Abstract
Introduction
The significant sex-disparity in sports-related knee injuries may be due to underlying differences in motor control. While the development of sex-specific movement patterns is likely multi-factorial, this study specifically focuses on the potential modulatory role of sex hormones.
Purpose
To investigate the muscle stretch reflex (MSR) across the menstrual cycle. We hypothesized that the MSR would fluctuate throughout the menstrual cycle, and that the lowest response would correspond with peak concentrations of estrogen.
Methods
Nineteen healthy women ages 18–35 participated in this study: 8 eumenorrheic women and 11 women taking oral contraceptives. Serum estradiol and progesterone concentrations, anterior knee laxity (AKL) and the MSR response of the quadriceps muscles were measured three times during the menstrual cycle.
Results
The MSR response of the RF varied significantly across the menstrual cycle in both groups. Specifically, the RF MSR response was 2.4 times lower during the peri-ovulatory phase when compared to the luteal phase (P = 0.007). The same trend was seen in the VM, but this did not reach statistical significance (P = 0.070). The MSR response of the VL did not change significantly across the menstrual cycle (P = 0.494). A mixed model comparison did not show an association between endogenous concentrations of estradiol and progesterone, exposure to hormonal contraceptives or AKL and the MSR response for any muscle.
Conclusions
Our results demonstrate that the RF MSR response varies throughout the menstrual cycle with the lowest response around the time of ovulation. Additional research is needed to clarify the exact relationship between sex hormones, AKL and the MSR response and to determine the specific origin of the change along the monosynaptic reflex arc.
Keywords: Injury Mechanism, Knee Biomechanics, Hormones, Connective Tissue
INTRODUCTION
The role of female sex hormones in reproductive tissues is well defined. However, there is mounting evidence that sex hormones also modulate a variety of non-reproductive tissues throughout the body (38). The presence of estrogen and progesterone receptors in bone, skeletal muscle, ligaments and the nervous system suggests that hormonal fluctuations may affect the structure and function of these tissues (5). However, a clear understanding of the underlying role of sex hormones on the neuromusculoskeletal system has not been defined. Knowledge about this interaction could provide insight into the sex disparity in musculoskeletal disorders. One of the most striking examples is injury to the anterior cruciate ligament (ACL), which occurs 2.78 and 3.6 times more often in female soccer and basketball players compared to males (1). Interestingly, prepubescent boys and girls display similar rates of injury and motor control strategies during functional tasks, but differences appear in both metrics during and after adolescence (13). The development of sex-specific patterns of neuromuscular control is likely a multi-factorial process with contributions from anatomical, biomechanical, hormonal and environmental factors. The collective knowledge that musculoskeletal tissues contain estrogen and progesterone receptors and that ACL injury seems to occur more frequently during the follicular and peri-ovulatory phases suggest that sex hormones may play an integral role in ACL injury in female athletes (21).
Liu et al were the first to document the presence of estrogen receptors on the human ACL (25). Subsequent research has shown these hormones can modulate the structural and mechanical properties of ligaments. Yu et al used an in vitro model of human ACL fibroblasts exposed to varying levels of estradiol and progesterone to demonstrate an estrogen-dependent suppression of fibroblast proliferation that was attenuated by rising progesterone concentrations (40). In addition, increased levels of estrogen have been shown to correlate with decreased ACL stiffness in humans (31) and decreased load to failure in rabbit ACLs (36). Estrogen and progesterone have also been shown to modulate protein synthesis in ligaments (25) and Hansen et al demonstrated a reduction in skeletal muscle protein synthesis in women using hormonal contraceptives (19). While a sex-hormone dependent effect on tendon collagen synthesis has not been definitively proven, there is some suggestion that rising serum estrogen translates into decreased stiffness of the hamstring myotendinous unit (2) as well as the entire lower limb (10). However, another study failed to demonstrate any significant differences in the maximal elongation or stiffness of the patellar tendon across the menstrual cycle (22). It also appears that these hormones might have neurosteroidal effects that can potentially alter neural transmission through the peripheral nervous system, spinal cord and brain (32, 37). In light of the potential for female sex hormones to modulate connective tissue mechanics and neural circuitry, a handful of studies have investigated changes in motor control throughout the menstrual cycle. While some have shown no change (14, 20), others have demonstrated fluctuations in muscle activation patterns, landing strategies and knee loads during sport-specific activities (6, 28). While interesting, the underlying mechanism for the observed changes in neuromuscular control remains unclear because these studies focused on gross motor control strategies that may reflect multiple processes. Accordingly, our study was designed to evaluate a basic unit of neuromuscular control: the muscle stretch reflex.
The muscle stretch reflex (MSR) is a monosynaptic volley involving the stretch-sensitive muscle spindles, Iα-afferent nerve, homonymous α-motoneuron and efferent motor nerve that results in a twitch response of the corresponding muscle (24). Our rationale for using the MSR to investigate changes in neuromuscular control was three-fold. First, by measuring the force applied to the tendon to elicit the reflex (input) and the corresponding muscle activation (output), we have a quantifiable measure of the basic neuromuscular response at the knee joint. Second, although the MSR is mediated through both the peripheral and central nervous systems, there is greater potential to minimize supraspinal influences (e.g. visual and auditory stimuli) while testing the muscle stretch reflex than during a more complicated motor task such as jumping or landing. Third, the sensitivity of the muscle spindle can change in response to the stiffness of the collagen fibers in the surrounding soft tissues, including the ligaments, joint capsule and myotendinous unit (18). Therefore, since sex hormones appear to alter the stiffness of collagen fibers, it is plausible that hormonal fluctuations throughout the menstrual cycle may indirectly modulate the MSR through changes in the musculotendinous afferent sensitivity.
Thus, the central goal of our study was to determine if the MSR at the knee joint changes throughout the menstrual cycle. We hypothesized that 1) if the mechanical properties of the tendon or neural circuitry vary with the hormonal milieu, then the MSR response will be significantly different during three key phases of menstrual cycle; 2) the MSR response will be lowest during the peri-ovulatory phase, which corresponds with peak serum concentrations of estrogen. We were also interested in whether or not the change in the MSR would be accompanied by a co-variant change in anterior knee laxity. Our hypothesis was that there would be an inverse relationship between anterior knee laxity and the MSR response throughout the menstrual cycle.
METHODS
Subjects
The Northwestern University institutional review board approved our study protocol. Participants were recruited from the Northwestern University graduate campus and written informed consent was obtained from all subjects. Twenty-four women, ages 18–35, volunteered to participate. Our preliminary data of five regularly menstruating women tested independently from this study demonstrated statistical significance. Thus, we targeted 12 women to give us ample statistical power even if we lost subjects due to unexpected abnormalities in menstrual cycle or scheduling conflicts. These women were defined as non-users because they had not taken hormonal contraceptives for ≥ 12 months prior to testing and they reported regular monthly menstrual cycles of 24–35 days. In addition, we recruited 12 women taking oral contraceptives (for at least 6 months prior to the testing). These women were defined as the user group and were included because they experience smaller magnitudes of hormonal fluctuations across the menstrual cycle than the non-users. Exclusion criteria included a history of musculoskeletal or orthopedic injury of the spine, hip, knee, ankle or foot, history of neurological injury or disease of the peripheral or central nervous system, current smoker, history of disordered eating, stress fracture, connective tissue disorder (Marfan’s syndrome, Ehlers-Danlos disease), menstrual dysfunction (primary or secondary amenorrhea, oligomenorrhea, anovulatory cycles, polycystic ovarian disease), current or prior pregnancy, starting or stopping oral contraceptives within the previous 6 months, use of an extended-cycle oral contraceptive that eliminates a monthly withdrawal period or use of an injectable or implantable contraceptive regimen. We also excluded those women exercising more than 7 hours per week or participating in competitive level sports due to the high rate of undiagnosed menstrual dysfunction in females of this population (16). As caffeine, alcohol and exercise have the potential to affect reflex excitability (11), subjects were asked to keep each of these variables stable in the 24 hours prior to each testing session.
Experimental Protocol
We aimed to test each non-user once during each of the three key menstrual phases: follicular (low estradiol and progesterone), peri-ovulatory (rising/peak estradiol and low progesterone) and luteal (high estrogen and progesterone – Figure 1). The testing dates were scheduled according to the onset and duration of each non-user’s menstrual cycle during the 3–4 months prior to data collection with the classical model of the menstrual cycle (duration 28 days, ovulation on day 14, peak estrogen 24–48 hours before ovulation and peak progesterone 7–10 days after ovulation) (15). Non-users with an average of a 27–29 day cycle underwent the following testing protocol: testing session #1 (days 1–3), testing session #2 (days 12–14) and testing session #3 (days 21–25). Non-users with an average of a 24–26 day cycle underwent the following protocol: testing session #1 (days 1–3), testing session #2 (days 10–12) and testing session #3 (days 19–23). Non-users with an average of a 30–35 day cycle underwent the following protocol: testing session #1 (days 1–3), testing session #2 (days 14–16) and testing session #3 (days 23–27). Day 1 was defined as the onset of menses. In order to minimize the effect of diurnal fluctuations of hormone levels, testing occurred at a consistent time of day (mean testing window was ≤ 2.4 hours SD of 1.26) throughout the month. Subjects were randomized to start testing in either the follicular, peri-ovulatory or luteal phase. Users were tested in an identical fashion to the non-users with a 27–29 day cycle; the first day of their withdrawal bleed designated as Day 1 of their cycle. The principal investigator, who performed all of the testing, was blinded to subjects’ phase and contraceptive use.
Figure 1.
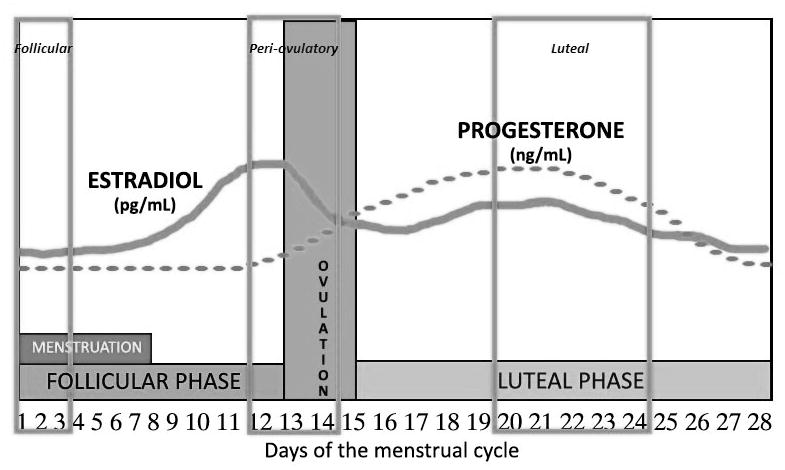
Sample testing schedule for a non-user with menstrual cycle duration of 27–29 days: follicular testing (days 1–3), peri-ovulatory testing (days 12–14) and luteal testing (days 20–24). Users were tested at similar time points. Day 1 was defined as the onset of menses (non-users) or withdrawal bleed (users).
Hormonal Levels
The principal investigator performed the venipuncture at each testing session. The samples were collected from the antecubital area of the subject’s arm, using a 25-gauge needle and a 3.5-mL vacutainer serum separator tube (BD, Franklin Lakes, New Jersey). Once the tube was completely filled, the sample was mixed well and allowed to clot for 15 minutes. The serum was then separated by centrifugation in a Heraeus Multifuge 3S Plus (Thermo Fisher Scientific), set at 3000 rpm for 5 minutes. Estradiol and progesterone levels were analyzed using the ADVIA chemiluminescence assay. Mean percentage coefficient of variations (%CV) ranged from 4–12.1% (within-run) and 4.5–8.1% (run-to-run) for estradiol and 2.5–12.4% (within-run) and 1.9–5.7% (run-to-run) for progesterone. The analysis was conducted at the Northwestern Memorial Hospital outpatient laboratory department.
Ligamentous Laxity Testing
Anterior knee laxity (AKL), the amount of anterior tibial displacement relative to the femur, was measured with the KT-1000™ knee arthrometer (MEDmetric® Corp; San Diego, CA). Using manufacturer’s guidelines, subjects were positioned in supine with a thigh support placed just proximal to the popliteal fossa to support the knees in 25–30° of flexion, the ankles were positioned in the manufacturer-provided foot cradle and a Velcro strap placed around the thighs to control rotation of the lower extremity. With the KT-1000™ properly positioned on the anterior tibia, the participants were instructed to relax the thigh muscles, and an anterior-to-posterior load was applied to the anterior tibia to identify a stable neutral point for the subsequent laxity measurements. Per the protocol described by Shultz et al, five trials at 30 pounds (133 N) of anterior directed force was performed at each testing session. The laxity measure was defined as the average of the middle three trials (33). The principal investigator, who performed all of the measurements, demonstrated strong intra-tester reliability [ICC (SEM) = 0.94 (0.33)]. The measurements used to determine intra-tester reliability were obtained prior to the onset of the study. Testing was performed on two female subjects on two separate occasions (once between days 1–2 and days 5–6 of their menstrual cycles). These time frames were chosen because they represent a similar hormonal milieu as they are both early in the follicular phase. Each subject underwent two trials at each of the testing sessions. The KT-1000 was removed from the subject and repositioned during the two trials obtained on the same day.
Muscle Stretch Reflex Testing
At each session, subjects were seated in an experimental chair with the right hip and knee positioned in 90° of flexion. Prior to electrode placement, an alcohol swab was used to abrade the skin. A pre-amplified sEMG electrode (DE-2.1 Bagnoli single differential) was placed along the vastus lateralis (VL) − 8cm proximal to the superolateral corner of the patella, the vastus medialis (VM) − 6cm proximal to the superomedial corner of the patella) and the rectus femoria (RF) − 1/3 of the distance from the anterior superior iliac spine to the superior border of the patella (8). In an effort to ensure electrode placement in the same location at subsequent testing sessions, each electrode was marked with a permanent marker and subjects were encouraged not to wash off the markings between sessions (Figure 2). The electrodes were connected to a 2 channel Delsys box (Bagnoli Handheld, 2-channel EMG system) and data acquisition hardware (National Instruments USB 6218 BNC board). Data acquisition was controlled with MATLAB software at a data acquisition rate of 1000Hz. A total of 3 MVC’s were recorded with a minimum of 10 seconds of rest between trials. Each MVC was held for 5 seconds and the entirety of the contraction was recorded. Strong verbal encouragement was provided to enhance subjects’ effort during the MVC. The MSR was evoked by tapping the patellar tendon with a hammer instrumented with a load cell (Kistler Instrument Corp., Amherst, New York), and the sEMG response was obtained for each muscle. A mark was placed on the patellar tendon to encourage the P.I. to aim for the same spot with each tap. A manually applied tap (with a target of 4.5 +/−0.5 lbs of force) was used to elicit the reflexive muscle contraction. Subjective underwent a total of 30 taps during each testing session. This number was based on the knowledge that every tap might not elicit a reflexive contraction in each muscle (35). The 30 taps were broken up into three sessions of 10 taps with a minimum of 5minutes of rest between sessions. The latency between individual taps was at least 10 seconds, and the latency was varied from 10–20 seconds between taps to limit subjects’ ability to anticipate the timing of the next tap (35). In an attempt to further minimize supraspinal modulation of the MSR, subjects were instructed to relax, maintain a standardized position (head resting on the head rest, hands on the handle bars, eyes closed), and auditory stimuli was limited to the sound of the hammer tapping the tendon (35). Surface EMG signals for the MVC and MSR responses were filtered offline using an eighth-order, low-pass Butterworth filter at 450Hz and then rectified. A baseline EMG signal and the corresponding standard deviation were calculated 100 ms before the peak of the tendon tap, to (defined as the time of the peak tap force). A window of 10 to 45 ms after the peak of the tendon tap was used to search for the onset of the reflex activity, t1 (average time for t1 was 25–35ms). Reflex activity onset was identified when five consecutive points in the EMG tracing were above three standard deviations of the pre-stimulus EMG activity (9). The MSR response to the tendon tap was computed across phases; only EMG responses that corresponded to tendon tap forces within 4–5 lbs of force were evaluated (on average 80% of tendon taps were in this range and elicited a quantifiable reflex). The MSR response was computed as follows (4):
| (1) |
where is the peak amplitude of the reflex tracing between t0 and t1, EMG MVC was defined as the maximum 500 ms time average of the EMG activity recorded per muscle during the subject’s maximal effort, and tendon tap force peak was defined as the largest amplitude of the tapper force profile at to (Figure 2). The principal investigator, who performed all of the measurements, demonstrated strong intra-tester reliability for determining the MSR response [ICC (SEM) for RF = 0.86 (0.14), VM = 0.81 (0.20) and RF 0.80 (0.11)]. The measurements used in the reliability calculation were on the first day of testing for each of the 19 subjects. Only these measurements were used to ensure that the hormonal milieu was identical for each of the measurements. There were comparable numbers of reflexes obtained in each of the three trials (range: 9.5–9.6 for VL, 9.2–9.3 for VM and 8.7–8.9 for RF).
Figure 2.
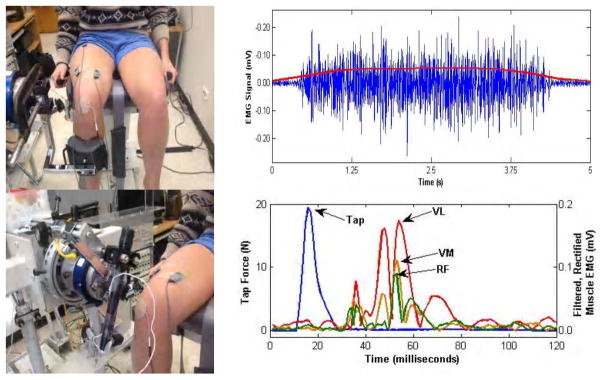
Typical set-up and signal analysis for the knee extensor maximum voluntary contraction and muscle stretch reflex response.
Statistical Analysis
Statistical analyses were conducted using SAS software 9.3 (Cary, NC). Mean and standard deviation were calculated for subject characteristics. To analyze the demographic difference between users and non-users, two-tailed independent samples t tests were utilized. Before conducting analyses, histograms of the distributions and normal probability plots of predictor and muscle reflex response outcome variables were studied to assess normality. As the distributions of the MSR response outcome variables were skewed, a log scale of the outcome variables was chosen to examine their relationship. One-way analysis of variance (ANOVA) was used to test the difference of the mean serum estradiol and progesterone levels as well as the mean AKL across the menstrual cycle phases (Figure 3). A mixed effects model accounting for repeated measures was used to compare the MSR response across the menstrual cycle, adjusting for hormonal contraceptive status, mean serum estradiol, mean serum progesterone and mean AKL (Figure 4). In addition, Spearman correlations between the MSR response and estrogen, progesterone and AKL were also performed. Bonferroni adjustment was used for the post-hoc pairwise comparisons. For all analyses, P-value was set at 0.05 for statistical significance.
Figure 3.
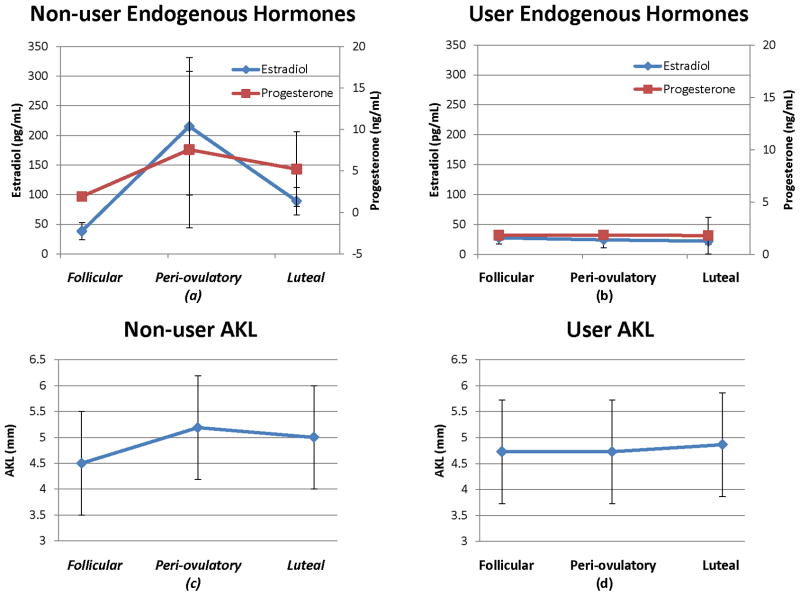
Mean endogenous concentrations of estradiol and progesterone and anterior knee laxity for non-users and users during the follicular, peri-ovulatory and luteal phases of the menstrual cycle.
Figure 4.
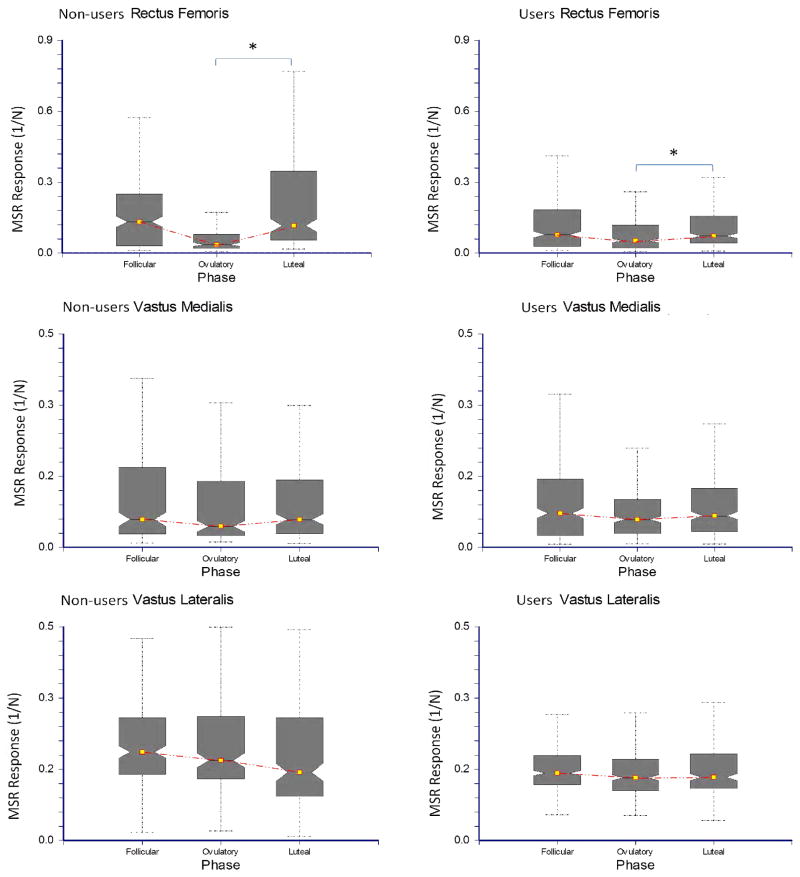
The relationship between the MSR response of the quadriceps muscles and the menstrual cycle in non-users and users. The box plots illustrate the minimum, lower quartile, median, upper quartile and maximum values for the MSR response.
RESULTS
Twenty-four women agreed to participate in the study. We had to exclude a total of 5 women: 3 due to inability to attend all three of the testing sessions and two women who had a menstrual cycle that lasted greater than 38 days. Thus, the data presented is based on 19 women (8 non-users and 11 users). There were no significant differences between the groups with respect to age (non-users 24.0 ± 2.2 years, users 24.1 ± 3.5 years, P = 0.58), body mass index (non-users 21.8 ± 1.9 kg/m2, users 22.7 ± 1.6 kg/m2, P = 0.28) or in exercise (non-users 2.2 ± 1.4 hours/week, users 2.0 ± 1.6 hours/week, P = 0.79). The users had been taking their respective contraceptive for an average of 44.9 months (see Table 1 for information on specific contraceptives used).
Hormonal analysis
There were statistically significant changes in the mean serum estradiol and progesterone across the menstrual cycle in the non-users (P = 0.006). The highest concentrations of estradiol (P < 0.0001) and progesterone (P < 0.0001) occurred in the phase we targeted as the peri-ovulatory phase (Figure 3). The users did not demonstrate any significant differences in the mean serum concentrations of endogenous estradiol (P = 0.900) or progesterone (P = 0.776). We did not measure the levels of exogenous estrogen or progesterone in the users (Figure 3).
Anterior knee laxity
Neither group demonstrated significant changes in AKL throughout the menstrual cycle. However, the non-users demonstrated a trend towards significance for increased AKL in the peri-ovulatory phase (P = 0.169 – Figure 3).
MSR Response
The MSR response of the RF varied significantly across the menstrual cycle in both groups. Specifically, the RF MSR response was 2.4 times lower during the peri-ovulatory phase when compared to the luteal phase (P = 0.007 – Fig 4). The MSR response of the VM showed a similar trend with decreased response in the peri-ovulatory phase, but the difference did not quite reach significance (P = 0.071 – Figure 4). The MSR in the VL did not fluctuate significantly across the menstrual cycle (P = 0.494). There was no significant association between serum estradiol (P = 0.647), serum progesterone (P = 0.734), exposure to hormonal contraception (P = 0.904) or AKL (P = 0.830) and the MSR response for any muscle. Furthermore, Spearman correlations did not indicate a significant correlation between the RF MSR response and estradiol (r = 0.059, P = 0.663), progesterone (r = 0.039, = 0.771) or AKL (r = −0.021, P = 0.876).
DISCUSSION
This is the first study to use the MSR to investigate hormonally mediated changes of neuromuscular control. Our primary goal was to determine if the MSR response varied significantly across the menstrual cycle. We found that the MSR response is modulated across the menstrual cycle, but the magnitude of the change likely varies by the individual subject and the particular muscle tested. The group comparison demonstrated a significant decrease in the MSR response of the RF during the peri-ovulatory phase when compared to the luteal phase in the non-users and users. The MSR response of the VM demonstrated a similar, but non-significant trend; whereas the MSR response of the VL did not fluctuate significantly. The reason for the different responses in the individual muscles is not entirely clear, but it is possible that inter-muscular architectural differences may play a role. The relative contribution of each of the quadriceps muscles to knee extension moment has been shown to depend on the volume and cross-sectional area of the muscle (3). Accordingly, it is possible that these differences in size or architecture may also affect the relative sensitivity of each muscle to a mechanical stretch. For example, the tendon-to-muscle ratio of the RF is two-times that of the VM or VL (27). Therefore, if the change we saw in the MSR response is due to hormonally based changes in tendon stiffness, the magnitude of the change might be larger in a muscle with a longer tendon. In addition, there is some suggestion that smaller muscles in an agonist group have a higher density of muscle spindles which allows them to function as a “kinesiological monitor” of joint position compared to the larger muscles responsible primarily for power (30). Since the volume of the RF is 2–2.5 times smaller than the vasti (3), it is possible that RF may have a higher density of muscle spindles and be more sensitive to changes in hormonal concentrations across the menstrual cycle.
Our findings support prior work demonstrating changes in gross motor tasks throughout the menstrual cycle (6, 28). Additionally, since our study focused on a basic unit of neuromuscular control, our results may shed light into the underlying mechanism for the observed change. The MSR is a monosynaptic transmission conducted through a variety of tissues, including connective tissue in the myotendinous unit and neural circuitry. Connective tissue metabolism has been shown to be sensitive to fluctuations in sex hormones, particularly in women (23). Specifically, estrogen has a variety of reported effects on collagen, including decreased synthesis, increased degradation and decreased load-to-failure (34, 36, 40). At least in the ligament, these effects are thought to be related to an estrogen-dependent suppression of fibroblast activity (40), and if there is a similar effect in the tendon, it could contribute to decreased musculotendinous stiffness around the time of ovulation (10). Decreased myotendinous stiffness has been shown to cause a neuromuscular adaptation where the sensitivity of the muscle spindle decreases with reduced stiffness in the collagen fibers within the tendon (18). Collectively, these findings support a possible connective tissue-based mechanism for an estrogen-induced decrease in the MSR response. However, another possible explanation is a hormonally mediated change in the transmission through neural circuitry. The neural components of the reflex include the Iα-afferent, the homonymous α-motoneuron in the spinal cord and the efferent fiber that results in a twitch response of the corresponding muscle (24). There is well-documented evidence of neurosteroidal effects of sex hormones on neural substrates in the brain and spinal cord (37). It has been proposed that estrogen enhances nerve membrane excitability and synaptic transmission whereas progesterone may play an inhibitory role. Thus, one could argue that the observed attenuation of the RF MSR response in the peri-ovulatory phase may be due to the combined effect of changes in transmission through neural substrates as well as changes in connective tissue mechanics.
Our second objective was to determine if the change in the MSR response was dependent on fluctuations in serum concentrations of estradiol and progesterone. Our analysis did not show any association between the hormonal milieu and the MSR, but did demonstrate a significant decrement in the RF MSR response during the peri-ovulatory phase. While we hypothesized that the MSR response would drop during the peri-ovulatory phase, we thought this change would be dependent on the peak levels of serum estradiol around the time of ovulation. However, we did not demonstrate a correlation between estradiol concentration and the change in the MSR response. One potential explanation for the lack of a correlation is the fact that we were not as accurate as we aimed to be in testing all of the non-users during the targeted menstrual cycle phase. We planned our testing schedule based on the average menstrual cycle duration per subject in the 3–4 months prior to testing. Since we did not use ovulation kits or any other objective measure to determine the length of the follicular and luteal phases or timing of ovulation, the only time point we can be certain of is the follicular phase because this data was collected within 2 days of the onset of menses. The classic definition of the menstrual cycle includes a duration of 28 days and ovulation on day 14 (15). However, several studies, including one by Fehring et al. (12) have demonstrated a high degree of variability within and across women. In his study of 140 women (1,060 menstrual cycles), the mean menstrual cycle length was 28.9 days (95% CI 22–36 days) and only 25% of participants had peak estrogen concentrations and ovulation occur between days 10–17. Our data is consistent with this high-degree of inter-subject variability as our subjects had peak estradiol and progesterone concentrations during the time we predicted would be the peri-ovulatory phase. When we reviewed each subject’s hormonal data, we found that 5 of the 12 non-users fit the targeted phases, but 2 subjects had higher than expected levels of progesterone in the peri-ovulatory phase and 1 subject had her peak estradiol in the luteal phase. It is likely that the peri-ovulatory testing was actually early luteal (rising progesterone and estrogen – Figure 1) and the luteal phase was late luteal (elevated progesterone and decreasing estradiol). In addition to missing our target menstrual phases in some subjects, the lack of correlation between serum hormonal concentrations and the change in the MSR response may also be due to the fact that this output is governed by more factors than absolute estradiol and progesterone concentrations at the time of testing. Potential such factors include a modulatory role for other hormones, including, relaxin (29), IGF-1 (34), and testosterone (26). Additionally, if the mechanism for the change in the MSR response is similar to change in anterior knee laxity demonstrated by Shultz et al., there may be a 3 to 5 day lag time effect of serum concentrations of estradiol, progesterone and testosterone that are responsible for some or all of the change we saw in the MSR response (33). Finally, since the MSR response behaved similarly across the menstrual cycle in both the users and non-users, it is unlikely that the absolute concentrations of estradiol and progesterone are solely responsible for the attenuated response in the peri-ovulatory phase. It is likely that a more refined protocol of daily testing and inclusion of additional hormones would enable us to definitely determine the role of hormonal fluctuations in the MSR response across the menstrual cycle.
We were also interested in whether or not the change in the MSR response would be accompanied by a co-variant change in anterior knee laxity. Since estrogen and progesterone appear to alter the stiffness of collagen fibers and AKL, and muscle spindle sensitivity can be modulated by the stiffness of the surrounding collagen fibers, it is plausible that hormonal fluctuations throughout the menstrual cycle may indirectly modulate the MSR through mechanoreceptor sensitivity. If this were the case, one would expect increased AKL and decreased MSR response during the peri-ovulatory or luteal phase of the menstrual cycle. While we did see this trend in the peri-ovulatory phase, the change in AKL was not statistically significant. Our AKL results are consistent with some studies (10, 39), but contradict others (7, 33). However, it is important to note that we did not power our study to look at AKL as our primary outcome and the P-value approaching significance (P = 0.169) suggests that a larger sample size may have demonstrated significantly increased AKL during the peri-ovulatory phase. In addition, we did not find any significant correlation between the RF MSR response and AKL, either through Spearman correlation or our mixed effects model. It is possible that there is truly no relationship between these variables, but other potential reasons for not finding a correlation include inadequate power for this calculation or that the variables are governed by different factors.
There are a variety of strengths and limitations to our study. Notable strengths include blinding of the examiner to subjects’ hormonal status, collection of serum hormonal concentrations, minimizing variables that can modulate reflex excitability (caffeine, alcohol, exercise prior to testing and the visual and auditory stimuli during the testing) and quantitative analysis of basic neuromuscular control with the MSR. However, the data we collected and our ability to interpret our findings is not without limitations, including small sample size and subject attrition due to the demand of testing at such specific time points. In addition, our exclusion of competitive athletes was necessary to limit women with subclinical menstrual dysfunction, but these women are at highest risk for knee injury. Our most critical limitation was in testing some of the non-users outside of the target phases of the menstrual cycle. We definitely tested everyone in the follicular phase, but our design of performing one testing session per menstrual phase based on historical tracking occasionally led to hormonal profiles that did not always match the targeted phase. As previously mentioned, there were 2 non-users who demonstrated peak progesterone concentrations during their “peri-ovulatory” testing session and one subject whose peak estrogen occurred in her “luteal” testing session. Thus we clearly missed the true hormonal peaks in some subjects and some of our testing was done “out of phase.” Measures to address this limitation in the future include daily testing and to document the time of ovulation for a few month prior to testing. These changes will not only ensure that true hormonal peaks are captured, but would allow investigation into complex interactions among a variety of hormones as well as capture any lag time effect of hormonal fluctuations.
CONCLUSION
One of the critical barriers in understanding the underlying mechanisms of sex-disparity in musculoskeletal injury is a lack of clarity regarding the relative contributions of anatomical, biomechanical, environmental and hormonal factors. This is the first study to use the MSR to investigate hormonally mediated changes of neuromuscular control. Our preliminary findings suggest that the muscle stretch reflex does change across the menstrual cycle. Specifically, we demonstrated that the MSR reflex of the RF was significantly lower in the peri-ovulatory phase than the luteal phase in users and non-users of oral contraceptives. Further exploration is necessary to determine the specific origin of this change as well as the interplay between estrogen and progesterone as well as additional hormones, such as testosterone and relaxin. Furthermore, additional investigations should be conducted to determine if the decreased MSR response seen in the quadriceps muscles is indicative of alterations in joint mechanics and stability that may affect risk of sports-related injury.
Supplementary Material
Table 1: Profile of Oral Contraceptives in the User Group (17)
Acknowledgments
This work was supported by a K-12 grant from the National Institutes of Health (5K12HD001097-15). No conflicts of interest, financial or otherwise, are declared by the authors. Our results do not constitute endorsement by the American College of Sports Medicine.
The authors would like to thank Jungwha Lee, PhD for her expertise in data analysis. This work was supported by a K-12 grant from the National Institutes of Health (5K12HD001097-15)
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors. Our results do not constitute endorsement by the American College of Sports Medicine.
References
- 1.Agel J, Arendt EA, Bershadsky B. Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33(4):524–30. doi: 10.1177/0363546504269937. [DOI] [PubMed] [Google Scholar]
- 2.Bell DR, Blackburn JT, Norcorss MF, Ondrak KS, Hudson JD, Hackney AC, et al. Estrogen and muscle stiffness have a negative relationship in females. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):361–7. doi: 10.1007/s00167-011-1577-y. Epub 2011/06/23. [DOI] [PubMed] [Google Scholar]
- 3.Blazevich AJ, Gill ND, Zhou S. Intra- and intermuscular variation in human quadriceps femoris architecture assessed in vivo. J Anat. 2006;209(3):289–310. doi: 10.1111/j.1469-7580.2006.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cammarata ML, Dhaher YY. Evidence of gender-specific motor templates to resist valgus loading at the knee. Muscle Nerve. 2010;41(5):614–23. doi: 10.1002/mus.21509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciocca DR, Roig LM. Estrogen receptors in human nontarget tissues: biological and clinical implications. Endocr Rev. 1995;16(1):35–62. doi: 10.1210/edrv-16-1-35. Epub 1995/02/01. [DOI] [PubMed] [Google Scholar]
- 6.Dedrick GS, Sizer PS, Merkle JN, Hounshell TR, Robert-McComb JJ, Sawyer SF, et al. Effect of sex hormones on neuromuscular control patterns during landing. J Electromyogr Kinesiol. 2008;18(1):68–78. doi: 10.1016/j.jelekin.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Deie M, Sakamaki Y, Sumen Y, Urabe Y, Ikuta Y. Anterior knee laxity in young women varies with their menstrual cycle. Int Orthop. 2002;26(3):154–6. doi: 10.1007/s00264-001-0326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delagi EFIJ, Perotto AO, Morrison D. Anatomic Guide for the Electromyographer: The Limbs. 2. Springfield, IL: 1980. [Google Scholar]
- 9.Dhaher YY, Tsoumanis AD, Rymer WZ. Reflex muscle contractions can be elicited by valgus positional perturbations of the human knee. J Biomech. 2003;36(2):199–209. doi: 10.1016/s0021-9290(02)00334-2. [DOI] [PubMed] [Google Scholar]
- 10.Eiling E, Bryant AL, Petersen W, Murphy A, Hohmann E. Effects of menstrual-cycle hormone fluctuations on musculotendinous stiffness and knee joint laxity. Knee Surg Sports Traumatol Arthrosc. 2007;15(2):126–32. doi: 10.1007/s00167-006-0143-5. [DOI] [PubMed] [Google Scholar]
- 11.Eke-Okoro ST. The H-reflex studied in the presence of alcohol, aspirin, caffeine, force and fatigue. Electromyogr Clin Neurophysiol. 1982;22(7):579–89. Epub 1982/12/01. [PubMed] [Google Scholar]
- 12.Fehring RJ, Schneider M, Raviele K. Variability in the phases of the menstrual cycle. J Obstet Gynecol Neonatal Nurs. 2006;35(3):376–84. doi: 10.1111/j.1552-6909.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- 13.Ford KR, Myer GD, Hewett TE. Longitudinal effects of maturation on lower extremity joint stiffness in adolescent athletes. Am J Sports Med. 2010;38(9):1829–37. doi: 10.1177/0363546510367425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friden C, Hirschberg AL, Saartok T, Backstrom T, Leanderson J, Renstrom P. The influence of premenstrual symptoms on postural balance and kinesthesia during the menstrual cycle. Gynecol Endocrinol. 2003;17(6):433–9. doi: 10.1080/09513590312331290358. Epub 2004/03/03. [DOI] [PubMed] [Google Scholar]
- 15.Fritz MA, Speroff L. The endocrinology of the menstrual cycle: the interaction of folliculogenesis and neuroendocrine mechanisms. Fertil Steril. 1982;38(5):509–29. doi: 10.1016/s0015-0282(16)46628-8. [DOI] [PubMed] [Google Scholar]
- 16.Gibbs JC, Williams NI, DES MJ. Prevalence of individual and combined components of the female athlete triad. Med Sci Sports Exerc. 2013;45(5):985–96. doi: 10.1249/MSS.0b013e31827e1bdc. [DOI] [PubMed] [Google Scholar]
- 17.Greer JB, Modugno F, Allen GO, Ness RB. Androgenic progestins in oral contraceptives and the risk of epithelial ovarian cancer. Obstet Gynecol. 2005;105(4):731–40. doi: 10.1097/01.aog.0000154152.12088.48. Epub 2005/04/02. [DOI] [PubMed] [Google Scholar]
- 18.Grigg P, Hoffman AH. Stretch-sensitive afferent neurons in cat knee joint capsule: sensitivity to axial and compression stresses and strains. J Neurophysiol. 1996;75(5):1871–7. doi: 10.1152/jn.1996.75.5.1871. Epub 1996/05/01. [DOI] [PubMed] [Google Scholar]
- 19.Hansen M, Langberg H, Holm L, Miller BF, Petersen SG, Doessing S, et al. Effect of administration of oral contraceptives on the synthesis and breakdown of myofibrillar proteins in young women. Scand J Med Sci Sports. 2011;21(1):62–72. doi: 10.1111/j.1600-0838.2009.01002.x. [DOI] [PubMed] [Google Scholar]
- 20.Hertel J, Williams NI, Olmsted-Kramer LC, Leidy HJ, Putukian M. Neuromuscular performance and knee laxity do not change across the menstrual cycle in female athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):817–22. doi: 10.1007/s00167-006-0047-4. [DOI] [PubMed] [Google Scholar]
- 21.Hewett TE, Zazulak BT, Myer GD. Effects of the menstrual cycle on anterior cruciate ligament injury risk: a systematic review. Am J Sports Med. 2007;35(4):659–68. doi: 10.1177/0363546506295699. [DOI] [PubMed] [Google Scholar]
- 22.Kubo K, Miyamoto M, Tanaka S, Maki A, Tsunoda N, Kanehisa H. Muscle and tendon properties during menstrual cycle. Int J Sports Med. 2009;30(2):139–43. doi: 10.1055/s-0028-1104573. Epub 2008/12/11. [DOI] [PubMed] [Google Scholar]
- 23.Lemoine S, Granier P, Tiffoche C, Rannou-Bekono F, Thieulant ML, Delamarche P. Estrogen receptor alpha mRNA in human skeletal muscles. Med Sci Sports Exerc. 2003;35(3):439–43. doi: 10.1249/01.MSS.0000053654.14410.78. [DOI] [PubMed] [Google Scholar]
- 24.Liddell EGT, Sherrington C. Reflexes in response to stretch (myotatic reflexes) Royal Society of London; 1924. pp. 212–42. [Google Scholar]
- 25.Liu SH, al-Shaikh R, Panossian V, Yang RS, Nelson SD, Soleiman N, et al. Primary immunolocalization of estrogen and progesterone target cells in the human anterior cruciate ligament. J Orthop Res. 1996;14(4):526–33. doi: 10.1002/jor.1100140405. Epub 1996/07/01. [DOI] [PubMed] [Google Scholar]
- 26.Mathor MB, Achado SS, Wajchenberg BL, Germek OA. Free plasma testosterone levels during the normal menstrual cycle. J Endocrinol Invest. 1985;8(5):437–41. doi: 10.1007/BF03348533. Epub 1985/10/01. [DOI] [PubMed] [Google Scholar]
- 27.O’Brien TD, Reeves ND, Baltzopoulos V, Jones DA, Maganaris CN. Muscle-tendon structure and dimensions in adults and children. J Anat. 2010;216(5):631–42. doi: 10.1111/j.1469-7580.2010.01218.x. Epub 2010/03/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park SK, Stefanyshyn DJ, Ramage B, Hart DA, Ronsky JL. Alterations in knee joint laxity during the menstrual cycle in healthy women leads to increases in joint loads during selected athletic movements. Am J Sports Med. 2009;37(6):1169–77. doi: 10.1177/0363546508330146. [DOI] [PubMed] [Google Scholar]
- 29.Pearson SJ, Burgess KE, Onambele GL. Serum relaxin levels affect the in vivo properties of some but not all tendons in normally menstruating young women. Exp Physiol. 2011;96(7):681–8. doi: 10.1113/expphysiol.2011.057877. Epub 2011/04/12. [DOI] [PubMed] [Google Scholar]
- 30.Peck D, Buxton DF, Nitz A. A comparison of spindle concentrations in large and small muscles acting in parallel combinations. J Morphol. 1984;180(3):243–52. doi: 10.1002/jmor.1051800307. [DOI] [PubMed] [Google Scholar]
- 31.Romani W, Patrie J, Curl LA, Flaws JA. The correlations between estradiol, estrone, estriol, progesterone, and sex hormone-binding globulin and anterior cruciate ligament stiffness in healthy, active females. Journal of Women’s Health. 2003;12(3):287–98. doi: 10.1089/154099903321667627. [DOI] [PubMed] [Google Scholar]
- 32.Schmitt PM, Kaufman MP. Estrogen’s attenuating effect on the exercise pressor reflex is more opioid dependent in gonadally intact than in ovariectomized female cats. J Appl Physiol. 2005;98(2):633–9. doi: 10.1152/japplphysiol.00788.2004. Epub 2004/09/28. [DOI] [PubMed] [Google Scholar]
- 33.Shultz SJ, Kirk SE, Johnson ML, Sander TC, Perrin DH. Relationship between Sex Hormones and Anterior Knee Laxity across the Menstrual Cycle. Med Sci Sports Exerc. 2004;36(7):1165–74. doi: 10.1249/01.mss.0000132270.43579.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shultz SJ, Wideman L, Montgomery MM, Beasley KN, Nindl BC. Changes in serum collagen markers, IGF-I, and knee joint laxity across the menstrual cycle. J Orthop Res. 2012;30(9):1405–12. doi: 10.1002/jor.22093. Epub 2012/03/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simons DG, DIMITRIJEVIC MR. Quantitative variations in the force of quadriceps responses to serial patellar tendon taps in normal man. Am J Phys Med Rehabil. 1972;51(5):240. [PubMed] [Google Scholar]
- 36.Slauterbeck J, Clevenger C, Lundberg W, Burchfield DM. Estrogen level alters the failure load of the rabbit anterior cruciate ligament. J Orthop Res. 1999;17(3):405–8. doi: 10.1002/jor.1100170316. [DOI] [PubMed] [Google Scholar]
- 37.Smith SS, Woolley CS. Cellular and molecular effects of steroid hormones on CNS excitability. Cleve Clin J Med. 2004;71(Suppl 2):S4. doi: 10.3949/ccjm.71.suppl_2.s4. [DOI] [PubMed] [Google Scholar]
- 38.Turgeon JL, Carr MC, Maki PM, Mendelsohn ME, Wise PM. Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: Insights from basic science and clinical studies. Endocr Rev. 2006;27(6):575–605. doi: 10.1210/er.2005-0020. Epub 2006/06/10. [DOI] [PubMed] [Google Scholar]
- 39.Van Lunen BL, Roberts J, Branch JD, Dowling EA. Association of menstrual-cycle hormone changes with anterior cruciate ligament laxity measurements. Journal of athletic training. 2003;38(4):298. [PMC free article] [PubMed] [Google Scholar]
- 40.Yu WD, Panossian V, Hatch JD, Liu SH, Finerman GA. Combined effects of estrogen and progesterone on the anterior cruciate ligament. Clin Orthop Relat Res. 2001;(383):268–81. doi: 10.1097/00003086-200102000-00031. Epub 2001/02/24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1: Profile of Oral Contraceptives in the User Group (17)