Abstract
Ischemia/reperfusion injury is the leading cause of acute tubular necrosis. Nitric oxide has a protective role against ischemia/reperfusion injury; however, the role of asymmetric dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide synthase, in ischemia/reperfusion injury remains unclear. ADMA is produced by protein arginine methyltransferase (PRMT) and is mainly degraded by dimethylarginine dimethylaminohydrolase (DDAH). Here we examined the kinetics of ADMA and PRMT and DDAH expression in the kidneys of ischemia/reperfusion-injured mice. After the injury, DDAH-1 levels were decreased and renal and plasma ADMA values were increased in association with renal dysfunction. Renal ADMA was correlated with 8-hydroxy-2′-deoxyguanosine, a marker of oxidative stress. An antioxidant, N-acetylcysteine, or a proteasomal inhibitor, MG-132, restored these alterations. Infusion of subpressor dose of ADMA exacerbated renal dysfunction, capillary loss, and tubular necrosis in the kidneys of ischemia/reperfusion-injured wild mice, while damage was attenuated in DDAH transgenic mice. Thus, ischemia/reperfusion injury–induced oxidative stress may reduce DDAH expression and cause ADMA accumulation, which may contribute to capillary loss and tubular necrosis in the kidney.
Keywords: ADMA, DDAH-1, ischemia/reperfusion injury, oxidative stress, renal capillary loss
Acute kidney injury (AKI) occurs in 5% of all hospitalized patients, and it is associated with 25 to over 90% of mortality in these subjects.1 A large cohort analysis has shown that AKI is associated with an odds ratio of death of 5.5.2 Ischemia/reperfusion (IR) injury is the leading cause of AKI/acute tubular necrosis (ATN), for which no specific therapy is currently available.3 Therefore, a further understanding of the pathophysiological mechanism of AKI will enable the design and development of therapeutic approaches for this devastating disorder.
Although various vasoactive factors and cytokines have been shown to have a role in AKI,4, 5 endothelial dysfunction and capillary loss due to reduced production and/or impaired function of nitric oxide (NO) are considered to be the key components that could elicit ATN and the progression of renal IR injury.6 Indeed, increased reactivity to vasoconstrictive agents and decreased vasodilatory responses were observed in the arterioles of the postischemic kidney.7 Peritubular capillary (PTC) loss was positively associated with tubular damage in the kidney of ischemic AKI, both of which were ameliorated by transplanted endothelial cell.8 NO precursor L-arginine has been shown to improve the postischemic AKI in rats,9 whereas inhibition of NO synthase (NOS) exacerbates the renal I/R injury.10 Treatment with NO donor has exerted remarkable protective effects against the postischemic AKI.11, 12 Further, Satake et al.13 found that estrogen not only augmented renal blood flow but also attenuated renal injury induced by IR in rats via activation of endothelial NOS (eNOS). These findings suggest the protective role of endothelium-derived NO against renal IR injury.
Asymmetric dimethylarginine (ADMA) is a degradation product of methylated protein, which is produced and metabolized by the enzymes protein arginine methyltrasnferase (PRMT) and dimethylarginine dimethylaminohydrolase (DDAH), respectively.14 It is a potent endogenous inhibitor of NOS, thus being involved in endothelial dysfunction, arterial stiffness, and renal injury.14, 15, 16, 17 Moreover, plasma ADMA level has been shown to be a predictor for graft failure, renal and cardiovascular events, and all-cause mortality in kidney transplant patients.18 As expression and/or activity of PRMT and DDAH are regulated by reactive oxygen species generation19 and oxidative stress has a role in IR injury,20, 21 it is conceivable that ADMA generation in the kidney is increased under oxidative stress conditions of IR injury and that beneficial effects of NO on renal microvasculature are compromised, which could lead to the development and progression of AKI. To address the issues, in this study, we first examined the kinetics of ADMA, and PRMT-1 and DDAH-1 levels in the kidney of IR-injured mice. Then, we investigated the effects of continuous infusion of subpressor dose of ADMA on renal IR injury in wild mice and also studied whether IR injury was attenuated in DDAH-1-overexpressed transgenic (DDAH-1 Tg) mice.
RESULTS
Renal function, ADMA values, and expression levels of DDAH-1 and PRMT-1 in IR-injured mice
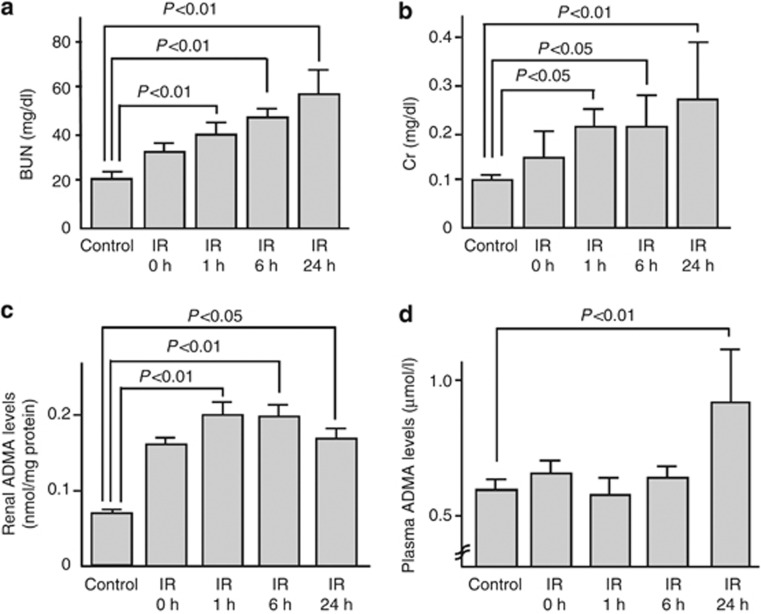
Compared with the control mice, serum blood urea nitrogen (BUN) and creatinine (Cr) levels were significantly elevated after IR injury (Figure 1a and b). As shown in Figure 1c, renal ADMA levels in IR-injured mice were increased in a bell-shaped manner; the values reached a maximum at 1 h after the IR injury and still significantly higher at 24 h compared with those of controls. Further, plasma levels of ADMA at 24 h after the injury were elevated to about two-fold of those of control mice (Figure 1d).
Figure 1.
Renal function and asymmetric dimethylarginine (ADMA) values in ischemia/reperfusion (IR)-injured mice. (a) Plasma blood urea nitrogen (BUN), (b) creatinine (Cr), (c) renal, and (d) plasma levels of ADMA in control (n=18) or IR-injured wild mice (n=48, including IR 0 h (n=12)). Mice were subjected to 45 min of bilateral renal ischemia. Reperfusion was allowed by means of clips removal for 0 h (n=12), 1 h (n=6), 6 h (n=8), and 24 h (n=22). Control mice were sham-operated (n=18). ADMA levels were measured by high-performance liquid chromatography.
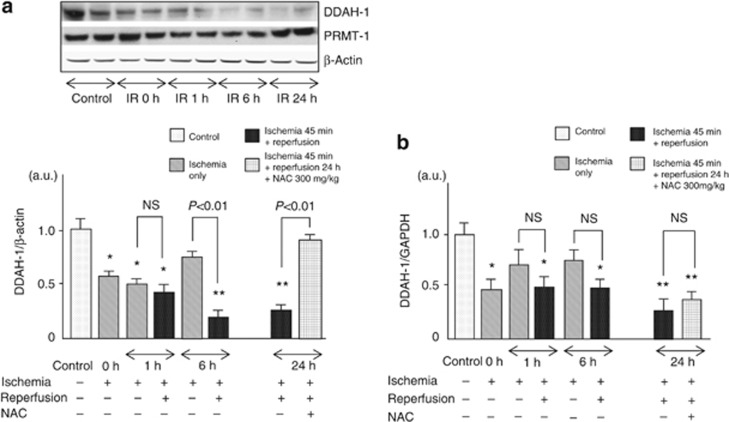
Western blot analysis revealed that renal levels of DDAH-1 were suppressed in a time-dependent manner after IR injury. DDAH-1 expression levels began to decrease at 1 h post reperfusion and reached a nadir at 6 h after IR injury; the values were about 1/4 of those of control mice (Figure 2a). Although PRMT-1 levels had a tendency to increase after IR injury, it was modest but not significant. In contrast to the response to IR, although ischemia for 45 min (0 h) and a further 15 min (1 h) decreased DDAH-1 expression, no further decrease in DDAH-1 levels was observed as a function of ischemia time; there was a significant difference of DDAH-1 levels at 6 h between the ischemia-only group and the IR-injured group (Figure 2a).
Figure 2.
Renal dimethylarginine dimethylaminohydrolase (DDAH)-1 and protein arginine methyltransferase (PRMT)-1 levels after ischemia with (n=36) or without reperfusion injury (n=24, including ischemia/reperfusion (IR) 0 h (n=12)). Wild mice (n=36) were subjected to 45 min of bilateral renal ischemia with reperfusion. Then, reperfusion was allowed by means of clips removal for 1 h (n=6), 6 h (n=8), and 24 h (n=22). Control mice were sham-operated (n=18). To examine the effects of ischemia only, wild mice (n=24) were subjected to 45 min (0 h, n=12), 1 h (n=6), and 6 h (n=6) of bilateral renal ischemia. N-acethylcysteine (NAC; 300 mg/kg of body weight) was administered intraperioneally at 1 day and 15 min before IR injury (n=6). The results of densitometric analysis are presented as a fold change compared with control mice. (a) Upper panel shows the representative bands of western blots. Lower panel shows the quantitative data. Data were normalized by the intensity of β-actin and related to the value of the control. (b) DDAH-1 mRNA levels after ischemia with or without reperfusion injury. *P<0.05 and **P<0.01 compared with control, respectively. a.u., arbitrary units; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NS, not significant.
Real-time reverse transcription PCR (RT-PCR) analysis showed that mRNA levels of DDAH-1 were rapidly decreased by ischemia itself (0 h), and no further decrease in DDAH-1 mRNA levels was observed. Moreover, pretreatment with N-acethylcysteine (NAC) did not restore the decrease in DDAH-1 mRNA levels in the IR-injured kidney (Figure 2b).
Relationship between renal ADMA and 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels
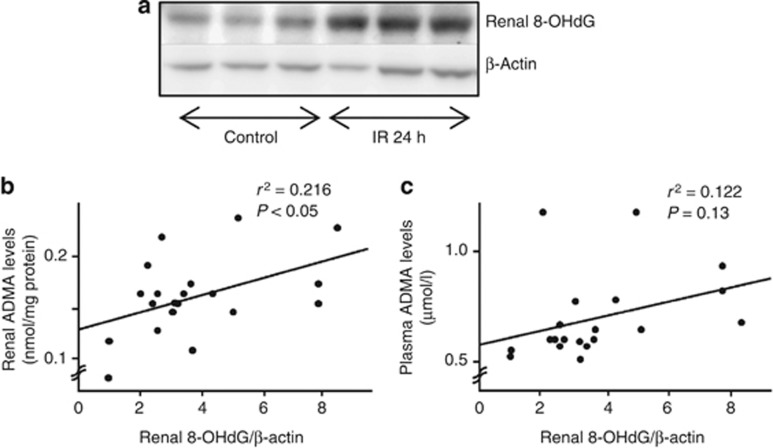
As shown in Figure 3a, renal levels of 8-OHdG, a marker of oxidative stress, were increased after IR injury. Although plasma ADMA levels were not correlated with renal 8-OHdG, there was a significant positive association between ADMA and 8-OHdG in the kidney of IR-injured mice (Figure 3b and c).
Figure 3.
Relationship between asymmetric dimethylarginine (ADMA) and 8-hydroxy-2′-deoxyguanosine (8-OHdG). Western blot analyses for 8-OHdG in the kidney of (a) ischemia/reperfusion (IR)-injured wild mice and its correlations with (b) renal (n=20) or (c) plasma levels of ADMA (n=20). ADMA levels were measured by high-performance liquid chromatography. Linear regression analysis was performed between renal or plasma levels of ADMA and renal levels of 8-OHdG.
Effects of NAC infusion on renal IR injury
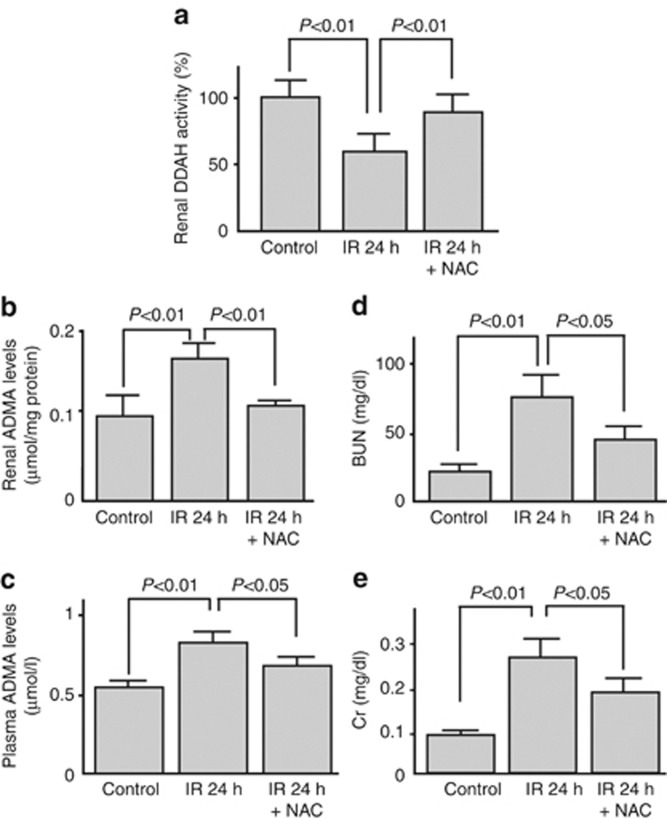
As the oxidative stress marker significantly correlated with renal ADMA levels (Figure 3b), we next investigated whether administration of the antioxidant NAC before the ischemic insult could block the IR injury. As shown in Figures 2a and 4, pretreatment of NAC significantly restored the decreased DDAH-1 levels and activity in the IR-injured kidney, which was associated with reduction of renal and plasma ADMA values and improvement of renal dysfunction.
Figure 4.
Effect of N-acethylcysteine (NAC) infusion on renal ischemia/reperfusion (IR) injury. Effect of an antioxidant, NAC, on (a) dimethylarginine dimethylaminohydrolase (DDAH) enzymatic activity, (b) renal, and (c) plasma levels of asymmetric dimethylarginine (ADMA) and (d, e) renal function. Wild mice (n=22) were subjected to 45 min of bilateral renal ischemia. Then, reperfusion was allowed by means of clips removal for 24 h. NAC (300 mg/kg of body weight) was administered intraperioneally at 1 day and 15 min before IR injury (n=6). Sham-operated mice (n=18) were used as a control. BUN, blood urea nitrogen; Cr, creatinine.
Mechanisms of renal DDAH-1 alteration in IR injury
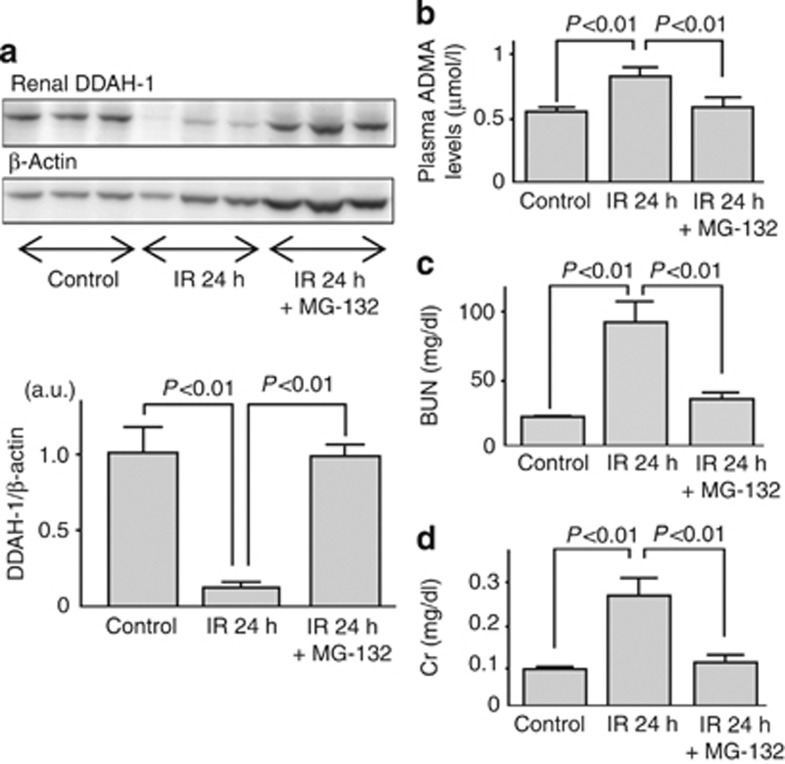
As shown in Figure 5, a proteasomal inhibitor, MG-132, treatment significantly restored the decreased DDAH-1 levels, which was associated with reduction of plasma ADMA levels and improvement of renal dysfunction.
Figure 5.
Mechanism of renal dimethylarginine dimethylaminohydrolase (DDAH)-1 alteration in ischemia/reperfusion (IR) injury. Effects of a proteasomal inhibitor MG-132 on (a) dimethylarginine dimethylaminohydrolase (DDAH)-1 levels, (b) plasma asymmetric dimethylarginine (ADMA) levels, and (c, d) renal function. Wild mice (n=11) were subjected to 45 min of bilateral renal ischemia. Then, reperfusion was allowed by means of clips removal for 24 h. Mice were treated with MG-132 (n=5) or dimethyl sulfoxide (n=6) before IR. Sham-operated mice were used as a control (n=18). (a) Upper panel shows the representative bands of western blots. Lower panel shows the quantitative data. Data were normalized by the intensity of β-actin and related to the value of the control. The results of densitometric analysis are presented as a fold change compared with control mice. a.u., arbitrary units; BUN, blood urea nitrogen; Cr, creatinine.
Effect of ADMA infusion and DDAH-1 overexpression on renal IR injury
To further elucidate the pathophysiological role of ADMA in renal IR injury, we first examined the effects of continuous infusion of subpressor dose of ADMA on IR-induced renal damage. As shown in Table 1, infusion of ADMA actually increased plasma ADMA levels and resultantly exacerbated renal dysfunction in IR-injured wild mice. Therefore, we next investigated whether IR injury was attenuated in DDAH-1 Tg mice. Although baseline levels of BUN and Cr were similar between control and DDAH-1 Tg mice, plasma ADMA levels and systolic blood pressure were siginificantly lower in DDAH-1 Tg mice compared with control mice (Table 1). Both elevation of plasma ADMA levels and renal dysfunction induced by IR injury were significantly prevented in DDAH-1 Tg mice. We confirmed that the expression levels of DDAH-1 in the kidney of DDAH-1 Tg mice was 1.5-fold higher than those of wild mice, which were unaffected by IR injury.
Table 1. Effect of ADMA infusion and DDAH-1 overexpression on renal IR injury.
| Characteristics of animals | Control mice (n=18) | DDAH-1Tg control mice (n=6) | IR-injured wild mice (n=22) | IR-injured ADMA-infused mice (n=10) | IR-injured DDAH-1 Tg mice (n=6) |
|---|---|---|---|---|---|
| Plasma ADMA (μmol/l) | 0.57±0.06 | 0.46±0.05* | 0.82±0.2** | 1.70±0.48**,# | 0.50±0.06# |
| Systolic blood pressure (mm Hg) | 112±5.5 | 103±8.1* | 111±7.3 | 110±7.4 | 101±8.2**,# |
| Mean blood pressure (mm Hg) | 74±7.4 | 75±7.4 | 77.6±7.1 | 72.3±11.0 | 70.0±3.4# |
| BUN (mg/dl) | 21.9±2.7 | 23.2±6.3 | 59.7±22.2** | 96.0±31.8**,# | 31.2±11.6# |
| Cr (mg/dl) | 0.10±0.03 | 0.10±0.02 | 0.26±0.13** | 0.37±0.1**,# | 0.15±0.74# |
Abbreviations: ADMA, asymmetric dimethylarginine; BUN, blood urea nitrogen; Cr, creatinine; DDAH, dimethylarginine dimethylaminohydrolase; DDAH-1Tg, DDAH-1 transgenic; IR, ischemia/reperfusion.
*P<0.05 and **P<0.01 compared with control mice, respectively; #P<0.05 compared with IR-injured wild mice.
Effects of IR-mediated DDAH–ADMA alteration on eNOS activity
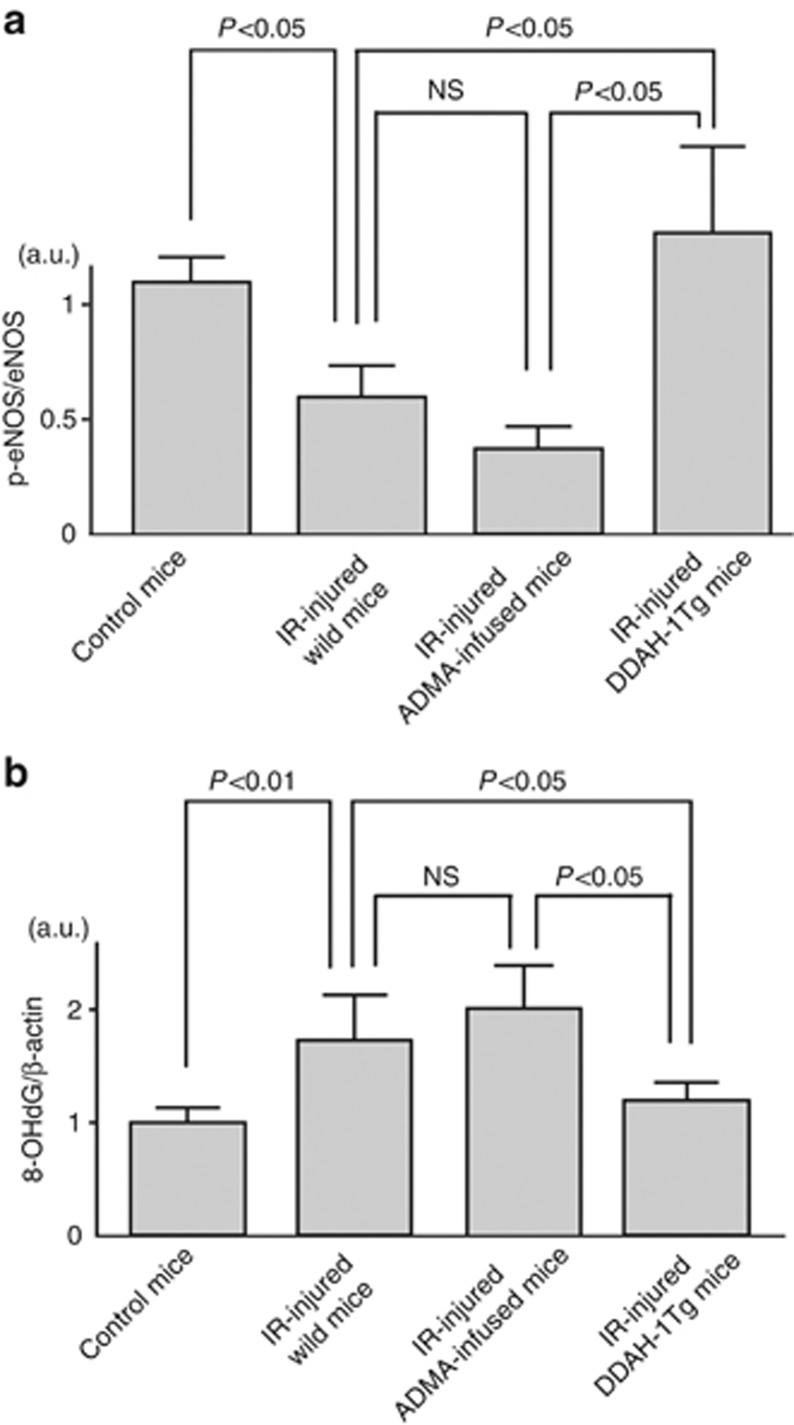
To confirm that increased ADMA could actually inhibit eNOS activity, we examined total and phosphorylated eNOS levels in the IR-injured kidney. Although IR did not affect total eNOS levels, the ratio of phosphorylated to total eNOS, a marker of eNOS activity,22 was significantly decreased by IR (Figure 6a). Infusion of ADMA to IR-injured mice further reduced the ratio, which was completely restored in DDAH-1 Tg mice (Figure 6a).
Figure 6.
Endothelial nitric oxide synthase (eNOS) phosphorylation and 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels in the kidney of control, ischemia/reperfusion (IR)-injured wild, asymmetric dimethylarginine (ADMA)-infused, or dimethylarginine dimethylaminohydrolase (DDAH)-1 gene-overexpressing mice. (a) The ratio of phosphorylated (Ser1177) to total eNOS levels were mesasured with western blot analysis. n=6 for each group. (b) Western blot analyses for 8-OHdG in the kidney in each group. Data were normalized by the intensity of β-actin. n=6 for each group. The results of densitometric analysis were presented as a fold change compared with control mice. a.u., arbitrary units; DDAH-1Tg, DDAH-1 transgenic; ; p-eNOS, phosphorylated eNOS; NS, not significant.
Effects of ADMA infusion and DDAH-1 overexpression on oxidative stress and IR-induced PTC and tubular damages
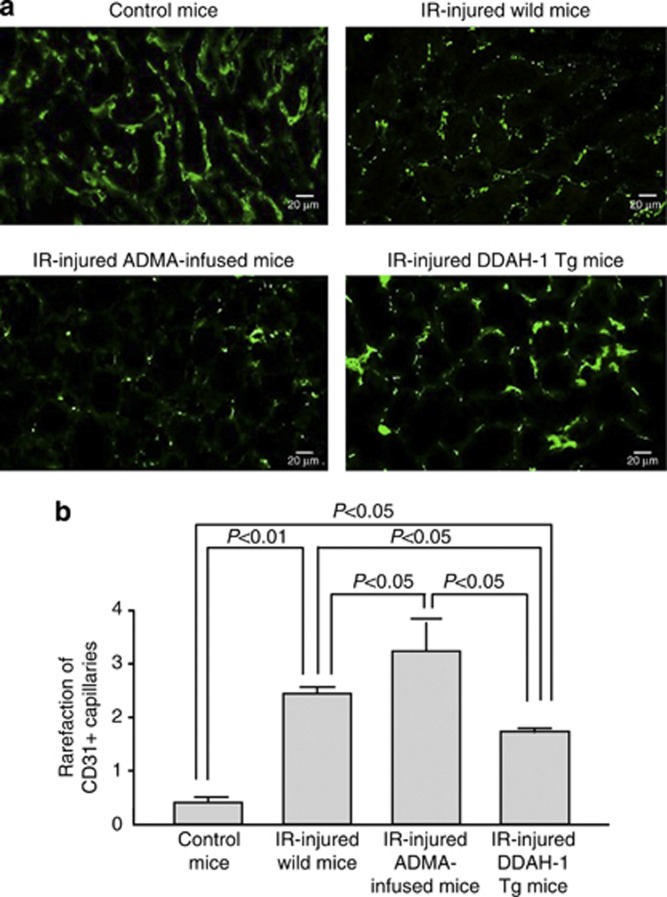
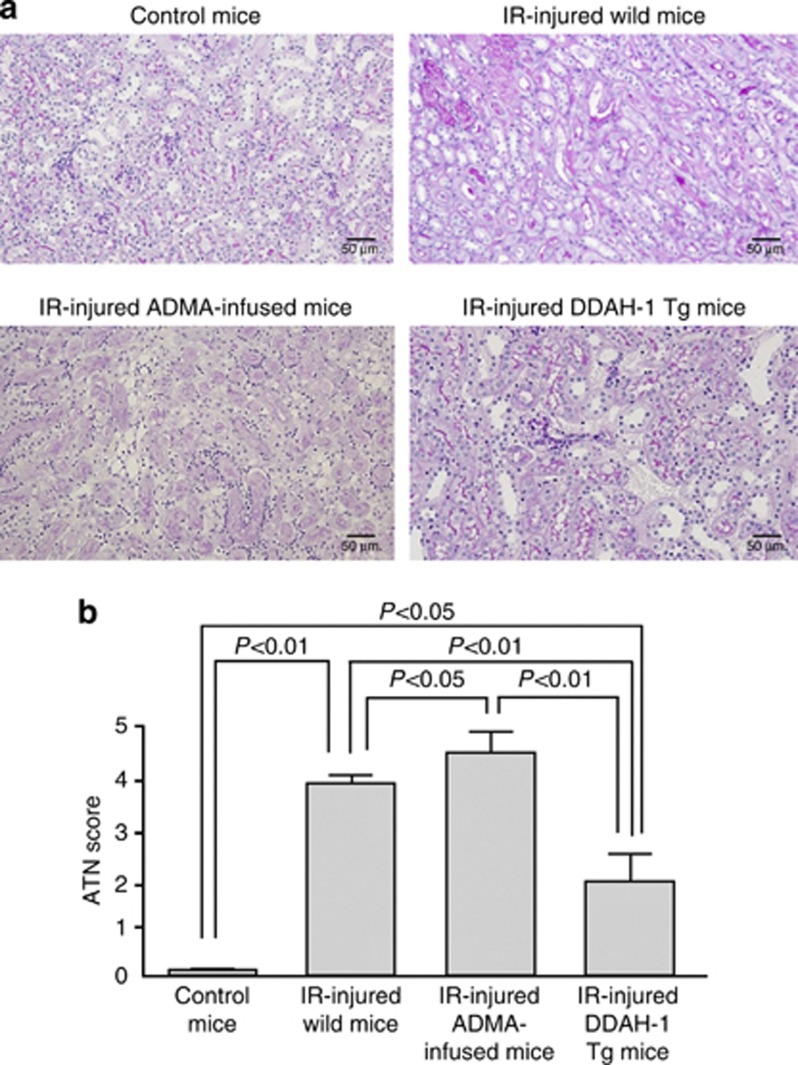
We next examined the role of ADMA in IR-induced oxidative stress generation and renal capillary and tubular damages. ADMA infusion tended to increase 8-OHdG accumulation in the IR-injured kidney, which was completely suppressed in DDAH-1 Tg mice (Figure 6b). As shown in Figure 7, consistent with previously reports,8, 23 capillary density assessed by CD31 staining was markedly reduced in IR-induced renal injury. Further, infusion of ADMA augmented the IR-induced PTC loss, whereas these IR-induced damages were significantly attenuated in DDAH-1 Tg mice. In addition, as shown in Figure 8, cellular necrosis, loss of brush border, cast formation, vacuolization, and tubule dilation were observed in IR-induced renal injury. These changes were significantly enhanced by ADMA infusion, whereas they were attenuated in DDAH-1 Tg mice.
Figure 7.
Effects of asymmetric dimethylarginine (ADMA) infusion and dimethylarginine dimethylaminohydrolase (DDAH)-1 overexpression on ischemia/reperfusion (IR)-induced peritubular capillary loss. (a) The upper panel shows the representative microphotographs of CD31-positive capillaries. (b) The lower panel shows the quantitative data. Magnification × 400. Staining for CD31 was performed on frozen kidney sections to evaluate endothelial injury and capillary rarefaction. Control kidneys demonstrated a very homogenous staining pattern. IR-injured kidneys demonstrated the absence of CD31 staining in many areas. ADMA infusion further reduced the CD31 staining, which was prevented in DDAH-1 transgenic (DDAH-1 Tg) mice. Rarefaction of CD31-positive capillaries was assessed semiquantitatively using a score from 0 to 4. A higher score, therefore, reflects a higher degree of capillary loss.
Figure 8.
Effects of asymmetric dimethylarginine (ADMA) infusion and dimethylarginine dimethylaminohydrolase (DDAH)-1 overexpression on ischemia/reperfusion (IR)-induced acute tubular necrosis (ATN). (a) The upper panel shows the representative renal histology with periodic acid–Schiff (PAS) staining. (b) The lower panel shows the quantitative data. Magnification × 200. The renal histology stained with PAS reagent. Renal damage included detachment of epithelial cells of the tubuli, interstitial edema, and many tubular cell casts. A higher score, therefore, reflects a higher degree of renal injury. IR-injured kidneys increased the intensity of renal damage. ADMA infusion further augmented the intensity, which was prevented in DDAH-1 transgenic (DDAH-1 Tg) mice.
DISCUSSION
The salient findings of this study are as follows: (1) decreased renal levels of DDAH-1 associated with increased levels of plasma and renal ADMA were observed in IR-injured mice; (2) renal levels of 8-OHdG, a marker of oxidative stress, were positively correlated to those of ADMA; (3) treatment with an antioxidant, NAC, or an inhibitor of proteosomal degradation, MG-132, restored the IR-induced decrease in DDAH-1 levels and increase in renal and plasma ADMA values; (4) continuous infusion of subpressor dose of ADMA actually increased plasma ADMA levels, decreased the ratio of phosphorylated eNOS to total eNOS levels, and further exacerbated renal dysfunction, PTC loss, and ATN in IR-injured mice; and (5) these IR-induced renal damages were significantly blocked in DDAH-1 Tg mice. Therefore, our present findings suggest that IR could elicit tubular damage and thereby contribute to the development of AKI by elevating ADMA levels in the kidney.
ADMA is generated by PRMT, whereas it is mainly degraded by DDAH.14 In this study, DDAH-1 expression was markedly suppressed in IR-injured kidneys, whereas the effects of IR on renal PRMT-1 expression were modest. Therefore, suppression of DDAH-1 rather than upregulation of PRMT-1 may be mainly involved in the elevation of renal and subsequent plasma levels of ADMA in IR-injured mice. There is accumulating evidence that DDAH expression is suppressed under oxidative stress conditions.21, 24 Indeed, we have recently found that advanced glycation end products, senescent macroprotein derivatives formed acceleratedly under diabetes, decrease DDAH-1 expression and subsequently increase ADMA generation in renal proximal tubular cells via oxidative stress generation.21 Homocysteine-induced oxidative stress increases ADMA generation in endothelial cells via the reduction of DDAH expression as well.24 Further, Luo et al.25 reported that angiotensin II increased cellular ADMA levels via NADPH-derived reactive oxygen species generation in cultured smooth muscle cells by reducing DDAH expression and activity. As renal levels of 8-OHdG were correlated with those of renal, but not plasma, levels of ADMA (Figure 3b and c), and NAC pretreatment completely restored the decreased levels of DDAH-1 (Figure 2a), the present study suggests that IR injury–induced oxidative stress generation could stimulate renal production of ADMA via suppression of DDAH-1.
There is accumulating evidence to show the active participation of endothelial dysfunction in the progression of renal IR injury.26 Reactivity to vasoconstrictive agents is increased, whereas responsiveness to vasodilators is impaired in the arterioles of postischemic kidney.7 Furthermore, NO donors have been shown to exert beneficial effects on IR-induced renal injury.11, 23 In this study, the ratio of phosphorylated to total eNOS, a marker of eNOS activity,22 was decreased in the IR-injured kidney. Infusion of subpressor dose of ADMA tended to decrease the ratio and increase 8-OHdG, and it further exacerbated renal dysfunction in IR-injured mice, all of which were completely blocked in DDAH-1 Tg mice. These observations suggest that, although the ratio of phosphorylated (ser1177) to total eNOS could not necessarily reflect total enzymatic activity of eNOS,22, 27 IR injury–induced oxidative stress generation could stimulate renal production of ADMA and subsequently inactivate eNOS via suppression of DDAH-1, which may lead to renal injury during IR. Therefore, our results are consistent with the possibility that NO levels are decreased along with changes in ADMA/DDAH, but direct measurements are needed to determine whether this is true, because renal levels of NO are increased rather than decreased during ischemia.28, 29 Further, we also confirmed that urinary excretion levels of cyclic guanosine monophosphate, another marker of renal production of NO,30 were significantly decreased in the IR-injured kidney (control vs. IR-injured mice; 10.1±1.22 vs. 0.34±0.24 nmol/mg Cr, P<0.01). However, there is another possibility to explain DDAH-1 decrease and renal increase in ADMA levels in the IR-injured kidney. Caglar et al.31 found a significant association between ADMA and proteinuria in CKD stage 1 patients, and improvement of proteinuria by blockade of the renin–angiotensin system has been shown to decrease ADMA levels in patients with CKD.32 Because DDAH is abundantly expressed in tubular cells, which might be inactivated by proteinuria-elicited oxidative stress, proteinuria could reduce tubular DDAH activity and subsequently enhance ADMA accumulation. Indeed, we have previously shown that proteinuria is associated with ADMA levels in an adriamycin-treated rat model of nephrotic syndrome.19 In this study, urinary albumin excretion levels were elevated by IR injury (control vs. IR-injured mice; 0.07±0.02 vs. 4.26±1.50 mg/mg Cr, P<0.01). Thus, although we could not examine here the relationship between albuminuria and renal ADMA levels because of the lack of samples, albuminuria might cause ADMA generation in the IR-injured kidney.
In the present study, we demonstrated for the first time that a proteasomal inhibitor, MG-132, treatment significantly restored the decrease in renal DDAH-1 levels, which was associated with a reduction of plasma ADMA levels and improvement of renal dysfunction (Figure 5). The proteasomal degradation system is activated under oxidative stress conditions,33 which could lead to the maintenance of cellular homeostasis by removing damaged, oxidized, and misfolded proteins.34 Therefore, the present findings suggest that protein degradation rather than gene suppression might be involved in DDAH-1 reduction in the IR-injured kidney. As DDAH is downregulated by cytokines, including tumor necrosis factor-α, MG-132 may be increasing DDAH-1 by suppressing the inflammation.35, 36 Several papers have reported that proteosomal inhibitors have a protective role against IR-induced renal dysfunction.37, 38 Therefore, suppression of proteasomal degradation of DDAH-1 might be a novel therapeutic target for preventing the IR injury in the kidney.
Our present study suggests that IR injury–induced oxidative stress generation may reduce DDAH-1 expression and resultantly cause ADMA accumulation, which could lead to capillary loss and tubular necrosis in the kidney, thereby being involved in renal IR injury. Recent evidence has suggested that, in addition to direct tubular cell damage, injury to the renal microvasculature also has a role in the development and progression of ischemic AKI.5 In particular, PTC loss could induce hypoxia and inflammatory reactions in the tubulointerstitium, which could promote the scarring and fibrotic processes in the damaged kidney.39 Indeed, a close correlation between progressive interstitial injury and the loss of PTC was shown in animal models of ischemic AKI.8, 23 In this study, infusion of ADMA enhanced the IR-induced PTC loss and ATN, whereas DDAH-1 Tg mice were resistant to IR and failed to exhibit PTC loss and ATN. NO inhibits apoptosis of endothelial cells40 and increases their proliferation and migration.41, 42 In addition, DDAH transfection into cultured endothelial cells enhances vascular endothelial growth factor mRNA expression and stimulates tube formation of these cells.43 Moreover, in a murine model of hindlimb ischemia, enhanced neovascularization and limb perfusion were observed in DDAH-1 Tg mice, which was associated with reduced plasma levels of ADMA.44
In summary, the present study has demonstrated for the first time that IR-elicited oxidative stress might contribute to the development and progression of AKI by promoting PTC loss and tubular necrosis through the elevation of ADMA in the kidney, probably via oxidative stress–induced proteosomal degradation of DDAH-1. Substitution of DDAH-1 protein or enhancement of its activity may become a novel therapeutic strategy for the treatment of renal IR injury.
MATERIALS AND METHODS
Animal preparation
Eight-week-old male C57BL/6J (wild) mice and DDAH-1 Tg mice were used. Mice (n=117) were subjected to ketamine anesthesia (45 mg/kg intraperitoneally, Sankyo, Tokyo, Japan), and then to 45 min of bilateral renal ischemia by means of occlusion of bilateral renal pedicle using vascular clips. Next, reperfusion was allowed for 1 h (n=6), 6 h (n=8), or 24 h (n=22). To examine the effects of ischemia only, wild mice (n=24) were subjected to 45 min (0 h, n=12), 1 h (n=6), and 6 h (n=6) of bilateral renal ischemia. In a second set of experiments, NAC (n=6) (Nakarai Tesque, Kyoto, Japan) was administered intraperitoneally 1 day (300 mg/kg of body weight) and 15 min (300 mg/kg of body weight) before the vascular clipping.45 In a third set of experiments, a proteasomal inhibitor MG-132 (n=5) (Calbiochem, Billerica, MA) or dimethyl sulfoxide (n=6) (Sigma, St Louis, MO) was administered 48 h (2 mg/kg body weight) and 24 h (1 mg/kg body weight) before the clipping.38 Control mice (n=18) were sham-operated with mobilization of kidney but no clamping of renal pedicles. Then, the mice were killed, plasma samples were obtained from each mice by retro-cardiac puncture under general anesthesia, and the kidneys were removed. Both plasma and kidney samples were frozen in liquid N2 and stored at −80 °C before using them for biochemical, western blot, immunofluorescence, or immunohistochemical analyses.
In a fourth set of experiments, 8-week-old male mice and human DDAH-1 Tg mice (n=12) were used. DDAH-1 Tg mice on the C57BL/6J background were purchased from Charles River (Charles River Laboratories Germany, Sulzfeld, Germany). Offspring were screened for transgene expression by PCR, as described previously.46 IR injury was performed in DDAH-1 Tg mice (n=6) and wild mice with (n=10) or without (n=22) ADMA infusion (0.0125 mg/kg/min).47 Saline or ADMA (Sigma) was infused via an implanted osmotic mini-pump (Alzet 1003D; DURECT, Cupertino, CA). The pump was placed into the subcutaneous space of the mice anesthetized with ketamine through a small incision in the back of the neck. Twenty-four hours after the implanted pump, mice were subjected to ketamine anesthesia, and then subjected to 45 min of bilateral renal ischemia. One day after the reperfusion, blood pressure (BP) was measured using a tail-cuff sphygmomanometer using an automated system with a photoelectric sensor (BP-98A; Softron, Tokyo, Japan). Next, the mice were killed. All experimental procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the ethical committee of our institution.
Chemical analysis
Renal and plasma levels of ADMA were measured by high-performance liquid chromatography as described previously.48 Serum BUN and Cr levels were measured with commercially available methods as described previously.49
Western blot analysis
The whole kidney tissues were homogenized and lysed with 25 mmol/l Tris–HCl (pH7.4) containing 1% Triton X-100, 0.1% sodium dodecyl sulfate (SDS), 2 mmol/l EDTA, and 1% protease inhibitor cocktail (Nakarai Tesque). Then, the supernatant was separated by SDS–PAGE (polyacrylamide gel electrophoresis) and transferred to PVDF membranes (Bio-Rad, Hercules, CA). The membranes were then blotted with anti-DDAH-1, anti-PRMT-1, anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA), anti-8-OHdG (Rockland Immunochemicals, Gilbertsville, PA), and anti-phosphorylated eNOS, anti-eNOS (Cell Signaling, Danvers, MA) antibodies. The aliquot of tissue homogenate was subjected to immunoblotting using a primary antibody (1:500 dilution) and a peroxidase-conjugated anti-goat, anti-rabbit, or anti-mouse secondary antibody (1:2000 dilution). The immune complexes were visualized with an enhanced chemiluminescence detection system (Amersham Bioscience, Buckinghamshire, UK).
Real-time RT-PCR
DDAH-1 gene expression levels were determined by quantitative real-time RT-PCR with Fast Real-Time PCR System and TaqMan Gene Expression Master Mix (Applied Biosystems, Foster City, CA) according to the manufacturer's protocols. GAPDH (glyceraldehydes-3-phosphate dehydrogenase) was used as an internal control. Applied primers and TaqMan probes were as follows: DDAH-1 Mm01319453_m1, GAPDH Mm99999915_g1 (Applied Biosystems).
DDAH activity
Total DDAH enzymatic activity was measured as described previously.50 In brief, homogenized kidney tissue (100 μl) was incubated with 0.5 mmol/l ADMA for 2.5 h at 37 °C, following preincubation with 50 units of urease for 15 min at 37 °C to remove urea. The reaction was stopped by the addition of equal volume of 10% trichloroacetic acid, and the supernatant was boiled with diacetyl monoxime (0.8% (wt/vol)) in 5% acetic acid) and antipyrine (0.5% (wt/vol) in 50% sulfuric acid). The amounts of L-citrulline formed were determined with the spectrophotometric analysis at 466 nm.
Immunofluorescence studies
Frozen tissues were sectioned at 4-μm intervals, fixed with acetone for 5 min, and mounted on glass slides. The sections were incubated with blocking reagent (Dako, Glostrup, Denmark) for 1 h and incubated overnight at 4 °C with rat monoclonal antibody raised against mouse CD31, a marker of endothelial cells (BD Pharmingen, Franklin Lakes, NJ) (1:50 dilution), as described previously.51 The sections were then incubated with Alexa-Flour 488 anti-rat antibody (Cell Signaling) (1:750 dilution) for 4 h at room temperature. Rarefaction of CD31-positive capillaries was assessed semiquantitatively by fluorescence intensity using a score from 0 to 4. A score of 0 was assigned if a homogenous positivity of all peritubular capillaries was present (normal finding). Scoring was 1 if a single CD31-negative segment was detected, 2 if CD31 was negative in up to 25% of the tissue section, 3 if negative in 25–50%, and 4 if CD31 staining was completely absent. A higher score, therefore, reflects a higher degree of capillary loss. PTC rarefaction was evaluated in at least 10 cortical fields.51, 52
Renal histological analysis
Kidneys were obtained from each mice, cut transversally, fixed in Bouin's solution, followed by 10% buffered formalin, and embedded in paraffin. Three-micrometer paraffin sections stained with periodic acid–Schiff were used for morphology analysis of kidneys with light microscopy. A semiquantitative score for ATN was assigned as described by Wang et al.53 For each mouse, at least 10 fields were examined. The percentages of tubules that displayed cellular necrosis, loss of brush border, cast formation, vacuolization, and tubule dilation were scored as follows: 0, none; 1, <10% 2, 11–25% 3, 26–45% 4, 46–75% and 5, >76%.
Statistical analyses
All data were expressed as mean±s.d. Experimental groups were compared by analysis of variance and, when appropriate, with Scheffe test for multiple comparisons. Linear regression analysis was performed between renal or plasma levels of ADMA and renal levels of 8-OHdG. A level of P<0.05 was accepted as statistically significant.
Acknowledgments
We thank Ms M. Shinkai for excellent technical support.
All the authors declared no competing interests.
Footnotes
Parts of this work were presented at the 43rd annual meeting of American Society of Nephrology, 16–21 November 2010; Denver, PA, USA.
References
- Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292–1298. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA. 1996;275:1489–1494. [PubMed] [Google Scholar]
- Xue JL, Daniels F, Star RA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17:1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002;62:1539–1549. doi: 10.1046/j.1523-1755.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199–2210. doi: 10.1097/01.asn.0000079785.13922.f6. [DOI] [PubMed] [Google Scholar]
- Liu H, Jia Z, Soodvilai S, et al. Nitro-oleic acid protects the mouse kidney from ischemia and reperfusion injury. Am J Physiol Renal Physiol. 2008;295:F942–F949. doi: 10.1152/ajprenal.90236.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger JD, Weil JV. Abnormal vascular function following ischemia-reperfusion injury. J Investig Med. 1995;43:431–442. [PubMed] [Google Scholar]
- Brodsky SV, Yamamoto T, Tada T, et al. Endothelial dysfunction in ischemic acute renal failure: rescue by transplanted endothelial cells. Am J Physiol Renal Physiol. 2002;282:F1140–F1149. doi: 10.1152/ajprenal.00329.2001. [DOI] [PubMed] [Google Scholar]
- Schramm L, Heidbreder E, Schmitt A, et al. Role of L-arginine-derived NO in ischemic acute renal failure in the rat. Ren Fail. 1994;16:555–569. doi: 10.3109/08860229409044885. [DOI] [PubMed] [Google Scholar]
- Chintala MS, Chiu PJ, Vemulapalli S, et al. Inhibition of endothelial derived relaxing factor (EDRF) aggravates ischemic acute renal failure in anesthetized rats. Naunyn Schmiedebergs Arch Pharmacol. 1993;348:305–310. doi: 10.1007/BF00169160. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Nishiura M, Deguchi S, et al. Protective effect of FK409, a spontaneous nitric oxide releaser, on ischemic acute renal failure in rats. J Pharmacol Exp Ther. 1998;287:1084–1091. [PubMed] [Google Scholar]
- Kurata H, Takaoka M, Kubo Y, et al. Protective effect of nitric oxide on ischemia/reperfusion-induced renal injury and endothelin-1 overproduction. Eur J Pharmacol. 2005;517:232–239. doi: 10.1016/j.ejphar.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Satake A, Takaoka M, Nishikawa M, et al. Protective effect of 17beta-estradiol on ischemic acute renal failure through the PI3K/Akt/eNOS pathway. Kidney Int. 2008;73:308–317. doi: 10.1038/sj.ki.5002690. [DOI] [PubMed] [Google Scholar]
- Cooke JP. Does ADMA cause endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2000;20:2032–2037. doi: 10.1161/01.atv.20.9.2032. [DOI] [PubMed] [Google Scholar]
- Ueda S, Yamagishi S, Okuda S. New pathways to renal damage: role of ADMA in retarding renal disease progression. J Nephrol. 2010;23:377–386. [PubMed] [Google Scholar]
- Zoccali C. The endothelium as a target in renal diseases. J Nephrol. 2007;20 (Suppl 12:S39–S44. [PubMed] [Google Scholar]
- Böger RH. The pharmacodynamics of L-arginine. J Nutr. 2007;137:1650S–1655S. doi: 10.1093/jn/137.6.1650S. [DOI] [PubMed] [Google Scholar]
- Abedini S, Meinitzer A, Holme I, et al. Asymmetrical dimethylarginine is associated with renal and cardiovascular outcomes and all-cause mortality in renal transplant recipients. Kidney Int. 2010;77:44–50. doi: 10.1038/ki.2009.382. [DOI] [PubMed] [Google Scholar]
- Kaida Y, Ueda S, Yamagishi S, et al. Proteinuria elevates asymmetric dimethylarginine levels via protein arginine methyltransferase-1 overexpression in a rat model of nephrotic syndrome. Life Sci. 2012;91:301–305. doi: 10.1016/j.lfs.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Lin KY, Ito A, Asagami T, et al. Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation. 2002;106:987–992. doi: 10.1161/01.cir.0000027109.14149.67. [DOI] [PubMed] [Google Scholar]
- Ishibashi Y, Yamagishi S, Matsui T, et al. Pravastatin inhibits advanced glycation end products (AGEs)-induced proximal tubular cell apoptosis and injury by reducing receptor for AGEs (RAGE) level. Metabolism. 2012;61:1067–1072. doi: 10.1016/j.metabol.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75:247–260. doi: 10.1016/j.cardiores.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Tada T, Brodsky SV, et al. Intravital videomicroscopy of peritubular capillaries in renal ischemia. Am J Physiol Renal Physiol. 2002;282:F1150–F1155. doi: 10.1152/ajprenal.00310.2001. [DOI] [PubMed] [Google Scholar]
- Tyagi N, Sedoris KC, Steed M, et al. Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Heart Circ Physiol. 2005;289:H2649–H2656. doi: 10.1152/ajpheart.00548.2005. [DOI] [PubMed] [Google Scholar]
- Luo Z, Teerlink T, Griendling K, et al. Angiotensin II and NADPH oxidase increase ADMA in vascular smooth muscle cells. Hypertension. 2010;56:498–504. doi: 10.1161/HYPERTENSIONAHA.110.152959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int. 2007;72:151–156. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Crespo I, Gerber NC, Ortiz de Montellano PR. Endothelial nitric-oxide synthase. Expression in Escherichia coli, spectroscopic characterization, and role of tetrahydrobiopterin in dimer formation. J Biol Chem. 1996;271:11462–11467. doi: 10.1074/jbc.271.19.11462. [DOI] [PubMed] [Google Scholar]
- Saito M, Miyagawa I. Real-time monitoring of nitric oxide in ischemia-reperfusion rat kidney. Urol Res. 2000;28:141–146. doi: 10.1007/s002400050153. [DOI] [PubMed] [Google Scholar]
- Rodríguez F, Nieto-Cerón S, Fenoy FJ, et al. Sex differences in nitrosative stress during renal ischemia. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1387–R1395. doi: 10.1152/ajpregu.00503.2009. [DOI] [PubMed] [Google Scholar]
- McDonald LJ, Murad F. Nitric oxide and cyclic GMP signaling. Proc Soc Exp Biol Med. 1996;211:1–6. doi: 10.3181/00379727-211-43950a. [DOI] [PubMed] [Google Scholar]
- Caglar K, Yilmaz MI, Sonmez A, et al. ADMA, proteinuria, and insulin resistance in non-diabetic stage I chronic kidney disease. Kidney Int. 2006;70:781–787. doi: 10.1038/sj.ki.5001632. [DOI] [PubMed] [Google Scholar]
- Yilmaz MI, Saglam M, Sonmez A, et al. Improving proteinuria, endothelial functions and asymmetric dimethylarginine levels in chronic kidney disease: ramipril vs. valsartan. Blood Purif. 2007;25:327–335. doi: 10.1159/000107410. [DOI] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- Eid HM, Lyberg T, Arnesen H, et al. Insulin and adiponectin inhibit the TNFalpha-induced ADMA accumulation in human endothelial cells: the role of DDAH. Atherosclerosis. 2007;194:e1–e8. doi: 10.1016/j.atherosclerosis.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Feng X, Yan J, Wang Y, et al. The proteasome inhibitor bortezomib disrupts tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression and natural killer (NK) cell killing of TRAIL receptor-positive multiple myeloma cells. Mol Immunol. 2010;47:2388–2396. doi: 10.1016/j.molimm.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Itoh M, Takaoka M, Shibata A, et al. Preventive effect of lactacystin, a selective proteasome inhibitor, on ischemic acute renal failure in rats. J Pharmacol Exp Ther. 2001;298:501–507. [PubMed] [Google Scholar]
- Siedlecki AM, Jin X, Thomas W, et al. RGS4, a GTPase activator, improves renal function in ischemia-reperfusion injury. Kidney Int. 2011;80:263–271. doi: 10.1038/ki.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton TA, Mang HE, Campos SB, et al. Injury of the renal microvascular endothelium alters barrier function after ischemia. Am J Physiol Renal Physiol. 2003;285:F191–F198. doi: 10.1152/ajprenal.00042.2003. [DOI] [PubMed] [Google Scholar]
- Suschek CV, Schnorr O, Hemmrich K, et al. Critical role of L-arginine in endothelial cell survival during oxidative stress. Circulation. 2003;107:2607–2614. doi: 10.1161/01.CIR.0000066909.13953.F1. [DOI] [PubMed] [Google Scholar]
- Ziche M, Parenti A, Ledda F, et al. Nitric oxide promotes proliferation and plasminogen activator production by coronary venular endothelium through endogenous bFGF. Circ Res. 1997;80:845–852. doi: 10.1161/01.res.80.6.845. [DOI] [PubMed] [Google Scholar]
- Murohara T, Witzenbichler B, Spyridopoulos I, et al. Role of endothelial nitric oxide synthase in endothelial cell migration. Arterioscler Thromb Vasc Biol. 1999;19:1156–1161. doi: 10.1161/01.atv.19.5.1156. [DOI] [PubMed] [Google Scholar]
- Smith CL, Birdsey GM, Anthony S, et al. Dimethylarginine dimethylaminohydrolase activity modulates ADMA levels, VEGF expression, and cell phenotype. Biochem Biophys Res Commun. 2003;308:984–989. doi: 10.1016/s0006-291x(03)01507-9. [DOI] [PubMed] [Google Scholar]
- Jacobi J, Sydow K, von Degenfeld G, et al. Overexpression of dimethylarginine dimethylaminohydrolase reduces tissue asymmetric dimethylarginine levels and enhances angiogenesis. Circulation. 2005;111:1431–1438. doi: 10.1161/01.CIR.0000158487.80483.09. [DOI] [PubMed] [Google Scholar]
- Di Giorno C, Pinheiro HS, Heinke T, et al. Beneficial effect of N-acetyl-cysteine on renal injury triggered by ischemia and reperfusion. Transplant Proc. 2006;38:2774–2776. doi: 10.1016/j.transproceed.2006.08.178. [DOI] [PubMed] [Google Scholar]
- Dayoub H, Achan V, Adimoolam S, et al. Dimethylarginine dimethylaminohydrolase regulates nitric oxide synthesis: genetic and physiological evidence. Circulation. 2003;108:3042–3047. doi: 10.1161/01.CIR.0000101924.04515.2E. [DOI] [PubMed] [Google Scholar]
- Kielstein JT, Impraim B, Simmel S, et al. Cardiovascular effects of systemic nitric oxide synthase inhibition with asymmetrical dimethylarginine in humans. Circulation. 2004;109:172–177. doi: 10.1161/01.CIR.0000105764.22626.B1. [DOI] [PubMed] [Google Scholar]
- Matsuguma K, Ueda S, Yamagishi S, et al. Molecular mechanism for elevation of asymmetric dimethylarginine and its role for hypertension in chronic kidney disease. J Am Soc Nephrol. 2006;17:2176–2183. doi: 10.1681/ASN.2005121379. [DOI] [PubMed] [Google Scholar]
- Ueda S, Kato S, Matsuoka H, et al. Regulation of cytokine-induced nitric oxide synthesis by asymmetric dimethylarginine: role of dimethylarginine dimethylaminohydrolase. Circ Res. 2003;92:226–233. doi: 10.1161/01.res.0000052990.68216.ef. [DOI] [PubMed] [Google Scholar]
- Yokoro M, Suzuki M, Murota K, et al. Asymmetric dimethylarginine, an endogenous NOS inhibitor, is actively metabolized in rat erythrocytes. Biosci Biotechnol Biochem. 2012;76:1334–1342. doi: 10.1271/bbb.120086. [DOI] [PubMed] [Google Scholar]
- Hohenstein B, Braun A, Amann KU, et al. A murine model of site-specific renal microvascular endothelial injury and thrombotic microangiopathy. Nephrol Dial Transplant. 2008;23:1144–1156. doi: 10.1093/ndt/gfm774. [DOI] [PubMed] [Google Scholar]
- Hagiwara M, Bledsoe G, Yang ZR, et al. Intermedin ameliorates vascular and renal injury by inhibition of oxidative stress. Am J Physiol Renal Physiol. 2008;295:F1735–F1743. doi: 10.1152/ajprenal.90427.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Faubel S, Ljubanovic D, et al. Endotoxemic acute renal failure is attenuated in caspase-1-deficient mice. Am J Physiol Renal Physiol. 2005;288:F997–1004. doi: 10.1152/ajprenal.00130.2004. [DOI] [PubMed] [Google Scholar]