Abstract
The present study compared the ability of sweet ageusic T1r3 knockout (KO) and Calhm1 KO mice to acquire preferences for a sucrose-paired flavor as well as for unflavored sucrose. The KO and wildtype (WT) mice were given 24-h one-bottle access to 8% sucrose containing one flavor (CS+, e.g., grape) and to water containing a different flavor (CS-, e.g., cherry) over 4 training days. In subsequent two-bottle tests with the flavors in water only, the T1r3 KO and Calhm1 KO mice, like WT mice, preferred the CS+ to the CS-. After training with flavored solutions, both KO groups also preferred unflavored 8% sucrose to water although Calhm1 KO mice required more sugar experience to match the preference of the T1r3 KO mice. These findings demonstrate that Calhm1 KO mice, like T1r3 KO mice and WT mice, are sensitive to the post-oral preference conditioning actions of sucrose and can discriminate sugar from water. Yet, despite their acquired sucrose preferences, the Calhm1 KO and T1r3 KO mice consumed only half as much sugar per day as did WT mice. Thus, sweet taste signaling elements are not needed in the gut for sugar conditioning, but sweet taste signaling in the mouth is essential for the full expression of sugar appetite.
Keywords: oral sugar conditioning, 24-h tests, post-oral sugar reward
1. Introduction
The sweet taste of sugar is a critical but not the only determinant of sugar appetite. This is demonstrated in sweet ageusic mice missing elements of the sweet taste signaling pathway including the T1r3 component of the sweet receptor and the downstream Ca2+-activated cation channel Trpm5 [4]. In brief access tests, knockout (KO) mice missing T1r3 or Trpm5 show no enhanced licking or preference for sucrose solutions relative to water [16,18,20]. The KO mice are also indifferent to dilute sucrose or glucose solutions in 24-h sugar vs. water choice tests [3,5,20,21,22]. However, T1r3 KO and Trpm5 KO develop strong preferences for concentrated sugar solutions (16-32%) in 24-h tests, which is attributed to post-oral nutrient conditioning effects [3,5,18,20,21,22]. Consistent with this interpretation, T1r3 KO and Trpm5 KO mice are similar to wild type (WT) mice in learning to prefer flavored solutions paired with intragastric (IG) infusions of sucrose or glucose [10,11].
Recently, Taruno et al. [15] reported that calcium homeostasis modulator 1 (CALHM1), a voltage-gated ATP release channel, is another critical downstream signaling element in sweet as well as umami and bitter taste perception. Like T1r3 and Trpm5 KO mice, Calhm1 KO mice failed to show enhanced licking to sucrose in brief access tests. In addition, Calhm1 KO mice were indifferent to dilute sucrose solutions in 24-h sugar vs. water choice tests. However, unlike T1r3 KO and Trpm5 KO mice [3,5,18,20,21,22], Calhm1 KO did not prefer concentrated sucrose solutions (∼10-34%) in 24-h choice tests. The latter finding suggests that Calhm1 KO mice are insensitive to post-oral sugar conditioning, which would be a novel finding.
To clarify the role of CALHM1 in sugar conditioning, the present study determined if Calhm1 KO mice develop sucrose-conditioned flavor preferences. We previously reported that T1r3 KO mice and WT mice acquire robust preferences for a flavored solution paired with IG self-infusions of sucrose [11]. Here we used an oral conditioning procedure in which mice are trained to drink, on separate days, a sucrose solution containing a novel flavor (the CS+) and water containing a different flavor (CS-), followed by a two-bottle choice test with the CS+ and CS- flavors presented in water. We first demonstrated the effectiveness of this oral conditioning protocol to produce sucrose-conditioned preferences in T1r3 KO mice and then evaluated the ability of Calhm1 KO mice to learn sucrose-conditioned preferences. Preferences for unflavored sucrose vs. water were also compared in the KO and WT mice.
2. Methods
2.1. Subjects
Adult female T1r3 KO mice (n=7) were used in Experiment 1; they were developed on a C57BL/6J background as previously described [5]. Age-matched (14 week old) C57BL/6J wildtype (WT, n=8) mice were derived from stock obtained from the Jackson Laboratory (Bar Harbor, ME). Experiment 2 used female Calhm1 KO mice (n=10) and age-matched (16 week old) wildtype controls (n=9) that were developed on a 129Sv × C57BL/6J genetic background as previously described [15]. The genotypes of the T1r3 KO and Calhm1 KO mice were confirmed by real-time PCR analysis of ear-punch biopsies (Transnetyx, Cordova, TN). The mice were singly housed in plastic tub cages with ad libitum access to chow (LabDiet 5001, PMI Nutrition, Brentwood, MO) and fluid in a room maintained at 22 degrees C with a 12:12 light-dark cycle (lights on 0900 h). The cage tops were modified to hold two drinking tubes with the sipper spouts positioned 3.7 cm apart. Experimental protocols were approved by the institutional animal care and use committee at Brooklyn College and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
2.2. Test solutions
The flavored sugar solution (CS+/Suc) used in training was prepared with deionized water, 8% w/w sucrose (Domino Sugar, Yonkers, NY) and 0.05% w/w cherry or grape Kool-Aid (unsweetened mix; Kraft Foods, White Plains, NY). The CS- solution contained 0.05% Kool-Aid flavor in water. Half the mice had grape as the CS+ and cherry as the CS-; the flavors were reversed for the remaining animals. In the two-bottle tests both the CS+ and CS- flavors were presented in plain water. Additional tests were conducted with unflavored 8% sucrose and water. Intakes were recorded to the nearest 0.1 g. Daily fluid spillage was estimated by recording the change in weight of two drinking tubes that were placed on an empty cage.
2.3. Procedure
CS Flavor Testing
The mice were to be trained with alternating one-bottle access to the CS- and CS+/Suc for four days. Due to an experimental error, they were given water on day 3 and therefore the training sequence was CS-, CS+/Suc, water, CS+/Suc, CS-. Then as planned, they were given a day of water only followed by two-bottle access to the CS+ vs. CS- flavors (in water only) for 4 consecutive days.
Sugar Testing
For 3 days after the last CS test, the mice were given water only followed by tests with unflavored 8% sucrose solution and water. On days 1 and 2 (Test 1) and 7 and 8 (Test 2) the mice were given two-bottle sucrose vs. water tests; on days 3-6 they were given one-bottle alternating access to sucrose and water for 2 days each. The left-right positions of the CS+ vs. CS- and sucrose vs. water bottles alternated daily during two-bottle tests and were counterbalanced on one-bottle training days. In addition, individual sipper spouts were fixed to a side rather than a solution to preclude the development of sipper spout preferences [7].
2.4. Data Analysis
CS+/Suc and CS- training intakes were averaged across days and evaluated with analysis of variance (Group × CS). Fluid intakes were averaged as 2-day blocks for the CS+ vs. CS- tests (Tests 1 and 2) and evaluated by ANOVA (Group × CS × Test). Preferences were also analyzed as percent intakes (CS+ intake/total intake × 100). Similarly, the absolute and percent intakes of unflavored sucrose and water were evaluated over Tests 1 and 2; one-bottle sucrose and water intakes were also averaged over 2-day blocks and analyzed as above.
3. Results
Experiment 1
Overall, the T1r3 KO and WT mice consumed more CS+/Suc than CS- during one-bottle training [F(1,14) = 83.4, P < 0.001] (Fig. 1). However, the WT mice consumed twice as much CS+/Suc as did the T1r3 KO mice (P < 0.001); CS- intakes did not differ [Group × CS interaction [F(1,14) = 26.6, P < 0.001]. In the two-bottle tests with the CS flavors presented in water, the T1r3 KO and WT mice consumed more CS+ than CS- in both Tests 1 and 2 [F(1,14) = 58.7, P < 0.001] and there were no group or test differences. The T1r3 KO and WT groups did not differ in their percent CS+ preferences in Test 1 but the preference of the T1r3 KO mice declined in Test 2 to a point that was marginally lower than that of the WT mice (P=0.054) [Group × Test interaction, F(1,4) = 6.1, P < 0.05] (Fig. 1).
Figure 1.
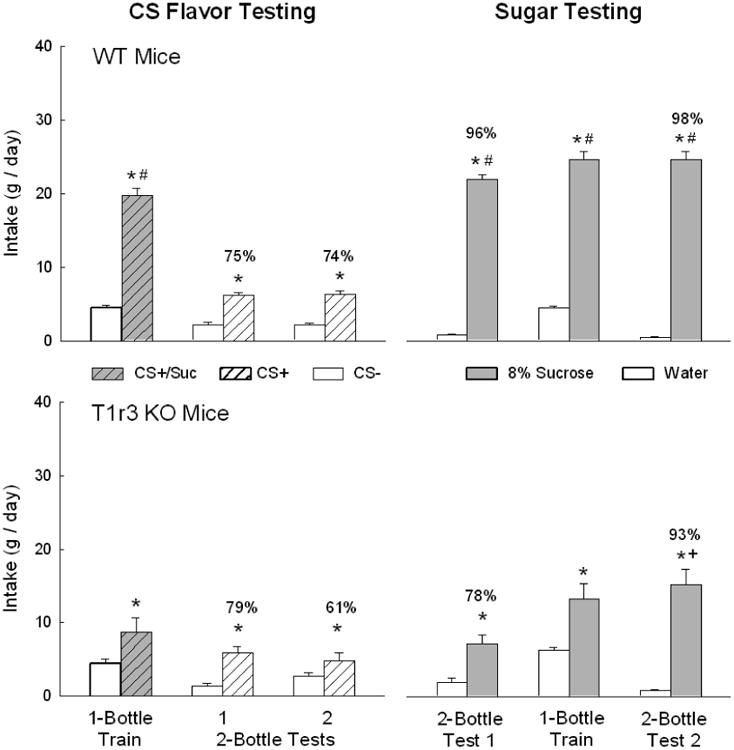
Mean (+sem) 24-h intakes of CS+/Suc and CS- in one-bottle training sessions and CS+ and CS- in two-bottle tests 1-2 (left panels) and of unflavored 8% sucrose and water in two-bottle tests 1-2 and one-bottle training days (right panels) of WT mice (top panels) and T1r3 KO mice (lower panels). Number atop bar represents mean percent preference for the CS+ or sucrose solution. An asterisk indicates a significant difference (P < 0.05) between the data within a pair of bars (CS+/Suc vs. CS-, CS+ vs. CS-, sucrose vs. water); a plus symbol indicates a significant difference between sucrose intake and preference between Test 1 vs. 2; a hash symbol indicates a significant difference between CS+/Suc or sucrose intake between WT and KO mice.
When offered the choice of unflavored 8% sucrose vs. water, the T1r3 KO and WT mice consumed more sucrose than water in both Tests 1 and 2 [F(1,14) = 415.6, P < 0.001], but overall the T1r3 KO mice drank less sucrose than did the WT mice, particularly in Test 1 [F(1,14) = 7.4, P < 0.05] (Fig. 1). The T1r3 KO mice also displayed a weaker sucrose preference in Test 1 but not in Test 2 than did the WT mice [F(1,14) = 8.9, P < 0.01]. During the one-bottle tests, both groups consumed more sugar than water [F(1,14) = 170.1, P < 0.001], but the WT mice consumed twice as much sucrose as did the T1r3 KO mice (P < 0.001); water intakes did not differ [Group × Fluid interaction, F(1,14) = 39.8, P < 0.001]. The one-bottle experience with sucrose increased the sugar preference of the T1r3 KO mice from Test 1 to 2 (78% to 93%, P < 0.001) whereas the preference of the WT mice was high in both tests (96-98%) and did not change [Group × Test interaction, F(1,14) = 8.9, P < 0.01].
Experiment 2
The pattern of results obtained with the Calhm1 KO mice was very similar to that observed with the T1r3 KO. During one-bottle CS training, the Calhm1 KO and WT mice consumed more CS+/Suc than CS- [F(1,17) = 73.7, P < 0.001] but the WT mice drank twice as much CS+/Suc as did the Calhm1 KO mice [Group × CS interaction, F(1,17) = 22.1, P < 0.001] (Fig. 2). In the two-bottle tests, the Calhm1 KO and WT mice consumed more CS+ than CS- in Tests 1 and 2 [F(1,17) = 165.6, P < 0.001] and overall CS+ intake declined from Test 1 to 2 [CS × Test interaction, F(1,17) = 5.9, P < 0.05]; there were no group differences in CS intakes. The Calhm1 KO and WT groups also did not differ in their percent CS+ preferences; preferences declined somewhat from Test 1 to 2 but this difference was not significant (Fig. 2).
Figure 2.
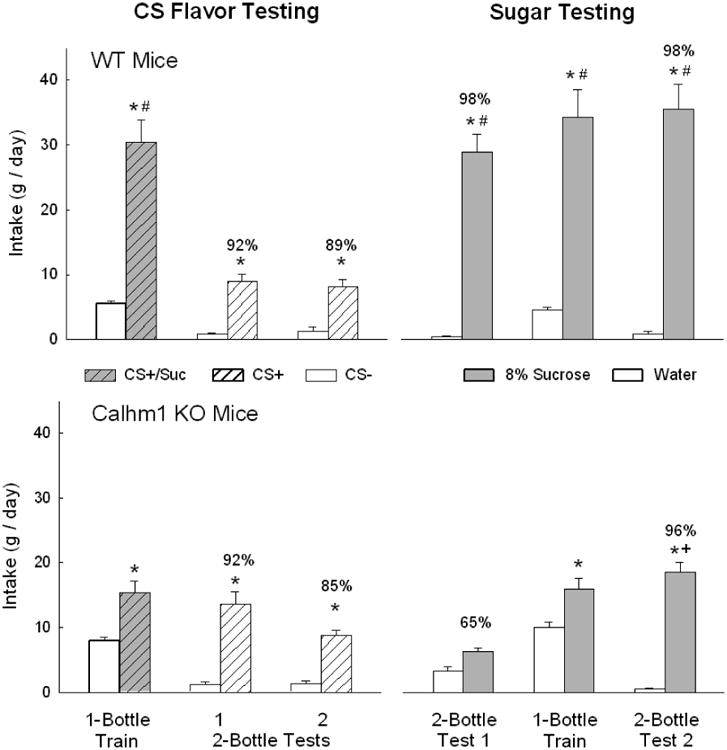
Mean (+sem) 24-h intakes of CS+/Suc and CS- in one-bottle training sessions and CS+ and CS- in two-bottle tests 1-2 (left panels) and of unflavored 8% sucrose and water in two-bottle tests 1-2 and one-bottle training days (right panels) of WT mice (top panels) and Calhm1 KO mice (lower panels). Number atop bar represents mean percent preference for the CS+ or sucrose solution. An asterisk indicates a significant difference (P < 0.05) between the data within a pair of bars (CS+/Suc vs. CS-, CS+ vs. CS-, sucrose vs. water); a plus symbol indicates a significant difference between sucrose intake and preference between Test 1 vs. 2; a hash symbol indicates a significant difference between CS+/Suc or sucrose intake between WT and KO mice.
In the unflavored 8% sucrose vs. water tests, overall the Calhm1 KO and WT mice consumed more sucrose than water but there was a Group × Test interaction [F(1,17) = 5.1, P < 0.001] (Fig. 2). In Test 1 the Calhm1 KO mice did not consume significantly more sucrose than water (p = 0.13) but did so in Test 2 (p < 0.001). In both tests the WT mice consumed substantially more sugar than did the Calhm1 KO mice (P < 0.001). The Calhm1 KO mice displayed a much weaker sucrose preference in Test 1 but not in Test 2 compared to the WT mice F(1,17) = 29.2, P < 0.001]. During one-bottle tests, the two groups consumed more sugar than water [F(1,17) = 75.2, P < 0.001], but the WT mice consumed twice as much sucrose as the Calhm1 KO mice (P < 0.001); water intakes did not differ [Group × fluid interaction, F(1,17) = 33.4, P < 0.001]. The one-bottle experience with sucrose significantly increased the sugar preference of the Calhm1 KO mice from Test 1 to 2 (65% to 96%, (P < 0.001) but the preference of the WT mice, near-total (98%) in both tests, did not change [Group × Test interaction, F(1,17) = 29.2, P < 0.001].
4. Discussion
As expected, the T1r3 KO mice acquired a significant preference for the CS+ flavor that was mixed into the 8% sucrose solution. This is consistent with our findings that T1r3 KO mice, like WT controls, learned to prefer a CS+ flavor that is paired with IG infusions of 16% sucrose, which was diluted to 8% sucrose in the stomach by the ingested CS+ solution [11]. In the IG study the T1r3 KO and WT mice displayed stronger CS+ preferences (92% and 87%) than those displayed in the present study. This can be attributed to several factors. First, the CS flavors (grape and cherry) in the IG study were mixed into a 1% fat solution to stimulate training intakes in T1r3 KO and WT mice. Second, the flavors remained the same during training and testing, whereas in the present study the CS+ flavor changed from training and testing due to the removal of the sucrose in the CS+ test solution. Third, the CS preference tests in the IG study were reinforced in that the CS+ and CS- remained paired with IG infusions of sucrose and water, respectively. However, this is not a critical factor because elsewhere we observed that IG sugar-conditioned CS+ flavor preferences in WT mice are similar whether the CS+ is paired with IG sugar or IG water during the tests [12]. Unlike the WT mice, the CS+ preference of the T1r3 KO mice in Experiment 1 declined from Test 1 to 2. The less persistent preference of the T1r3 KO mice may have occurred because they consumed substantially less CS+/Suc during training than did the WT mice.
The T1r3 KO mice also consumed less of the unflavored sucrose in Experiment 1 than did the WT mice but nevertheless displayed a significant sucrose preference. This confirms earlier findings that 24-h experience with sucrose solutions can induce robust sugar preferences in T1r3 KO mice [20]. In this earlier study, T1r3 KO mice given 24-h sucrose vs. water tests with ascending sugar concentrations (0.5 – 32%) initially failed to prefer 8% sucrose, but after developing preferences for 16% and 32% sucrose the KO mice displayed preferences for 8% and lower sugar concentrations. Thus, the initial 78% (Test 1) sucrose preference displayed by the T1r3 KO mice in Experiment 1 can be attributed to their prior one-bottle experience with the CS+/Sucrose solution. Their one-bottle experience with the unflavored sucrose then enhanced their sugar preference to 93% in Test 2.
In contrast to the T1r3 KO mice, the ability of sucrose to condition preferences in Calhm1 KO mice was uncertain given their reported failure to display a sucrose preference in 24-h sugar vs. water tests with 0.1-34.2% (0.003-1 M) sucrose solutions [15]. However, Experiment 2 revealed that, like T1r3 KO mice, the Calhm1 KO mice developed a significant preference for the sucrose-paired CS+ flavor as well as for 8% sucrose after one-bottle training with the CS+/Sucrose and sucrose solutions. In fact, the Calhm1 KO and WT mice displayed stronger CS+ preferences (85-92%) than did the T1r3 KO and WT mice (61-79%). This may be secondary to their higher training intakes of the CS+/Sucrose solution compared to the T1r3 KO and WT mice. Background strain differences may have contributed to the different training intakes and preferences observed in the T1r3 KO and Calhm1 KO mice. In contrast to the T1r3 KO mice, however, the Calhm1 KO mice did not display a significant preference for unflavored 8% sucrose in Test 1. Only after experiencing the unflavored sugar on one-bottle test days did the Calhm1 KO mice display a sucrose preference (96%) comparable to that of the T1r3 KO mice (93%) as well as their WT controls (98%). The initially attenuated sucrose preference of the Calhm1 KO mice may have occurred because they are more taste ageusic than are T1r3 KO mice. That is, the T1r3 KO mice may have some residual sensitivity to sucrose because of their intact T1r2 component of the sweet taste receptor [18], as well as from sugar signaling by the T1r1 receptor component [9]. The Calhm1 KO mice, in contrast, have a much more global deficit that blocks signaling from all T1R and T2R taste receptors [15]. These differences may account for the ability of T1r3 KO mice but not Calhm1 KO mice to develop sucrose preferences in 24-h sugar vs. water tests [15]. Nevertheless, the present findings demonstrate that with one-bottle exposure to 8% sucrose Calhm1 KO mice, like T1r3 KO mice, display sucrose preferences as strong as those of WT mice. Note that there is no discrepancy between the sucrose preference observed in the present study but not in the Taruno et al. [15] study because only the Calhm1 KO mice in the present study were given one-bottle experience with sucrose. Thus, Calhm1 KO mice, like other KO mice with impaired sweet taste signaling (T1r3 KO, gustducin KO, Trpm5 KO) [10] are sensitive to the post-oral flavor conditioning effects of sugars.
Similar to T1R3 KO and Calhm1 KO mice, mice missing the ATP receptor (P2X2/P2X3) on gustatory nerves are indifferent to dilute sucrose solutions (1-3.4%) in 24-h two-bottle tests [8]. The preference response of P2X KO mice for higher sucrose concentrations was not investigated, but based on the present findings, they would be expected to learn to prefer sucrose after one-bottle sugar experience. Related to the present flavor conditioning results, P2X KO mice are indifferent to 100-300 mM monosodium glutamate (MSG) in 24-h two-bottle tests [8], but learned to prefer a flavor added to a 150 mM MSG solution [14] based on the post-oral actions of glutamate [2,17]. Similarly, we observed that T1r3 KO and Trpm5 KO mice display preferences for a CS+ flavor paired with MSG [1]. Thus, P2X KO mice would be expected to learn sucrose-conditioned flavor preferences as well.
Although the T1R3 KO and Calhm1 KO mice ultimately expressed near-total preferences for 8% sucrose, they consumed only half as much sugar solution as did the WT mice. This demonstrates that there are two components to sugar appetite: an unlearned attraction mediated by the presumably innate connection between the sweet taste receptors and brain reward systems and a learned preference mediated by the post-oral actions of the sugar on brain reward systems [6,13]. In sweet ageusic KO mice, this learned component enhances their attraction to the non-sweet flavor properties (e.g., odor, viscosity) of sugar solutions [22] but does not stimulate them to drink as much sugar as do WT mice. Consistent with this finding, WT mice learn strong preferences for a non-sweet CS+ flavor (e.g., grape) paired with IG sucrose infusions, but consume considerably more of the CS+ flavor when it is sweetened with saccharin [12]. Thus, sugar appetite is driven by both the sweet taste and post-oral actions of sugars.
The present and prior findings showing that T1r3 KO mice acquire significant preferences for sucrose and sucrose-paired flavors indicate that the sweet taste signaling proteins in the gut are not essential for sugar conditioning [3,5,11,12,18,20,21,22]. There is no evidence that Calhm1 is found in gut cells, and the normal flavor conditioning displayed by Calhm1 KO mice demonstrates that Calhm1 signaling in the mouth or elsewhere is not required for post-oral sugar conditioning. Instead, other recent findings suggest that gut glucose sensing by sodium-glucose transporter 1 (SGLT1) and perhaps SGLT3 are critically involved in the post-oral appetite-stimulating actions of glucose and glucose-containing carbohydrates (sucrose, maltose, maltodextrin) [19].
Highlights.
T1r3 and Calhm1 knockout (KO) mice are insensitive to sweet taste
Yet the KO mice learn to prefer a flavor added to 8% sucrose with 24-h training
The KO mice also learn to prefer sucrose but drink much less than normal mice
Taste signaling is required for normal sugar appetite but not post-oral learning
Acknowledgments
This research was supported by grant DK-31135 from the National Institutes of Diabetes and Digestive and Kidney Diseases. We thank Robert F. Margolskee for providing us with the T1r3 KO mouse stock, and Martin Zartarian, Mohammed Riad and Valérie Vingtdeux for their expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ackroff K, Sclafani A. Flavor preference conditioned by oral monosodium glutamate in mice. Chem Senses. 2013;38:745–758. doi: 10.1093/chemse/bjt049. [DOI] [PubMed] [Google Scholar]
- 2.Ackroff K, Sclafani A. Flavor preferences conditioned by intragastric monosodium glutamate in mice. Chem Senses. 2013;38:759–769. doi: 10.1093/chemse/bjt042. [DOI] [PubMed] [Google Scholar]
- 3.Brasser SM, Norman MB, Lemon CH. T1r3 taste receptor involvement in gustatory neural responses to ethanol and oral ethanol preference. Physiol Genomics. 2010;41:232–243. doi: 10.1152/physiolgenomics.00113.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol. 2010;190:285–296. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- 6.de Araujo IE. Sweet taste signaling and the formation of memories of energy sources. Front Syst Neurosci. 2011;5 doi: 10.3389/fnsys.2011.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elizalde G, Sclafani A. Flavor preferences conditioned by intragastric Polycose infusions: A detailed analysis using an electronic esophagus preparation. Physiol Behav. 1990;47:63–77. doi: 10.1016/0031-9384(90)90043-4. [DOI] [PubMed] [Google Scholar]
- 8.Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 9.Kusuhara Y, Yoshida R, Ohkuri T, Yasumatsu K, Voigt A, Hübner S, Maeda K, Boehm U, Meyerhof W, Ninomiya Y. Taste responses in mice lacking taste receptor subunit T1R1. J Physiol. 2013;591:1967–1985. doi: 10.1113/jphysiol.2012.236604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sclafani A, Ackroff K. The role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1119–R1133. doi: 10.1152/ajpregu.00038.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sclafani A, Glass DS, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1643–R1650. doi: 10.1152/ajpregu.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: Oral and postoral interactions. Am J Physiol Regul Integr Comp Physiol. 2005;289:R712–R720. doi: 10.1152/ajpregu.00176.2005. [DOI] [PubMed] [Google Scholar]
- 13.Sclafani A, Touzani K, Bodnar RJ. Dopamine and learned food preferences. Physiol Behav. 2011;104:64–68. doi: 10.1016/j.physbeh.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stratford JM, Finger TE. Central representation of postingestive chemosensory cues in mice that lack the ability to taste. J Neurosci. 2011;31:9101–9110. doi: 10.1523/JNEUROSCI.0404-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, Koppel J, Davies P, Civan MM, Chaudhari N, Matsumoto I, Hellekant G, Tordoff MG, Marambaud P, Foskett JK. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 2013;495:223–226. doi: 10.1038/nature11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Treesukosol Y, Blonde G, Spector AC. The T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to Polycose: Implications for saccharide taste receptors in mice. Am J Physiol Regul Integr Comp Physiol. 2009;296:R855–R865. doi: 10.1152/ajpregu.90869.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uematsu A, Tsurugizawa T, Kondoh T, Torii K. Conditioned flavor preference learning by intragastric administration of l-glutamate in rats. Neurosci Lett. 2009;451:190–193. doi: 10.1016/j.neulet.2008.12.054. [DOI] [PubMed] [Google Scholar]
- 18.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJP, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 19.Zukerman S, Ackroff K, Sclafani A. Post-oral appetite stimulation by sugars and non-metabolizable sugar analogs in mice: Role of intestinal SGLT sugar sensors. Am J Physiol Regul Integr Comp Physiol. 2013 doi: 10.1152/ajpregu.00297.2013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. T1R3 taste receptor is critical for sucrose but not Polycose taste. Am J Physiol Regul Integr Comp Physiol. 2009;296:R866–R876. doi: 10.1152/ajpregu.90870.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. Impact of deleting T1r3 or Trpm5 on carbohydrate preference and acceptance in C57BL/6 mice. Chem Senses. 2013;38:421–437. doi: 10.1093/chemse/bjt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zukerman S, Touzani K, Margolskee RF, Sclafani A. Role of olfaction in the conditioned sucrose preference of sweet-ageusic T1R3 knockout mice. Chem Senses. 2009;34:685–694. doi: 10.1093/chemse/bjp055. [DOI] [PMC free article] [PubMed] [Google Scholar]


