Abstract
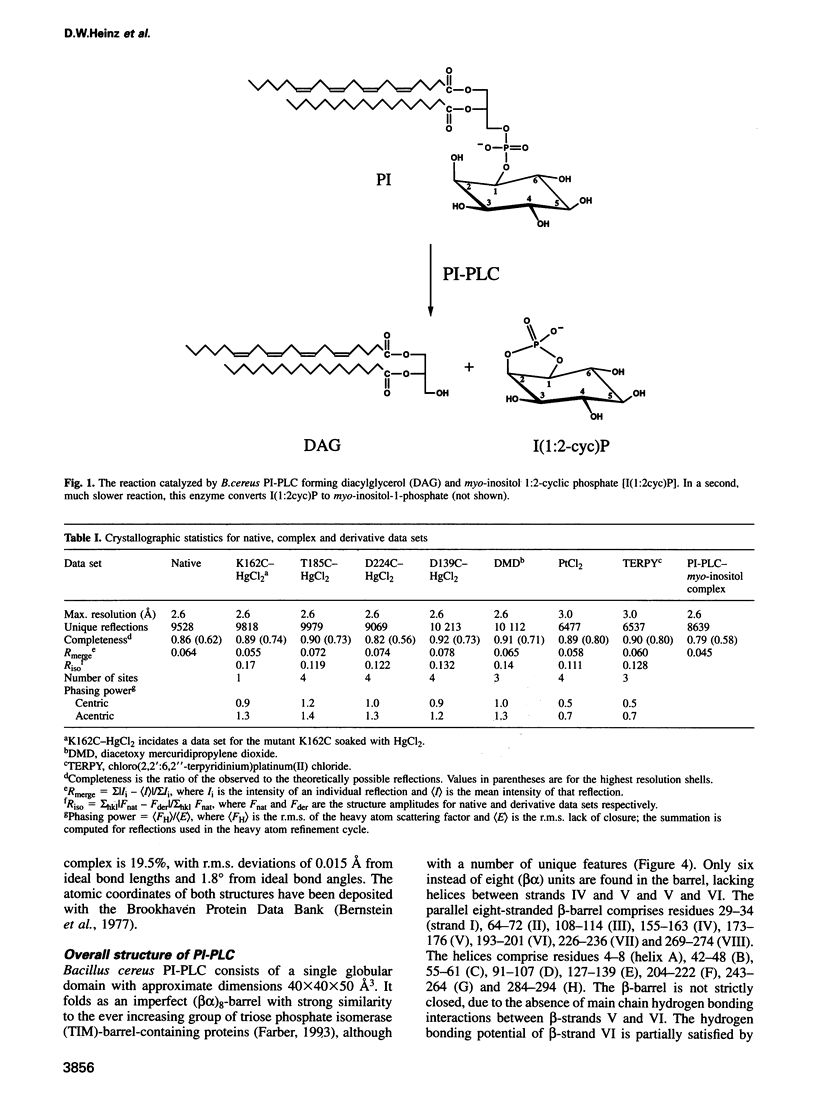
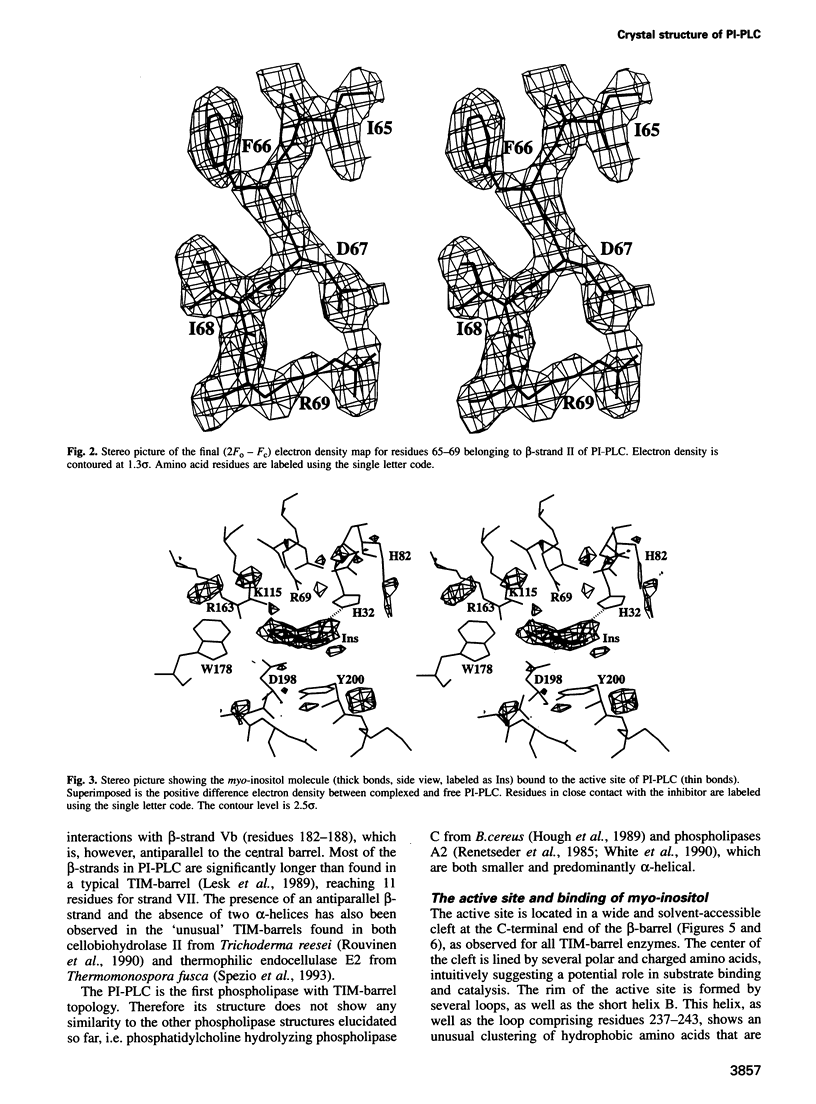
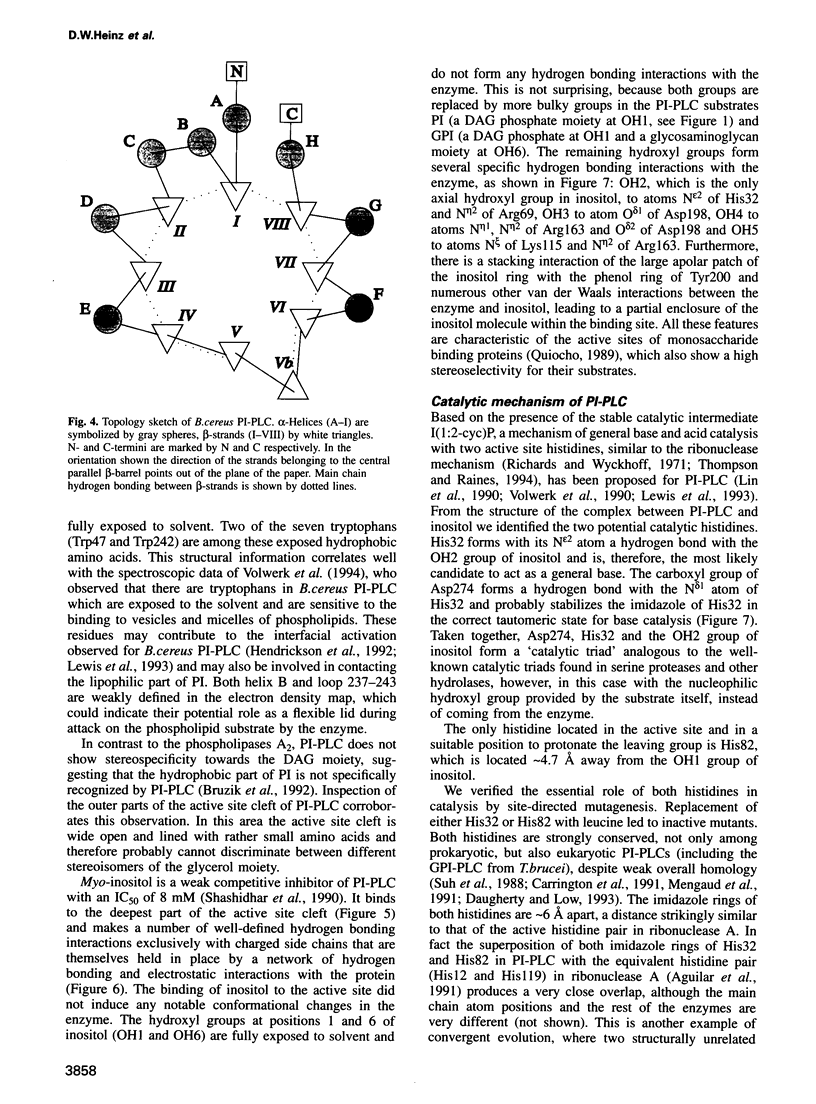
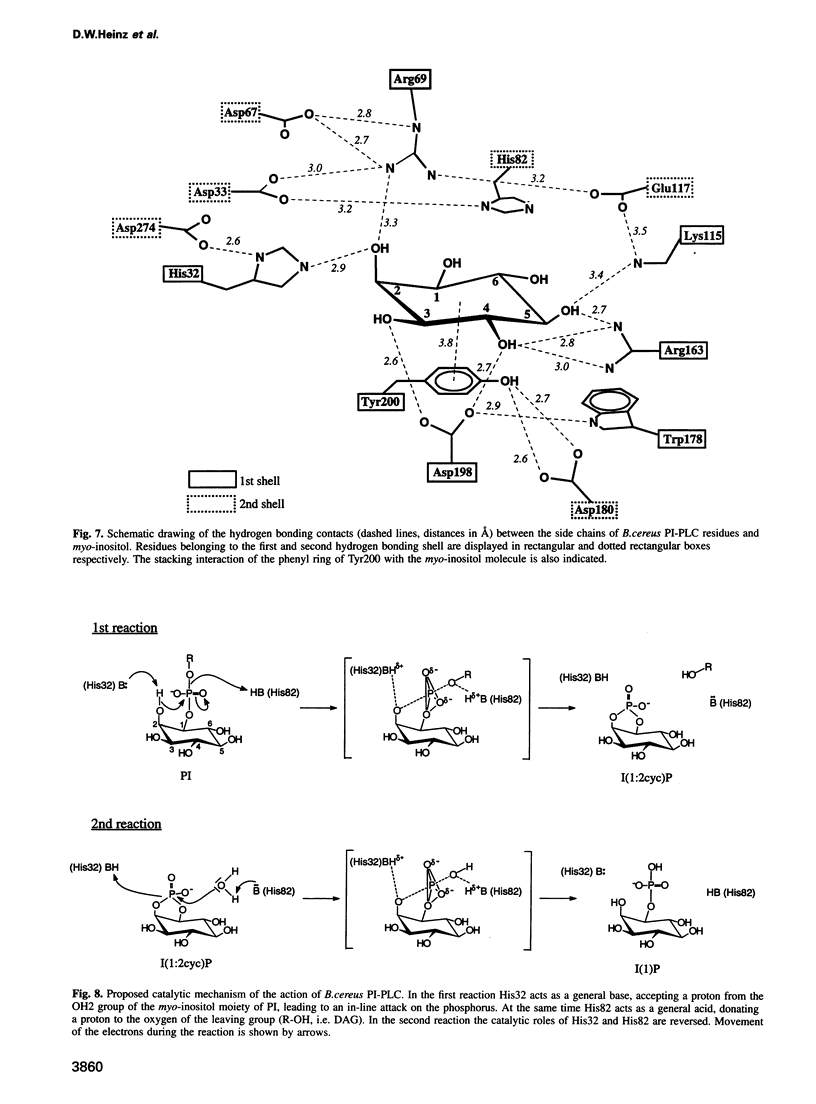
Phosphatidylinositol (PI), once regarded as an obscure component of membranes, is now recognized as an important reservoir of second messenger precursors and as an anchor for membrane enzymes. PI-specific phospholipase C (PI-PLC) is the enzyme that cleaves PI, invoking numerous cellular responses. The crystal structure of PI-PLC from Bacillus cereus (EC 3.1.4.10) has been solved at 2.6 A resolution and refined to a crystallographic R factor of 18.7%. The structure consists of an imperfect (beta alpha)8-barrel similar to that first observed for triose phosphate isomerase and does not resemble any other known phospholipase structure. The active site of the enzyme has been identified by determining the structure of PI-PLC in complex with its inhibitor, myo-inositol, at 2.6 A resolution (R factor = 19.5%). This substrate-like inhibitor interacts with a number of residues highly conserved among prokaryotic PI-PLCs. Residues His32 and His82, which are also conserved between prokaryotic and eukaryotic PI-PLCs, most likely act as general base and acid respectively in a catalytic mechanism analogous to that observed for ribonucleases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar C. F., Thomas P. J., Moss D. S., Mills A., Palmer R. A. Novel non-productively bound ribonuclease inhibitor complexes--high resolution X-ray refinement studies on the binding of RNase-A to cytidylyl-2',5'-guanosine (2',5'CpG) and deoxycytidylyl-3',5'-guanosine (3',5'dCpdG). Biochim Biophys Acta. 1991 Dec 11;1118(1):6–20. doi: 10.1016/0167-4838(91)90435-3. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bruzik K. S., Morocho A. M., Jhon D. Y., Rhee S. G., Tsai M. D. Phospholipids chiral at phosphorus. Stereochemical mechanism for the formation of inositol 1-phosphate catalyzed by phosphatidylinositol-specific phospholipase C. Biochemistry. 1992 Jun 9;31(22):5183–5193. doi: 10.1021/bi00137a014. [DOI] [PubMed] [Google Scholar]
- Bruzik K. S., Tsai M. D. Toward the mechanism of phosphoinositide-specific phospholipases C. Bioorg Med Chem. 1994 Feb;2(2):49–72. doi: 10.1016/s0968-0896(00)82002-7. [DOI] [PubMed] [Google Scholar]
- Bullock T. L., Ryan M., Kim S. L., Remington S. J., Griffith O. H. Crystallization of phosphatidylinositol-specific phospholipase C from Bacillus cereus. Biophys J. 1993 Mar;64(3):784–791. doi: 10.1016/S0006-3495(93)81439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystroff C., Baker D., Fletterick R. J., Agard D. A. PRISM: application to the solution of two protein structures. Acta Crystallogr D Biol Crystallogr. 1993 Sep 1;49(Pt 5):440–448. doi: 10.1107/S0907444993004020. [DOI] [PubMed] [Google Scholar]
- Carrington M., Walters D., Webb H. The biology of the glycosylphosphatidylinositol-specific phospholipase C of Trypanosoma brucei. Cell Biol Int Rep. 1991 Nov;15(11):1101–1114. doi: 10.1016/0309-1651(91)90058-q. [DOI] [PubMed] [Google Scholar]
- Cheng H. F., Jiang M. J., Chen C. L., Liu S. M., Wong L. P., Lomasney J. W., King K. Cloning and identification of amino acid residues of human phospholipase C delta 1 essential for catalysis. J Biol Chem. 1995 Mar 10;270(10):5495–5505. doi: 10.1074/jbc.270.10.5495. [DOI] [PubMed] [Google Scholar]
- Daugherty S., Low M. G. Cloning, expression, and mutagenesis of phosphatidylinositol-specific phospholipase C from Staphylococcus aureus: a potential staphylococcal virulence factor. Infect Immun. 1993 Dec;61(12):5078–5089. doi: 10.1128/iai.61.12.5078-5089.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis M. V., U S., Katan M. Mutations within a highly conserved sequence present in the X region of phosphoinositide-specific phospholipase C-delta 1. Biochem J. 1995 Apr 1;307(Pt 1):69–75. doi: 10.1042/bj3070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S. V. SETOR: hardware-lighted three-dimensional solid model representations of macromolecules. J Mol Graph. 1993 Jun;11(2):134-8, 127-8. doi: 10.1016/0263-7855(93)87009-t. [DOI] [PubMed] [Google Scholar]
- Hamlin R. Multiwire area X-ray diffractometers. Methods Enzymol. 1985;114:416–452. doi: 10.1016/0076-6879(85)14029-2. [DOI] [PubMed] [Google Scholar]
- Hatfull G. F., Sanderson M. R., Freemont P. S., Raccuia P. R., Grindley N. D., Steitz T. A. Preparation of heavy-atom derivatives using site-directed mutagenesis. Introduction of cysteine residues into gamma delta resolvase. J Mol Biol. 1989 Aug 20;208(4):661–667. doi: 10.1016/0022-2836(89)90156-3. [DOI] [PubMed] [Google Scholar]
- Hendrickson H. S., Hendrickson E. K., Johnson J. L., Khan T. H., Chial H. J. Kinetics of Bacillus cereus phosphatidylinositol-specific phospholipase C with thiophosphate and fluorescent analogs of phosphatidylinositol. Biochemistry. 1992 Dec 8;31(48):12169–12172. doi: 10.1021/bi00163a028. [DOI] [PubMed] [Google Scholar]
- Hereld D., Hart G. W., Englund P. T. cDNA encoding the glycosyl-phosphatidylinositol-specific phospholipase C of Trypanosoma brucei. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8914–8918. doi: 10.1073/pnas.85.23.8914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough E., Hansen L. K., Birknes B., Jynge K., Hansen S., Hordvik A., Little C., Dodson E., Derewenda Z. High-resolution (1.5 A) crystal structure of phospholipase C from Bacillus cereus. Nature. 1989 Mar 23;338(6213):357–360. doi: 10.1038/338357a0. [DOI] [PubMed] [Google Scholar]
- Howard A. J., Nielsen C., Xuong N. H. Software for a diffractometer with multiwire area detector. Methods Enzymol. 1985;114:452–472. doi: 10.1016/0076-6879(85)14030-9. [DOI] [PubMed] [Google Scholar]
- Ikezawa H. Bacterial PIPLCs-unique properties and usefulness in studies on GPI anchors. Cell Biol Int Rep. 1991 Nov;15(11):1115–1131. doi: 10.1016/0309-1651(91)90059-r. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Koke J. A., Yang M., Henner D. J., Volwerk J. J., Griffith O. H. High-level expression in Escherichia coli and rapid purification of phosphatidylinositol-specific phospholipase C from Bacillus cereus and Bacillus thuringiensis. Protein Expr Purif. 1991 Feb;2(1):51–58. doi: 10.1016/1046-5928(91)90009-8. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kuppe A., Evans L. M., McMillen D. A., Griffith O. H. Phosphatidylinositol-specific phospholipase C of Bacillus cereus: cloning, sequencing, and relationship to other phospholipases. J Bacteriol. 1989 Nov;171(11):6077–6083. doi: 10.1128/jb.171.11.6077-6083.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimeister-Wächter M., Domann E., Chakraborty T. Detection of a gene encoding a phosphatidylinositol-specific phospholipase C that is co-ordinately expressed with listeriolysin in Listeria monocytogenes. Mol Microbiol. 1991 Feb;5(2):361–366. doi: 10.1111/j.1365-2958.1991.tb02117.x. [DOI] [PubMed] [Google Scholar]
- Lesk A. M., Brändén C. I., Chothia C. Structural principles of alpha/beta barrel proteins: the packing of the interior of the sheet. Proteins. 1989;5(2):139–148. doi: 10.1002/prot.340050208. [DOI] [PubMed] [Google Scholar]
- Lewis K. A., Garigapati V. R., Zhou C., Roberts M. F. Substrate requirements of bacterial phosphatidylinositol-specific phospholipase C. Biochemistry. 1993 Aug 31;32(34):8836–8841. doi: 10.1021/bi00085a014. [DOI] [PubMed] [Google Scholar]
- Lin G. L., Bennett C. F., Tsai M. D. Phospholipids chiral at phosphorus. Stereochemical mechanism of reactions catalyzed by phosphatidylinositide-specific phospholipase C from Bacillus cereus and guinea pig uterus. Biochemistry. 1990 Mar 20;29(11):2747–2757. doi: 10.1021/bi00463a018. [DOI] [PubMed] [Google Scholar]
- Lüthy R., Bowie J. U., Eisenberg D. Assessment of protein models with three-dimensional profiles. Nature. 1992 Mar 5;356(6364):83–85. doi: 10.1038/356083a0. [DOI] [PubMed] [Google Scholar]
- Mengaud J., Braun-Breton C., Cossart P. Identification of phosphatidylinositol-specific phospholipase C activity in Listeria monocytogenes: a novel type of virulence factor? Mol Microbiol. 1991 Feb;5(2):367–372. doi: 10.1111/j.1365-2958.1991.tb02118.x. [DOI] [PubMed] [Google Scholar]
- Morris A. L., MacArthur M. W., Hutchinson E. G., Thornton J. M. Stereochemical quality of protein structure coordinates. Proteins. 1992 Apr;12(4):345–364. doi: 10.1002/prot.340120407. [DOI] [PubMed] [Google Scholar]
- Renetseder R., Brunie S., Dijkstra B. W., Drenth J., Sigler P. B. A comparison of the crystal structures of phospholipase A2 from bovine pancreas and Crotalus atrox venom. J Biol Chem. 1985 Sep 25;260(21):11627–11634. [PubMed] [Google Scholar]
- Rhee S. G., Suh P. G., Ryu S. H., Lee S. Y. Studies of inositol phospholipid-specific phospholipase C. Science. 1989 May 5;244(4904):546–550. doi: 10.1126/science.2541501. [DOI] [PubMed] [Google Scholar]
- Rouvinen J., Bergfors T., Teeri T., Knowles J. K., Jones T. A. Three-dimensional structure of cellobiohydrolase II from Trichoderma reesei. Science. 1990 Jul 27;249(4967):380–386. doi: 10.1126/science.2377893. [DOI] [PubMed] [Google Scholar]
- Scott D. L., White S. P., Otwinowski Z., Yuan W., Gelb M. H., Sigler P. B. Interfacial catalysis: the mechanism of phospholipase A2. Science. 1990 Dec 14;250(4987):1541–1546. doi: 10.1126/science.2274785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashidhar M. S., Volwerk J. J., Keana J. F., Griffith O. H. Inhibition of phosphatidylinositol-specific phospholipase C by phosphonate substrate analogues. Biochim Biophys Acta. 1990 Feb 23;1042(3):410–412. doi: 10.1016/0005-2760(90)90172-t. [DOI] [PubMed] [Google Scholar]
- Sippl M. J. Recognition of errors in three-dimensional structures of proteins. Proteins. 1993 Dec;17(4):355–362. doi: 10.1002/prot.340170404. [DOI] [PubMed] [Google Scholar]
- Spezio M., Wilson D. B., Karplus P. A. Crystal structure of the catalytic domain of a thermophilic endocellulase. Biochemistry. 1993 Sep 28;32(38):9906–9916. doi: 10.1021/bi00089a006. [DOI] [PubMed] [Google Scholar]
- Suh P. G., Ryu S. H., Moon K. H., Suh H. W., Rhee S. G. Cloning and sequence of multiple forms of phospholipase C. Cell. 1988 Jul 15;54(2):161–169. doi: 10.1016/0092-8674(88)90548-x. [DOI] [PubMed] [Google Scholar]
- Volwerk J. J., Filthuth E., Griffith O. H., Jain M. K. Phosphatidylinositol-specific phospholipase C from Bacillus cereus at the lipid-water interface: interfacial binding, catalysis, and activation. Biochemistry. 1994 Mar 29;33(12):3464–3474. doi: 10.1021/bi00178a002. [DOI] [PubMed] [Google Scholar]
- Volwerk J. J., Shashidhar M. S., Kuppe A., Griffith O. H. Phosphatidylinositol-specific phospholipase C from Bacillus cereus combines intrinsic phosphotransferase and cyclic phosphodiesterase activities: a 31P NMR study. Biochemistry. 1990 Sep 4;29(35):8056–8062. doi: 10.1021/bi00487a010. [DOI] [PubMed] [Google Scholar]
- White S. P., Scott D. L., Otwinowski Z., Gelb M. H., Sigler P. B. Crystal structure of cobra-venom phospholipase A2 in a complex with a transition-state analogue. Science. 1990 Dec 14;250(4987):1560–1563. doi: 10.1126/science.2274787. [DOI] [PubMed] [Google Scholar]