Abstract
Lipid lateral segregation into specific domains in cellular membranes is associated with cell signaling and metabolic regulation. This phenomenon partially arises as a consequence of the very distinct bilayer-associated lipid physico-chemical properties that give rise to defined phase states at a given temperature. Until now lamellar gel (Lβ) phases have been described in detail in single or two-lipid systems. Using x-ray scattering, differential scanning calorimetry, confocal fluorescence microscopy, and atomic force microscopy, we have characterized phases of ternary lipid compositions in the presence of saturated phospholipids, cholesterol, and palmitoyl ceramide mixtures. These phases stabilized by direct cholesterol-ceramide interaction can exist either with palmitoyl sphingomyelin or with dipalmitoyl phosphatidylcholine and present intermediate properties between raft-associated phospholipid-cholesterol liquid-ordered and phospholipid-ceramide Lβ phases. The present data provide novel, to our knowledge, evidence of a chemically defined, multicomponent lipid system that could cooperate in building heterogeneous segregated platforms in cell membranes.
Introduction
Sphingolipids are important biomolecules in the onset of physiological events such as cell proliferation, signal transduction, and programmed cell death (1). Ceramides in particular consist of a sphingosine molecule to which a fatty acyl chain is linked through an amide group. Ceramides are strongly connected with programmed cell death or apoptosis (2). In addition, they represent the main hydrophobic building block of more complex sphingolipids such as sphingomyelin, which presents a polar phosphorylcholine group linked to ceramide at the C1 position. Membrane component lateral segregation into specific domains appears to be a characteristic step in sphingolipid-dependent metabolic signaling (3). Palmitoyl sphingomyelin (pSM) is an abundant sphingolipid in cell plasma membranes, giving rise to a lamellar gel (Lβ) phase in model membranes at physiological temperatures. Its special affinity for cholesterol (Chol) via hydrogen bonding at its amide group drives the formation of liquid-ordered (lo) phases (4–6), structures related to the short-lived membrane rafts (7). Furthermore, pSM association with its relative sphingolipid palmitoyl ceramide (pCer) renders highly ordered bilayers in a Lβ phase (8). The in vivo activation of a sphingomyelinase enzymatic activity on sphingomyelin under stress conditions is considered as the main mechanism for ceramide synthesis in signaling events (9). As a consequence, sphingomyelin- and cholesterol-rich membrane compartments arise as putative loci for sphingomyelinase action, generating platforms enriched in sphingomyelin, cholesterol, and ceramide in cell membranes. In this regard, both cholesterol and ceramide have been considered as competitors for their close interaction with sphingomyelin (10,11), the former governing their lateral organization in a concentration-dependent manner (12,13). Interestingly, in ternary mixtures with palmitoyl-oleoyl-phosphatidylcholine (POPC) or dioleoyl-phosphatidylcholine (DOPC), cholesterol and ceramide have been observed to coexist with no apparent displacement effects between the two latter lipids (14). The higher ceramide solubility in Chol-rich membranes would support the generation of compositionally heterogeneous bilayers in the studied model systems.
Cell membranes exist mainly in the lamellar liquid-crystalline or fluid disordered (Lα) phase (15). In recent years, the presence of cell membrane domains in the lamellar lo phase has been proposed (7,16). However, gel (Lβ) phases have been characterized in detail only for pure lipids, typically DPPC or SM at room temperature (15), and in certain binary mixtures, e.g., SM/ceramide (8). Characteristically, gel phases undergo a thermotropic transition to liquid-crystalline (fluid) phases (15,17). A number of ternary gel phases have been mentioned, or their existence indicated in phase diagrams, e.g., in mixtures DOPC:DPPC:Chol (18,19), POPC:SM:Cer (12,14,20), SM:Cer:Chol (21), or in stratum corneum lipid mixtures containing Cer and Chol (22,23). However such phases were not structurally characterized in depth and were often present in systems with fluid-phase coexistence. In this study, using a combination of established physico-chemical approaches, we describe the generation of novel, to our knowledge, stable Lβ phases of ternary lipid compositions, based on sphingo- or phospholipid/Chol/pCer mixtures. The new phases are stabilized by direct Chol-pCer interactions and occur in the presence of either pSM or of its glycerophospholipid analog dipalmitoyl phosphatidyllcholine DPPC. The lack of requirement of the amide group at the phospholipid interface for the formation of ternary phases underlines the capacity of highly hydrophobic Chol and pCer to organize and self-interact among phospholipid molecules, perhaps according to the umbrella model (24). The ternary systems exhibit intermediate properties between Chol-rich lo and pCer-rich gel (Lβ) phases and they undergo cooperative thermotropic transitions to Lα phases. Our ternary phases are completely destabilized by substitution of either Chol by 5-cholestane-3-one (Chne) or of pCer by dipalmitoyl glycerol (DPG). This confirms the importance of the Chol polar hydroxyl group and of the pCer interfacial moiety in structure stabilization through direct hydrogen bonding. The characterization of Lβ phases of ternary lipid compositions opens the way to the existence of putative gel domains in the cell membrane, perhaps related to membrane platform formation and signaling.
Materials and Methods
Chemicals
N-palmitoyl-D-erythro-sphingosylphosphorylcholine (pSM), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), cholesterol (Chol), N-palmitoyl-D-erythro-sphingosine (pCer), and 1,2-dipalmitoyl-sn-glycerol (DPG) were purchased from Avanti Polar Lipids (Alabaster, AL). 5-cholesten-3-one (Chne), naphtho[2,3-a]pyrene (NAP) and Triton X-100 (TX100) were from Sigma-Aldrich (St. Louis, MO). The lipophilic fluorescent probe 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) was purchased from Molecular Probes (Eugene, OR). NBD-C6-Cer was a kind gift from Dr. G. Fabrias (IIQAB, CSIC, Barcelona, Spain). Assay buffer solution was 20 mM PIPES, 1 mM EDTA, 150 mM NaCl, pH 7.4. All other reagents were of the highest commercially available purity.
Confocal microscopy of giant unilamellar vesicles (GUVs)
Giant vesicles were prepared using the electroformation method (25) at 70°C. Observation of vesicles containing sucrose was carried out at room temperature (22 ± 1°C) using a PRET-GUV 4 Chamber supplied by Industrias Técnicas ITC (Bilbao, Spain). Fluorescent probe concentrations were 0.4 mol % DiI, 0.5 mol % NAP, and 0.7 mol % NBD-C6-Cer. AC field conditions were [1), 10 Hz, 2.5 V for 2 h; 2), 1 Hz, 2.5 V for 10 min]. Imaging was performed in a Leica TCSSP5 confocal fluorescence microscope (Leica Microsystems, Wetzlar, Germany). The excitation wavelength was 458 nm for NAP, 488 nm for NBD-C6-Cer, and 543 nm for DiI. Emission was recovered between 478–520 nm for NAP, 505–525 nm for NBD-C6-Cer, and 563–700 nm for DiI. Other details are as described previously (13).
DSC
The measurements were performed in a VP-DSC high-sensitivity scanning microcalorimeter (MicroCal, Northampton, MA). DSC was performed on multilamellar vesicle suspensions in assay buffer as described in (8). Lipid hydration was performed at a temperature well above that of the phase transitions for all mixtures. A final amount of 0.5 ml at 1.3 mM total lipid concentration was loaded into the calorimeter, performing three heating scans at a 45°C/h rate, between 10 and 105°C for all samples. Phospholipid concentration was determined by means of lipid phosphorus, and used together with data from the third scan, to obtain normalized thermograms. The software Origin 7.0 (MicroCal) was used to determine the different thermodynamic parameters from the scans. The transition enthalpies (ΔH) were obtained per mol of phospholipid. Endotherm decomposition was performed using the PeakFit software (Systat Software, Chicago, IL). The resulting peaks were fitted using a [Gauss + Lor Amp] function. The thermograms were fitted to the minimum number of peaks rendering a coefficient of determination of R2 > 0.985.
X-ray diffraction (XRD)
Simultaneous small (SAX)- and wide (WAX)-angle XRD measurements were carried out using a modified Kratky compact camera (Hecus MBraum-Graz-Optical Systems, Graz, Austria), which uses two coupled linear position sensitive detectors (PSD, MBraun, Garching, Germany) to monitor the s-ranges (s = 2 sinθ/λ, 2θ = scattering angle, λ = 1.54 Å) between 0.0075 and 0.07 and 0.20 and 0.29 Å−1, respectively. Nickel-filtered Cu Kα x-rays were generated by a Philips PW3830 X-ray Generator operating at 50 kV and 30 mA. The position calibration of the detectors was performed by using Ag-stearate (small-angle region, d-spacing at 48.8 Å) and lupolen (wide-angle region, d-spacing at 4.12 Å) as reference materials. Samples for XRD were prepared as multilamellar vesicles as described previously for DSC measurements, with the unique exception of leaving the samples dry overnight to remove any solvent traces. Final volume was 800 μl, and the samples contained a minimum of 20 mg of phospholipid. The pellets were measured in a thin walled high quality quartz capillary (1 mm diameter) held in a steel cuvette, which provides good thermal contact to the Peltier heating unit. XRD profiles were obtained for 10 min exposure times after 10 min of temperature equilibration. Data analysis and d-spacing calculations were performed with 3D-VIEW v4.1 software (Hecus MBraun, Graz, Austria).
Background corrected SAXS data were analyzed using the program GAP (Global Analysis Program) written by Georg Pabst and obtained from the author (26). GAP allowed retrieving the membrane thickness [dB = 2(zH + 2σH)] from a full q-range analysis of the SAXS patterns (27). The parameters zH and σH are position and width, respectively, of the Gaussian used to describe the electron-dense headgroup regions within the electron density model. The water layer was then given by [dW = d-dB].
Supported planar bilayer (SPB) preparation
Planar bilayer preparation on mica substrate for force spectroscopy measurements was performed using either the vesicle adsorption (28) or the spin coating (29) procedures. In particular, all pCer-containing samples were prepared through spin-coating as they presented very low vesicle-adsorption efficiency. For the vesicle adsorption technique, SPBs were prepared as described in (13). Lipid hydration and vesicle adsorption was performed at 70°C.
For the spin-coating procedure, lipids were directly dissolved at the desired ratio in an isopropanol/hexane/H2O (3:1:1) solution at 10 mM total lipid concentration. 0.4 mol % DiI and 0.2 mol % DiI-0.5 mol % NAP were included for the binary phospholipid/pCer and ternary phospholipid/Chol/pCer mixtures, respectively. 20 μl of the lipid mixture were directly pipetted onto a freshly cleaved mica substrate and rotated at 3000 rpm for 40 s using a KW-4A spin-coater (Chemat Technology, Northridge, CA). The substrate was then left under high vacuum overnight, mounted onto the Biocell, and hydrated with 400 μl assay buffer. Multiple bilayers were then generated. The temperature was raised to 70°C for 30 min and the sample washed throughout with assay buffer at 70°C. In this way, bilayers that nondirectly adhered to the mica substrate were discarded and the one on top of the mica left to perform the desired measurement. Finally, the Biocell was set to 22°C and the planar bilayers left to equilibrate for 1 h before measurements.
Force spectroscopy
The measurements were performed on a NanoWizard II AFM (JPK Instruments) at 22°C. Atomic force microscopy (AFM) imaging was initially carried out in contact mode (constant vertical deflection) to localize the SPBs and combined with direct epifluorescence microscopy to localize the desired bilayer areas. MLCT Si3N4 tips (Veeco Instruments, Plainview, NY) with spring constants of 0.1 or 0.5 N/m were used. No differences in the indentation patterns were observed regardless of the tip used. Each of the cantilevers was initially calibrated in a lipid-free solid substrate before imaging. The spring constant was determined using the thermal noise method. Force spectroscopy was finally performed onto the planar bilayers at 1 μm/s. Force steps were manually determined for each of the indentation curves within the extended traces. Resulting histograms were generated from at least three independent sample preparations with at least three independently calibrated cantilevers.
Results
pSM/Chol/pCer mixtures at a 54:23:23 mol ratio gives rise to homogeneously DiI-stained GUVs (Fig. 1 A). DiI is a lipophilic fluorescent probe unable to partition within highly ordered phospholipid/ceramide mixtures (8,13) (Chart 1). The ternary mixture renders a nearly symmetric gel (Lβ) to fluid (Lα) endothermal transition (Tm = 56°C) when analyzed by DSC (Fig. 2 A). The combined GUV and DSC data suggest that the ternary mixture, selected from data in a previous study (13), gives rise to a Lβ phase in excess water. In this mixture, no pCer-mediated cholesterol displacement from pSM was observed (30). To assess the driving force allowing a ternary phase to be formed, a series of studies using confocal microscopy and DSC were performed. The amide group of sphingomyelin presents a favorable interaction with the cholesterol hydroxyl group, perhaps through hydrogen bonding (4–6). Accordingly the amide group influence on the putative ternary phase stabilization was studied by replacing pSM with its glycerophospholipid counterpart DPPC. Although the amide group of sphingomyelin can act as both hydrogen bond donor and acceptor, dipalmitoyl phosphatidylcholine presents an ester group that is only an H-bond acceptor. Fig. 1 D shows a homogeneous DiI fluorescent probe distribution in DPPC/Chol/pCer giant vesicles at a 54:23:23 lipid ratio. As seen with pSM (13), no probe-depleted domains are observed. Dark regions would be expected if pCer displaced Chol for a close binary interaction with DPPC, as phospholipid/ceramide mixtures present strong intermolecular packing preventing DiI partition within (8,31). A fingerprint for the generation of phospholipid/pCer binary mixtures in model membranes is the appearance of high-temperature melting asymmetric endotherms in calorimetric studies (8,31–33). Note that in this study we discuss the shape of the overall endothermal transition to be symmetric or asymmetric considering as asymmetric those endotherms that present clearly visible shoulders. In Fig. 2 B it can be observed that the DPPC/Chol/pCer ternary mixture presents an essentially symmetric gel-fluid endothermal transition (Tm = 54°C). Again, the combination of microscopy and DSC data shows that DPPC/Chol/pCer (54:23:23 mol ratio) gives rise to an apparently homogeneous gel phase, in the absence of large phospholipid/pCer domains. No displacement of Chol by pCer is observed, independently of the chemical nature of the phospholipid accompanying the two latter lipids. The ternary mixtures are in a gel phase at both physiological and room temperatures.
Figure 1.
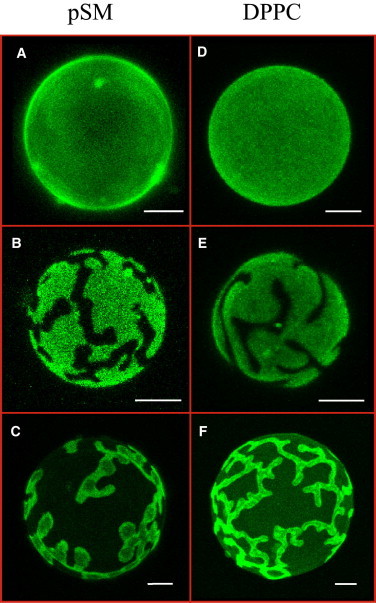
Confocal microscopy of DiIC18-stained pSM/Chol/pCer (A), pSM/Chne/pCer (B), pSM/Chol/DPG (C), DPPC/Chol/pCer (D), DPPC/Chne/pCer (E) and DPPC/Chol/DPG (F) GUVs at a 54:23:23 lipid ratio. Temperature = 22°C. Bars = 5 μm. To see this figure in color, go online.
Chart 1.
Chemical structure of the lipids and fluorescent probes used in this study.
Figure 2.
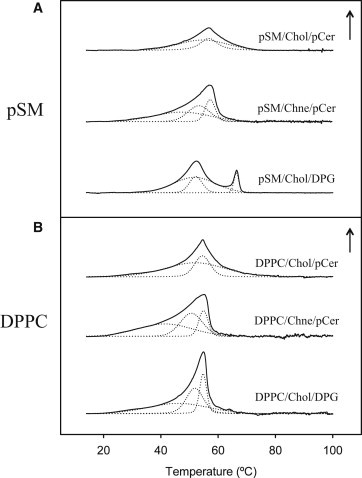
DSC representative thermograms of pSM- (A) and DPPC-based (B) multilamellar vesicles of ternary lipid mixtures at a 54:23:23 ratio. Dotted lines represent peak fitted endotherms. Arrows correspond to 0.4 kcal/mol/°C.
The lamellarity of the ternary mixtures was further examined under small (SAXD)- and wide (WAXD)-angle XRD measurements (Fig. 3). SAXD patterns for the pSM/Chol/pCer mixtures at a 54:23:23 ratio (Fig. 3 A) show sharp Bragg reflections with distances related as 1:1/2:1/4, characteristic of a multilamellar lipid organization. Moreover WAXD data (Fig. 3 B) evidence a broad peak centered at 4.1 Å at 20°C, which is not further observed at higher temperatures, i.e., 50 and 70°C. This constitutes a consistent evidence of a thermotropic gel-fluid phase transition, and a confirmation for the pSM-based ternary phase to be in the gel phase at room temperature. Note that a lo phase in WAXD shows just a very broad and diffuse scattering with a maximum at 4.4 Å at all temperatures (34). A further observation from the SAXD data is that the d-spacings do not change with temperature for the pSM-based ternary mixture, nor does the dp-p interphosphate group distances (Fig. S1 and Table S1 in the Supporting Material). This suggests that, in these mixtures, the phase transition occurs with little or no bilayer thickness or hydration variation. For the DPPC-based ternary mixture, a similar behavior is observed in WAXD measurements. (Fig. 3 B). SAXD data show for the DPPC/Chol/pCer sample lamellar d-spacings related as 1:1/2, the first order peak decreasing from 69.8 to 67.4 Å along the phase transition. The data suggest that the ternary mixtures under study present properties that deserve further study. Still, the most relevant conclusion from the XRD data is that the ternary mixtures are in a Lβ phase at room temperature.
Figure 3.
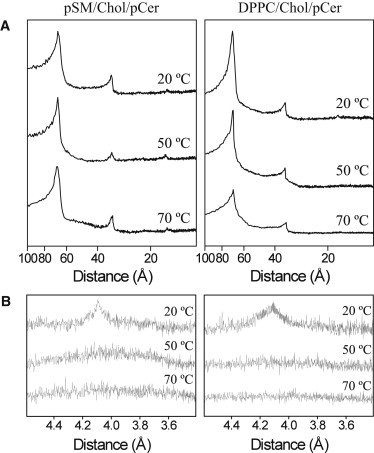
Small-angle (A) and wide-angle (B) XRD profiles of phospholipid/Chol/pCer (54:23:23) multilamellar vesicles at increasing temperatures.
The molar ratios of the previous mixtures were chosen from our previous study of a set of ternary mixtures, to test high enough Chol and pCer concentrations to favor any possible displacement effects (13), thus we cannot fully discard that phase segregation is occurring in those mixtures. In this regard, a series of fluorescent probes were independently incorporated into pSM- or DPPC-containing ternary mixtures to detect possible domain segregation within the vesicles, not detectable with DiI. Experiments with NAP provided an interesting observation. NAP is a polycyclic aromatic fluorescence molecule with high affinity for Chol-containing lipid-phases (37,38). Vesicles formed by ternary mixtures containing both NAP and DiI showed the generation of a minor discontinuous phase in the GUVs, not detectable with other fluorescent probes (Fig. 4). Thus, the phospholipid/cholesterol/ceramide mixtures at those proportions were not giving rise to completely homogeneous laterally segregated vesicles, which should be as well observed within calorimetric endotherms. Accordingly, we decomposed the obtained thermograms, setting a filtering coefficient of determination (R2) value that determined the minimum number of peaks that we considered to reflect the experimental transition. As can be observed in Fig. 2, the most reliable fitting for the Chol- and Cer-containing ternary mixtures reflected two contributions, a major, broad and a minor narrow component. Thus, the observed NAP-depleted minor domains may correspond to the narrow component observed in the DSC endotherms after fitting (Fig. 2, A and B, upper scans) and would account for ∼25–30% of the overall calorimetric contribution in either pSM or DPPC mixtures (Table S2). Thus, the pSM- or DPPC/Chol/pCer ternary mixtures at a 54:23:23 ratio appear to give rise to two kinds of laterally segregated microdomains, potentially reflecting the generation of two ternary phases of different composition in each of the samples, the most abundant ones corresponding to the broader endotherm component, and possibly higher Chol contents. These ternary-based structures appear to occur independently of the SM amide group, because a similar behavior is observed with DPPC-based ternary mixtures. Note however that the segregated phases seen in Fig. 4 could as well be constituted by immiscible binary and ternary mixtures, or by immiscibility among lipid mixtures and single components, in addition to the proposed explanation of coexisting ternary phases. Presently, the different possibilities cannot be discerned. Also relevant in this respect is the observation by Fanani et al. (39) that pCer as a single component can show coexistence of condensed phases with different structure and thickness; this could constitute a basis for phase segregation in the gel state.
Figure 4.
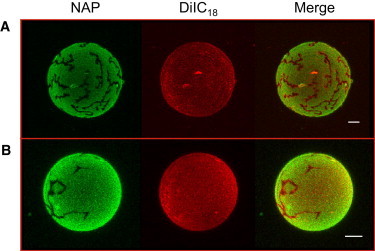
Confocal microscopy of NAP- and DiIC18-stained pSM/Chol/pCer (A) and DPPC/Chol/pCer (B) GUVs at a 54:23:23 lipid ratio. Temperature = 22°C. Bars = 5 μm.
Because both Chol and pCer present hydrogen bond donor and acceptor groups at their small polar heads, we next tested the possible stabilization of the ternary phases through a direct hydrogen bond-mediated pCer-Chol interaction. First, we substituted Chol for its analog Chne, which presents a carbonyl instead of a hydroxyl group at its polar headgroup. Thus, Chne can only act as a hydrogen bond acceptor molecule. This apparently simple substitution in the vesicles causes drastic alterations on the lipid behavior. Ternary GUVs containing Chne exhibit clear phase segregation of DiI-depleted microdomains with either pSM or DPPC (Fig. 1, B and E). Moreover, the corresponding calorimetric endotherms undergo a transformation into complex, asymmetric patterns (Fig. 2, A and B, middle curves). These results illustrate a destabilization of the ternary phases, and the generation of DiI-depleted phospholipid/pCer-rich binary phases within the vesicles. This is confirmed in Fig. S2 A and B by 7-nitro-2-1,3-benzoxadiazol-4-yl-amino-ceramide (NBD-Cer), a fluorescently labeled ceramide analog unable to partition into highly ordered phospholipid/Cer mixtures (30), which in Chne-containing ternary vesicles does not localize within DiI-depleted microdomains. The calorimetric data (Fig. 2) provide further information on the observed effect. The asymmetric endotherms can be decomposed into not less than three components, the first of which is a broad peak centered at 47.2 and 41.5°C for the pSM- and DPPC-containing ternary mixtures, respectively, representing ∼50% of the total absorbed heat (Table S2). The other two contributions, melting at higher temperatures, account for the other half of the absorbed heat and present an increased cooperativity reflected by the decrease of their transition widths (Fig. 2). The higher temperature melting and the increased cooperativity come along with phase transitions of pCer-rich phases (8,13,30,32), whereas broad transitions at around 40–45°C are generally obtained for high Chol-containing phospholipid/Chol binary mixtures (40,41). Overall, upon Chol substitution by Chne both the confocal microscopy and the calorimetric data show a destabilization of the ternary phases and the generation of phospholipid/Chne-rich and of at least two phospholipid/pCer-rich mixtures instead. Cer demixing and gel phase formation upon replacement of Chol by Chne have also been observed by Castro et al. (14).
As a further, complementary approach, pCer was substituted by DPG. DPG is the glycerolipid most similar to pCer, lacking the hydrogen bond donor and acceptor amide and hydroxyl groups at positions C2 and C3, respectively. Instead, DPG presents two ester groups that can act as hydrogen bond acceptors. DPG is able to form segregated microdomains in phospholipid/DPG binary GUVs and generates high-temperature melting asymmetric endotherms in DSC (30). Its behavior is thus similar to that of pCer. Similar to Chol replacement by Chne, pCer substitution by DPG in either pSM- or DPPC-containing ternary vesicles with Chol completely destabilizing the ternary phases. Fig. 1, C and F, shows the large micron-sized domains generated in the presence of DPG. In this case and in contrast to pCer-containing mixtures, DiI partitions into both phases but with different intensities. When NBD-Cer is incorporated into the vesicles, it does only partition into the less intense DiI-stained areas. It is known that NBD-Cer does not partition within highly ordered phases (30), thus the more intense DiI-stained areas in this mixture would correspond to the most ordered DPG-rich domains (Fig. S2, C and D). DSC analysis supports the destabilization of the ternary phases. In DPPC/Chol/DPG vesicles, a highly asymmetric endotherm is observed, which can be decomposed into at least three components (Fig. 2). The behavior resembles the one observed for Chne-containing vesicles with a clear segregation of a phospholipid/Chol-rich and at least two phospholipid/DPG-rich phases. In the presence of pSM the system is somewhat more complex. Two clearly separated endotherms are observed, the one at the lower temperature presenting at least two components after peak fitting, namely a broad transition and a more cooperative one centered at 51°C (Fig. 2 and Table S2). NAP-fluorescence GUV staining (not shown) shows dye partition within the less intense DiI-stained phase. Due to the higher affinity of NAP toward Chol-enriched phases, the latter could thus correspond to a pSM/Chol-rich phase. The other two endotherms components observed, at 66 and 51°C, respectively, would most probably reflect the presence of two pSM/DPG-rich phases of different DPG content. Indeed, these two phases can be observed within DiI-containing vesicles (Fig. S3) inside the most fluorescent phase as distinct shades of DiI-staining. In summary, we have shown that in the presence of either pSM or DPPC and both Chol and pCer, ternary phases are being formed; these phases are probably stabilized by direct Chol-pCer hydrogen bonding.
To identify the generation of new phases of ternary compositions in a different membrane model, we next performed force spectroscopy measurements in supported planar bilayers (SPBs) under an atomic force microscope. Specifically, AFM tip-mediated indentation curves were carried out onto the planar bilayers to quantitatively characterize their mechanical resistance to deformation. The indentation process ideally renders a breakthrough force at which the AFM tip jumps into the bilayer and this force can be used as a parameter to classify chemically distinct bilayers (42). In the present experiment, ternary gel phase-forming phospholipid/Chol/pCer planar bilayers at a 54:23:23 mol ratio were analyzed. Various SPBs were as well examined as controls: i), pure pSM and DPPC bilayers in a gel phase, ii), pSM and DPPC bilayers containing high enough Chol contents (30 mol %) to ensure the formation of homogeneous bilayers in a lo phase, and iii), pSM and DPPC bilayers with 30 mol % pCer, a concentration rendering nearly homogeneous phospholipid/pCer SPBs in a gel (Lβ) phase. Initially, AFM imaging was performed in combination with direct epifluorescence microscopy to control proper bilayer areas for indentation processes to be carried out. From the studied SPBs, binary phospholipid/pCer mixtures showed DiI-based phase segregation of phospholipid- and pCer-rich (DiI-depleted) areas (8). Force spectroscopy was performed within pCer-rich DiI-depleted areas. Similarly, for the ternary phases indentation was performed within the major DiI- and NAP-rich phase. Fig. S4 shows representative indentation curves for each of the previously mentioned SPBs. A broad spectrum of indentation patterns was obtained. Some of the bilayers showed a single force step during indentation, whereas others displayed two reproducible steps. As AFM-piezo-displacement before and after indentation was always in the 5–6 nm range, ensuring piercing of single bilayers, the two reproducible steps most probably reflect breaking through each of the SPB monolayers. In our setup and in good agreement with previous reports (42,43), DPPC bilayers exhibit a single force step at 16 nN (see Fig. 5 e). Chol incorporation results in an increased mechanical stability, showing two force steps centered at 9 and 20 nN (see Fig. 5 f). DPPC/pCer (7:3) SPBs show a further increased resistance to tip penetration, in accordance with its strong intermolecular packing. Two force steps are detected at 26 and 37 nN (see Fig. 5 h). Ternary phase-forming DPPC/Chol/pCer bilayers exhibit a strong mechanical resistance; with two force steps at 21 and 39 nN (see Fig. 5 g). The mixture exhibits a higher resistance to penetration than pure DPPC or lo phase-forming DPPC/Chol bilayers. Its piercing behavior shows a certain proximity to that of DPPC/pCer, however with two important differences: i), the ternary phase bilayer presents a reduction in bilayer thickness of around 0.6 nm (data not shown), and ii), DiI partitioned into the ternary phase forming SPBs, at variance with the DPPC/pCer bilayers. Hence, the piercing pattern observed for the ternary mixture corresponds to a chemically distinct bilayer phase.
Figure 5.
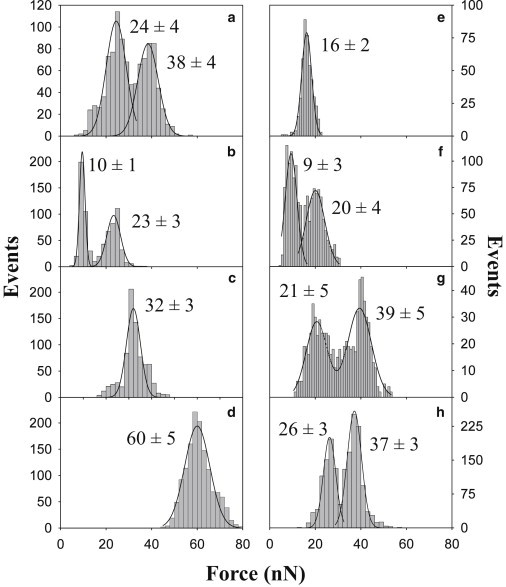
Force step distribution from AFM tip indentation curves on pSM- (left) and DPPC-based (right) supported planar bilayers. Samples were pSM (a) (n = 543), pSM/Chol (7:3) (b) (n = 384), pSM/Chol/pCer (54:23:23) (c) (n = 791), pSM/pCer (7:3) (d) (n = 1384), DPPC (e) (n = 447), DPPC/Chol (7:3) (f) (n = 722), DPPC/Chol/pCer (54:23:23) (g) (n = 472) and DPPC/pCer (7:3) (h) (n = 997). Continuous lines represent Gaussian fittings. Data average ± SD (n = 350–1800) (each histogram is based on three samples analyzed with three independent tips).
The analysis of pSM-based bilayers provides slightly different results. No single, marked breakthrough is obtained for pure pSM SPBs. Instead, two relatively smooth force steps are detected at 24 and 38 nN (Fig. 5 a and Fig. S4 a). The higher forces observed in comparison with DPPC; when both lipids are in a Lβ phase at 22°C, will probably reflect a stronger intermolecular interaction for the case of pSM. pSM bilayers containing Chol exhibit a force indentation pattern similar to DPPC/Chol in a lo phase (Fig. 5 b and Fig. S4 b). Force spectroscopy in pSM/pCer bilayers reveals the highest breakthrough force for the analyzed samples with a single force step centered at 60 nN, demonstrating the previously described firm intermolecular packing of the mixture (8). Finally, the ternary pSM/Chol/pCer mixture shows a completely different pattern with a clear force step at 32 nN. Interestingly, this bilayer presents a breakthrough force between those of the lo pSM/Chol and the gel pSM/pCer bilayers. The observation of two separate force distributions in some bilayers, including pure SM is surprising, and deserves further study. It could be due to uncoupling between the two leaflets of the bilayer, although the samples are kept in the fluid phase during preparation, to avoid uncoupling (44). In general, the force spectroscopy data in the phospholipid/Chol/pCer planar bilayers further support the generation of novel, to our knowledge, ternary phases with characteristic features.
Discussion
Ternary phase driving force
In our earlier report (13) we proposed the generation of cholesterol- and palmitoyl ceramide-enriched lipid mixtures in the pSM/Chol/pCer ternary phase at a 54:23:23 ratio. Opposite to what was expected, pCer did not displace cholesterol as previously proposed (10–12). Here, we confirm not only that the system is giving rise to stable ternary mixtures in which no displacement effects are occurring, but also that the ternary mixture is sphingomyelin-independent. Taking advantage of the specific exclusion of the fluorescent probe DiIC18 from highly ordered phospholipid/ceramide mixtures, and of their characteristic thermograms in calorimetric studies, we show that the phospholipid accompanying the two competitors (either pSM or DPPC) might just structurally accommodate Chol and pCer for the ternary mixtures to occur in a stable form. In terms of solubilization, earlier reports have likewise shown the inability of phospholipid/ceramide domains to be formed in mixtures with high cholesterol contents and the lamellar fluid (Lα) phase-forming POPC (14,31).
The specific dependence of these ternary phases on both Chol and pCer arises from samples in which small chemical substitutions have been individually performed on both lipids. The simple introduction of a carbonyl instead of a hydroxyl group in cholesterol C3, making the sterol even more hydrophobic, completely destabilizes the ternary mixtures on both phospholipid-containing samples. The latter is of great interest because it points to direct Chol-pCer interactions to be at the origin of the presently described ternary phases. Indeed, experiments of binary Chol-pCer monolayers have shown a favorable interaction between the two lipids (45), stabilizing the generation of mixed homogeneous configurations at various proportions, including the 1:1 mixture. Such interaction could be due to hydrogen bonding between the Chol OH in C3 and the SM amide carbonyl. However, Kan et al. (46) have stressed the importance of van der Waals forces in SM:Chol interactions. Furthermore, the difference between Chol and Chne observed in our work might also be due to the less polar Chne residing deeper in the bilayer than Chol. In this study, the possible Chol- and pCer-mediated ternary phase formation is further strengthened by comparing pCer behavior with that of DPG. Albeit the exchange of pCer by DPG is far more complex than that of Chol by Chne, it is the closer chemically related glycerolipid lacking the amide group, which we assume to stabilize the Chol-pCer interaction. The glycerol moiety of DPG substitutes the double bond at C4-C5, the hydroxyl group at C3, and the amide group at C2 of the pCer sphingosine backbone. Therefore, although pCer presents three H-bond donor/acceptor groups and a further carbonyl acceptor group, DPG presents a single H-bond donor/acceptor and two acceptor groups. The lower DPG propensity for hydrogen bonding with Chol could thus explain the nonhomogeneous DiI partition within the giant vesicles and the complex disruption of the broad initial thermotropic transition. From the presently studied analogs, it becomes apparent that if an intermolecular hydrogen bonding network between pCer and Chol is stabilizing the ternary phases, the hydroxyl group of cholesterol and both the amide and/or hydroxyl groups of palmitoyl ceramide are potentially involved.
Lamellar structure of the gel ternary phases
Due to the high cross-sectional area of their hydrophobic tails, both Chol and pCer are molecules that could possibly favor the formation of nonlamellar structures when present in high amounts, as is the present case. In our previous study we demonstrated the presence of both hydrophobic lipids in the ternary pSM/Chol/pCer system by following DiI partition before and after Chol extraction by methyl-β-cyclodextrin (mβCD) (13). The appearance of highly DiI-depleted domains in GUVs after extraction confirmed the presence of the two lipids within the vesicles. Here, we present additional data to corroborate the lamellar disposition of both ternary mixtures by means of XRD and AFM experiments. The most reliable method for this purpose is XRD, which provides information on the overall structure of the sample being analyzed. The clear lamellar organization found for the two phospholipid-based ternary mixtures supports the absence of large proportions of nonlamellar structures at the studied ratio. XRD at increasing temperatures also allowed the assessment of a thermotropic transition to occur, sustaining the lamellar structures to be in a gel phase at room temperature.
In support of the above the ternary mixtures under study exhibited clear endothermic signals by DSC, indicative of gel-fluid transitions. The experimental ΔH values found for the ternary phases (Table S2) are comparable with the literature data for pure phospholipids, e.g., ΔH values for the main gel-fluid transitions of DPPC and pSM are respectively around 35 kJ/mol and 28 kJ/mol (13,17). These values are expected to be modified both by the presence of ceramide and of cholesterol, the former tending to rise the transition enthalpy, the latter tending to decrease it (13). When both lipids are present in the ternary mixtures it appears as if their respective effects are counterbalanced.
The AFM-based force spectroscopy experiments give further details on the nature of the ternary phases. By following the nanomechanical resistance to tip penetration of the different studied bilayers, i.e., their breakthrough forces (Fb), we were able to clearly characterize each lipid mixture. In this way we could use the Fb values and the indentation patterns in a fingerprint-like manner. Both ternary phases gave unique results when compared to the control bilayers, showing the generation of chemically distinct lamellae. We find of interest the observation of an intermediate Fb value for the pSM-containing ternary mixture in between the raft-resembling pSM/Chol binary mixture in a lo phase and the pSM/pCer binary mixture in a gel phase. In terms of intermolecular ordering, chain packing, and lateral motion, this might reflect halfway properties of the pSM-based ternary phase. In the case of the DPPC-containing mixture this cannot be confirmed as the ternary phase presents similar Fb values as the binary DPPC/pCer mixture. Although DiI partition allows clearly setting apart these two samples, their mechanical properties cannot be distinguished by force spectroscopy.
Biological impact
The generation of ceramide-driven laterally segregated domains in cellular membranes is a potential upstream event during programmed cell death. However, the ceramide-generated intermolecular packing in simple model membranes arises as a clear drawback for specific proteins to freely diffuse and interact within such platforms before signal transduction. Even if under most conditions Cer makes <1% of membrane lipids, in some circumstances its concentration may transiently reach 20% of phosphatidylcholine, i.e., 6–8% of total lipids (47). This is compatible with the presence of localized domains in which the local Cer concentration can easily reach the levels used in this study. Recent studies have shown the formation of ceramide-enriched microdomains upon stress in physiological membranes such as erythrocyte ghost (48) and mitochondrial outer membranes (49). These promising results, where ceramide is recruited into specific platforms, point to the constitution of complex lipidic segregated domains allowing protein partition within. In this regard, cholesterol may turn an important biomolecule. Indeed, if we keep our attention on the previously mentioned examples, erythrocyte membranes are known to be highly cholesterol-enriched (∼40 mol % of total lipid), whereas Lee and collaborators (49) directly observed cholesterol to be colocalized within ceramide-rich macrodomains. An additional study discerned an accumulation of both cholesterol and ceramide into mitochondrial detergent-resistant fractions in reperfused rat hearts together with proapoptotic Bcl-2 family proteins (50). The close-contact between the two lipids in membranes is further supported by experiments in which high cholesterol contents prevented enzymatically generated ceramide to give rise to phospholipid/Cer-rich gel domains (21). All these examples would support the accumulation of ceramide- and cholesterol-rich compositionally heterogeneous platforms within cell membranes.
Additionally, the generation and stability of such lipid-enriched structures could be as well connected with the stratum corneum, which presents lamellae highly enriched in cholesterol, ceramides, and free fatty acids but practically lacking phospholipids (51). Cholesterol-ceramide interaction might thus favor the generation of highly packed platforms of various sources. Nevertheless, in our case the possible formation of pure Chol/pCer phases within the ternary phases is ruled out, as there is no evidence of pure pSM or DPPC phases to occur using any of the various approaches. In conclusion, this study constitutes a strong support for cholesterol and ceramide to cooperate rendering ternary (or even more complex) lipid platforms/microdomains with potential biological impact.
Conclusion
This study illustrates how lipid bilayers in a gel phase can be originated from chemically complex lipid mixtures. We show that the two hydrophobic lipids Chol and pCer, intensively studied for the past 15 years due to their strong domain-forming ability, collaborate with certain phospholipids to render bilayers in a Lβ phase. The high sensitivity of these mixtures toward specific chemical modifications of both Chol and pCer at their small polar headgroups supports a potential stabilization of these ternary phases based on intermolecular hydrogen bonding. The present data constitute a clear example of how cholesterol and ceramide, under certain conditions, collaborate rather than compete in the generation of chemically defined phospholipid/Chol/pCer lamellae in a gel phase. This work is of importance in the field of membrane organization in response to the manifold cellular signaling events, and more specifically in the lateral segregation of its components.
Acknowledgments
The authors are indebted to Dr. G. Fabrias for her gift of NBD-Cer.
This work was supported in part by grants from the Spanish Ministerio de Economía (BFU 2011-28566 to A.A. and BFU 2012-36241 to F.M.G.), from the Basque Government (IT838-13 to A.A. and IT849-13 to F.M.G.) and from the Ministerio de Economía y Competitividad (BFU 2011-22828 to J.C.G.-F.). J.V.B. was a postdoctoral fellow of the University of the Basque Country. A.G.-A. was a predoctoral student supported by the Basque Government.
Footnotes
Jon V. Busto’s present address is Institute of Cell Dynamics and Imaging, Von-Esmarch-Straße-56, 48149 Münster, Germany.
Supporting Material
References
- 1.Bartke N., Hannun Y.A. Bioactive sphingolipids: metabolism and function. J. Lipid Res. 2009;50(Suppl):S91–S96. doi: 10.1194/jlr.R800080-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taha T.A., Mullen T.D., Obeid L.M. A house divided: ceramide, sphingosine, and sphingosine-1-phosphate in programmed cell death. Biochim. Biophys. Acta. 2006;1758:2027–2036. doi: 10.1016/j.bbamem.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goñi F.M., Alonso A. Effects of ceramide and other simple sphingolipids on membrane lateral structure. Biochim. Biophys. Acta. 2009;1788:169–177. doi: 10.1016/j.bbamem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Veiga M.P., Arrondo J.L., Marsh D. Interaction of cholesterol with sphingomyelin in mixed membranes containing phosphatidylcholine, studied by spin-label ESR and IR spectroscopies. A possible stabilization of gel-phase sphingolipid domains by cholesterol. Biochemistry. 2001;40:2614–2622. doi: 10.1021/bi0019803. [DOI] [PubMed] [Google Scholar]
- 5.Ramstedt B., Slotte J.P. Membrane properties of sphingomyelins. FEBS Lett. 2002;531:33–37. doi: 10.1016/s0014-5793(02)03406-3. [DOI] [PubMed] [Google Scholar]
- 6.Collado M.I., Goñi F.M., Marsh D. Domain formation in sphingomyelin/cholesterol mixed membranes studied by spin-label electron spin resonance spectroscopy. Biochemistry. 2005;44:4911–4918. doi: 10.1021/bi0474970. [DOI] [PubMed] [Google Scholar]
- 7.Simons K., Gerl M.J. Revitalizing membrane rafts: new tools and insights. Nat. Rev. Mol. Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 8.Busto J.V., Fanani M.L., Alonso A. Coexistence of immiscible mixtures of palmitoylsphingomyelin and palmitoylceramide in monolayers and bilayers. Biophys. J. 2009;97:2717–2726. doi: 10.1016/j.bpj.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cremesti A.E., Goñi F.M., Kolesnick R. Role of sphingomyelinase and ceramide in modulating rafts: do biophysical properties determine biologic outcome? FEBS Lett. 2002;531:47–53. doi: 10.1016/s0014-5793(02)03489-0. [DOI] [PubMed] [Google Scholar]
- 10.Megha E., London Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function. J. Biol. Chem. 2004;279:9997–10004. doi: 10.1074/jbc.M309992200. [DOI] [PubMed] [Google Scholar]
- 11.Alanko S.M., Halling K.K., Ramstedt B. Displacement of sterols from sterol/sphingomyelin domains in fluid bilayer membranes by competing molecules. Biochim. Biophys. Acta. 2005;1715:111–121. doi: 10.1016/j.bbamem.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Silva L.C., de Almeida R.F., Prieto M. Ceramide-domain formation and collapse in lipid rafts: membrane reorganization by an apoptotic lipid. Biophys. J. 2007;92:502–516. doi: 10.1529/biophysj.106.091876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busto J.V., Sot J., Alonso A. Cholesterol displaces palmitoylceramide from its tight packing with palmitoylsphingomyelin in the absence of a liquid-disordered phase. Biophys. J. 2010;99:1119–1128. doi: 10.1016/j.bpj.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castro B.M., Silva L.C., Prieto M. Cholesterol-rich fluid membranes solubilize ceramide domains: implications for the structure and dynamics of mammalian intracellular and plasma membranes. J. Biol. Chem. 2009;284:22978–22987. doi: 10.1074/jbc.M109.026567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luzzati V. X-ray diffraction studies of lipid-water systems. In: Chapman D., editor. Biological Membranes. Academic Press; London: 1968. pp. 71–123. [Google Scholar]
- 16.Goñi F.M., Alonso A., Thewalt J.L. Phase diagrams of lipid mixtures relevant to the study of membrane rafts. Biochim. Biophys. Acta. 2008;1781:665–684. doi: 10.1016/j.bbalip.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byrner P., Chapman D. Liquid crystalline nature of phospholipids. Nature. 1964;202:987–988. doi: 10.1038/202987a0. [DOI] [PubMed] [Google Scholar]
- 18.Veatch S.L., Keller S.L. Seeing spots: complex phase behavior in simple membranes. Biochim. Biophys. Acta. 2005;1746:172–185. doi: 10.1016/j.bbamcr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Davis J.H., Clair J.J., Juhasz J. Phase equilibria in DOPC/DPPC-d62/cholesterol mixtures. Biophys. J. 2009;96:521–539. doi: 10.1016/j.bpj.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castro B.M., de Almeida R.F., Prieto M. Formation of ceramide/sphingomyelin gel domains in the presence of an unsaturated phospholipid: a quantitative multiprobe approach. Biophys. J. 2007;93:1639–1650. doi: 10.1529/biophysj.107.107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva L.C., Futerman A.H., Prieto M. Lipid raft composition modulates sphingomyelinase activity and ceramide-induced membrane physical alterations. Biophys. J. 2009;96:3210–3222. doi: 10.1016/j.bpj.2008.12.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White S.H., Mirejovsky D., King G.I. Structure of lamellar lipid domains and corneocyte envelopes of murine stratum corneum. An X-ray diffraction study. Biochemistry. 1988;27:3725–3732. doi: 10.1021/bi00410a031. [DOI] [PubMed] [Google Scholar]
- 23.McIntosh T.J. Organization of skin stratum corneum extracellular lamellae: diffraction evidence for asymmetric distribution of cholesterol. Biophys. J. 2003;85:1675–1681. doi: 10.1016/S0006-3495(03)74597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J., Feigenson G.W. A microscopic interaction model of maximum solubility of cholesterol in lipid bilayers. Biophys. J. 1999;76:2142–2157. doi: 10.1016/S0006-3495(99)77369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angelova M.I., Dimitrov D.S. Liposome electroformation. Faraday Discuss. Chem. Soc. 1986;81:303–311. [Google Scholar]
- 26.Pabst G., Rappolt M., Laggner P. Structural information from multilamellar liposomes at full hydration: full q-range fitting with high quality x-ray data. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Topics. 2000;62(3 Pt B):4000–4009. doi: 10.1103/physreve.62.4000. [DOI] [PubMed] [Google Scholar]
- 27.Pabst G. Global properties of biomimetic membranes: perspectives on molecular features. Biophys. Rev. Lett. 2006;1:57–84. [Google Scholar]
- 28.McConnell H.M., Watts T.H., Brian A.A. Supported planar membranes in studies of cell-cell recognition in the immune system. Biochim. Biophys. Acta. 1986;864:95–106. doi: 10.1016/0304-4157(86)90016-x. [DOI] [PubMed] [Google Scholar]
- 29.Simonsen A.C., Bagatolli L.A. Structure of spin-coated lipid films and domain formation in supported membranes formed by hydration. Langmuir. 2004;20:9720–9728. doi: 10.1021/la048683+. [DOI] [PubMed] [Google Scholar]
- 30.Sot J., Ibarguren M., Alonso A. Cholesterol displacement by ceramide in sphingomyelin-containing liquid-ordered domains, and generation of gel regions in giant lipidic vesicles. FEBS Lett. 2008;582:3230–3236. doi: 10.1016/j.febslet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Fidorra M., Duelund L., Bagatolli L.A. Absence of fluid-ordered/fluid-disordered phase coexistence in ceramide/POPC mixtures containing cholesterol. Biophys. J. 2006;90:4437–4451. doi: 10.1529/biophysj.105.077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrer D.C., Maggio B. Phase behavior and molecular interactions in mixtures of ceramide with dipalmitoylphosphatidylcholine. J. Lipid Res. 1999;40:1978–1989. [PubMed] [Google Scholar]
- 33.Veiga M.P., Arrondo J.L., Alonso A. Ceramides in phospholipid membranes: effects on bilayer stability and transition to nonlamellar phases. Biophys. J. 1999;76:342–350. doi: 10.1016/S0006-3495(99)77201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ausili A., Torrecillas A., Gómez-Fernández J.C. Edelfosine is incorporated into rafts and alters their organization. J. Phys. Chem. B. 2008;112:11643–11654. doi: 10.1021/jp802165n. [DOI] [PubMed] [Google Scholar]
- 35.Reference deleted in proof.
- 36.Reference deleted in proof.
- 37.Baumgart T., Hunt G., Feigenson G.W. Fluorescence probe partitioning between Lo/Ld phases in lipid membranes. Biochim. Biophys. Acta. 2007;1768:2182–2194. doi: 10.1016/j.bbamem.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juhasz J., Davis J.H., Sharom F.J. Fluorescent probe partitioning in giant unilamellar vesicles of ‘lipid raft’ mixtures. Biochem. J. 2010;430:415–423. doi: 10.1042/BJ20100516. [DOI] [PubMed] [Google Scholar]
- 39.Fanani M.L., Maggio B. Phase state and surface topography of palmitoyl-ceramide monolayers. Chem. Phys. Lipids. 2010;163:594–600. doi: 10.1016/j.chemphyslip.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Maulik P.R., Shipley G.G. N-palmitoyl sphingomyelin bilayers: structure and interactions with cholesterol and dipalmitoylphosphatidylcholine. Biochemistry. 1996;35:8025–8034. doi: 10.1021/bi9528356. [DOI] [PubMed] [Google Scholar]
- 41.Mannock D.A., McIntosh T.J., McElhaney R.N. Effects of natural and enantiomeric cholesterol on the thermotropic phase behavior and structure of egg sphingomyelin bilayer membranes. Biophys. J. 2003;84:1038–1046. doi: 10.1016/S0006-3495(03)74920-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Manyes S., Redondo-Morata L., Sanz F. Nanomechanics of lipid bilayers: heads or tails? J. Am. Chem. Soc. 2010;132:12874–12886. doi: 10.1021/ja1002185. [DOI] [PubMed] [Google Scholar]
- 43.Redondo-Morata L., Oncins G., Sanz F. Force spectroscopy reveals the effect of different ions in the nanomechanical behavior of phospholipid model membranes: the case of potassium cation. Biophys. J. 2012;102:66–74. doi: 10.1016/j.bpj.2011.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seeger H.M., Di Cerbo A., Facci P. Supported lipid bilayers on mica and silicon oxide: comparison of the main phase transition behavior. J. Phys. Chem. B. 2010;114:8926–8933. doi: 10.1021/jp1026477. [DOI] [PubMed] [Google Scholar]
- 45.Scheffer L., Solomonov I., Addadi L. Structure of cholesterol/ceramide monolayer mixtures: implications to the molecular organization of lipid rafts. Biophys. J. 2005;88:3381–3391. doi: 10.1529/biophysj.104.051870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kan C.C., Ruan Z.S., Bittman R. Interaction of cholesterol with sphingomyelin in bilayer membranes: evidence that the hydroxy group of sphingomyelin does not modulate the rate of cholesterol exchange between vesicles. Biochemistry. 1991;30:7759–7766. doi: 10.1021/bi00245a013. [DOI] [PubMed] [Google Scholar]
- 47.Obeid L.M., Linardic C.M., Hannun Y.A. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 48.Montes L.R., López D.J., Alonso A. Ceramide-enriched membrane domains in red blood cells and the mechanism of sphingomyelinase-induced hot-cold hemolysis. Biochemistry. 2008;47:11222–11230. doi: 10.1021/bi801139z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee H., Rotolo J.A., Kolesnick R. Mitochondrial ceramide-rich macrodomains functionalize Bax upon irradiation. PLoS ONE. 2011;6:e19783. doi: 10.1371/journal.pone.0019783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martínez-Abundis E., Correa F., Zazueta C. Bax distribution into mitochondrial detergent-resistant microdomains is related to ceramide and cholesterol content in postischemic hearts. FEBS J. 2009;276:5579–5588. doi: 10.1111/j.1742-4658.2009.07239.x. [DOI] [PubMed] [Google Scholar]
- 51.Weerheim A., Ponec M. Determination of stratum corneum lipid profile by tape stripping in combination with high-performance thin-layer chromatography. Arch. Dermatol. Res. 2001;293:191–199. doi: 10.1007/s004030100212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


