Abstract
Objective
Alopecia is a common disorder affecting more than half of the population worldwide. Androgenetic alopecia, the most common type, affects 50% of males over the age of 40 and 75% of females over 65. Only two drugs have been approved so far (minoxidil and finasteride) and hair transplant is the other treatment alternative. This review surveys the evidence for low-level laser therapy (LLLT) applied to the scalp as a treatment for hair loss and discusses possible mechanisms of actions.
Methods and Materials
Searches of PubMed and Google Scholar were carried out using keywords alopecia, hair loss, LLLT, photobiomodulation.
Results
Studies have shown that LLLT stimulated hair growth in mice subjected to chemotherapy-induced alopecia and also in alopecia areata. Controlled clinical trials demonstrated that LLLT stimulated hair growth in both men and women. Among various mechanisms, the main mechanism is hypothesized to be stimulation of epidermal stem cells in the hair follicle bulge and shifting the follicles into anagen phase.
Conclusion
LLLT for hair growth in both men and women appears to be both safe and effective. The optimum wavelength, coherence and dosimetric parameters remain to be determined.
Keywords: alopecia, androgenetic alopecia, hair loss, LLLT, low level laser (light)
INTRODUCTION
It has long been known that red or near-infrared laser light promotes tissue repair and regeneration and low-intensity light called low-level laser therapy (LLLT) stimulates cellular activity [1]. After the discovery of lasers in the 1960s, there has been tremendous interest in using these laser devices to treat various medical conditions. The most commonly used devices have wavelengths in the range 500–1,100 nm (the so-called optical window of tissue) and they deliver fluences of 1–10 J/cm2 with a power density of 3–90 mW/cm2. LLLT has shown beneficial effects for a variety of medical conditions such as wound healing, nerve regeneration, joint pain relief, stroke recovery, and the prevention and treatment of mucositis [2–8]. Home-use LLLT devices that emit low power coherent monochromatic red light have been developed for various skin conditions, including hair growth [9]. In this review, we will focus on the use of LLLT as a potential treatment for several types of hair loss.
HAIR AND TYPES OF HAIR LOSS
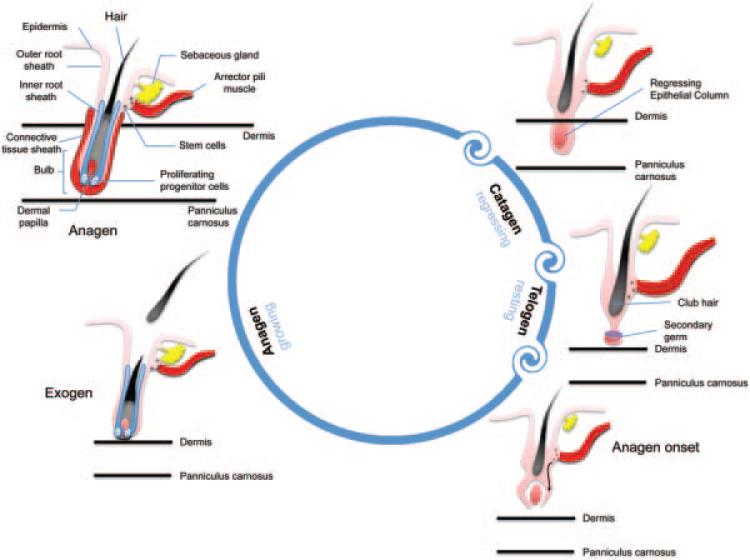
Hair is one of the fastest growing tissues of the human body and the hair follicle, which is a unique characteristic of mammals, represents a stem cell-rich, prototypic neuroectodermal–mesodermal interaction system [10]. Hair follicles undergo repetitive regenerative cycles and each of these cycles consists of three stages: anagen (rapid growth, active stage), catagen (apoptosis-driven regression, physiological involution stage), and telogen (resting stage) (Fig. 1) [10]. Bulge stem cells are found in the region of the outer root sheath located just below the sebaceous gland, coinciding with the point of anchorage of the arrector pili muscle [11]. During the telogen to anagen transition, there is a tightly controlled activation of these epithelial bulge stem cells and within the same period, secondary hair germ cells give rise to transient amplifying (TA) progeny cells [12]. Throughout the entire anagen phase, there is a robust proliferation of the TA cells within the epithelial matrix of the hair follicle. Consequently, proliferating trichocytes terminally differentiate to form the bulk of the hair filament which is the final product of the hair cycle. The dermal papilla of the hair follicle is believed to be the key regulatory element in progenitor cell activation, hair matrix cell proliferation and terminal differentiation of trichocytes [13].
Fig. 1.
Stages of hair cycle. Anagen stage is the growth stage which may last 2–6 years. In cagaten stage, club hair transitions upwards towards the skin pore and the dermal papilla begins to separate from the follicle. This phase usually lasts from 1 to 2 weeks. In telogen stage, the dermal papilla fully separates from the follicle and it takes about 5–6 weeks. Lastly, the dermal papilla moves upward to meet hair follicle once again and the hair matrix begins to form new hair, which represents the return to anagen stage.
Androgenetic alopecia (AGA) is the most common form of hair loss in men affecting almost 50% of the male population [14]. AGA refers to hair loss in genetically susceptible individuals caused by effects of androgens such as testosterone and its derivative dihydrotestosterone (DHT). Testosterone is a lipophilic compound that diffuses across the cell membrane. Testosterone is converted by the cytoplasmic enzyme 5-α reductase to DHT, which is its more active form. There are two types of 5-α reductase; Type 1 is found in keratinocytes, fibroblasts, sweat glands, and sebocytes, and Type 2 is found in skin and the inner root sheath of hair follicles [15]. DHT binds the nuclear androgen receptor which regulates gene expression [15]. Disruption of epithelial progenitor cell activation and TA cell proliferation due to abnormal androgen signaling forms the essential pathophysiological component of this condition which in turn leads to continuous miniaturization of sensitive terminal hair follicles, and their conversion to vellus hair follicles [16,17]. Although the exact genes involved in hair loss are not clearly known, some of the proposed genes responsible for hair growth are desmoglein, activin, epidermal growth factor (EGF), fibroblast growth factor (FGF), lymphoid-enhancer factor-1 (LEF-1), and sonic hedgehog [15]. As of today, the most common methods used for treating AGA are topical minoxidil, finasteride (males only), and surgical hair transplantation [14]. Unfortunately, current therapies are not efficacious for all patients with AGA. Medical therapies require indefinite use and are limited by patient adherence; surgical options (hair transplants) are limited by cost, each patient's supply of donor hair, and possible scarring in donor sites [18]. Due to a need for more efficacious therapies, LLLT has emerged as a new therapeutic approach to treat AGA. The Hairmax Lasercomb® was approved by the US Food and Drug Administration (FDA) and received 510 K clearance as a safe therapy for the treatment of male AGA in 2007 and female AGA in 2011 [19]. There has been a recent review [20] on the use of lasers and light therapies for alopecia that covered 308 nm excimer laser, fractional photothermolysis, and UV phototherapy, but did not cover LLLT mediated by red laser which is the main subject of the present review.
There are several other forms of hair loss such as alopecia areata (AA), telogen effluvium (TE), and chemotherapy-induced alopecia. AA is an autoimmune inflammatory condition, which presents with non-scarring alopecia and is characterized on histology by intra- or perifollicular lymphocytic infiltrates composed of CD4+ and CD8+ T-cells [19]. There are severe variants of AA: alopecia totalis, a total loss of scalp hair and alopecia universalis, total loss of scalp and body hair [21]. The most common treatment modality is intralesional corticosteroid injections; however, other treatments include topical and systemic corticosteroids, minoxidil, anthralin, contact sensitizers, psoralen plus ultraviolet A, cyclosporine, tacrolimus, and biologics such as alefacept, efalizumab, etanercept, infliximab, and adalimumab [15]. TE is abnormal hair cycling causing excessive loss of telogen hair [15]. Some common causes include acute severe illness, surgery, iron deficient anemia, thyroid disease, malnutrition, chronic illness, and medications such as oral contraceptives, lithium, and cimetidine. Chemotherapy works by destroying rapidly dividing cancer cells, however, at the same time, other rapidly dividing cells of the body such as hair follicles are also destroyed, and this unwanted effect leads to chemotherapy-induced alopecia starting 1–3 weeks and peaking at 1–2 months of treatment [22].
LLLT for Prevention and Reversal of Hair Loss
In the late 1960s, Endre Mester, a Hungarian physician, began a series of experiments on the carcinogenic potential of lasers by using a low-power ruby laser (694 nm) on mice. Mice were shaved as a part of the experimental protocol. To Mester's surprise, the laser did not cause cancer but instead improved hair growth around the shaved region on the animal's back [23]. This was the first demonstration of “photobiostimulation” with LLLT, and it opened a new path in the field of medicine [24].
Recently, attention has been drawn towards an uncommon but striking adverse effect of lasers being used for hair removal. It has been noticed in some cases that, increase in hair density, color or coarseness or a combination of these occurs at or around sites treated for hair removal [19,25–27]. The name given for this phenomenon is “Paradoxical Hypertrichosis” and the incidence varies from 0.6% to 10% [19]. A group of researchers also observed transformation of small vellus hairs into larger terminal hairs upon low fluence diode laser treatment and named this phenomenon “terminalization” of vellus hair follicles [28,29]. Until today, different mechanisms have been proposed to explain paradoxical hypertrichosis. In one study, this was attributed to presence of polycystic ovarian syndrome in 5 out of 49 females undergoing IPL laser treatment for facial hirsutism [27]. Another group of researchers suggested that although the heat produced by the laser is less than the temperature necessary for thermolysis of the hair follicle, this heat may be sufficient to induce follicular stem cell proliferation and differentiation by increasing the level of heat shock proteins (HSPs) such as HSP27, which plays a role in regulation of cell growth and differentiation [19]. Sub-therapeutic injury caused by the laser could also result in the release of certain factors which could potentially induce follicular angiogenesis and affect the cell cycling [29].
LLLT for Hair Regrowth, Proposed Mechanisms
As previously mentioned, in 2007 and 2011, LLLT mediated by a laser comb was approved by the FDA as a safe treatment for male and female pattern hair loss respectively [19]. Laser phototherapy is assumed to stimulate anagen re-entry in telogen hair follicles, prolong duration of anagen phase, increase rates of proliferation in active anagen hair follicles and to prevent premature catagen development [19,30]. The exact mechanism of action of LLLT in hair growth is not known; however, several mechanisms have been proposed. Evidence suggests that LLLT acts on the mitochondria and may alter cell metabolism through photodissociation of inhibitory nitric oxide (NO) from cytochrome c oxidase (CCO) [31] (Unit IV in the respiratory chain of mitochondria), causing increased ATP production, modulation of reactive oxygen species, and induction of transcription factors such as nuclear factor kappa B, and hypoxia-inducible factor-1 [32]. These transcription factors in return cause protein synthesis that triggers further effects down-stream, such as increased cell proliferation and migration, alteration in the levels of cytokines, growth factors and inflammatory mediators, and increased tissue oxygenation [32]. Moreover, NO is known to be a potent vasodilator via its effect on cyclic guanine monophosphate production and it can be speculated that LLLT may cause photodissociation of NO not only from CCO but also from intracellular stores such as nitrosylated forms of both hemoglobin and myoglobin leading to vasodilation and increased blood flow which was reported in several studies [32–34]. Yamazaki and coworkers observed an upregulation of hepatocyte growth factor (HGF) and HGF activator expression following irradiation of the backs of Sprague Dawley rats with linear polarized infrared laser [35].
Some authors have drawn comparisons between the mechanism of action of LLLT and the mechanism of minoxidil. Even though the mechanism by which minoxidil promotes hair growth is not fully understood, it is known that minoxidil contains an N-oxide group which may be able to release NO, which is an important cellular signaling molecule involved in many physiological and pathological processes [36] and is also a vasodilator [37]. Furthermore, minoxidil is an ATP sensitive K+ channel opener which in turn cause hyperpolarization of cell membranes [38]. Since ATP sensitive K+ channels in mitochondria and increased levels of NO [39–41] may have some role to play in effects of LLLT in brain and heart [41–43], given what is known about the role of K-ATP channels and NO in hair regrowth mediated by minoxidil, a mechanistic overlap can be identified. Weiss and coworkers, by using RT-PCR and microarray analysis, demonstrated that depending on the treatment parameters, LLLT modulates 5-α reductase expression, which converts testosterone into DHT, alters vascular endothelial growth factor gene expression as wells as matrix metalloproteinase (MMP-2) which have significant roles in hair follicle growth, and in turn the group reported stimulation of hair growth on human dermal papillae cells [44–47]. Notably, similar changes have also been reported with topical minoxidil use [47]. Furthermore, LLLT has been demonstrated to modulate inflammatory processes and immunological responses, which may also have an effect in hair regrowth [32,48]. A study conducted by Wikramanayake et al. [19] on C3H/HeJ mouse model of AA supported this assumption wherein the mice treated with laser comb, increased number of hair follicles with majority in anagen phase were noted with decreased inflammatory infiltrates. Considering that inflammatory infiltrates are highly disruptive to hair follicle biology and multiple cytokines such as IFN-γ, IL-1α and β, TNF-α, MHC and Fas-antigen and macrophage migration inhibitory factor are all involved in the cyclic hair growth and have been shown to play a role in the pathogenesis of AA, modulatory effects of LLLT on inflammation might have a significant role in treatment of AA [19].
LLLT for Hair Regrowth in Animal Models
Wikramanayake et al. [19] demonstrated the hair growth effects of LLLT on C3H/HeJ mouse model of AA, using HairMax Laser Comb® (emits nine beams and attached combs help to part the hairs and improve delivery of laser light to scalp), 655 nm for 20 seconds daily three times per week for a total of 6 weeks [19]. At the end of the treatment, hair regrowth was observed in all the laser treated mice but no difference was observed in the sham-treated group (control group undergoing similar treatment procedures without administration of the key therapeutic element, such as application of light that has no therapeutic effect) [19]. On histology, while an increased number of anagen hair follicles was observed in laser-treated mice, sham-treated mice demonstrated telogen follicles with absent hair shafts [19].
Shukla et al. [49] investigated the effect of helium–neon (He–Ne) laser (632 nm, at doses of 1 and 5 J/cm2 at 24-hour intervals for 5 days) on the hair follicle growth cycle of testosterone-treated and un-treated Swiss albino mice skin. Testosterone treatment led to the inhibition of hair growth which was characterized by a significant increase in catagen follicles [49]. The results showed that exposure of testosterone treated mice to the He–Ne laser at a dose of 1 J/cm2 led to significant increase in the number of hair follicles in anagen phase when compared to the other groups. However, the 5 J/cm2 treated group showed a significant decrease in the number of anagen hair and an increase in telogen hair follicles. This is consistent with the biphasic effect of LLLT wherein low irradiation doses may cause biostimulation and high irradiation doses may cause inhibition [32,49]. Since hair growth promoting effect of He–Ne laser (1 J/cm2) was much higher for the testosterone-treated mice than the non-testosterone treated mice, it can be suggested that cells growing at slower rate or under stress conditions respond better to the stimulatory effects of LLLT. Another notable observation in this study is that in He–Ne laser (1 J/cm2) irradiated skin, some of the anagen follicles appeared from deeper layers of the skin and possessed a different orientation which both represent the late anagen stage in the hair cycle that in turn suggests that laser irradiation prolongs the anagen phase [50,51]. Furthermore, in testosterone-treated and He–Ne (1 J/cm2) irradiated skin, hair follicles were seen to originate from the middle of the dermis, and these follicles represent early anagen phase [49]. Based on this observation, it may be proposed that the majority of catagen and telogen follicles re-enter into anagen phase as a result of low-level laser irradiation at 1 J/cm2.
The incidence of alopecia related to cancer treatments such as chemotherapy is close to 65% and it has severe negative psychological effects [22]. LLLT has been suggested as a treatment modality to promote hair regrowth for chemotherapy-induced alopecia. In a rat model, different regimens of chemotherapy were given to each rat in conjunction with an LLLT device which had the laser unit and switch from the HairMax LaserComb®, but without the comb or handle [52]. Hair regrowth occurred 5 days earlier in all laser treated rats when compared to control and sham-treated rats. Histology results demonstrated large anagen hair bulbs penetrating deeper into the subcutaneous adipose tissue in LLLT-treated skin. Furthermore, it did not compromise the efficacy of chemotherapy by causing localized protection of the cancer cells [52].
LLLT for Hair Growth in Clinical Trials
In order to test the effect of linear polarized infrared irradiation in treatment of AA, a study was conducted with 15 patients (6 men, 9 women) using Super Lizer™, a medical instrument emitting polarized pulsed linear light with a high output (1.8 W) of infrared radiation (600–1,600 nm) that is capable of penetrating into deep subcutaneous tissue [53]. The scalp was irradiated for 3 minutes either once every week or once every other week until vellus hair regrowth in at least 50% of the affected area was observed. Additionally, carpronium chloride 5% was applied topically twice daily to all the lesions in combination with oral antihistamines, cepharanthin and glycyrrhizin (extracts of Chinese medicine herbs) [53]. As a result of this study, in 47% of the patients’ hair growth occurred 1.6 months earlier in irradiated areas than in non-irradiated areas [53]. However, 1 year after irradiation, all the lesions disappeared; hair density, length and diameter of hair shafts were the same both in irradiated and non-irradiated lesions; suggesting that LLLT only accelerates the process of hair regrowth in AA patients. It is worth mentioning that the method for assessment of hair regrowth, density and thickness was not clearly stated, which was one of the main limitations of this study.
Using 655 nm red light and 780 nm infrared light once a day for 10 minutes, 24 male AGA patients were treated and evaluated by a group of investigators [54]. Evaluation has been performed via global photography and phototrichogram [54]. Following 14 weeks of treatment, increase in hair density on both the vertex (145.1/cm2 vs. 137.3/cm2 pre-treatment, P < 0.005) and occiput (163.3/cm2 vs. 153.3/cm2, P < 0.005) as well as anogen/telogen ratio (vertex: 84.7 vs. 79.7 pre-treatment and occiput: 91.9 vs. 89.6 pre-treatment) was observed, and 83% of the patients reported to be satisfied with the treatment [54].
Satino et al. [55] tested the efficacy of LLLT on hair growth and tensile strength on 28 male and 7 female AGA patients. Each patient was given a HairMax LaserComb® 655 nm, to use at home for 6 months for 5–10 minutes every other day [55]. Tensile strength was measured by VIP HairOSCope (Belson Imports, Hialeah, FL) through removal of three typical terminal hairs from a one square centimeter area. Hair count was performed within one centimeter square space created within a mold that was prepared around the area of greatest alopecia. A surgical hook and magnification has been used while counting the number of hair. In terms of hair tensile strength, the results revealed greater improvement in the vertex area for males and temporal area for females; however, both sexes benefited in all areas significantly [55]. In terms of hair count, both sexes and all areas had substantial improvement (for temporal area: 55% in women, 74% in men, in vertex area: 65% in women, 120% in men) with vertex area in males having the best outcome [55]. The HairMax LaserComb® device was tested by Leavitt et al. in a double-blind, sham device-controlled, multicenter, 26-week trial randomized study among 110 male AGA patients [30]. Patients used the device three times per week for 15 minutes for a total of 26 weeks [30]. Significantly greater increase in mean terminal hair density compared to subjects in the sham device group has been reported [30]. Significant improvements in overall hair regrowth, slowing of hair loss, thicker feeling hair, better scalp health and hair shine were also demonstrated in terms of patients’ subjective assessment at 26 weeks over baseline [30].
Recently, a double-blind randomized controlled trial by Lanzafame et al. [56] using a helmet containing 21, 5 mW lasers and 30 LEDs (655±5 nm, 67.3 J/cm2, 25 minutes treatment) every other day for 16 weeks reported 35% increase in hair growth among male AGA patients. Another recent study by Kim et al. [57] designed a 24 weeks randomized, double-blind, sham device-controlled multicenter trial among both male and female AGA patients in order to investigate the efficacy of a helmet type LLLT device combining 650 nm laser with 630 and 660 nm LEDs (total energy density—92.15 mW/cm2, 47.90 J/cm2 for 18 minutes). Even though mean hair thickness (12.6±9.4 vs. 3.9±7.3 in control group, P = 0.01) hair density (17.2±12.1 vs. –2.1±18.3 in control group, P = .003) increased significantly in the treatment group, there was no prominent difference in global appearance between the two groups [57]. Findings from a different study by Avram and Rogers [58] were in accordance with these results where LLLT increased hair count and shaft diameter, however, blinded global images did not support these observations.
Safety and Possible Side Effects
LLLT has demonstrated a remarkably low incidence of adverse effects when it has been used over 50 years for diverse medical conditions and in a variety of anatomical sites. In the specific area of LLLT for hair growth, the only adverse reports in humans, was the temporary onset of TE developing in the first 1–2 months after commencing LaserComb treatment [55], but disappearing on continued application. Some other possible considerations are presence of dysplastic or malignant lesions on the scalp which could be stimulated to grow by proliferative effects of LLLT [59].
CONCLUSION
LLLT was discovered serendipitously in the 1960s when mice irradiated with a low fluence red laser grew hair. Since that time LLLT has demonstrated promise in conditions from wound healing to stroke recovery, from treatment of musculoskeletal pain to prevention of mucositis. Animal and human data have slowly accumulated supporting LLLT for hair growth (Table 1). LLLT appears to improve a variety of non-scarring alopecias—AGA, AA, and chemotherapy-induced alopecia. Based on the studies demonstrating LLLT's effects on promoting graft survival, it may be further suggested to have a potential to be used during the immediate period of post-hair transplant surgery to facilitate the healing process and enhance viability and earlier growth of the grafts [60,61]. While mechanisms are still emerging, LLLT may increase anagen hairs through release of NO from CCO by photodissociation and LLLT may reduce inflammation in AA. However, more studies are needed to optimize treatment parameters and determine long-term efficacy as well as safety of emerging LLLT technologies. Most studies investigating effects of LLLT on hair growth have used wavelengths that range from 635 to 650 nm, but as of today no study has compared the effect of near-infrared wavelengths such as 810 nm, which have deeper penetrating capacities, to red light. Moreover, further studies are required to compare efficacy of different light sources (continuous vs. pulsed) and methods of light delivery (laser vs. LED).
TABLE 1.
Summary of the Studies That Investigated the Efficacy of LLLT for Hair Growth
| Author, year | Subject group | Alopecia type | Device type, parameters and treatment regimen | Refs. |
|---|---|---|---|---|
| Wikramanayake et al., 2012 | C3H/HeJ mice | Alopecia areata | HairMaxLaserComb®, 655 nm, 20 seconds daily, 3 times/week, for 6 weeks | [19] |
| Shukla et al., 2010 | Swiss albino mice | Testosterone treated (increase in catagen follicles) vs. non-treated | 632 nm, 1 and 5 J/cm2 at 24-hour intervals for 5 days | [49] |
| Trueb, 2009 | Rat model for chemotherapy-induced alopecia | Chemotherapy-induced alopecia | Laser unit and switch from the HairMaxLaserComb®, but without the comb or handle | [52] |
| Yamazaki et al., 2003 | 6 male and 9 female patients | Alopecia areata | Super Lizer™ emitting polarized pulsed linear light, 600–1,600 nm, 1.8 W. 3 minutes/week or every other week. Additional supplements and medications have been given. Treated until vellus hair regrowth in at least 50% of the affected area was observed. | [53] |
| Kim et al., 2007 | 24 male patients | Androgenetic alopecia | 655 and 780 nm, once a day for 10 minutes, for 14 weeks | [54] |
| Satino et al., 2003 | 28 male and 7 female patients | Androgenetic alopecia | HairMaxLaserComb® 655 nm, 5–10 minutes every other day, for 6 months | [55] |
| Lanzafame et al., 2013 | 44 male patients | Androgenetic alopecia | Helmet (TOPHAT655) containing 21, 5 mW lasers and 30 LEDs, 655 ± 5 nm, 67.3 J/cm2 25 minutes every other day, for 16 weeks | [56] |
| Kim et al., 2013 | 40 patients | Androgenetic alopecia | Helmet type LLLT device, 650 nm laser with 630 and 660 nm LEDs, 92.15 mW/cm2, 47.90 J/cm2, 18 minutes/day, for 24 weeks | [57] |
| Leavitt et al., 2009 | 110 male patients | Androgenetic alopecia | HairMaxLaserComb, 3 times/week for 15 minutes, for 26 weeks | [30] |
ACKNOWLEDGMENT
Research in the Hamblin Laboratory is supported by US NIH Grant R01AI050875.
Contract grant sponsor: US NIH; Contract grant number: R01AI050875.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Michael R. Hamblin is on the scientific advisory board and holds stock in Transdermal Cap Inc. He has been on the scientific advisory board and has received sponsored research funding from Lexington Int. He has been an expert witness for Advanced Hair Studio Australia. Other authors reported no conflict of interest.
REFERENCES
- 1.Schindl A, Schindl M, Pernerstorfer-Schon H, Schindl L. Low-intensity laser therapy: A review. J Investig Med. 2000;48(5):312–326. [PubMed] [Google Scholar]
- 2.Bjordal JM, Couppe C, Chow RT, Tuner J, Ljunggren EA. A systematic review of low level laser therapy with location-specific doses for pain from chronic joint disorders. Aust J Physiother. 2003;49(2):107–116. doi: 10.1016/s0004-9514(14)60127-6. [DOI] [PubMed] [Google Scholar]
- 3.Brosseau L, Welch V, Wells G, deBie R, Gam A, Harman K, Morin M, Shea B, Tugwell P. Low level laser therapy (classes I, II and III) in the treatment of rheumatoid arthritis. Cochrane Database Syst Rev. 2000;(2):CD002049. doi: 10.1002/14651858.CD002049. [DOI] [PubMed] [Google Scholar]
- 4.Cauwels RG, Martens LC. Low level laser therapy in oral mucositis: A pilot study. Eur Arch Paediatr Dent. 2011;12(2):118–123. doi: 10.1007/BF03262791. [DOI] [PubMed] [Google Scholar]
- 5.Christie A, Jamtvedt G, Dahm KT, Moe RH, Haavardsholm EA, Hagen KB. Effectiveness of nonpharmacological and nonsurgical interventions for patients with rheumatoid arthritis: An overview of systematic reviews. Phys Ther. 2007;87(12):1697–1715. doi: 10.2522/ptj.20070039. [DOI] [PubMed] [Google Scholar]
- 6.Jamtvedt G, Dahm KT, Holm I, Flottorp S. Measuring physiotherapy performance in patients with osteoarthritis of the knee: A prospective study. BMC Health Serv Res. 2008;8:145. doi: 10.1186/1472-6963-8-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schubert MM, Eduardo FP, Guthrie KA, Franquin JC, Bensadoun RJ, Migliorati CA, Lloid CM, Eduardo CP, Walter NF, Marques MM, Hamdi M. A phase III randomized double-blind placebo-controlled clinical trial to determine the efficacy of low level laser therapy for the prevention of oral mucositis in patients undergoing hematopoietic cell transplantation. Support Care Cancer. 2007;15(10):1145–1154. doi: 10.1007/s00520-007-0238-7. [DOI] [PubMed] [Google Scholar]
- 8.Silva GB, Mendonca EF, Bariani C, Antunes HS, Silva MA. The prevention of induced oral mucositis with low-level laser therapy in bone marrow transplantation patients: A randomized clinical trial. Photomed Laser Surg. 2011;29(1):27–31. doi: 10.1089/pho.2009.2699. [DOI] [PubMed] [Google Scholar]
- 9.Metelitsa AI, Green JB. Home-use laser and light devices for the skin: An update. Semin Cutan Med Surg. 2011;30(3):144–147. doi: 10.1016/j.sder.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Paus R, Foitzik K. In search of the “hair cycle clock”: A guided tour. Differentiation. 2004;72(9–10):489–511. doi: 10.1111/j.1432-0436.2004.07209004.x. [DOI] [PubMed] [Google Scholar]
- 11.Braun KM, Niemann C, Jensen UB, Sundberg JP, Silva-Vargas V, Watt FM. Manipulation of stem cell proliferation and lineage commitment: Visualisation of label-retaining cells in wholemounts of mouse epidermis. Development. 2003;130(21):5241–5255. doi: 10.1242/dev.00703. [DOI] [PubMed] [Google Scholar]
- 12.Tiede S, Kloepper JE, Bodo E, Tiwari S, Kruse C, Paus R. Hair follicle stem cells: Walking the maze. Eur J Cell Biol. 2007;86(7):355–376. doi: 10.1016/j.ejcb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Plikus MV, Sundberg JP, Chuong CM. Mouse skin ectodermal organs. In: Fox JBS, Davisson M, editors. The mouse in biomedical research. Academic Press; New York: 2006. pp. 691–694. [Google Scholar]
- 14.Otberg N, Finner AM, Shapiro J. Androgenetic alopecia. Endocrinol Metab Clin North Am. 2007;36(2):379–398. doi: 10.1016/j.ecl.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Ghanaat M. Types of hair loss and treatment options, including the novel low-level light therapy and its proposed mechanism. South Med J. 2010;103(9):917–921. doi: 10.1097/SMJ.0b013e3181ebcf71. [DOI] [PubMed] [Google Scholar]
- 16.Itami S, Inui S. Role of androgen in mesenchymal epithelial interactions in human hair follicle. J Investig Dermatol Symp Proc. 2005;10(3):209–211. doi: 10.1111/j.1087-0024.2005.10107.x. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann R, Happle R. Current understanding of androgenetic alopecia. Part I: Etiopathogenesis. Eur J Dermatol. 2000;10(4):319–327. [PubMed] [Google Scholar]
- 18.Rogers NE, Avram MR. Medical treatments for male and female pattern hair loss. J Am Acad Dermatol. 2008;59(4):547–566. doi: 10.1016/j.jaad.2008.07.001. quiz 567–548. [DOI] [PubMed] [Google Scholar]
- 19.Wikramanayake TC, Rodriguez R, Choudhary S, Mauro LM, Nouri K, Schachner LA, Jimenez JJ. Effects of the Lexington LaserComb on hair regrowth in the C3H/HeJ mouse model of alopecia areata. Lasers Med Sci. 2012;27(2):431–436. doi: 10.1007/s10103-011-0953-7. [DOI] [PubMed] [Google Scholar]
- 20.Rangwala S, Rashid RM. Alopecia: A review of laser and light therapies. Dermatol Online J. 2012;18(2):3. [PubMed] [Google Scholar]
- 21.Wasserman D, Guzman-Sanchez DA, Scott K, McMichael A. Alopecia areata. Int J Dermatol. 2007;46(2):121–131. doi: 10.1111/j.1365-4632.2007.03193.x. [DOI] [PubMed] [Google Scholar]
- 22.Trueb RM. Chemotherapy-induced alopecia. Semin Cutan Med Surg. 2009;28(1):11–14. doi: 10.1016/j.sder.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Mester E, Ludany G, Sellyei M, Szende B, Gyenes G, Tota GJ. Studies on the inhibiting and activating effects of laser beams. Langenbecks Arch Chir. 1968;322:1022–1027. doi: 10.1007/BF02453990. [DOI] [PubMed] [Google Scholar]
- 24.Barolet D. Light-emitting diodes (LEDs) in dermatology. Semin Cutan Med Surg. 2008;27(4):227–238. doi: 10.1016/j.sder.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Vlachos SP, Kontoes PP. Development of terminal hair following skin lesion treatments with an intense pulsed light source. Aesthetic Plast Surg. 2002;26(4):303–307. doi: 10.1007/s00266-002-2002-1. [DOI] [PubMed] [Google Scholar]
- 26.Moreno-Arias GA, Castelo-Branco C, Ferrando J. Side-effects after IPL photodepilation. Dermatol Surg. 2002;28(12):1131–1134. doi: 10.1046/j.1524-4725.2002.02117.x. [DOI] [PubMed] [Google Scholar]
- 27.Moreno-Arias G, Castelo-Branco C, Ferrando J. Paradoxical effect after IPL photoepilation. Dermatol Surg. 2002;28(11):1013–1016. doi: 10.1046/j.1524-4725.2002.02101.x. discussion 1016. [DOI] [PubMed] [Google Scholar]
- 28.Bernstein EF. Hair growth induced by diode laser treatment. Dermatol Surg. 2005;31(5):584–586. doi: 10.1111/j.1524-4725.2005.31168. [DOI] [PubMed] [Google Scholar]
- 29.Bouzari N, Firooz AR. Lasers may induce terminal hair growth. Dermatol Surg. 2006;32(3):460. doi: 10.1111/j.1524-4725.2006.32092.x. [DOI] [PubMed] [Google Scholar]
- 30.Leavitt M, Charles G, Heyman E, Michaels D. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: A randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig. 2009;29(5):283–292. doi: 10.2165/00044011-200929050-00001. [DOI] [PubMed] [Google Scholar]
- 31.Eells JT, Wong-Riley MT, VerHoeve J, Henry M, Buchman EV, Kane MP, Gould LJ, Das R, Jett M, Hodgson BD, Margolis D, Whelan HT. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion. 2004;4(5–6):559–567. doi: 10.1016/j.mito.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 32.Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40(2):516–533. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lohr NL, Keszler A, Pratt P, Bienengraber M, Warltier DC, Hogg N. Enhancement of nitric oxide release from nitrosyl hemoglobin and nitrosyl myoglobin by red/near infrared radiation: Potential role in cardioprotection. J Mol Cell Cardiol. 2009;47(2):256–263. doi: 10.1016/j.yjmcc.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Makihara E, Masumi S. Blood flow changes of a superficial temporal artery before and after low-level laser irradiation applied to the temporomandibular joint area. Nihon Hotetsu Shika Gakkai Zasshi. 2008;52(2):167–170. doi: 10.2186/jjps.52.167. [DOI] [PubMed] [Google Scholar]
- 35.Miura Y, Yamazaki M, Tsuboi R, Ogawa H. Promotion of rat hair growth by irradiation using Super LizerTM. Jpn J Dermatol. 1999;109(13):2149–2152. [Google Scholar]
- 36.Hou YC, Janczuk A, Wang PG. Current trends in the development of nitric oxide donors. Curr Pharm Des. 1999;5(6):417–441. [PubMed] [Google Scholar]
- 37.Proctor PH. Endothelium-derived relaxing factor and minoxidil: Active mechanisms in hair growth. Arch Dermatol. 1989;125(8):1146. [PubMed] [Google Scholar]
- 38.Rossi A, Cantisani C, Melis L, Iorio A, Scali E, Calvieri S. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6(2):130–136. doi: 10.2174/187221312800166859. [DOI] [PubMed] [Google Scholar]
- 39.Karu TI, Pyatibrat LV, Afanasyeva NI. Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg Med. 2005;36(4):307–314. doi: 10.1002/lsm.20148. [DOI] [PubMed] [Google Scholar]
- 40.Tuby H, Maltz L, Oron U. Modulations of VEGF and iNOS in the rat heart by low level laser therapy are associated with cardioprotection and enhanced angiogenesis. Lasers Surg Med. 2006;38(7):682–688. doi: 10.1002/lsm.20377. [DOI] [PubMed] [Google Scholar]
- 41.Karu TI. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem Photobiol. 2008;84(5):1091–1099. doi: 10.1111/j.1751-1097.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- 42.Karu TI, Pyatibrat LV, Afanasyeva NI. A novel mitochondrial signaling pathway activated by visible-to-near infrared radiation. Photochem Photobiol. 2004;80(2):366–372. doi: 10.1562/2004-03-25-RA-123. [DOI] [PubMed] [Google Scholar]
- 43.Ignatov YD, Vislobokov AI, Vlasov TD, Kolpakova ME, Mel'nikov KN, Petrishchev IN. Effects of helium-neon laser irradiation and local anesthetics on potassium channels in pond snail neurons. Neurosci Behav Physiol. 2005;35:871–875. doi: 10.1007/s11055-005-0137-7. [DOI] [PubMed] [Google Scholar]
- 44.Castex-Rizzi N, Lachgar S, Charveron M, Gall Y. Implication of VEGF, steroid hormones and neuropeptides in hair follicle cell responses. Ann Dermatol Venereol. 2002;129(5 Pt 2):783–786. [PubMed] [Google Scholar]
- 45.Weiss R, McDaniel DH, Geronemus RG, Weiss M. LED photomodulation induced hair growth stimulation. 2005;36(S17):27. [Google Scholar]
- 46.Yano K, Brown LF, Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest. 2001;107:409–417. doi: 10.1172/JCI11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamazaki M, Tsuboi R, Lee YR, Ishidoh K, Mitsui S, Ogawa H. Hair cycle-dependent expression of hepatocyte growth factor (HGF) activator, other proteinases, and proteinase inhibitors correlates with the expression of HGF in rat hair follicles. J Investig Dermatol Symp Proc. 1999;4(3):312–315. doi: 10.1038/sj.jidsp.5640236. [DOI] [PubMed] [Google Scholar]
- 48.Meneguzzo DT, Lopes LA, Pallota R, Soares-Ferreira L, Lopes-Martins RA, Ribeiro MS. Prevention and treatment of mice paw edema by near-infrared low-level laser therapy on lymph nodes. Lasers Med Sci. 2013;28(3):973–980. doi: 10.1007/s10103-012-1163-7. [DOI] [PubMed] [Google Scholar]
- 49.Shukla S, Sahu K, Verma Y, Rao KD, Dube A, Gupta PK. Effect of helium-neon laser irradiation on hair follicle growth cycle of Swiss albino mice. Skin Pharmacol Physiol. 2010;23(2):79–85. doi: 10.1159/000265678. [DOI] [PubMed] [Google Scholar]
- 50.Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, Stenn KS, Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117(1):3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- 51.Philp D, Nguyen M, Scheremeta B, St-Surin S, Villa AM, Orgel A, Kleinman HK, Elkin M. Thymosin beta4 increases hair growth by activation of hair follicle stem cells. FASEB J. 2004;18(2):385–387. doi: 10.1096/fj.03-0244fje. [DOI] [PubMed] [Google Scholar]
- 52.Wikramanayake TC, Villasante AC, Mauro LM, Nouri K, Schachner LA, Perez CI, Jimenez JJ. Low-level laser treatment accelerated hair regrowth in a rat model of chemotherapy-induced alopecia (CIA). Lasers Med Sci. 2013;28(3):701–706. doi: 10.1007/s10103-012-1139-7. [DOI] [PubMed] [Google Scholar]
- 53.Yamazaki M, Miura Y, Tsuboi R, Ogawa H. Linear polarized infrared irradiation using Super Lizer is an effective treatment for multiple-type alopecia areata. Int J Dermatol. 2003;42(9):738–740. doi: 10.1046/j.1365-4362.2003.01968.x. [DOI] [PubMed] [Google Scholar]
- 54.Kim SS, Park MW, Lee CJ. Phototherapy of androgenetic alopecia with low level narrow band 655-nm red light and 780-nm infrared light.. J Am Acad Dermatolog; American Academy of Dermatology 65th Annual Meeting.2007. p. AB112. [Google Scholar]
- 55.Satino JL, Markou M. Hair regrowth and increased hair tensile strength using the HairMax LaserComb for Low-Level Laser Therapy. Int J Cos Surg Aest Dermatol. 2003;5:113–117. [Google Scholar]
- 56.Lanzafame R, Blanche R, Bodian A, Chiacchierini R, Fenandez-Obregon A, Kazmirek E, Raymond J. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45(S25):12. doi: 10.1002/lsm.22173. [DOI] [PubMed] [Google Scholar]
- 57.Kim H, Choi JW, Kim JY, Shin JW, Lee SJ, Huh CH. Low-level light therapy for androgenetic alopecia: A 24-week, randomized, double-blind. Sham Device-Controlled Multicenter Trial. Dermatol Surg. 2013;39(8):1177–1183. doi: 10.1111/dsu.12200. [DOI] [PubMed] [Google Scholar]
- 58.Avram MR, Rogers NE. The use of low-level light for hair growth: Part I. J Cosmetic Laser Ther. 2009;11(2):110–117. doi: 10.1080/14764170902842531. [DOI] [PubMed] [Google Scholar]
- 59.Frigo L, Luppi JS, Favero GM, Maria DA, Penna SC, Bjordal JM, Bensadoun RJ, Lopes-Martins RA. The effect of low-level laser irradiation (In-Ga–Al–AsP—660 nm) on melanoma in vitro, in vivo. BMC Cancer. 2009;9:404. doi: 10.1186/1471-2407-9-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pinfildi CE, Hochman BS, Nishioka MA, Sheliga TR, Neves MA, Liebano RE, Ferreira LM. What is better in TRAM flap survival: LLLT single or multi-irradiation? Lasers Med Sci. 2013;28(3):755–761. doi: 10.1007/s10103-012-1130-3. [DOI] [PubMed] [Google Scholar]
- 61.Prado RP, Garcia SB, Thomazini JA, Piccinato CE. Effects of 830 and 670 nm laser on viability of random skin flap in rats. Photomed Laser Surg. 2012;30(8):418–424. doi: 10.1089/pho.2011.3042. [DOI] [PubMed] [Google Scholar]