Serum levels of IL-6, IL-10 and TNF-α in patients with bipolar disorder and schizophrenia: differences in pro- and anti-inflammatory balance. Rev Bras Psiquiatr. 2011;33:268–274. doi: 10.1590/s1516-44462011000300010. [DOI] [PubMed] [Google Scholar]
- Lin A, Kenis G, Bignotti S, Tura GJ, De Jong R, Bosmans E, et al. The inflammatory response system in treatment-resistant schizophrenia: increased serum interleukin-6. Schizophr Res. 1998;32:9–15. doi: 10.1016/s0920-9964(98)00034-6. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Zhou DF, Zhang PY, Wu GY, Cao LY, Shen YC. Elevated interleukin-2, interleukin-6 and interleukin-8 serum levels in neuroleptic-free schizophrenia: association with psychopathology. Schizophr Res. 2002;57:247–258. doi: 10.1016/s0920-9964(01)00296-1. [DOI] [PubMed] [Google Scholar]
- Kim Y-K, Jung H-G, Myint A-M, Kim H, Park S-H. Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. J Affect Disord. 2007;104:91–95. doi: 10.1016/j.jad.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Brietzke E, Stertz L, Fernandes BS, Kauer-Sant'anna M, Mascarenhas M, Escosteguy Vargas A, et al. Comparison of cytokine levels in depressed, manic and euthymic patients with bipolar disorder. J Affect Disord. 2009;116:214–217. doi: 10.1016/j.jad.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Tze WJ, Tai J. Successful intracerebral allotransplantation of pancreatic endocrine cells in spontaneous diabetic BB rats without immunosuppression. Metabolism. 1984;33:785–789. doi: 10.1016/0026-0495(84)90103-3. [DOI] [PubMed] [Google Scholar]
- Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37:26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Beumer W, Gibney SM, Drexhage RC, Pont-Lezica L, Doorduin J, Klein HC, et al. The immune theory of psychiatric diseases: a key role for activated microglia and circulating monocytes. J Leukoc Biol. 2012;92:959–975. doi: 10.1189/jlb.0212100. [DOI] [PubMed] [Google Scholar]
- Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18:206–214. doi: 10.1038/mp.2012.110. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Trojanowski JQ, Gur RE, Blackwell P, Han LY, Choi C. Absence of neurodegeneration and neural injury in the cerebral cortex in a sample of elderly patients with schizophrenia. Arch Gen Psychiatry. 1998;55:225–232. doi: 10.1001/archpsyc.55.3.225. [DOI] [PubMed] [Google Scholar]
- Takano A, Arakawa R, Ito H, Tateno A, Takahashi H, Matsumoto R, et al. Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET study with [11C]DAA1106. Int J Neuropsychopharmacol. 2010;13:943–950. doi: 10.1017/S1461145710000313. [DOI] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Rao JS, Harry GJ, Rapoport SI, Kim HW. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry. 2010;15:384–392. doi: 10.1038/mp.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorduin J, de Vries EFJ, Willemsen ATM, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med. 2009;50:1801–1807. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- Schwab SG, Hallmayer J, Freimann J, Lerer B, Albus M, Borrmann-Hassenbach M, et al. Investigation of linkage and association/linkage disequilibrium of HLA A-, DQA1-, DQB1-, and DRB1-alleles in 69 sib-pair- and 89 trio-families with schizophrenia. Am J Med Genet. 2002;114:315–320. doi: 10.1002/ajmg.10307. [DOI] [PubMed] [Google Scholar]
- Xu M, He L. Convergent evidence shows a positive association of interleukin-1 gene complex locus with susceptibility to schizophrenia in the Caucasian population. Schizophr Res. 2010;120:131–142. doi: 10.1016/j.schres.2010.02.1031. [DOI] [PubMed] [Google Scholar]
- Papiol S, Rosa A, Gutiérrez B, Martín B, Salgado P, Catalán R, et al. Interleukin-1 cluster is associated with genetic risk for schizophrenia and bipolar disorder. J Med Genet. 2004;41:219–223. doi: 10.1136/jmg.2003.012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmans P, Green EK, Pahwa JS, Ferreira MAR, Purcell SM, Sklar P, et al. Gene ontology analysis of GWA study data sets provides insights into the biology of bipolar disorder. Am J Hum Genet. 2009;85:13–24. doi: 10.1016/j.ajhg.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P, Holmans PA, et al. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev Neurobiol. 2012;72:1272–1276. doi: 10.1002/dneu.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, McKeague I, Bao Y, Pollak SV, Shen L, Schaefer CA.Are Maternal Cytokine Elevations Specific to Schizophrenia Among Offspring With Psychiatric Disorders Schizophrenia Bulletin 201339S59Orlando, FL, USA, 2013. [Google Scholar]
- Yolken RH, Torrey EF. Viruses, schizophrenia, and bipolar disorder. Clin Microbiol Rev. 1995;8:131–145. doi: 10.1128/cmr.8.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair D, Tsai S-Y, Woon HG, Weickert CS. Abnormal glucocorticoid receptor mRNA and protein isoform expression in the prefrontal cortex in psychiatric illness. Neuropsychopharmacology. 2011;36:2698–2709. doi: 10.1038/npp.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E, Guest PC, Rahmoune H, Harris LW, Wang L, Leweke FM, et al. Identification of a biological signature for schizophrenia in serum. Mol Psychiatry. 2011;17:494–502. doi: 10.1038/mp.2011.42. [DOI] [PubMed] [Google Scholar]
- Perlman WR, Webster MJ, Kleinman JE, Weickert CS. Reduced glucocorticoid and estrogen receptor alpha messenger ribonucleic acid levels in the amygdala of patients with major mental illness. BPS. 2004;56:844–852. doi: 10.1016/j.biopsych.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- Steiner RW, Awdishu L. Steroids in kidney transplant patients. Semin Immunopathol. 2011;33:157–167. doi: 10.1007/s00281-011-0259-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:112–119. doi: 10.1016/j.pnpbp.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Waterman WR, Xu LL, Tetradis S, Motyckova G, Tsukada J, Saito K, et al. Glucocorticoid inhibits the human pro-interleukin lbeta gene (ILIB) by decreasing DNA binding of transactivators to the signal-responsive enhancer. Mol Immunol. 2006;43:773–782. doi: 10.1016/j.molimm.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Mukaida N, Morita M, Ishikawa Y, Rice N, Okamoto S, Kasahara T, et al. Novel mechanism of glucocorticoid-mediated gene repression. Nuclear factor-kappa B is target for glucocorticoid-mediated interleukin 8 gene repression. J Biol Chem. 1994;269:13289–13295. [PubMed] [Google Scholar]
- Nadeau S, Rivest S. Glucocorticoids play a fundamental role in protecting the brain during innate immune response. J Neurosci. 2003;23:5536–5544. doi: 10.1523/JNEUROSCI.23-13-05536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoog NJD, Du Toit A, Avenant C, Hapgood JP. Glucocorticoid-independent repression of tumor necrosis factor (TNF) alpha-stimulated interleukin (IL)-6 expression by the glucocorticoid receptor: a potential mechanism for protection against an excessive inflammatory response. J Biol Chem. 2011;286:19297–19310. doi: 10.1074/jbc.M110.193672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage A, Slupphaug G, Shalaby R. Glucocorticoids inhibit the production of IL6 from monocytes, endothelial cells and fibroblasts. Eur J Immunol. 1990;20:2439–2443. doi: 10.1002/eji.1830201112. [DOI] [PubMed] [Google Scholar]
- Päth G, Bornstein SR, Ehrhart-Bornstein M, Scherbaum WA. Interleukin-6 and the interleukin-6 receptor in the human adrenal gland: expression and effects on steroidogenesis. J Clin Endocrinol Metab. 1997;82:2343–2349. doi: 10.1210/jcem.82.7.4072. [DOI] [PubMed] [Google Scholar]
- van der Meer MJ, Sweep CG, Pesman GJ, Tilders FJ, Hermus AR. Chronic stimulation of the hypothalamus-pituitary-adrenal axis in rats by interleukin 1beta: central and peripheral mechanisms. Cytokine. 1996;8:910–919. doi: 10.1006/cyto.1996.0122. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Sheedy D, Rothmond DA, Dedova I, Fung S, Garrick T, et al. Selection of reference gene expression in a schizophrenia brain cohort. Aust N Z J Psychiatry. 2010;44:59–70. doi: 10.3109/00048670903393662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair D, Fillman SG, Webster MJ, Shannon Weickert C. Dysregulation of glucocorticoid receptor co-factors FKBP5, BAG1, PTGES3 in the dorsolateral prefrontal cortex in psychotic illness. Sci Rep. 2013;3:3539–3549. doi: 10.1038/srep03539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs BW, Elashoff M, Richman S, Barci B. An online database for brain disease research. BMC Genomics. 2006;7:70. doi: 10.1186/1471-2164-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechter K, Reiber H, Herzog S, Fuchs D, Tumani H, Maxeiner HG. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: Identification of subgroups with immune responses and blood–CSF barrier dysfunction. J Psychiatr Res. 2010;44:321–330. doi: 10.1016/j.jpsychires.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Fan X, Pristach C, Liu EY, Freudenreich O, Henderson DC, Goff DC. Elevated serum levels of C-reactive protein are associated with more severe psychopathology in a subgroup of patients with schizophrenia. Psychiatry Res. 2007;149:267–271. doi: 10.1016/j.psychres.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Wilke I, Arolt V, Rothermundt M, Weitzsch C, Hornberg M, Kirchner H. Investigations of cytokine production in whole blood cultures of paranoid and residual schizophrenic patients. Eur Arch Psychiatry Clin Neurosci. 1996;246:279–284. doi: 10.1007/BF02190280. [DOI] [PubMed] [Google Scholar]
- Mrozek S, Vardon F, Geeraerts T. Brain temperature: physiology and pathophysiology after brain injury. Anesthesiol Res Pract. 2012;2012:989487. doi: 10.1155/2012/989487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br J Psychiatry. 2009;195:194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, et al. A.E. Bennett Research Award. Developmental traumatology. Part II: brain development. Biol Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. NeuroImage. 2007;35:795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampa J, Westman M, Kadetoff D, Agréus AN, Le Maître E, Gillis-Haegerstrand C, et al. Peripheral inflammatory disease associated with centrally activated IL-1 system in humans and mice. Proc Natl Acad Sci USA. 2012;109:12728–12733. doi: 10.1073/pnas.1118748109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirshing DA. Schizophrenia and obesity: impact of antipsychotic medications. J Clin Psychiatry. 2004;65:13–26. [PubMed] [Google Scholar]
- Saetre P, Emilsson L, Axelsson E, Kreuger J, Lindholm E, Jazin E. Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry. 2007;7:46. doi: 10.1186/1471-244X-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arion D, Unger T, Lewis DA, Levitt P, Mirnics K. Molecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia. Biol Psychiatry. 2007;62:711–721. doi: 10.1016/j.biopsych.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C, Belbin O, Kalsheker N, Morgan K. SERPINA3 (aka alpha-1-antichymotrypsin) Front Biosci. 2007;12:2821–2835. doi: 10.2741/2275. [DOI] [PubMed] [Google Scholar]
- Montesinos-Rongen M, Brunn A, Bentink S, Basso K, Lim WK, Klapper W, et al. Gene expression profiling suggests primary central nervous system lymphomas to be derived from a late germinal center B cell. Leukemia. 2007;22:400–405. doi: 10.1038/sj.leu.2405019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C, Nielsen HM, Minthon L, Wright HT, Chappell S, Okyere J, et al. Effects of Alzheimer's peptide and α1-antichymotrypsin on astrocyte gene expression. Neurobiol Aging. 2007;28:51–61. doi: 10.1016/j.neurobiolaging.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Abraham CR, Selkoe DJ, Potter H. Immunochemical identification of the serine protease inhibitor alpha 1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell. 1988;52:487–501. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- Travis J, Salvesen GS. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Cooperman BS. Identification of lysines within [IMAGE]1-antichymotrypsin important for DNA binding. J Biol Chem. 1995;270:14548–14555. doi: 10.1074/jbc.270.24.14548. [DOI] [PubMed] [Google Scholar]
- Lannan EA, Galliher-Beckley AJ, Scoltock AB, Cidlowski JA. Proinflammatory actions of glucocorticoids: glucocorticoids and TNF coregulate gene expression in vitro and in vivo. Endocrinology. 2012;153:3701–3712. doi: 10.1210/en.2012-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean B, Gibbons AS, Tawadros N, Brooks L, Everall IP, Scarr E. Different changes in cortical tumor necrosis factor-α-related pathways in schizophrenia and disorders mood. Mol Psychiatry. 2012;18:767–773. doi: 10.1038/mp.2012.95. [DOI] [PubMed] [Google Scholar]
- Liu L, Jia F, Yuan G, Chen Z, Yao J, Li H, et al. Tyrosine hydroxylase, interleukin-1beta and tumor necrosis factor-alpha are overexpressed in peripheral blood mononuclear cells from schizophrenia patients as determined by semi-quantitative analysis. Psychiatry Res. 2010;176:1–7. doi: 10.1016/j.psychres.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Fabrazzo M, Tortorella A, Maj M. Plasma levels of interleukin-6 and tumor necrosis factor alpha in chronic schizophrenia: effects of clozapine treatment. Psychiatry Res. 1997;71:11–17. doi: 10.1016/s0165-1781(97)00036-x. [DOI] [PubMed] [Google Scholar]
- Bradley AJ, Dinan TG. Review: a systematic review of hypothalamic-pituitary-adrenal axis function in schizophrenia: implications for mortality. J Psychopharmacol. 2010;24:91–118. doi: 10.1177/1359786810385491. [DOI] [PubMed] [Google Scholar]
- Watson S, Gallagher P, Ritchie JC, Ferrier IN, Young AH. Hypothalamic-pituitary-adrenal axis function in patients with bipolar disorder. Br J Psychiatry. 2004;184:496–502. doi: 10.1192/bjp.184.6.496. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Makino S, Matsumoto R, Nakayama S, Nishiyama M, Terada Y, et al. Regulation of glucocorticoid receptor transcription and nuclear translocation during single and repeated immobilization stress. Endocrinology. 2010;151:4344–4355. doi: 10.1210/en.2010-0266. [DOI] [PubMed] [Google Scholar]
- Patel PD, Katz M, Karssen AM, Lyons DM. Stress-induced changes in corticosteroid receptor expression in primate hippocampus and prefrontal cortex. Psychoneuroendocrinology. 2008;33:360–367. doi: 10.1016/j.psyneuen.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Stress down-regulates corticosterone receptors in a site-specific manner in the brain. Endocrinology. 1984;114:287–292. doi: 10.1210/endo-114-1-287. [DOI] [PubMed] [Google Scholar]
- Diaz Heijtz R, Fuchs E, Feldon J, Pryce CR, Forssberg H. Effects of antenatal dexamethasone treatment on glucocorticoid receptor and calcyon gene expression in the prefrontal cortex of neonatal and adult common marmoset monkeys. Behav Brain Funct. 2010;6:18. doi: 10.1186/1744-9081-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DYM, Bloom JW.Update on glucocorticoid action and resistance J Allergy Clin Immunol 20031113–22.quiz 23. [DOI] [PubMed] [Google Scholar]
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Hashimoto K, Suzuki Y, Higuchi T. Correlation of plasma neurosteroid levels to the severity of negative symptoms in male patients with schizophrenia. Schizophr Res. 2002;58:69–74. doi: 10.1016/s0920-9964(01)00367-x. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, McBride JL, Han I, Berry-Kravis EM, Zhou L, Herzog CD, et al. Intrastriatal CERE-120 (AAV-Neurturin) protects striatal and cortical neurons and delays motor deficits in a transgenic mouse model of Huntington's disease. Neurobiol Dis. 2009;34:40–50. doi: 10.1016/j.nbd.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Neumann H, Misgeld T, Matsumuro K, Wekerle H. Neurotrophins inhibit major histocompatibility class II inducibility of microglia: involvement of the p75 neurotrophin receptor. Proc Natl Acad Sci USA. 1998;95:5779–5784. doi: 10.1073/pnas.95.10.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Hyde TM, Cassano HL, Deep-Soboslay A, Kleinman JE, Weickert CS. Promoter specific alterations of brain-derived neurotrophic factor mRNA in schizophrenia. Neuroscience. 2010;169:1071–1084. doi: 10.1016/j.neuroscience.2010.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2003;8:592–610. doi: 10.1038/sj.mp.4001308. [DOI] [PubMed] [Google Scholar]
- Grosse G, Djalali S, Deng DR, Höltje M, Hinz B, Schwartzkopff K, et al. Area-specific effects of brain-derived neurotrophic factor (BDNF) genetic ablation on various neuronal subtypes of the mouse brain. Brain Res Dev Brain Res. 2005;156:111–126. doi: 10.1016/j.devbrainres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Ligons DL, Romanczyk T, Ungaro G, Hyde TM, Herman MM, et al. Reductions in neurotrophin receptor mRNAs in the prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2005;10:637–650. doi: 10.1038/sj.mp.4001678. [DOI] [PubMed] [Google Scholar]
- Caberlotto L, Hurd YL. Reduced neuropeptide Y mRNA expression in the prefrontal cortex of subjects with bipolar disorder. Neuroreport. 1999;10:1747–1750. doi: 10.1097/00001756-199906030-00022. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Duncan CE, Webster MJ, Rothmond DA, Bahn S, Elashoff M, Shannon Weickert C. Prefrontal GABA(A) receptor alpha-subunit expression in normal postnatal human development and schizophrenia. J Psychiatr Res. 2010;44:673–681. doi: 10.1016/j.jpsychires.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, et al. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci USA. 1998;95:15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdurand M, Fillman SG, Weickert CS, Zavitsanou K. Increases in [3 H]Muscimol and [3 H]Flumazenil binding in the dorsolateral prefrontal cortex in schizophrenia are linked to α4 and γ2 S mRNA levels respectively. PLoS One. 2013;8:e52724. doi: 10.1371/journal.pone.0052724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brietzke E, Kapczinski F. TNF-alpha as a molecular target in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1355–1361. doi: 10.1016/j.pnpbp.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Catts VS, Shannon Weickert C. Gene expression analysis implicates a death receptor pathway in schizophrenia pathology. PLoS One. 2012;7:e35511. doi: 10.1371/journal.pone.0035511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L, Vawter MP. Shared gene expression alterations in schizophrenia and bipolar disorder. Biol Psychiatry. 2008;64:89–97. doi: 10.1016/j.biopsych.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg AM, Ellman LM. Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Prog Neurobiol. 2013;73:951–966. doi: 10.1016/j.biopsych.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone JH, Oliver B. Microarrays, deep sequencing and the true measure of the transcriptome. BMC Biol. 2011;9:34. doi: 10.1186/1741-7007-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-H, Kim K, Kwagh JG, Dicker DT, Herlyn M, Rustgi AK, et al. Death induction by recombinant native TRAIL and its prevention by a caspase 9 inhibitor in primary human esophageal epithelial cells. J Biol Chem. 2004;279:40044–40052. doi: 10.1074/jbc.M404541200. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.