Abstract
Beach sands can sustain indigenous and introduced populations of enterococci. The objective of this study was to evaluate wave action in promoting the release of introduced bacteria. To accomplish this objective this study developed a method to assess attachment and identified conditions under which introduced bacteria are integrated into the sand. A new “shearing assay” showed that attachment of the introduced spike mimicked that of the natural sand when the spike was allowed to integrate into the sand for 24 hours at room temperature at a sand moisture content of 20%. Experiments in a wave flume showed that waves were capable of releasing about 60% of the total bacteria added. This suggests that for the range of wave conditions evaluated (height: 1.9-10.5 cm, period:1-2.7 s), waves were incapable of releasing all of the bacteria. Further study is needed to evaluate bacteria attachment mechanisms.
Keywords: enterococci, waves, beach sand, attachment, wave flume
1. Introduction
Enterococci are recommended by the U.S. Environmental Protection Agency (EPA) to evaluate the safety of recreational marine beaches (U.S. EPA, 1976). Enterococci are associated with sewage, and while not commonly pathogenic, their levels have been found to correlate with negative human health risks at sewage impacted beaches (Cabelli et al., 1982; Dufour, 1984; Wade et al., 2003) and at urban runoff impacted beaches (Colford et al. 2007; Haile et al. 1999). Enterococci can also impact beaches that are not exposed to sewage or urban runoff through sustained populations within beach sediments (Fujioka et al. 1998; Byappanahalli and Fujioka 2004; Wright et al., 2011; Beversdorf et al., 2007; Yamahara et al., 2007; Feng et al., 2010; Imamura et al. 2011). These populations can impact water quality (Phillips et al., 2011a; Abdelzaher et al. 2011) and may represent an increased risk to bathers (Fleisher et al., 2010; Sinigalliano et al., 2010; Heaney et al. 2012). Shah et al. (2011) suggests that these increased risks are due to correlations between enterococci and human pathogens in beach sand. The sources of enterococci and ultimately pathogens can include the deposition of microbes from human and animal sources (Elmir et al. 2009; Whitman and Nevers 2003; Edge and Hill 2007; Lu et al. 2011), transport and deposition of contaminated sand through wave action (Gast et al. 2011; Boehm et al. 2002; Ishii et al. 2007; Ge et al. 2010, 2012), and the transport of microbes to the sand from the water through rainwater runoff (Roll and Fujioka 1997; Haack et al. 2003; Beversdorf et al. 2007) and tidal washing (Boehm and Weisberg 2005; Solo-Gabriele et al. 2000; Feng et al., 2013). These sources can then be potentially supplemented through the regrowth of microbes in the sand (Fujioka et al., 1999; Ishii and Sadowsky, 2008; Yamahara et al. 2009; Byappanahalli et al. 2012). Thus there is an inter-play between microbes entering the sand from external sources and their persistence and possible regrowth.
Currently there are no standardized methods to evaluate microbial attachment to beach sands nor have the conditions conducive to bacterial attachment or detachment under wave conditions been evaluated. Several studies have identified wave-induced sediment resuspension as a significant mechanism for transporting bacteria from beach sediments to the water column (Ge et al., 2012; Feng et al., 2013), release of bacteria from the sediment grains due to shear effects (Ishii et al. 2007), transfer of bacteria trapped in the pore water through diffusion (Feng et al., 2013), and groundwater transport (Boehm et al., 2004; Phillips et al., 2011b). These mechanisms are difficult to quantify in situ due to the complexity of hydrodynamics and a large number of environmental variables. The spatial variability of enterococci in the beach sand and their rapid dilution and dispersion into the water column (Yamahara et al., 2007; Enns et al., 2012, Shibata et al., 2004; Wright et al., 2011) also makes it challenging to quantify bacterial movement and to make meaningful measurements to elucidate mechanisms for release. The transient nature of environmental conditions adds to the difficulty of studying bacterial release on recreational beaches.
Detachment of enterococci from beach sands through wave action has not been studied under controlled laboratory conditions. The objective of this study was to develop a method to assess attachment of enterococci to beach sand, identify conditions under which the bacteria are integrated into the sand, and to ultimately evaluate wave action in promoting releases. This study utilized an artificial beach which was seeded with enterococci and placed into a wave flume. This allowed for control over wave conditions as well as control over the location and amount of microbes in the sand. Since the wave flume simulated a narrow section of the beach, the impact from alongshore movement and subsequent dilution of enterococci was eliminated thereby isolating the impacts from wave action.
2. Methods
Due to the inherent variability of enterococci levels in sand collected from the environment (Alm et al. 2003; Bonilla et al. 2007; Yamahara et al. 2007; Enns et al. 2012), methods were needed to establish a consistent level of “attached” enterococci to sand. To address this need, this study included the development of a bench-top “shearing assay” to evaluate the release of bacteria from sand. This assay provided a consistent level of shear energy and results were used to check the degree to which bacteria were integrated into the sand.
This study also required identifying optimum conditions for preparing the sand used in the wave flume experiments. This included identifying the optimum beach from which to harvest sand and the sand moisture content for adding the bacterial seed. These conditions were then used for subsequent experiments aimed at identifying the optimum dye for visualization of mixing processes and tracking the location of the enterococci within the water column.
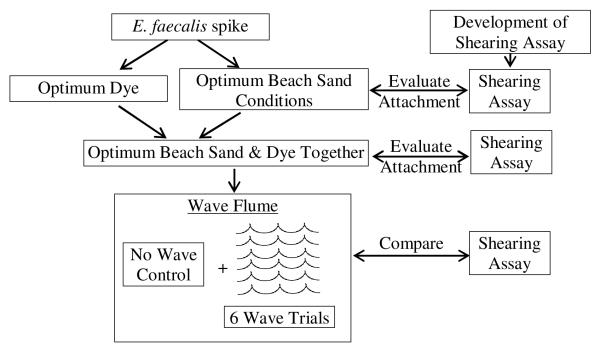
Ultimately, these efforts were integrated into the wave flume experiments which assessed the release of enterococci from the sand under wave conditions (see Figure 1).
Figure 1. Methods development.
Approach for developing the method for seeding the sand with enterococci, points where shearing assay was used, and conceptual approach for wave flume evaluation.
2.1. Development of the “Shearing Assay”
In order for the seeded sand to be useful for experimentation, it should mimic the natural, hydraulically-induced release of bacteria. Thus the seeded sand should show a comparable degree of bacterial attachment to that of natural beach sands. Since there is no established assay to assess the attachment of bacteria to sand grains, one was developed using a gyratory shaker (New Brunswick Scientific G24 Environmental Incubator Shaker) to induce a standardized amount of shear forces for a set time that would serve to detach (or release) the bacteria from the sand grains. The shearing assay applied the same shear force to all sand samples, and this method allowed for the same relative measure of bacterial attachment across all samples including those used for the flume experiments. Multiple different settings were tested on the gyratory shaker (swirling action) in order to define the time and revolutions per minute (RPMs) needed to produce consistent results over multiple trials. Once this setting was determined (300 seconds at 200 RPMs) it was used to test native sand from multiple local beaches in order to establish a baseline attachment of enterococci to sand grains.
The final protocol developed for the “shearing assay” consisted of placing three, 20 g aliquots of seeded sand into 250 ml beakers and inundating the sand with 150 ml of aseptic sea water. One beaker, designated as the control, was set aside and not shaken in the gyratory shaker. The bacteria measured in the water supernatant in this control are considered to represent the unattached bacteria, which likely represent bacteria residing within the interstitial space between sand grains. The two other beakers were placed into a gyratory shaker and were agitated for 300 seconds at 200 RPMs. After the agitation process was completed, samples of both the supernatant and sediment were collected for analysis. The bacteria measured in the supernatant of the “sheared” samples are considered to represent the fraction of the “attached” bacteria that was released by the shearing assay. The fraction of bacteria released was computed as the ratio of the attached bacteria that were released by shearing, and the total attached bacteria. The total attached bacteria was computed as the sum of the attached bacteria that was released plus the remaining bacteria that remained in the sand after the shearing assay. The bacteria that remained in the sand was measured using a vigorous shaking method (see laboratory analysis below).
2.2. Optimum Conditions for the Addition of the Bacteria Seed to the Sand
A procedure was developed to seed the sand. The procedure should result in the attachment of the bacterial spike in a fashion similar to the indigenous bacteria. Dye was to be added to the spiked sand in order to visualize both the seeded sand on the beach and the mixing of the interstitial water into the water column. This dye should not significantly interfere with the bacterial growth, survival or adherence to the seeded sand.
The stock bacteria suspension used for the spike consisted of Enterococcus faecalis (ATCC 29212) grown in suspension in BBLTM Brain Heart Infusion Broth (Becton, Dickinson and Company) for 48 hours then centrifuged for 10 minutes at 4500 RPMs. The pellet was then transferred to sterile phosphate buffered saline (PBS) solution and serial dilutions were performed and plated (on mEI agar) in order to determine the concentration of enterococci within the stock.
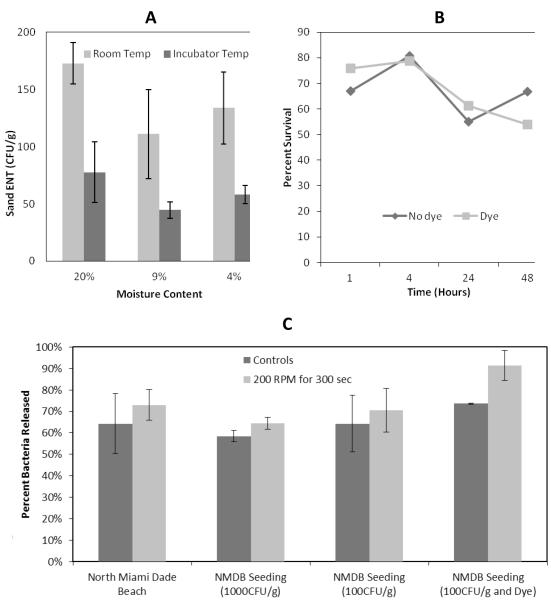
Once the stock was created, it was added to the sand and thoroughly mixed. Sands from multiple beaches were evaluated to determine which beach sand best harbored the growth of enterococci. Sands from different beaches were evaluated (supplemental Figure 2). These sands were processed at different moisture contents and before and after autoclaving in an effort to determine optimal conditions for attachment and integration of the enterococci. Attachment was measured using the shearing assay. The optimal sand was collected from a North Miami-Dade Beach (NMDB) and the optimal moisture content was 20% (Figure 2A). The bacteria were integrated into the sand after about 24 hours at room temperature.
Figure 2. Development of sand seeding method.
A, Seeded sand (at a 20% moisture content and subject to overnight incubation (41°C) and at room temperature (20 °C) was most conducive to bacterial growth. B, this growth was not significantly retarded by the addition of dye into the sand. C, in testing this seeded sand with the shearing assay there was no significant difference in the percentage of bacteria released from the seeded sand, seeded sand with dye and the natural sand taken from NMDB(p=0.62). All error bars correspond to one standard deviation.
Three different types of dyes (Risk Reactor™ IFWB-33 Orange Water Based Tracer, Risk Reactor™ IFWB-14 Non-Fluorescent Blue Tracer, and Fluorescein) were tested for their toxicity to enterococci. Exposure studies were conducted with concentrations of dye ranging from 10 ppt to100 ppb. A known quantity of enterococci was added to each of these solutions and the die off was measured to assess toxicity (Supplemental Figure 2). The dye that was found to be least toxic to enterococci (non-fluorescent blue tracer) was then used for further testing of the surrogate contaminated sand (NMDB sand under 20% moisture) to assess bacterial survival and to ensure that the dye did not interfere with adherence of the bacteria to the sand. Over a period of 48 hours, no significant differences were observed in seeded-sand enterococci numbers with or without dye (Figure 2B). Moreover, the dye did not significantly interfere with the attachment of the seeded bacteria to the sand (Figure 2C). This attachment was evaluated under both control conditions (no shaking) and under shearing assay conditions. For the NMDB sand, the percentage release of enterococci from the native sand was very similar to the release of enterococci from the seeded sand (Figure 2C).
These methodological results were used in preparing the seeded sand used for the wave flume experiments. The method included the collection of 10 L of new NMDB sand the day before each experiment. Upon receipt at the laboratory this sand was spiked with approximately 1000 CFU/g of enterococci and 0.25% (V/V) of the non-florescent blue tracer dye resulting in a moisture content near 20%. After the addition of the enterococci and dye, the sand was placed in a sterile, sealed container for 24 hours at room temperature to allow bacteria time to grow and integrate with the sand. After the 24 hours the seeded sand was homogenized for 5 minutes, sampled, and placed into the wave flume.
2.3. Wave Flume Set Up
The experiments were conducted in the 15 m × 1 m × 1 m Air-Sea Interaction Saltwater Tank (ASIST) at the Rosenstiel School of Marine and Atmospheric Science, University of Miami. The wave flume was equipped with a mechanical wave generator that consisted of a hydraulically driven ram with a vertical face extending from above the water surface to the flume bottom (see Savelyev et al., 2011 for wave flume specifics). The wave generator was used to generate waves with a wave height between 1.9 to 10.5 cm and a wave period between 1 and 2.7 seconds. Within a few meters of the paddle the vertical distribution of the wave orbital velocity is no longer effected by the unrealistic initial vertical velocity profile (Savelyev et al., 2011). These small amplitude waves were equivalent in full scale to those observed at a local beach on Biscayne Bay (Feng et al., 2013). A “no wave” control was conducted to evaluate the release from filling and emptying the wave flume. A model beach, the base made of wooden plates, was created to represent the same local recreational beach with a model scale (3.7 degrees beach slope) similar to the shallow slope of the actual beach profile (2.3 degrees beach slope) (Enns et al. 2012). The upper extent of the beach base (42 cm high) was placed at the end farthest from the wave generator. Approximately 300 L of dry unspiked “background” NMDB sand was evenly spread out to form a 5 cm layer on top of the beach base. A 20 cm strip that spanned across the width of the flume was cleared for the seeded sand. The flume was filled with natural seawater that was pumped from intakes on Bear Cut which connects Biscayne Bay and the Atlantic Ocean. The seawater was pumped into rooftop settling tanks to remove large particles then passed through a sand filter and finally a 10 μm cartridge filter. The test section was filled to a mean water depth of 40 cm. The salinity in the test section ranged from 32 to 35 ppt (Criales et al., 2013). Between trials the sand was rinsed with tap water and then with aseptic seawater.
2.3.1. Sediment Sampling
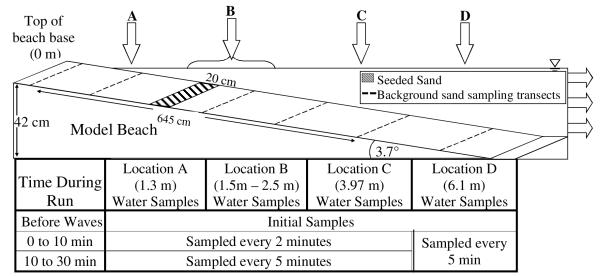
Before each trial, “initial” sand samples were collected along six “background” transects (Figure 3) along the length of the model beach. At each transect, a composite sample was prepared from six core samples (2.54 cm diameter, 5 cm deep) spaced equidistant across the width of the flume. For each respective transect, the composite samples were homogenized and processed for initial concentrations of enterococci. Prior to placement in the flume, the seeded sand was homogenized and a composite sample was also taken in a fashion equivalent to that used for the “background” transects. This seeded sand composite was split, with one part utilized to measure the initial concentration of enterococci in the spiked sand and the other part used for the shearing assay (see above) to measure the attachment of the spiked bacteria.
Figure 3. Beach base and the locations of water and sediment samples.
The horizontal reference point for the water samples corresponds to the top of the beach base. Dotted lines across the top of the beach base represent sampling transects where initial and final samples of the background sand were collected. The shaded box shows the 20 cm wide strip of seeded sand. Water sample location B was always taken from above the strip of seeded sand. The seeded sand was placed in predetermined locations along the sloping beach to simulate wave interactions with the sediment on the beach.
After each trial, “final” sand samples were collected at each of the 6 transects in the same manner that the “initial” sand was sampled. The method of sample collection of the seeded sand, after the trial, was different. For the seeded sand sample, three larger core samples (10.16 cm diameter) were taken horizontally along the location of the seeded sand strip. The top and bottom halves of these larger cores were analyzed separately for enterococci levels. To do this the depth of the sand was noted and as the sand was removed from the core, half the total depth was aseptically scraped into one Whirl-pak™ bag and the remainder was placed in a second thus separating the top and bottom halves of the sand core.
2.3.2. Water Sampling
Prior to the experiment, a water sample was taken from the incoming seawater to determine “background” levels of enterococci in the water. In the wave flume, during each trial, water samples were taken at about a 2 cm depth (below mean water level) at four designated horizontal locations using sterile pipettes. A sample was collected from each of the four locations once the flume was filled to a 40 cm depth prior to the initiation of waves. These samples were called “prior” water samples. Upon initiation of the experiment, all sampling equipment and personnel were set, the wave generator was turned on, and another set of water samples (“time-zero” water samples) were collected. The designated sampling locations and their sampling intervals are shown in Figure 3.
2.3.3. Laboratory Analysis
Water samples were analyzed for enterococci within 2 hours of sample collection using a membrane filtration method that utilized mEI agar media (Method 1600, US EPA 1997). The amount of blue dye in the water was measured using a UV/Vis spectrophotometer (Beckman Coulter DU720, calibrated against 5000 ppb, 2500 ppb, 1000 ppb, 500 ppb, 100 ppb, 50 ppb and 0 ppb standards) set at 630 nm.
Sediment samples were analyzed for moisture content and enterococci levels. Before analysis, each sand sample was homogenized. Moisture content was measured gravimetrically (before and after drying for 24 hours at 110°C). In order to extract microbes from the sand samples, approximately 10 grams of sand were aseptically transferred into a sterile bottle and shaken vigorously for 2 minutes in 100 mL of sterile phosphate buffered saline solution (Shibata et al. 2004; Boehm et al, 2009). The shaking consisted of agitating the bottle through an intense wrist action in the direction from the top to the bottom of the bottle (as opposed to swirling) causing significant turbulence within the bottle. After allowing the sediment to settle, two volumes of the eluent (25ml and 3ml) were then analyzed in the same fashion as the water samples (Method 1600, US EPA 1997). For seeded sand samples collected prior to placement in the wave flume, a 1:10 dilution was performed on the eluent prior to plating in order to obtain countable colonies.
2.3. Statistical Analysis
Statistical analysis was performed using Microsoft Excel, XL STAT (Addinsoft USA, New York, NY). Data were tested for normality. Correlations for normally distributed data were acquired using Pearson’s Test (r). Correlations for non-normally distributed data were computed using Spearman’s Rank Order Test (rs). An alpha of 0.05 was used for all tests. The relationships between the percentages of enterococci released by the shearing assay trials versus the wave flume experiments were analyzed using a single variable ANOVA and Fisher’s F-test.
3. Results
3.1. Relationships between Enterococci and Dye
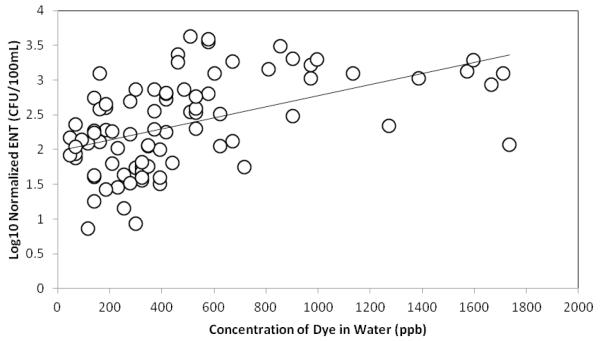
Log normalized levels of enterococci in the water column correlated with the concentrations of blue dye in the water at all zones. These results were statistically significant (r=0.49, p<0.001) for the first 6 minutes of waves (Figure 4). After the first 6 minutes the trend dissipated.
Figure 4. Concentrations of dye versus levels of enterococci.
Concentration of dye generally tracks the levels of ENT in the water for the first 6 minutes of waves. r=0.49, p<0.001
3.2. Release of Bacteria from Seeded Sand
The enterococci released from the sediment and the total concentration in the water (Supplemental Figure 3) exhibited a consistent increasing linear trend during the first 6 minutes of the experiment (Figure 5). Both quantities then varied significantly between trials for the last 24 minutes of the runs (Figure 6). For the wave conditions evaluated, no correlation was observed, however, between either wave height or wave period and detachment. This is likely due to the spatial and temporal variability in enterococci measures.
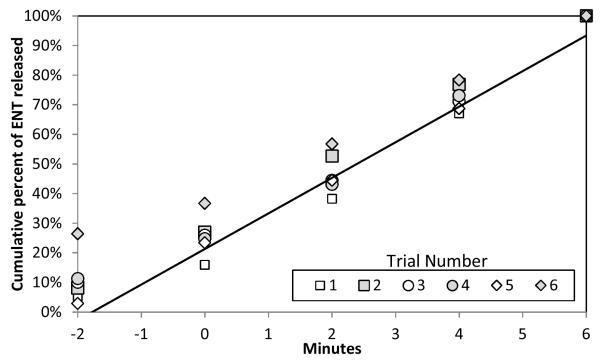
Figure 5. Percentage release of total enterococci released from the sand, normalized by the total number of released enterococci in the first 6 minutes.
In this experiment ENT were observed to have a linear release from the sand as shown by the data points on the graph. The time “−2” represents the sampling before the initiation of waves but after the seeded sand had been fully inundated with water. The percent was calculated as the total number of bacteria released in the first 6 minutes, divided by the total number of bacteria released at 2-minute intervals.
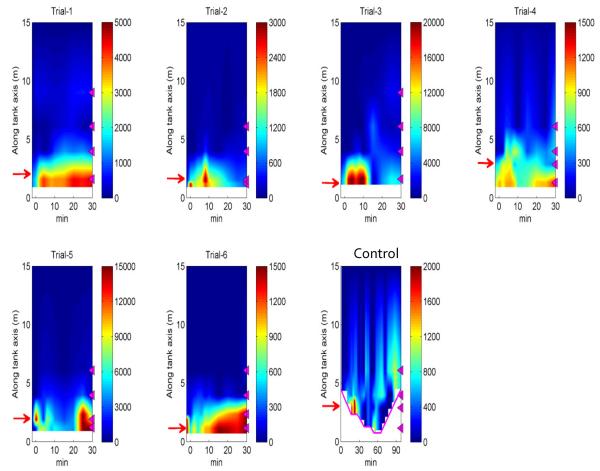
Figure 6. Gross release of enterococci for the entire 30-minute trial.
Contours of enterococci concentrations (unit of color bar scale: CFU/100 mL) in the flume water using linear interpolation between sampling locations. The magenta triangles on the right of each panel illustrate the water sampling locations. Red arrows indicate locations of seeded sand strip. The magenta line in the “no wave” control trial shows the location of waterline.
After the initial 6 minutes of the experiment, the bacteria levels in the water column became sporadic likely due to dilution, advection, and mixing effects within the wave flume coupled with more intermittent releases of bacteria from the sand (Figure 6).
Some patterns were observed in the vertical distribution of the enterococci released from the sand. For trials with waves, analysis of the top and bottom layers of sediment in the “final” seeded sand revealed that more enterococci were removed from the top layer (78% removal and standard deviation of 17% ) as compared to the bottom layer (58% removal and standard deviation of 28%) (p=0.05, average difference between top and bottom layers =194 CFU/g dry sand). Although the trial lacking waves showed a larger removal from the top layer as compared to the bottom, this difference was not significant (p=0.57, average difference between top and bottom layer =121 CFU/g dry sand). Comparing the trials with waves to the trial without waves indicated that the trials with waves removed more bacteria from the top of the sediment than the trial without waves (p=0.045). However there was no difference in the bacteria removed from the bottom half of the sediment between trials with and without waves (p=0.44).
3.3. Shearing Assay
A similar pattern was observed for the percent removal of sand enterococci from the shearing assay as compared to the corresponding removal from the wave flume experiments (Figure 7A). There was no significant difference (p=0.6) between the average percentage of enterococci removed by the shearing assay (62%±12%) and the average percentage of enterococci removed during the wave flume experiments (65%±15%). The variances of both the percent removal from the shearing assay (1.4%) and the wave flume (2.3%) were not shown to be unequal using an F-Test for equality of variances (p=0.57). There was a significant positive correlation between the percent removals (r=0.63, p=0.037) despite the observations being independent of one another (the percent removal from the shearing assay was not the cause of the percent removal from the wave flume). The experimental data (within 90% confidence, r=0.52, p=0.08) was statistically not different than a theoretical 1:1(y=x) relationship between the two percentages (figure 7B). This indicates that the shearing assay removed statistically similar percentages of enterococci as the wave action within the wave flume.
Figure 7. Percent released for wave flume trials and percent released for corresponding shearing assays.
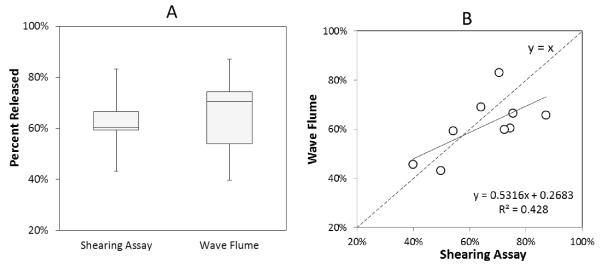
A. The percent released is similar between the shearing assay and wave flume for all trials. Mean is displayed as an X on each box. There was no statistical difference between the two data sets (p=0.6) B. Dotted line represents a theoretical 1:1 relationship between the percent released in the wave flume and percent released in the shearing assay. The solid line represents the linear regression for the observed relationship. The linear regression from the data is not statistically dissimilar from a 1:1 ratio and fits this theoretical 1:1 linear regression (r=0.52, p=0.08). The percentages released from sand during the wave flume and shearing assay are correlated (r=0.63, p=0.037).
The percentage of bacteria liberated from the sand in the control trial with no waves was similar to that from the controls from the shearing assay, (e.g. 50% from control trial with no waves as compared to 43% in the shearing assay control). Both the bacteria liberated from the sand in the control trial and the bacteria released in the control from the shearing assay were well below the average from the other trials’ shearing assay and wave flume experiments (64±11% and 67±15% respectively).
4. Discussion
4.1. Dye
The concentration of dye within the water generally mirrored the levels of bacteria (Figure 4) during the first 6 minutes of wave action. This correlation was seen for all sampling locations. This suggests that both the dye and bacteria are initially released in a similar fashion and once released from the sediment, bacteria are passively transported with the flow of water. This is consistent with prior studies modeling microbe and dye release from the sediment-water interface (Grant et al., 2011). The release from the sand is of interest as the dye and enterococci spike were both added to the sand and allowed to air dry at room temperature for a period of 24 hours. Just like the enterococci, the dye was given the opportunity to dry on to the sand grains thereby also providing some adherence of the dye to the sand. The possible differences in the attachment between the dye and enterococci can account for some of the deviations from the linear relationship between the two, especially during time periods after initial 6 minutes. The dye apparently follows the more loosely bound bacteria that are released earlier during experimentation.
4.2. Release of Bacteria from Sand
During the first 6 minutes of each trial, bacteria were released from the sand at a steady, linear rate (Figure 5). This was the case regardless of wave period or wave height. Trials with higher energy conditions did not release bacteria from the sand any faster than less energetic conditions. This suggests that for bacteria which are loosely attached to the sand grains, the release of bacteria, for the wave conditions evaluated, may not solely be controlled by kinetic conditions such as waves but may be more highly controlled by other intrinsic factors of the bacteria or the sand itself.
The difference in release between the top and bottom half of the seeded sand was due to the difference in energy acting on the bacteria contained within each half. Enterococci contained within the top half of the seeded sand were exposed to the bed shear stirring action of waves. The lower half of the sediment was, at least initially, protected from this mechanical stirring and the contained bacteria were primarily mobilized by the upward movement of pore water. Prior studies using naturally stratified beach sands have found that pore water flow is only a minor contributor to indigenous bacterial release, mobilizing on average only 10% of bacteria contained within sand similar to that used in this study (Phillips et al., 2011b). Our experiments removed on average 58% of the bacteria contained within the bottom half of the seeded sand. The difference in percent released could be attributed to differences in the flow environment. In the wave flume the pore water in the lower sediment layers moves back and forth through the sand whereas pore water flow under the experimental conditions of Phillips et al. (2011b) was unidirectional. Further variation could be attributed to differences in characteristics of the beach sands used for each study. This variation was seen in shearing assays done on sands from two different beaches (Supplemental Figure 1).
4.3. Shearing Assay
Attachment and release of bacteria from sand has been shown to be affected by the characteristics of the media from which the bacteria are being removed and the pore water velocity in which the bacteria are being mobilized (Stevik et al., 2004). Pore water velocity in our experimentation would be driven by the energy from waves and agitation (Precht and Huettel, 2004). The similarity between percent removal in the wave flume and percent removal in the shearing assay is noteworthy because while the amount of energy in the wave flume changed depending on the sand’s location and the wave characteristics; the amount of energy in the shearing assay remained unchanged for all trials of the experiment. This further supports that there is some intrinsic property of the sand or the bacteria that governs the percentage of bacteria that can be removed via agitation. Since there was no time series data collected for the shearing assay, it is possible that the agitation, created by both the shearing assay and the wave flume, reached or exceeded a certain maximum threshold, after which no more bacteria can be released from the sand (within the ranges of energy used during the experiment).
Intrinsic properties of sand that have been shown to affect bacterial removal and attachment include factors such as saturation of the media (Schäfer et al., 1998), dissolved organic matter, sediment organic matter (Johnson and Logan, 1999; Morales et al., 2011), grain size, surface area, biofilms, concentration of bacteria, electrostatic charges and characteristics of the liquid media such as pH (Stevik et al., 2004). There was no correlation seen between percentage removed and concentration of bacteria (r=0.07, p=0.74). Characteristics of the salt water and sand, such as saturation, grain size and surface area could have had some impact on the amount of bacteria removed but most likely remained relatively constant throughout the trials as the sources of all sand and water were constant.
Biofilms could potentially explain the consistency in the percentage of bacteria removed from the sand for each trial. Bacteria such as enterococci can persist without a substrate, loosely attached to a substrate (such as sand) and contained within biofilms (Piggot et al, 2012; Mohamed and Huang, 2007). These biofilms can interconnect sand grains to stabilize the sediment (Yallop et al., 2000; Kim and Pachepsky, 2008) and protect bacteria from shear forces caused by currents and wave action making them harder to remove from the sediments (Decho, 2000). The percentage of bacteria that were not removed from the sediment could potentially represent the percentage of bacteria that had become assimilated within biofilms. The fraction assimilated would remain relatively unchanged between aliquots of homogenized sands. Thus the same percentages of bacteria remained adhered for both the wave flume and the shearing assay. During analysis (see methods) these remaining bacteria within the biofilms were removed and quantified through vigorous shaking as found by Boehm et al. (2009) to be the most effective way of removing enterococci from sands.
5. Conclusions
Enterococci within the sand pose a unique problem to beach regulators as they are difficult to remediate when they cause exceedences in regulatory standards. Knowledge of enterococci attachment and mobilization into the water column would be helpful to better predict and possibly manage exceedences as they occur. Under the controlled settings evaluated in this study, bacterial release was linear during the first few minutes of the experiment and more sporadic thereafter. More bacteria were released from the surface of the sand in comparison to the deeper layers. No trend was observed between the amount of bacteria released and the wave heights and periods evaluated in this study (wave heights from 1.9 to 10.5 cm and wave periods from 1 to 2.7 s). Although waves represent a mechanism by which bacteria are released from sands (Ge et al. 2012; Ge et al. 2010; Whitman and Nevers 2003, Phillips et al. 2011b), the fraction released was dominated by other factors including the relative attachment or adhesion of the bacteria to the sand.
This study emphasizes that wave action releases a portion of the bacteria from contaminated sands. The release due to wave action is intermittent after an initial few-minute period. Even after 30 minutes, wave-action alone was not sufficient to remove all bacteria from shoreline beach sand. If the remaining bacteria are capable of regrowth, intermittent bursts of bacteria releases can potentially occur as waves impact the beach sand environment.
6. Recommendations
More work is needed to investigate the intrinsic characteristics of sand and the environment that govern the attachment of bacteria to sand. The role of biofilms should be more fully explored and the relationships between wave energy and biofilm removal should be more fully evaluated.
Supplementary Material
Highlights.
Artificial seeded sand was successfully created to mimic attachment of bacteria to natural beach sand.
A wave tank and shearing assay were developed to measure the release of bacteria from sand.
Percentage released by waves was not different than bench top shearing assay.
Maximum percentage of microbes released governed by factors other than agitation.
Biofilms and adhesion of bacteria to sand grains are suspected to control release.
Acknowledgements
Funding for this project was received through the National Science Foundation (NSF) and the National Institute of Environmental Health Sciences (NIEHS) Oceans and Human Health Center at the University of Miami Rosenstiel School [NSF 0CE0432368/0911373/1127813] and [NIEHS P50 ES12736]. Supplemental support was provided by the NSF-REU program and NSF grant OCE1127813. We would like to thank the following individuals for their assistance during sample collection and analysis: Malia Carpio, Noha Abdel-Mottaleb, Amir Abdelzaher, Nasly Jimenez, Vincent Warger, Yasiel Hernandez, and Rafael Hernandez. We also appreciate the assistance received from the City of Sunny Isles Beach, Florida and from Dr. Samir Elmir of Miami-Dade Department of Health. We also appreciate Dr. Troy Scott’s advice as we were developing the sand spiking method for the dye and enterococci.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelzaher AM, Wright ME, Ortega C, Hasan AR, Shibata T, Solo-Gabriele HM, Kish J, Withum K, He G, Elmir SM, Bonilla JA, Bonilla TD, Palmer CJ, Scott TM, Lukasik J, Harwood VJ, McQuaig S, Sinigalliano CD, Gidley ML, Wanless D, Plano LRW, Garza AC, Zhu X, Stewart JR, Dickerson JW, Yampara-Iquise H, Carson C, Fleisher JM, Fleming LE. Daily measures of microbes and human health at a non-point source marine beach. J. Water Health. 2011;9(3):443–457. doi: 10.2166/wh.2011.146. DOI 10.2166/wh.2011.146. [DOI] [PubMed] [Google Scholar]
- Alm E, Burke J, Spain A. Fecal indicator bacteria are abundant in wet sand at freshwater beaches. Water Res. 2003;37(16):3978–82. doi: 10.1016/S0043-1354(03)00301-4. DOI:10.1016/S0043-1354(03)00301-4. [DOI] [PubMed] [Google Scholar]
- Beversdorf LJ, Bornstein-Forst SM, McLellan SL. The potential for beach sand to serve as a reservoir for Escherichia coli and the physical influences on cell die-off. J. Appl. Microbiol. 2007;102(5):1372–81. doi: 10.1111/j.1365-2672.2006.03177.x. DOI: 10.1111/j.1365-2672.2006.03177.x. [DOI] [PubMed] [Google Scholar]
- Boehm AB, Weisberg SB. Tidal Forcing of Enterococci at Marine Recreational Beaches at Fortnightly and Semidiurnal Frequencies. Environ. Sci. Technol. 2005;39:5575–5583. doi: 10.1021/es048175m. DOI: 10.1021/es048175m. [DOI] [PubMed] [Google Scholar]
- Boehm AB, Grant SB, Kim JH, Mowbray SL, McGee CD, Clark CD, Foley DM, Wellman DE. Decadal and shorter period variability of surf-zone water quality at Huntington Beach, California. Environ. Sci. Technol. 2002;36(18):3885–3892. doi: 10.1021/es020524u. DOI 10.1021/es020524u. [DOI] [PubMed] [Google Scholar]
- Boehm AB, Shellenbarger GG, Paytan A. Groundwater discharge: Potential association with fecal indicator bacteria in the surf zone. Environ. Sci. Technol. 2004;38(13):3558–3566. doi: 10.1021/es035385a. DOI: 10.1021/es035385a. [DOI] [PubMed] [Google Scholar]
- Boehm AB, Griffith J, McGee C, Edge TA, Solo-Gabriele HM, Whitman R, Cao Y, Getrich M, Jay JA, Ferguson D, Goodwin KD, Lee C, Madison M, Weisberg SB. Faecal indicator bacteria enumeration in beach sand: a comparison study of extraction methods in medium to coarse sands. J. Appl. Microbiol. 2009;107:1740–1750. doi: 10.1111/j.1365-2672.2009.04440.x. DOI: 10.1111/j.1365-2672.2009.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla TD, Nowosielski K, Cuvelier M, Hartz A, Green M, Esiobu N, McCorquodale DS, Fleisher JM, Rogerson A. Prevalence and distribution of fecal indicator organisms in South Florida beach sand and preliminary assessment of health effects associated with beach sand exposure. Mar. Pollut. Bull. 2007;54(9):1472–82. doi: 10.1016/j.marpolbul.2007.04.016. DOI: 10.1016/j.marpolbul.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Byappanahalli M, Fujioka R. Indigenous soil bacteria and low moisture may limit but allow faecal bacteria to multiply and become a minor population in tropical soils. Water Sci. Technol. 2004;50(1):27–32. [PubMed] [Google Scholar]
- Byappanahalli MN, Nevers MB, Korajkic A, Staley ZR, Harwood VJ. Enterococci in the environment. Microbiol. Mol. Biol. Rev. 2012;76(4):685–706. doi: 10.1128/MMBR.00023-12. DOI: 10.1128/MMBR.00023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabelli VJ, Dufour AP, McCabe LJ, Levin MA. Swimming associated gastroenteritis and water quality. Am. J. Epidemiol. 1982;115(4):606–616. doi: 10.1093/oxfordjournals.aje.a113342. [DOI] [PubMed] [Google Scholar]
- Colford JM, Jr., Wade TJ, Schiff KC. Water quality indicators and the risk of illness at beaches with non point sources of fecal contamination. Epidemiology. 2007;18(1):27–35. doi: 10.1097/01.ede.0000249425.32990.b9. DOI:10.1097/01.ede.0000249425.32990.b9. [DOI] [PubMed] [Google Scholar]
- Criales MM, Zink IC, Haus BK, Wylie J, Browder JA. Effect of turbulence on the behavior of pink shrimp postlarvae and implications for selective tidal stream transport behavior. Mar. Ecol. Prog. Ser. 2013;477:161–176. DOI: 10.3354/meps10141. [Google Scholar]
- Decho AW. Microbial biofilms in intertidal systems: an overview. Cont. Shelf Res. 2000;20(10-11):1257–1273. DOI: 10.1016/S0278-4343(00)00022-4. [Google Scholar]
- Dufour AP. Health effects criteria for fresh recreational waters. Office of Research and Development, US Environmental Protection Agency; Cincinnati, OH: 1984. EPA-600/1-84-004. [Google Scholar]
- Edge TA, Hill S. Multiple lines of evidence to identify the sources of fecal pollution at a freshwater beach in Hamilton Harbour, Lake Ontario. Water Res. 2007;41:3585–3594. doi: 10.1016/j.watres.2007.05.012. DOI: 10.1016/j.watres.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Elmir SM, Shibata T, Solo-Gabriele HM, Sinigalliano CD, Gidley ML, Miller G, Plano L, Kish J, Withum K, Fleming L. Quantitative evaluation of enterococci and Bacteroidales released by adults and toddlers in marine water. Water Res. 2009;43(18):4610–4616. doi: 10.1016/j.watres.2009.07.006. DOI: 10.1016/j.watres.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enns AA, Vogel LJ, Abdelzaher AM, Solo-Gabriele HM, Plano LRW, Gidley ML, Phillips MC, Klaus JK, Piggot AM, Feng Z, Reniers AJHM, Haus BK, Elmir SM, Zhang Y, Jimenez NH, Abdel-Mottaleb N, Schoor ME, Brown A, Khan SQ, Dameron AS, Salazar NC, Fleming LE. Spatial and temporal variation in indicator microbe sampling is influential in beach management decisions. Water Res. 2012;46:2237–2246. doi: 10.1016/j.watres.2012.01.040. DOI: 10.1016/j.watres.2012.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng F, Goto D, Yan T. Effects of autochthonous microbial community on the die-off of fecal indicators in tropical beach sand. FEMS Microbiol. Ecol. 2010;74(1):214–225. doi: 10.1111/j.1574-6941.2010.00921.x. DOI: 10.1111/j.1574-6941.2010.00921.x. [DOI] [PubMed] [Google Scholar]
- Feng Z, Reniers A, Haus BK, Solo-Gabriele HM. Modeling sediment-related enterococci release, transport, and inactivation at an embayed nonpoint source beach. Water Resour. Res. 2013;49:693–712. doi:10.1029/2012WR012432, 2013. [Google Scholar]
- Fleisher JM, Fleming LE, Solo-Gabriele HM, Jonathan KK, Sinigalliano CD, Plano LRW, Elmir SM, Wang JD, Withum K, Shibata T, Gidley ML, Abdelzaher AM, He G, Ortega C, Zhu X, Wright M, Hollenbeck J, Backer LC. The BEACHES Study: health effects and exposures from non-point source microbial contaminants in subtropical recreational marine waters. Int. J. Epidemiol. 2010;39(5):1291–1298. doi: 10.1093/ije/dyq084. DOI 10.1093/ije/dyq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka R, Sian-Denton C, Borja M, Castro J, Morphew K. Indigenous soil bacteria and low moisture may limit but allow faecal bacteria to multiply and become a minor population in tropical soils. J Appl Microbiol. 1998;85(Suppl 1):83S–89S. doi: 10.1111/j.1365-2672.1998.tb05286.x. DOI: 10.1111/j.1365-2672.1998.tb05286.x. [DOI] [PubMed] [Google Scholar]
- Fujioka R, Sian-Denton C, Borja M, Castro J, Morphew K. Soil: the environmental source of Escherichia coli and enterococci in Guam’s streams. J. Appl. Microbiol. Symp. Suppl. 1999;85:83S–89S. doi: 10.1111/j.1365-2672.1998.tb05286.x. [DOI] [PubMed] [Google Scholar]
- Gast RJ, Gorrell L, Raubenheimer B, Elgar S. Impact of erosion and accretion on the distribution of enterococci in beach sands. Continental shelf research. 2011;31(14):1457–1461. doi: 10.1016/j.csr.2011.06.011. doi:10.1016/j.csr.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant SB, Litton-Mueller RM, Ahn JH. Measuring and modeling the flux of fecal bacteria across the sediment-water interface in a turbulent stream. Water Resour. Res. 2011;47:1–13. DOI: 10.1029/2010WR009460. [Google Scholar]
- Ge Z, Whitman RL, Nevers MB, Phanikumar MS, Byappanahalli MN. Nearshore hydrodynamics as loading and forcing factors for Escherichia coli contamination at an embayed beach. Limnol. Oceanogr. 2012;57(1):362–381. doi:10.4319/lo.2012.57.1.0362. [Google Scholar]
- Ge Z, Nevers MB, Schwab DJ, Whitman RL. Coastal loading and transport of Escherichia coli at an embayed beach in Lake Michigan. Environ Sci Technol. 2010;44(17):6731–7. doi: 10.1021/es100797r. DOI: 10.1021/es100797r. [DOI] [PubMed] [Google Scholar]
- Haack SK, Fogarty LR, Wright C. Escherichia coli and Enterococci at Beaches in the Grand Traverse Bay, Lake Michigan: Sources, Characteristics, and Environmental Pathways. Environ Sci Technol. 2003;37:3275–3282. doi: 10.1021/es021062n. DOI: 10.1021/es021062n. [DOI] [PubMed] [Google Scholar]
- Haile RW, Witte JS, Gold M. The health effects of swimming in ocean water contaminated by storm drain runoff. Epidemiology. 1999;10(4):355–363. [PubMed] [Google Scholar]
- Heaney CD, Sams E, Dufour AP, Brenner KP, Haugland RA, Chern E, Wing S, Marshall S, Love DC, Serre M, Noble R, Wade TJ. Fecal Indicators in Sand, Sand Contact, and Risk of Enteric Illness Among Beachgoers. Epidemiology. 2012;23(1):95–106. doi: 10.1097/EDE.0b013e31823b504c. DOI: 10.1097/EDE.0b013e31823b504c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura GJ, Thompson RS, Boehm AB, Jay JA. Indigenous soil bacteria and low moisture may limit but allow faecal bacteria to multiply and become a minor population in tropical soils. FEMS Microbiol Ecol. 2011;77(1):40–49. DOI: 10.1111/j.1574-6941.2011.01082.x. [Google Scholar]
- Ishii S, Hansen DL, Hicks RE, Sadowsky MJ. Beach Sand and Sediments are Temporal Sinks and Sources of Escherichia coli at Lake Superior. Environ Sci Technol. 2007;41:2203–2209. doi: 10.1021/es0623156. DOI: 10.1021/es0623156. [DOI] [PubMed] [Google Scholar]
- Ishii S, Sadowsky MJ. Escherichia coli in the Environment: Implications for Water Quality and Human Health. Microbes Environ. 2008;23(2):101–108. doi: 10.1264/jsme2.23.101. DOI: 10.1264/jsme2.23.101. [DOI] [PubMed] [Google Scholar]
- Johnson WP, Logan BE. Enhanced transport of bacteria in porous media by sediment569 phase and aqueous-phase natural organic matter. Water res. 1999;30(4):923–931. DOI:10.1016/0043-1354(95)00225-1. [Google Scholar]
- Kim J, Choi H, Pachepsky YA. Biofilm morphology as related to the porous media clogging. Water Res. 2008;44(4):1193–1201. doi: 10.1016/j.watres.2009.05.049. DOI:10.1016/j.watres.2009.05.049. [DOI] [PubMed] [Google Scholar]
- Lu JR, Ryu HD, Hill S, Schoen M, Ashbolt N, Edge TA, Domingo JS. Distribution and potential significance of a gull fecal marker in urban coastal and riverine areas of southern Ontario, Canada. Water Res. 2011;45:3960–3968. doi: 10.1016/j.watres.2011.05.003. DOI:10.1016/j.watres.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Mohamed JA, Huang DB. Biofilm formation by enterococci. J. Med Microbio. 2007;56:1581–1588. doi: 10.1099/jmm.0.47331-0. DOI: 10.1099/jmm.0.47331-0. [DOI] [PubMed] [Google Scholar]
- Morales VL, Zhang W, Gao B, Lion LW, Bisogni JJ, Jr., McDonough BA, Steenhuis TS. Impact of dissolved organic matter on colloid transport in the vadose zone: Deterministic approximation of transport deposition coefficients from polymeric coating characteristics. Water res. 2011;45(4):1691–1701. doi: 10.1016/j.watres.2010.10.030. DOI:10.1016/j.watres.2010.10.030. [DOI] [PubMed] [Google Scholar]
- Phillips MC, Solo-Gabriele HM, Piggot AM, Klaus JS, Zhang Y. Relationship between sand and water quality at recreational beaches. Water Res. 2011a;45:6763–6769. doi: 10.1016/j.watres.2011.10.028. DOI:10.1016/j.watres.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MC, Solo-Gabriele HM, Reniers AJHM, Wang JD, Kiger RT, Abdel-Mottaleb N. Pore water transport of enterococci out of beach sediments. Mar. Pollut. Bull. 2011b;62:2293–2298. doi: 10.1016/j.marpolbul.2011.08.049. DOI:10.1016/j.marpolbul.2011.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot AM, Klaus JS, Johnson S, Phillips MC, Solo-Gabriele HM. Relationship between enterococcal levels and sediment biofilms at recreational beaches in south Florida. Appl. Environ. Microbiol. 2012;78(17):5973–5982. doi: 10.1128/AEM.00603-12. DOI: 10.1128/AEM.00603-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Precht E, Huettel M. Rapid wave-driven advective pore water exchange in a permeable costal sediment. J. Sea Res. 2004;51:93–107. DOI:10.1016/j.seares.2003.07.003. [Google Scholar]
- Roll BM, Fujioka RS. Sources of faecal indicator bacterica in a brackish, tropical stream and their impact on recreational water quality. Water Sci. Technol. 1997;35:179–186. DOI: 10.1016/S0273-1223(97)00255-2. [Google Scholar]
- Savelyev IB, Haus BK, Donelan MA. Experimental study on wind-wave momentum flux in strongly forced conditions. J. Phys. Oceanogr. 2011;41:1328–1344. DOI: 10.1175/2011JPO4577.1. [Google Scholar]
- Schäfer A, Ustohal P, Harms H, Stauffer F, Dracos T, Zehnder AJB. Transport of bacteria in unsaturated porous media. Journal of Contaminant Hydrology. 1998;33(1-2):149–169. DOI:10.1016/S0169-7722(98)00069-2. [Google Scholar]
- Shah A, Abdelzaher AM, Phillips M, Hernandez R, Solo-Gabriele HM, Kish J, Scorzetti G, Fell JW, Diaz MR, Scott TM, Lukasik J, Harwood VJ, McQuaig S, Sinigalliano CD, Gidley ML, Wanless D, Ager A, Lui J, Stewart JR, Plano LRW, Fleming LE. Indicator microbes correlate with pathogenic bacteria, yeast, and helminthes in sand at a subtropical recreational beach site. J. Applied Microb. 2011;110(6):1571–1583. doi: 10.1111/j.1365-2672.2011.05013.x. DOI: 10.1111/j.1365-2672.2011.05013.x. [DOI] [PubMed] [Google Scholar]
- Shibata T, Solo-Gabriele HM, Fleming L, Elmir S. Monitoring marine recreational water quality using multiple microbial indicators in an urban tropical environment. Water Res. 2004;38:3119–3131. doi: 10.1016/j.watres.2004.04.044. DOI:10.1016/j.watres.2004.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinigalliano CD, Fleisher JM, Gidley ML, Solo-Gabriele HM, Shibata T, Plano L, Elmir SM, Wang JD, Wanless D, Bartowiak J, Boiteau R, Withum K, Abdelzaher A, He G, Ortega C, Zhu X, Wright M, Kish J, Hollenbeck J, Backer LC, Fleming LE. Traditional and molecular analyses for fecal indicator bacteria in non-point source subtropical recreational marine waters. Water Res. 2010;44(13):3763–3772. doi: 10.1016/j.watres.2010.04.026. DOI 10.1016/j.watres.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevik TK, Aa K, Ausland G, Hanssen JF. Retention and removal of pathogenic bacteria in wastewater percolating through porous media: A review. Water Res. 2004;38:1355–1367. doi: 10.1016/j.watres.2003.12.024. DOI: 10.1016/j.watres.2003.12.24. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency . Method 1600: membrane filter test method for enterococci in water. U.S. Environmental Protection Agency; Washington, DC: 1997. EPA 821/R-97/004. [Google Scholar]
- U.S. Environmental Protection Agency . Quality Criteria for Water. U.S. Environmental Protection Agency; Washington, DC: 1976. EPA-440976023. [Google Scholar]
- Wade TJ, Pai N, Eisenberg JN, Colford JMJ. Do U.S. Environmental Protection Agency Water Quality Guidelines for Recreational Waters Prevent Gastrointestinal Illness? A Systematic Review and Meta-analysis. Environ. Health Persp. 2003;111:1102–1109. doi: 10.1289/ehp.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman R,L, Nevers MB. Indigenous soil bacteria and low moisture may limit but allow faecal bacteria to multiply and become a minor population in tropical soils. Appl Environ Microbiol. 2003;69(9):5555–5562. [Google Scholar]
- Wright ME, Solo-Gabriele HM, Abdelzaher AM, Elmir S, Fleming LE. The inter634 tidal zone is the geographic location of elevated concentrations of enterococci. Water Sci. Technol. 2011;63(3):542–549. doi: 10.2166/wst.2011.255. [DOI] [PubMed] [Google Scholar]
- Yallop ML, Paterson DM, Wellsbury P. Interrelationships between rates of microbial production, exopolymer production, microbial biomass, and sediment stability in biofilms of intertidal sediments. Microb. Ecol. 2000;39(2):116–127. doi: 10.1007/s002489900186. DOI: 10.1007/s002489900186. [DOI] [PubMed] [Google Scholar]
- Yamahara KM, Layton BA, Santoro AE, Boehm AB. Beach sands along the California coast are diffuse sources of fecal bacteria to coastal waters. Environ. Sci. Technol. 2007;41(13):4515–4521. doi: 10.1021/es062822n. DOI: 10.1021/es062822n. [DOI] [PubMed] [Google Scholar]
- Yamahara KM, Walters SP, Boehm AB. Growth of enterococci in unaltered, unseeded sands subjected to tidal wetting. Appl. Environ. Microbiol. 2009;75:1517–1524. doi: 10.1128/AEM.02278-08. DOI: 10.1128/AEM.02278-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.