Abstract
Two virulence factors produced by Vibrio cholerae, cholera toxin (CT) and toxin-corregulated pilus (TCP), are indispensable for cholera infection. ToxT is the central regulatory protein involved in activation of CT and TCP expression. We previously reported that lack of a respiration-linked sodium-translocating NADH–ubiquinone oxidoreductase (Na+-NQR) significantly increases toxT transcription. In this study, we further characterized this link and found that Na+-NQR affects toxT expression only at the early-log growth phase, whereas lack of Na+-NQR decreases CT production after the mid-log growth phase. Such decreased CT production was independent of toxT and ctxB transcription. Supplementing a respiratory substrate, L-lactate, into the growth media restored CT production in the nqrA-F mutant, suggesting that decreased CT production in the Na+-NQR mutant is dependent on electron transport chain (ETC) activity. This notion was supported by the observations that two chemical inhibitors, a Na+-NQR specific inhibitor 2-n-Heptyl-4-hydroxyquinoline N-oxide (HQNO) and a succinate dehydrogenase (SDH) inhibitor, thenoyltrifluoroacetone (TTFA), strongly inhibited CT production in both classical and El Tor biotype strains of V. cholerae. Accordingly, we propose the main respiratory enzyme of V. cholerae, as a potential drug target to treat cholera because human mitochondria do not contain Na+-NQR orthologs.
Keywords: anti-virulence drug, Vibrio cholerae, Na+-NQR, electron transport chain, cholera toxin
1. Introduction
Vibrio cholerae is the etiological agent of cholera, a life-threatening diarrheal disease. Toxin-coregulated pilus (TCP) and cholera toxin (CT) are critical determinants of the pathogenicity of V. cholerae. TCP is a Type IV pilus that is required for colonization in the small intestine [1], whereas CT is a secreted enterotoxin responsible for inducing severe watery diarrhea, a hallmark feature of cholera [2]. The expression levels of TCP and CT are positively regulated by an AraC-type transcriptional regulator, ToxT [3].
The sodium-translocating NADH–ubiquinone oxidoreductase (Na+-NQR) is a unique redox-driven sodium pump and is found in the electron transport chain (ETC) of a number of pathogenic and marine bacteria [4]. Na+-NQR is predicted to play a vital role in the ETC of these organisms, including V. cholerae, that do not possess an ortholog of the mitochondrial Complex I, which is typically the main ETC-linked NADH-dehydrogenase (http://gsc.jcvi.org/projects/msc/vibrio/). It is well known that the genes encoding NQR are strongly repressed at anaerobic conditions [5,6]. Since some parts of the small intestine are anaerobic, one might speculate that lack of Na+-NQR does not affect V. cholerae O1 virulence. However, a previous study revealed that Na+-NQR is essential for V. cholerae O1 colonization in the small intestine of mice and in acid tolerance response (ATR) [7]. This suggested that Na+-NQR is essential for V. cholerae O1 virulence and could be used as a molecular target to develop new therapeutic treatments for cholera. In this study, we further aimed to examine the link between Na+-NQR and virulence factor production as a first step to evaluate Na+-NQR as a molecular target for anti-cholera drug development.
2. Materials and Methods
2.1. Bacterial strains, plasmids and media
V. cholerae O1 classical biotype strains, O395N1 and CA401, their ΔnqrA-F mutant strains, and El Tor biotype strain, N16961, were used in this study. The ΔnhaA mutant, the ΔnhaB mutant and the ΔmrpA-F mutant strains of V. cholerae O395N1 (Quinn et. al. unpublished) were also used in this study. All bacterial strains were kept at −80°C in 20% glycerol stocks. The classical biotype strains were grown overnight in Luria-Bertani (LB) medium (Difco) at 37°C, washed, diluted to OD 600 = 0.05 in LB (initial pH 6.5), and grown at 30°C. The pH of the LB medium was adjusted to pH 6.5 with HCl. The El Tor biotype strain, N16961, was grown overnight in LB medium at 37°C and then grown in Yeast Extract Peptone water (YEP) as described previously (i.e., AKI growth conditions) [8]. HQNO and TTFA were added at 2.5 μM. L-lactate was added at 40 mM. L-lactate was also added to the pre-cultures to induce L-lactate dehydrogenase activity. Streptomycin was supplemented at 100 μg/ml.
2.2 Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis
Cells of V. cholerae O1 grown in LB (initial pH 6.5) at 30°C for 2, 4, 6, and 8 hours were treated with RNA Protect Bacteria Reagent (Qiagen). RNA was extracted using the QIAGEN RNeasy Mini Kit (Qiagen) and treated with TURBO DNA-free™ Kit (Invitrogen). Primers used for qRT-PCR are 5Vc16SrRNAqRT: GATCATGGCTCAGATTGAACG, 3Vc16SrRNAqRT: TCGCCACCCAAGGAACA, 5VcToxTqRT: GCTGTCCTTTCTGAAGTGGTAAATG, 3VCToxTqRT: TTCTACTTTCGAGAAGAACCCTGAA, 5VcCtxBqRT: AGCGATTGAAAGGATGAAGGA, 3VcCtxBqRT: CGCATGAGGCGTTTTATTATTC, 5VcTcpAqRT: CGTAATGCAGCAGCTAATAAAGCA, 3VcTcpAqRT: GGAACATATCACCGACACTGGTAA. Real-time qRT-PCR reactions were performed using the SuperScript® III Platinum® SYBR® Green One-Step qRT-PCR Kit (Invitrogen) and an ABI PRISM 7500 FAST Sequence Detection System (Applied Biosystems) at the Center for Genome Research and Biocomputing Core Laboratory at Oregon State University.
2.3 Measurements of CT production
CT production was determined by GM1-based enzyme linked immunosorbent assays (CT-ELISA) essentially as described [9]. In brief, CT-ELISA was performed using a cholera toxin-specific monoclonal antibody (Abcam) and Goat-Anti-Mouse (GAM)-HRP Conjugated antibodies (Bio-Rad). An HRP Substrate kit (Bio-Rad) was used to detect the HRP activity and the plates were read at 415 nm on an iMark microplate reader (Bio-Rad). The amount of CT was quantified using known amounts of purified cholera toxin B subunit (Sigma) as the standard.
3. Results
3.1 Growth phase dependent effects of Na+-NQR on toxT expression and cholera toxin production
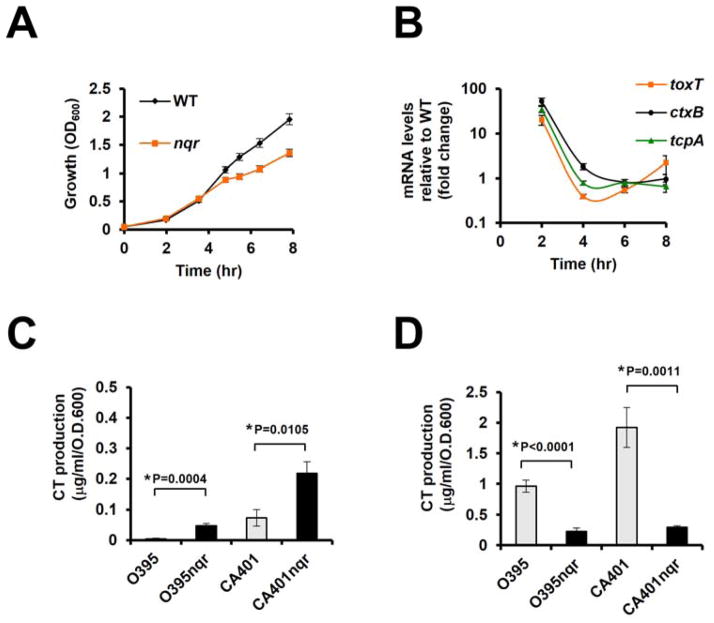
Because we previously reported that Na+-NQR affects toxT transcription [10], we monitored the growth and virulence gene expressions using V. cholerae parent and isogenic O395N1ΔnqrA-F mutant strains cultured under conditions typically used for in vitro induction of virulence gene expression [LB (initial pH 6.5) at 30°C] [9]. Initially, both strains displayed very similar growth rates, although the O395N1ΔnqrA-F mutant transitioned to a slower growth rate starting approximately from the mid- to late-exponential growth phase (Fig. 1A). Measurements of toxT, ctxB and tcpA expression levels in the O395N1ΔnqrA-F mutant were compared with the parent strain by qRT-PCR. Consistent with our previous findings [10], the O395N1ΔnqrA-F mutant showed higher toxT, ctxB and tcpA expression levels than the isogenic parent strain, however, this effect was only observed at the very early exponential growth phase (2 hr growth) (Fig. 1B). In agreement with the gene expression data, extracellular CT levels in the O395N1ΔnqrA-F mutant were higher than in the parent strain at the early exponential growth phase (Fig. 1C). In contrast, the O395N1ΔnqrA-F mutant showed significantly lower extracellular CT levels compared to the parent strain at the late exponential growth phase (Fig. 1D), even though transcriptional levels of the toxT and ctxB genes were similar in both strains during late exponential growth phase (6 hr and 8 hr growth) (Fig. 1B). Similar extracellular CT level patterns were also observed in another V. cholerae O1 classical strain, CA401 and its ΔnqrA-F deletion derivative (Fig. 1C and 1D).
Figure 1.
Growth-phase dependent virulence gene expression and CT production. Overnight cultures of V. cholerae O395N1, CA401, and their respective isogenic ΔnqrA-F mutants were washed and diluted in LB (initial pH 6.5) to OD 600 = 0.05 and shaken in LB (initial pH 6.5) at 30°C. All experiments were repeated three times. The error bars indicate standard deviations. (A) Bacterial growth was measured by OD600. (B). Total RNA was extracted and analyzed by qRT-PCR. Gene expression levels were normalized between the samples by using 16S ribosomal RNA. (C, D) The cell-free culture supernatants were prepared from 4hr growth (C) and 8hr growth (D) and assayed for CT production by CT-ELISA. P values were calculated by Student’s t test and * indicates P< 0.05.
The autoagglutination phenotype is correlated with TCP production [11]. Here, we observed that both the O395N1ΔnqrA-F and the CA401ΔnqrA-F strains did not show a full autoagglutination phenotype after overnight growth in LB (initial pH 6.5) at 30°C, whereas both of the parent strains did (data not shown). This suggested that the lack of functional Na+-NQR also negatively affects overall TCP production, although tcpA transcription levels were comparable between the O395N1 parent and ΔnqrA-F mutant strains (Fig. 1B).
3.2 Other sodium-translocating enzymes do not affect CT production
We next aimed to gain a better understanding of the effects of loss of Na+-NQR on CT production in V. cholerae O1. The Na+-NQR has two major functions: it is a primary sodium pump and a major component in the V. cholerae ETC [4]. To examine the role of sodium pumping, we investigated the effects of loss of other sodium-translocating enzymes on CT production. Genetic inactivation of various sodium/proton antiporters, including NhaA, NhaD, and Mrp, did not have any effects on extracellular CT levels (data not shown), suggesting that loss of sodium pumping per se does not affect extracellular CT levels. Furthermore, we noted that the addition of L-lactate into the growth media “rescued” extracellular CT levels in the O395N1ΔnqrA-F mutant to the levels observed in the parent strain (Fig. 2). Since L-lactate is a respiration substrate for the L-lactate-ubiquinone oxidoreductase activity, these data suggested that reduced ETC activity might be responsible for the decreased extracellular CT levels in the V. cholerae O1 Na+-NQR mutants.
Figure 2.
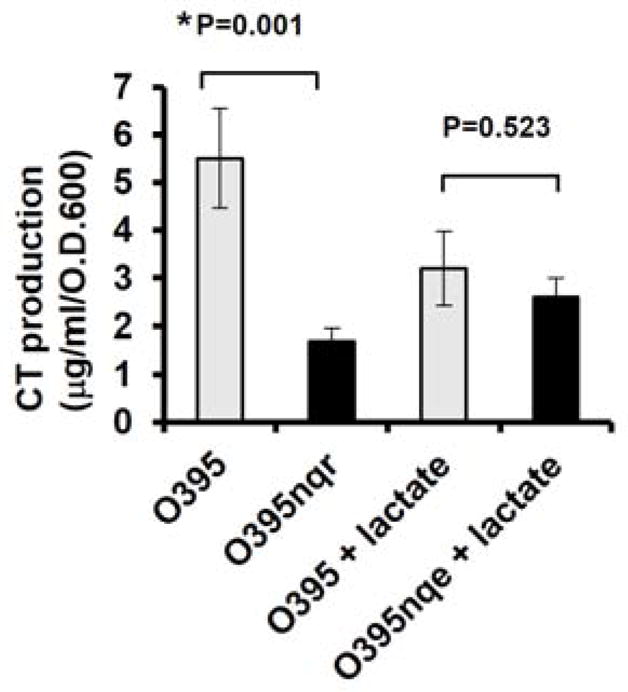
The effects of L-lactate on CT production. Overnight cultures of V. cholerae O395N1 and its respective isogenic ΔnqrA-F mutant were washed and diluted in LB (initial pH 6.5) to OD 600 = 0.05 and shaken in LB (initial pH 6.5) at 30°C for overnight. The cell-free culture supernatants were prepared after growth and assayed for CT production by CT-ELISA. All experiments were repeated at least three times. The error bars indicate standard errors. P values were calculated by one-way ANOVA followed by post hoc comparisons using the Bonferroni test and * indicates statistical significance (P< 0.05).
3.3 ETC inhibitors inhibit CT production
To further investigate the role of ETC on CT production, we investigated the effects of the Na+-NQR-specific inhibitor, 2-n-Heptyl-4-hydroxyquinoline N-oxide (HQNO), and a succinate dehydrogenase (SDH) inhibitor, thenoyltrifluoroacetone (TTFA), on CT production. In agreement with the data from the O395N1ΔnqrA-F and the CA401ΔnqrA-F strains, HQNO inhibited CT production in two classical biotype wild-type strains of V. cholerae O1, O395N1 and CA401 (Fig. 3). Similarly, TTFA also inhibited CT production in these two strains.
Figure 3.
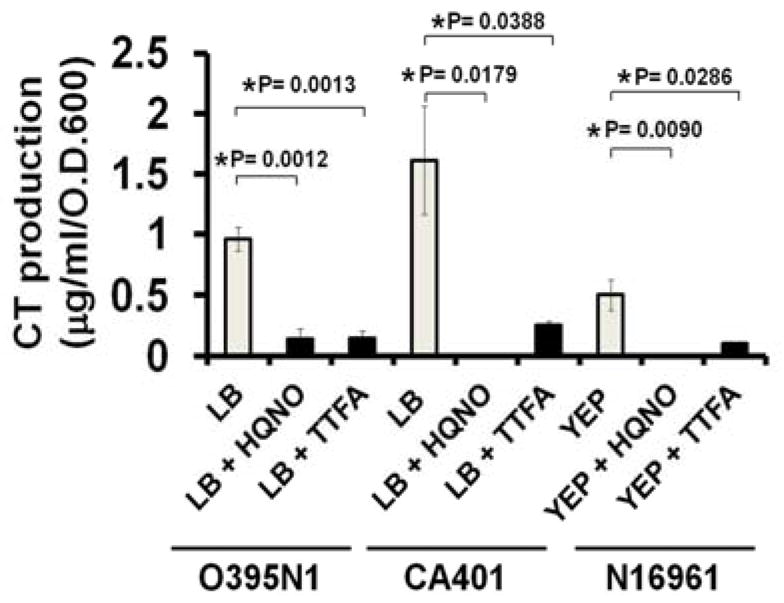
The effects of HQNO and TTFA on CT production. Overnight cultures of V. cholerae O395N1 and CA401 were washed and diluted in LB (initial pH 6.5) to the initial OD 600 = 0.05 and cultured in LB (initial pH 6.5) at 30°C for 8hr. Cultures of V. cholerae N16961 were grown in YEP medium under AKI conditions. HQNO and TTFA were added at 2.5 μM. The cell-free culture supernatants were prepared after growth and assayed for CT production by CT-ELISA. All experiments were repeated three times. The error bars indicate standard errors. P values were calculated by one-way ANOVA followed by post hoc Bonferroni test and * indicates P< 0.05.
To investigate whether ETC inhibitors also inhibited CT production in an El Tor biotype strain, we tested the effect of these chemicals on CT production of V. cholerae N16961. When grown under AKI conditions, a specific growth condition for the El Tor biotype strain to produce measurable CT in vitro [8], the V. cholerae N16961 strain produced detectable amounts of CT as expected. However, addition of HQNO or TTFA to the growth media strongly inhibited CT production of V. cholerae N16961 (Fig. 3). These data suggested that the ETC activities are essential for CT production in both classical and El Tor biotype strains.
4. Discussion
In this study, we found that inhibition of V. cholerae O1 Na+-NQR inhibited CT production. Further studies revealed that such decreased CT production is not a Na+-NQR specific effect and inhibition of other ETC components, such as SDH, also inhibited CT production. In general, many of the ETC inhibitors are highly toxic to human cells due to their inhibitory effect on the mitochondrial ETC [12]. Thus, for future antimicrobial drug development, it is important to target bacterial ETC components that do not exist in the human mitochondrial ETC. Comparison of the V. cholerae and the human mitochondrial ETCs revealed that Na+-NQR, two bd-type oxidases and three ETC-linked dehydrogenases (DHs) are present in V. cholerae that are not in found the human mitochondrial ETC (Fig. 4). Thus, apart from Na+-NQR, such bd-type oxidases and ET-linked DHs might also be potential drug targets to inhibit CT and TCP production in V. cholerae O1. We are currently investigating this possibility.
Figure 4.
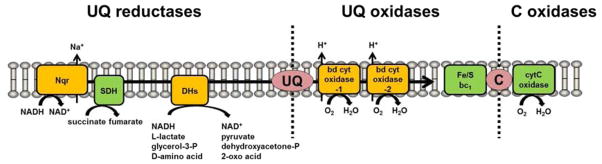
Putative reductases and oxidases of ubiquinone (UQ) predicted from the V. cholerae O395N1 and N16961 genomes are shown. Green represents the components present both in V. cholerae and human ETC. Orange represents the components that are not present in human ETC. The UQ reductase activity of NQR (VC2290-95) is coupled by the NADH oxidation and sodium extrusion activity. The UQ reductase activities of succinate dehydrogenase (SDH) (VC2088-91) and glycerol-3-phosphate dehydrogenase (GlpD) (VCA0657) are coupled to the oxidation of succinate and glycerol-3-P to fumarate and dehyroxyacetone-P, respectively. Other bacteria-specific respiration-linked dehydrogenases [such as Ndh2 (VC1890), L-lactate dehydrogenase (VCA0984), and D-amino acid dehydrogenase (VC0786)] are shown collectively (DHs). The bd-type cytochrome (bd cyt) oxidase-1 (VC1843-44) and oxidase-2 (VCA0872-73) oxidize the reduced form of UQ coupled to oxygen reduction and proton efflux activities. The ubiquinol-cytochrome C reductase (Fe/S bc1) (VC0573-75) also oxidizes the reduced form of UQ and the reaction is coupled with the cytochrome c4 (C) reduction and proton efflux activities. The reduced form of C is oxidized by cytochrome C (cytC) oxidase and the reaction is coupled to oxygen reduction and proton efflux activities.
We found that the V. cholerae O1 ΔnqrA-F mutant showed growth defects when grown in LB media. Similar growth kinetics have previously been observed for an Escherichia coli Complex I (NADH:ubiquinone oxidoreductase, nuoB) mutant in tryptone medium [13]. Furthermore, like the E. coli nuoB mutant [13], the O395N1ΔnqrA-F mutant showed decreased acetate utilization [14], suggesting a depletion of available NAD+. Taken together with the fact that the V. cholerae genome does not encode nuo genes, these observations suggested that Na+-NQR is the main ETC-linked NADH dehydrogenase in V. cholerae. Similar to V. cholerae, some other important pathogenic bacteria, such as Vibrio parahaemolyticus, Vibrio vulnificus, and Haemophilus influenza do not have Nuo but instead have Na+-NQR, suggesting that Na+-NQR plays major roles in the ETC in these pathogenic bacteria. On the other hand, some pathogenic bacteria, such as Yersinia pestis and Pseudomonas aeruginosa, have both Na+-NQR and Nuo. In contrast to V. cholerae, the Y. pestis and P. aeruginosa strains that lack functional Na+-NQR did not show growth defects when grown in LB media (Minato et.al. unpublished data), suggesting that lack of Na+-NQR significantly affects bacterial ETC activity only when Nuo is absent. It is also important to note that lack of Na+-NQR does not affect Y. pestis virulence [15].
The temporal increase in toxT expression in the V. cholerae O1 ΔnqrA-F mutant might be caused by a combination of multiple factors. Our recent study suggested that lack of Na+-NQR increases toxT expression via affecting acetyl-CoA [14]. Interestingly, it was known that intracellular acetyl-CoA levels are higher only at the early-log growth in E. coli [16]. Thus, it is tempting to speculate that lack of Na+-NQR increases toxT expression only at the early-log growth phase because intracellular acetyl-CoA levels are high only at the early-log growth phase.
The overall CT production of the V. cholerae O1 ΔnqrA-F mutant was much less than the parent strain. Since ctxB gene expressions in the ΔnqrA-F mutant were similar to the parent strain after the mid-log growth phase, these data indicated that lack of Na+-NQR has a negative impact on CT levels via affecting either translation or secretion. Future studies will be necessary for understanding the molecular mechanism of Na+-NQR mediated CT production.
In summary, our data raised the possibility of using Na+-NQR as a potential novel molecular target for the development of new therapeutic interventions against cholera for several reasons: 1) Na+-NQR appears to be the major ETC-linked enzyme in V. cholerae; 2) genetic or chemical inactivation of Na+-NQR significantly diminishes the overall levels of CT; 3) the V. cholerae ΔnqrA-F mutant has a severe to moderate growth defect; 4) the V. cholerae ΔnqrA-F mutant was previously found to be attenuated in a mouse model [7]; and 5) Na+-NQR has no orthologs in the human cells. Because inhibition of Na+-NQR does not kill bacteria, targeting Na+-NQR would be expected to produce less pressure to evolve bacterial resistance compared to the traditional antimicrobial agents, similar to the other “anti-virulence” drug strategies [17].
Highlights.
Lack of Na+-NQR negatively affects cholera toxin (CT) production in V. cholerae at the late exponential growth phase.
Chemical Inhibitors for electron transport chain (ETC) also inhibits CT production.
Decreased CT production in the Na+-NQR mutant is linked to ETC.
Acknowledgments
We thank Erin J. Lind, Frances M. Biel and Dr. Alisha M. Aagesen for their excellent technical assistances. This research was supported by grants from the National Institutes of Health to C.C.H. [AI-063121-02]. S.R.F was partially supported by the OSU Undergraduate Research, Innovation, Scholarship & Creativity (URISC) fund and the OSU Howard Hughes Medical Institute Summer Undergraduate Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Taylor RK, Miller VL, Furlong DB, Mekalanos JJ. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci USA. 1987;84:2833–7. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanden Broeck D, Horvath C, De Wolf MJ. Vibrio cholerae: cholera toxin. Int J Biochem Cell Biol. 2007;39:1771–5. doi: 10.1016/j.biocel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 3.DiRita VJ, Parsot C, Jander G, Mekalanos JJ. Regulatory cascade controls virulence in Vibrio cholerae. Proc Natl Acad Sci USA. 1991;88:5403–7. doi: 10.1073/pnas.88.12.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Häse CC, Fedorova ND, Galperin MY, Dibrov PA. Sodium ion cycle in bacterial pathogens: evidence from cross-genome comparisons. Microbiol Mol Biol Rev. 2001;65:353–70. doi: 10.1128/MMBR.65.3.353-370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fadeeva MS, Yakovtseva EA, Belevich GA, Bertsova YV, Bogachev AV. Regulation of expression of Na+ -translocating NADH:quinone oxidoreductase genes in Vibrio harveyi and Klebsiella pneumoniae. Arch Microbiol. 2007;188:341–48. doi: 10.1007/s00203-007-0254-5. [DOI] [PubMed] [Google Scholar]
- 6.Isabella VM, Clark VL. Deep sequencing-based analysis of the anaerobic stimulon in Neisseria gonorrhoeae. BMC Genomics. 2011;12:51. doi: 10.1186/1471-2164-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merrell DS, Hava DL, Camilli A. Identification of novel factors involved in colonization and acid tolerance of Vibrio cholerae. Mol Microbiol. 2002;43:1471–91. doi: 10.1046/j.1365-2958.2002.02857.x. [DOI] [PubMed] [Google Scholar]
- 8.Iwanaga M, Kuyyakanond T. Large production of cholera toxin by Vibrio cholerae O1 in yeast extract peptone water. J Clin Microbiol. 1987;25:2314–6. doi: 10.1128/jcm.25.12.2314-2316.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardel CL, Mekalanos JJ. Regulation of cholera toxin by temperature, pH, and osmolarity. Methods Enzymol. 1994;235:517–26. doi: 10.1016/0076-6879(94)35167-8. [DOI] [PubMed] [Google Scholar]
- 10.Häse CC, Mekalanos JJ. Effects of changes in membrane sodium flux on virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci USA. 1999;96:3183–7. doi: 10.1073/pnas.96.6.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiang SL, Taylor RK, Koomey M, Mekalanos JJ. Single amino acid substitutions in the N-terminus of Vibrio cholerae TcpA affect colonization, autoagglutination, and serum resistance. Mol Microbiol. 1995;17:1133–42. doi: 10.1111/j.1365-2958.1995.mmi_17061133.x. [DOI] [PubMed] [Google Scholar]
- 12.Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu Rev Biochem. 1985;54:1015–69. doi: 10.1146/annurev.bi.54.070185.005055. [DOI] [PubMed] [Google Scholar]
- 13.Prüss BM, Nelms JM, Park C, Wolfe AJ. Mutations in NADH:ubiquinone oxidoreductase of Escherichia coli affect growth on mixed amino acids. J Bacteriol. 1994;176:2143–50. doi: 10.1128/jb.176.8.2143-2150.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minato Y, Fassio SR, Wolfe AJ, Häse CC. Central metabolism controls transcription of a virulence gene regulator in Vibrio cholerae. Microbiology. 2013;159:792–802. doi: 10.1099/mic.0.064865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minato Y, Ghosh A, Faulkner WJ, Lind EJ, Schesser Bartra S, Plano GV, et al. Na+/H+ antiport is essential for Yersinia pestis virulence. Infect Immun. 2013;81:3163–72. doi: 10.1128/IAI.00071-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfe AJ. The acetate switch. Microbiol Mol Biol Rev. 2005;69:12–50. doi: 10.1128/MMBR.69.1.12-50.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasko DA, Sperandio V. Anti-virulence strategies to combat bacteria-mediated disease. Nat Rev Drug Discov. 2010;9:117–28. doi: 10.1038/nrd3013. [DOI] [PubMed] [Google Scholar]