Abstract
Purpose
In an effort to identify molecular markers of tumor aggressiveness and therapeutic targets in lung adenocarcinoma (ADC), we investigated the expression of mesothelin (MSLN) in lung ADC, as well as its biological and clinical relevance.
Experimental Design
In a training and validation set of patients with early-stage (I–III) lung ADC (n=1209), a tissue microarray consisting of tumors and normal lung tissue was used to examine the association between MSLN expression and recurrence-free survival (RFS) and overall survival (OS). The influence of MSLN overexpression on lung ADC was investigated in vitro and in vivo by use of clinically relevant orthotopic and metastatic xenogeneic and syngeneic mouse models.
Results
MSLN was expressed in 69% of lung ADC tumors, with one in five patients strongly expressing MSLN and no expression in normal lung tissue. Increased MSLN expression was associated with reduced OS (HR, 1.78 [95% CI, 1.26–2.50]; P<0.01) and RFS (HR, 1.67 [95% CI, 1.21–2.27]; P<0.01) in multivariate analyses, even after adjustment for currently known markers of tumor aggressiveness in lung ADC: male sex, smoking history, increasing stage, morphologic pattern, visceral pleural invasion, lymphatic or vascular invasion, and mutation status. In vitro, lung ADC cells overexpressing MSLN demonstrated increased cell proliferation, migration, and invasion; in vivo, mice with MSLN(+) tumors demonstrated decreased survival (P=0.001).
Conclusions
MSLN expression in patients with early-stage lung ADC is associated with increased risk of recurrence and reduced OS, indicating that MSLN expression is a molecular marker of tumor aggressiveness and a potential target for therapy.
Keywords: Mesothelin, lung adenocarcinoma, prognosis, targeted therapy, non-small cell lung cancer
Introduction
For patients with lung adenocarcinoma (ADC), the most frequent subtype of lung cancer (1), prognosis is stage dependent: 5-year survival is 73% for stage IA patients, 58% for stage IB patients, and 24% for stage IIIA patients, even after combined chemotherapy and resection with curative intent (2). Currently, targeted therapy is available for EGFR mutant tumors, which constitute 15% of lung ADC tumors (3–5). For patients with EGFR wild-type tumors, no clinical or molecular biomarker (other than stage) has been prospectively demonstrated to further inform decision-making. While molecular-targeted approaches such as targeted cellular immunotherapy are promising (6), candidate target antigens in lung ADC are limited and require further investigation.
Mesothelin (MSLN) is a cell-surface glycoprotein overexpressed in mesothelioma and pancreatic and ovarian carcinomas (7) and is associated with poor prognoses (8, 9). Furthermore, MSLN has been shown to promote peritoneal metastasis in ovarian ADC via its binding interaction with CA-125 (10) and its suppression of cell death (11). MSLN expression is associated with neoplastic progression in Barrett’s-associated esophageal ADC (12) and in triple-negative breast cancer (13). Although the expression of MSLN in lung ADC has been previously described in a small cohort of patients (14), its clinical and biological significance remain undefined. To further delineate the role of MSLN in lung ADC, we examined its expression, clinical characteristics, and patient survival in the largest series to date. On the basis of our clinical observations, we hypothesized that MSLN expression in lung ADC promotes an aggressive tumor phenotype, resulting in poor outcomes.
Materials and Methods
Patient selection
With institutional review board approval at Memorial Sloan-Kettering Cancer Center (MSKCC), we investigated 1252 patients diagnosed with stage I to III lung ADC who underwent surgical resection at MSKCC from 1995 to 2009. Overall survival (OS) and recurrence-free survival (RFS) were examined in the clinical cohort, from the time of surgical resection until the time of death (OS) or until the first relapse or death, whichever came first (RFS). First relapse was confirmed by pathologic diagnosis of the biopsy specimen. Patients who did not experience the event of interest by the end of the study were censored at the time of the last available follow-up.
Tissue microarray (TMA) and immunohistochemistry
Two pathologists independently reviewed hematoxylin and eosin (H&E)–stained slides (1–12 slides per patient) and reported (a) histologic subtypes according to the seventh edition of the IASLC/ATS/ERS classification, (b) visceral-pleural invasion (VPI) as either absent (PLX, PL0) or present (PL1, PL2, PL3), and (c) lymphatic and vascular invasion. Four to six representative tumor areas were marked on H&E-stained slides, and four cylindrical, 0.6-mm cores were arrayed into a block by use of an automated arrayer. Paraffin sections 5 μm in thickness were cut from the TMA and stained for MSLN immunohistochemical analysis using specific antibodies (Vector clone 5B2, 1:200 dilution) (15). Grading of MSLN staining intensity was performed by a pathologist who was blinded to the clinical data: 0 (staining absent), 1 (weak expression), 2 (moderate expression), and 3 (strong expression). The distribution of MSLN-positive tumor cells, among the tumor cells in each core, was graded as 0 (staining absent), 1 (1%–50%), or 2 (51%–100%). The sum of the MSLN stain intensity and the distribution grade determined the total MSLN score, ranging from 0 to 5. The MSLN score for each patient was then determined using the average of all of the patient’s tumor cores (16).
Mutation and gene expression analysis
After histologic confirmation of lung ADC, DNA was extracted from tumor specimens. EGFR exon 19 deletions and exon 21 L858R mutations were identified by polymerase chain reaction, as described previously (17). Standard direct sequencing was used to identify KRAS codon 12 and 13 mutations (18).
In vitro assays
Human lung ADC cell lines H1299 and A549 and Lewis lung carcinoma (LLC) mouse lung ADC cells were purchased from the American Type Culture Collection, grown in standard media, and used for cell flow cytometric analyses, cell proliferation assays (using a Countess automated cell counter; Invitrogen, Carlsbad, CA), and invasion/migration Boyden chamber assays (using BD Matrigel Chambers; BD Biosciences, San Jose, CA). Green fluorescent protein (GFP)–firefly luciferase fusion and human MSLN genes were transduced into lung ADC cells by use of SFG retroviral vectors, as described previously(15). The mouse MSLN sequence was obtained from the Mammalian Gene Collection (Invitrogen, Carlsbad, CA), subcloned into a monocistronic SFG retroviral vector, and confirmed by sequencing. For experiments comparing MSLN-transduced cells with MSLN-negative cells, a transduction control was performed with the GFP-luciferase vector, to control for any direct effects of retroviral transduction.
Orthotopic and metastatic lung ADC mouse models
Female SCID/beige mice (for experiments using H1299 cells) or female NOD-SCID-γC mice (for experiments using A549 cells), 6 to 10 weeks old, were used. For the orthotopic lung ADC mouse model, a H1299 cell suspension mixed with ice-cold Matrigel at a ratio of 1:1, for a final concentration of 10×106 cells/mL, was directly injected into the left lung of anesthetized mice, in a sterile fashion under direct visualization, using a 30-gauge needle attached to a 0.1-mL Hamilton syringe (Reno, NV). The bioluminescence imaging (BLI) methodology has been described previously (19). For the intravenous lung ADC mouse model, NOD-SCID-γC mice were injected with 1×106 A549 cells in a volume of 200 μL of phosphate buffered saline. For in vivo tumor flow cytometric analysis, minced lung tumor was incubated in 0.2 mg/mL collagenase IV (Sigma-Aldrich, St. Louis, MO). The lysate was then strained using 100-um and 40-um filters. Erythrocytes were lysed using 500 μL of ACK lysing buffer (Invitrogen, Carlsbad, CA) and stained using APC-conjugated antihuman MSLN antibody.
Male C57BL/6 mice 6–8 weeks old were used in an intravenous model of lung cancer with LLC mouse lung ADC cells. Mice were injected with 1×105 cells in a volume of 200 μL of phosphate buffered saline.
Statistical analysis
Associations between clinicopathologic variables and TMA findings were analyzed using Pearson’s χ2 test (or Fisher’s exact test, when applicable) for categorical variables and the nonparametric Wilcoxon rank sum test for continuous variables. To determine the MSLN score that best discriminates patients with good or poor prognosis, we randomly split the cohort into a training set (66% of the data) and a validation set, stratified by surgery interval. The optimal score was identified in the training set by use of the minimum P value approach (20) and confirmed in the validation set. Once the cutoff was determined in this manner, the remaining results were reported for the entire cohort. RFS and OS were estimated using the Kaplan-Meier method. A Cox proportional hazards model was used to evaluate the independent association between MSLN expression and OS. The model was stratified by stage, to account for subpopulation differences, and adjustment was made for clinicopathologic variables that were correlated with OS in univariate analysis. All P values were based on two-tailed statistical analysis, and P<0.05 was considered to indicate statistical significance. All clinical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). Preclinical data were analyzed with GraphPad Prism (La Jolla, CA).
Results
Lung ADC MSLN expression and RFS and OS
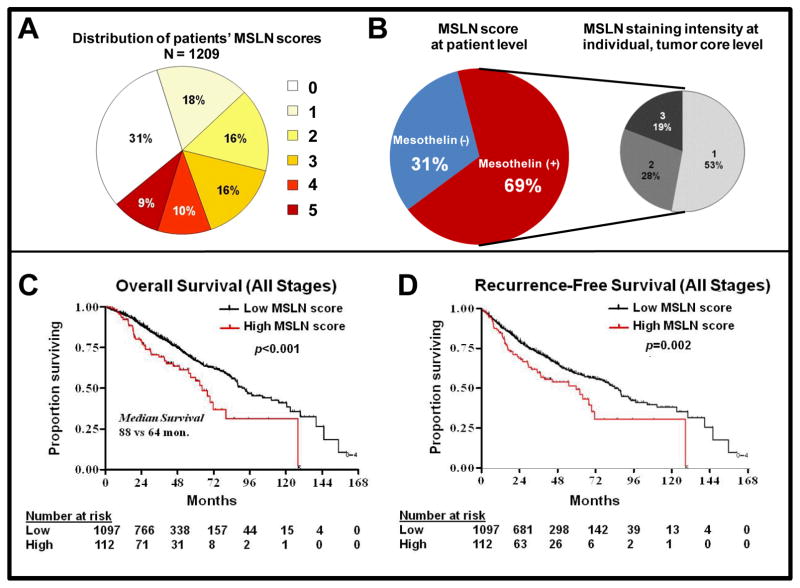
Of the 1209 patients who underwent anatomic resection and had an adequate specimen available for immunohistochemical analysis (Table 1), 69% had detectable levels of MSLN in their tumor, with no MSLN staining detected in normal lung tissue or tumor stroma. Specifically, we observed that individual tumor cells demonstrate strong cytoplasmic staining and mixed membranous staining with varying intensity and distribution (Supplementary Fig. S1A&B). This pattern of mesothelin staining is similar to what has been previously described (21). The distribution of MSLN scores among all patients is presented in Figure 1A to 1B.
Table 1.
Patient Characteristics According to Low and High Mesothelin (MSLN) Scores
| Patient Characteristic | All (N=1209) | MSLN Score Low (N=1097) | MSLN Score High (N=112) | P |
|---|---|---|---|---|
| Age, years, mean ± SD | 67.9 ± 6.9 | 67.8 ± 9.9 | 68.6 ± 9.2 | 0.50 |
| Sex | <0.001 | |||
| Male | 469 (39) | 405 (37) | 64 (57) | |
| Female | 740 (61) | 692 (63) | 48 (43) | |
| Smoking history | <0.001 | |||
| Current | 168 (14) | 147 (14) | 21 (19) | |
| Former | 828 (69) | 740 (68) | 88 (78) | |
| Never | 208 (17) | 205 (19) | 3 (3) | |
| Pack-years, median | 30 (10–54) | 30 (6–53) | 40 (25–60) | <0.001 |
| Stage | 0.466 | |||
| I | 987 (82) | 900 (82) | 87 (78) | |
| II | 129 (11) | 115 (11) | 14 (13) | |
| III | 93 (7) | 82 (7) | 10 (9) | |
| Histologic subtype | <0.001 | |||
| Solid/micropapillary | 295 (26) | 276 (26) | 19 (18) | |
| Acinar/papillary | 741 (64) | 665 (63) | 76 (74) | |
| Lepidic | 120 (10) | 112 (11) | 8 (8) | |
| Lymphatic invasion | 0.92 | |||
| Yes | 487 (40) | 441 (40) | 46 (41) | |
| No | 722 (60) | 656 (60) | 66 (59) | |
| Vascular invasion | 0.16 | |||
| Yes | 366 (30) | 339 (31) | 27 (24) | |
| No | 843 (70) | 758 (69) | 85 (76) | |
| Visceral pleural invasion | 0.39 | |||
| PLO | 779 (65) | 699 (64) | 80 (71) | |
| PL1 | 336 (28) | 309 (28) | 27 (24) | |
| PL2 | 77 (6) | 73 (7) | 4 (4) | |
| PL3 | 17 (1) | 16 (1) | 1 (1) | |
| Mutation status | <0.001 | |||
| No EGFR or KRAS | 471 (60) | 421 (60) | 50 (63) | |
| KRAS mutation | 200 (25) | 171 (24) | 29 (36) | |
| EGFR mutation | 116 (15) | 115 (16) | 1 (1) |
Note. Smoking history, histologic subtype, and mutation status were available from 1204, 1156, and 787 patients, respectively. Data are presented as no. (%), unless otherwse noted. SD, standard deviation.
Fig. 1. Mesothelin (MSLN) expression and clinicopathologic characteristics.
(A) The sum of MSLN staining intensity and distribution grades was used to determine the total MSLN score, ranging from 0 to 5, at the core level; all cores per patient were averaged to yield an MSLN score at the patient level. (B) Overall, 69% of patients had detectable levels of MSLN in their tumor cores; among MSLN(+) patients, 53%, 28%, and 19% of cores had MSLN staining intensity of 1 (weak), 2 (moderate), and 3 (strong), respectively. Overall survival (C) (P< 0.001) and recurrence-free survival (D) (P=0.002) of patients with stage I to III lung adenocarcinoma were reduced in those with high-level MSLN expression.
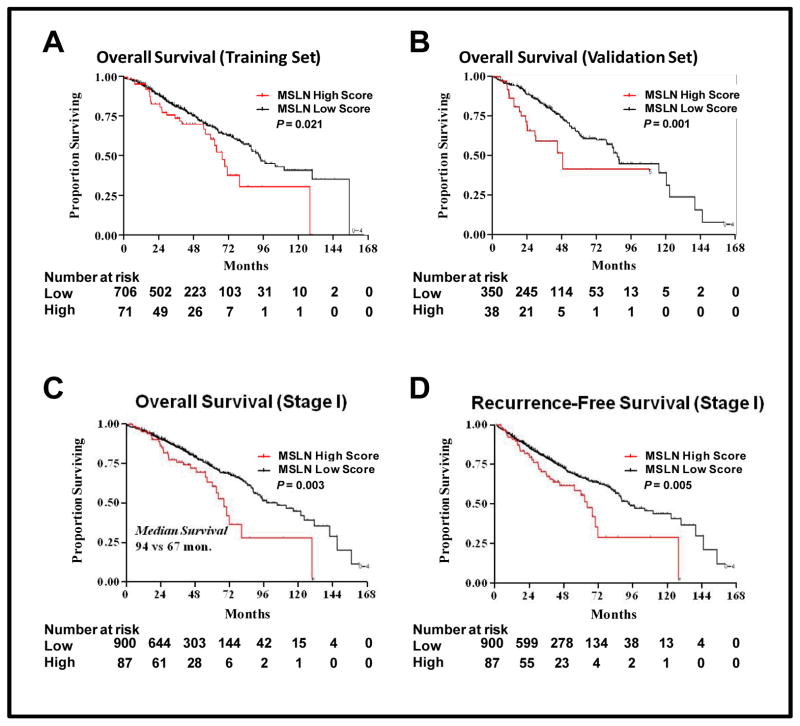
The systematic search for an optimal cutoff point in the training set indicated that the best separation, with respect to OS, was provided by categorizing low MSLN (score, 0–4) versus high MSLN (score, 5) (χ2=5.35; adjusted P=0.003; Fig. 2A). Testing in the validation set confirmed that patients with a high MSLN score had worse OS, compared with patients with a low MSLN score (five-year OS, 41% [95% CI, 18%–63%] vs 64% [95% CI, 56%–70%]; P=0.001; Fig. 2B). When the 44 patients who had received neoadjuvant chemotherapy were excluded from the overall cohort, patients with low-level MSLN expression had a survival advantage, compared with patients with high-level expression (five-year OS, 67% vs 54%; P<0.001; Fig. 1C). Patients with high-level MSLN expression had reduced RFS, compared with patients with low-level expression (five-year RFS, 48% [95% CI, 36%–59%] vs 59% [95% CI, 54%–62%]; P=0.002; Fig. 1D).
Fig. 2. Mesothelin (MSLN) expression and patient survival.
The optimal cutoff for comparison of overall survival (OS) was identified in a randomly split cohort. In the training set (A), patients with an MSLN score of 5 (MSLN high score) had reduced OS (P=0.021), compared with patients with an MSLN score of 0 to 4 (MSLN low score), which was confirmed in the validation set (B) (P=0.001). In patients with stage I lung adenocarcinoma, OS (C) (P=0.003) and RFS (D) (P=0.005) remained significantly reduced in patients with high-level MSLN expression.
Among patients with stage I disease (n=987), five-year OS was 72% (95% CI, 67%–75%) for patients with low-level MSLN expression and 60% (95% CI, 43%–72%) for patients with high-level expression (P=0.003; Fig. 2C). RFS was similarly improved in patients with stage I lung ADC with low MSLN (P=0.005; Fig. 2D). In the multivariate analysis (Table 2), high-level MSLN expression remained a significant independent predictor of RFS after adjustment for smoking history, stage, histologic subtype, VPI, and lymphatic and vascular invasion (hazard ratio [HR], 1.78 [95% CI, 1.26–2.50]; P <0.01).
Table 2.
Univariate and Multivariate Overall Survival (OS) Analysis
| Variable | No. | Univariate OS Analysis | Multivariate OS Analysis | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| HR | 95% CI | P | HRa | 95% CI | P | ||
| Mesothelin score | |||||||
| High (score, 5) | 112 | 1.74 | (1.28–2.38) | <0.001 | 1.78 | (1.26–2.50) | <0.01 |
| Low (score, 0–4) | 1097 | 1.00 | 1.00 | ||||
| Sex | |||||||
| Male | 469 | 1.55 | (1.25–1.91) | <0.001 | 1.48 | (1.18–1.86) | <0.01 |
| Female | 740 | 1.00 | |||||
| Smoking history | 0.05 | 0.21 | |||||
| Current | 168 | 1.57 | (1.07–2.31) | 1.40 | (0.92–2.13) | ||
| Former | 828 | 1.16 | (0.85–1.58) | 1.10 | (0.78–1.56) | ||
| Never | 208 | 1.00 | |||||
| Stage | <0.001 | ||||||
| III | 93 | 3.99 | (2.93–5.44) | ||||
| II | 129 | 2.08 | (1.53–2.83) | ||||
| I | 987 | 1.00 | |||||
| Tumor morphologic pattern | <0.001 | 0.021 | |||||
| Solid/micropapillary | 295 | 3.31 | (1.93–5.68) | 1.91 | (1.08–3.37) | ||
| Acinar/papillary | 741 | 1.98 | (1.17–3.34) | 1.41 | (0.82–2.40) | ||
| Lepidic | 120 | 1.00 | |||||
| Lymphatic invasion | <0.001 | 0.023 | |||||
| Yes | 487 | 2.05 | (1.66–2.54) | 1.35 | (1.04–1.74) | ||
| No (reference) | 722 | 1.00 | 1.00 | ||||
| Vascular invasion | <0.001 | 0.016 | |||||
| Yes | 366 | 1.96 | (1.58–2.43) | 1.35 | (1.05–1.72) | ||
| No | 843 | 1.00 | 1.00 | ||||
| Visceral pleural invasion | 0.008 | <0.001 | |||||
| PL3/PL2 | 94 | 1.23 | (0.88–1.73) | 1.43 | (1.00–2.05) | ||
| PL1 | 336 | 1.43 | (1.14–1.81) | 1.57 | (1.23–2.01) | ||
| PL0 (reference) | 779 | 1.00 | |||||
| Mutation | 0.25 | ||||||
| KRAS mutation | 200 | 1.53 | (0.92–2.57) | ||||
| Wild-type EGFR/KRAS | 471 | 1.34 | (0.85–2.11) | ||||
| EGFR mutation | 116 | 1.00 | |||||
The hazard ratio (HR) for the multivariate OS analysis was stratified by stage and adjusted for sex, smoking history, tumor morphologic pattern, lymphatic invasion, vascular invasion, and visceral pleural invasion. CI, confidence interval.
Lung ADC MSLN expression and clinicopathologic characteristics
Table 1 presents characteristics of patients according to high versus low MSLN level. Male sex was associated with high-level MSLN expression (P<0.001). Smoking history was associated with MSLN expression (P<0.001): 97% of patients with high-level MSLN expression were current or former smokers. Patients with high-level MSLN expression were found to have a higher number of pack-years, compared with patients with low-level expression (average, 45 vs 36 pack-years; P=0.008). Among smokers (current and former), a higher MSLN score was associated with increased average number of pack-years (P<0.001). High-level MSLN expression was associated with papillary-predominant lung ADC (P<0.001). More importantly, the currently known markers of tumor aggressiveness in lung ADC—solid or micropapillary morphologic pattern, VPI, lymphatic invasion, and vascular invasion—were not associated with high-level MSLN expression. Tumor EGFR and KRAS mutation status was available for 787 patients in our cohort. Patients with high-level MSLN expression were more likely to have KRAS mutations, compared with patients with low-level expression (36% vs 24%; P=0.018). In contrast, patients with low-level MSLN expression were more likely to have EGFR mutations (16% vs 1%).
In this cohort, the median follow-up among survivors was 37 months. In the univariate OS analysis, high-level MSLN expression (HR, 1.74; P<0.001), male sex (HR, 1.55; P<0.001), smoking history (current: HR, 1.57; former: HR, 1.16; P=0.052), increasing disease stage (stage III: HR, 3.99; stage II: HR, 2.08; P<0.001), aggressive morphologic pattern (solid/micropapillary: HR, 3.31; acinar/papillary: HR, 1.98; P<0.001), lymphatic invasion (HR, 2.05; P<0.001), vascular invasion (HR, 1.96; P<0.001), and VPI (PL3/PL2: HR, 1.23; PL1: HR, 1.43; P=0.008) were associated with reduced OS (Table 2). When these factors were adjusted for, high MSLN score remained an independent predictor of OS in the multivariate analysis (HR, 1.78; P<0.001). Subgroup analysis of the 787 patients whose tumors underwent mutation testing demonstrated that MSLN remained an independent predictor of OS, even after adjustment for mutation status (HR, 1.89; P=0.007).
MSLN expression promotes proliferation, invasion, and migration in lung ADC cells
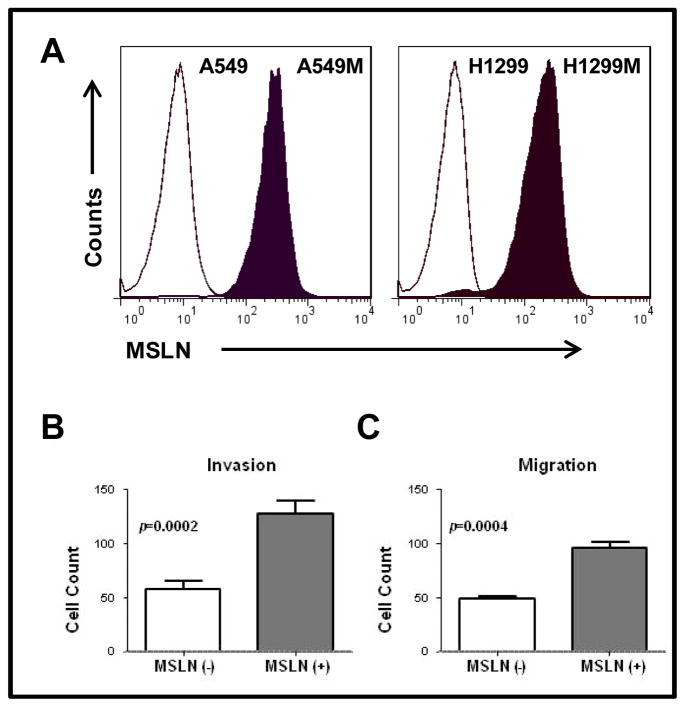
We noted no morphologic differences between cultured cells overexpressing MSLN and parental, untransduced cells and controls. Cell-counting assays demonstrated increased proliferation of MSLN(+) H1299M cells—approximately 1.6-fold greater at 48 hours (P<0.001)—compared with MSLN(−) H1299 cells transduced with the control vector. MSLN expression increased both cell invasion (2.21-fold increase; P=0.0002) and migration (1.98-fold increase; P=0.0004), compared with MSLN(−) control H1299 cells (Fig. 3).
Fig. 3. Mesothelin (MSLN) expression promotes lung adenocarcinoma (ADC) aggressiveness in vitro.
Fluorescence-activated cell sorting (FACS) was performed, after retroviral transduction, using FACSAria (BD Biosciences, San Jose, CA) to selectively obtain adequately transduced cells. FACS for green fluorescent protein (GFP) positivity demonstrated high-level expression of GFP–firefly luciferase. Human MSLN cell-surface expression was detected using allophycocyanin-conjugated antihuman MSLN rat IgG2A (R&D Systems, Minneapolis, MN). Subsequent flow cytometry for analysis of GFP and MSLN expression was performed using either FACSCaliber or LSRII flow cytometers (BD Biosciences, San Jose, CA). Resulting FACS data were analyzed using FlowJo (Tree Star, Ashland, OR) analysis software. (A) A549 and H1299 cells transduced to overexpress MSLN are designated “A549M” and “H1299M,” respectively. Human lung ADC cell lines transduced to overexpress MSLN were compared with cells transduced with control vector. For invasion assays, Matrigel-coated polyethylene terephthalate inserts with 8-um pores were used in 24-well plates. For migration assays, inserts without Matrigel coating were used. (B) MSLN overexpression in H1299 lung ADC cells (H1299M) increased invasion at 24 hours (44 vs 99 cells [2.21-fold increase]; P = 0.0002) and (C) migration at 12 hours (48 vs 96 cells [1.98-fold increase]; P = 0.0004).
MSLN expression promotes increased tumor progression and decreased survival in mouse models of lung ADC
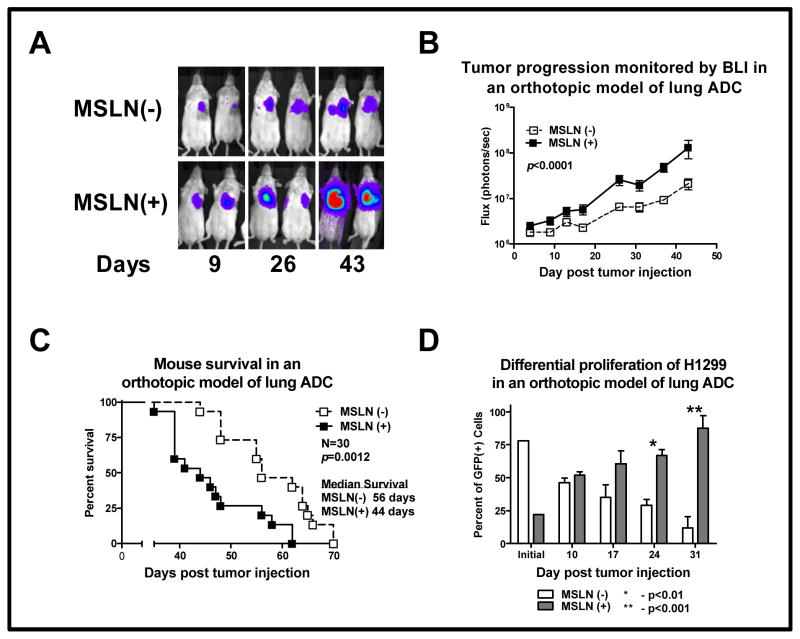
Mice with MSLN(+) H1299M tumors accumulated a greater BLI signal over time, suggesting an increased rate of tumor progression in vivo (Fig. 4A–B). This increased in vivo tumor progression correlated with decreased survival of mice with MSLN(+) H1299M orthotopic tumors (median OS, 44 vs 56 days; P=0.001; Fig. 4C); these results were reproduced in three independent experiments. In addition, in a metastatic mouse model with intravenous administration of MSLN(+) A549M lung ADC cells, mice demonstrated an increase in lung signal emission, signifying an increased tumor burden, compared with mice administered a similar number of MSLN(−) A549 cells at serial time points (P<0.05; Supplementary Fig. S2). MSLN(+) A549M mice had decreased median survival, compared with mice administered MSLN(−) A549 cells (P<0.0061). To determine whether increased tumor progression was related to in vivo tumor proliferation, mice were injected with a heterogeneous population of MSLN-expressing H1299 cells. The initial tumor injection contained 22% MSLN(+) H1299M cells. H1299M tumor cells, identified by GFP and MSLN positivity on flow cytometric analysis, proliferated at a greater rate in vivo than did MSLN(−) H1299 cells; H1299M became the dominant tumor cell population over time (Fig. 4D). This result was significant at days 24 (66% vs 29%; P<0.01) and 31 (87% vs 12%; P<0.001).
Fig. 4. Mesothelin (MSLN) expression promotes lung adenocarcinoma (ADC) tumor progression and decreased survival in vivo.
All control tumors were developed from cells transduced with control vector. An orthotopic mouse model of lung ADC was established in the left lung of mice by a single intrapulmonary injection of H1299 cells. (A) Mice were serially imaged using bioluminescence imaging (BLI) to noninvasively assess tumor progression over time. (B) Mice bearing MSLN(+) H1299M tumors accumulated a greater BLI signal over time (P<0.0001), suggesting increased tumor progression with MSLN overexpression. (C) This correlated with the decreased survival of mice bearing MSLN(+) H1299M orthotopic tumors (44 vs 56 days; P=0.0012). (D) To evaluate the relative in vivo proliferation of H1299 tumor cells with and without MSLN expression, we orthotopically injected mice with a heterogeneous population of MSLN-expressing H1299 cells. The initial tumor inoculum was 22% MSLN(+) H1299M on flow cytometry. Tumor cells, identified by green fluorescent protein expression by use of flow cytometry, were then gated for MSLN positivity. H1299M became the dominant tumor cell population over time; these results were statistically significant at days 24 (66% vs 29%; P< 0.01) and 31 (87% vs 12%; P<0.001).
We sought to confirm these findings in an immunocompetent syngeneic model of metastatic lung ADC. LLC cells stably transduced to overexpress murine MSLN were designated “LLCmM.” We observed an increase in cell invasion in vitro with MSLN overexpression (Supplementary Fig. S2) as well as demonstrated a decreased survival (38 vs 29 days; P =.0012; Supplementary Fig. S2) in mice with intravenously induced LLCmM tumors.
Discussion
In this study, we observed that MSLN is commonly expressed in lung ADC, even in stage I patients. In the largest series to date of patients with stage I to III lung ADC, MSLN expression correlated with decreased RFS and OS. In the multivariate analysis, high-level MSLN expression remained an independent predictor of survival. With these clinical observations, we hypothesized that MSLN expression was an independent driver of an aggressive tumor phenotype. The impetus to explore the expression and biological influence of MSLN on lung ADC is driven by (a) the lack of a clinically applicable molecular biomarker for the majority of lung ADC cases (other than EGFR mutation status), (b) the lack of targeted therapy for the majority of patients with lung ADC, (c) the evidence that MSLN-specific immune responses prolong survival in patients with pancreatic cancer, and (d) the promising preclinical and ongoing early-phase clinical studies that have demonstrated that MSLN may be an effective cancer antigen to target with immunotherapy in solid tumors (22–24).
In our study cohort, high-level MSLN expression was associated with male sex, history of smoking, and papillary-predominant lung ADC histologic subtype. More importantly, our data identify a unique cohort of patients with lung ADC with poor prognosis by MSLN expression; these patients did not possess current known features of aggressive lung ADC (VPI and lymphovascular invasion). We also observed that MSLN expression was a marker of worse clinical prognosis, independent of smoking or KRAS mutation status. More importantly, our data suggest that the influence of MSLN on increased cell proliferation and aggressiveness in vitro and in vivo can be seen in multiple human and mouse lung ADC cells, including in KRAS-negative H1299 cells. The identification of MSLN expression in 69% of stage I to III lung ADC tumors is significant in itself, given the low frequency of candidate target antigens and genes in lung ADC. Furthermore, detection of elevated levels of serum MSLN may provide a noninvasive marker for the detection of lung ADC recurrence (9, 12). A prospective clinical trial is currently under way at our institution to evaluate the prognostic utility of tissue and serum MSLN in lung ADC. While heterogeneous expression does occur, a significant percentage of MSLN(+) tumors had high-level expression of MSLN. The association between high-level MSLN expression and KRAS mutations lends rationale to investigating MSLN-targeted therapies in lung ADC, as this subset of patients possesses a tumor phenotype that is resistant to currently approved targeted therapies (3).
Our group has previously reported the role of MSLN in promoting invasion and matrix metallopeptidase–9 expression in malignant pleural mesothelioma, both in an orthotopic mouse model and in patients (25). In addition, we have documented the role of serum and tissue MSLN as a biomarker of neoplastic progression in Barrett’s-associated esophageal ADC (12). We and others have demonstrated MSLN expression in triple-negative breast cancer. Although our goal in this explorative study was not to elucidate the explicit biological pathway of MSLN in lung ADC, our preclinical observations show an association between MSLN expression and increased tumor aggressiveness (Supplementary Fig. S3). Furthermore, MSLN expression remains present in lung ADC from stage I to stage III. Perhaps most importantly, targeting MSLN-expressing lung ADC, which is resistant to the current standard-of-care therapy, provides a new therapeutic opportunity. Targeting MSLN with the immunotoxin SS1P has shown in vivo specificity and antitumor activity (26–28), including in patients with non-small cell lung cancer (29).
The immunogenicity of MSLN as a viable cellular therapy target has been demonstrated in vaccine trials using pancreatic ADC cells and granulocyte-macrophage colony–stimulating factor. MSLN-targeted adoptive T-cell therapies have demonstrated efficacy in preclinical models of mesothelioma and ovarian cancer (23, 30), and this approach is currently being tested in clinical trials for other malignancies (6).
MSLN is a molecular marker commonly expressed in lung ADC, and as an independent predictor of worse survival, it identifies a tumor phenotype with increased aggressiveness and invasive properties. As MSLN overexpression provides a likely advantage to lung ADC cells, this antigen is possibly important to the tumor’s growth and dissemination. Thus, MSLN expression provides a potential therapeutic target in patients with aggressive and therapy-resistant lung ADC for future clinical trials.
Supplementary Material
Statement of translational relevance.
Given the lack of prognostic markers and cancer-antigen targets in lung adenocarcinoma (ADC), we sought to determine whether mesothelin, a cell-surface cancer-associated antigen expressed in lung ADC, could influence patient outcomes and tumor phenotype. Mesothelin was expressed in 69% of early-stage lung ADC, with strong expression in one of five patients. High-level expression of mesothelin was associated with reduced overall and recurrence-free survival in a multivariate analysis. In orthotopic and metastatic mouse models of lung ADC, human and murine lung ADC cells overexpressing mesothelin demonstrated increased in vivo proliferation and tumor progression and decreased survival. In summary, mesothelin is a potential biomarker and a candidate for targeted therapy in lung ADC.
Acknowledgments
Financial Support
This work was supported, in part, by the International Association for the Study of Lung Cancer Young Investigator Award; National Lung Cancer Partnership/LUNGevity Foundation Research Grant; American Association for Thoracic Surgery Third Edward D. Churchill Research Scholarship; William H. Goodwin and Alice Goodwin, the Commonwealth Foundation for Cancer Research and the Experimental Therapeutics Center;AACR Stand Up to Cancer and Cancer Research Institute Immunology Translational Research Dream Team; the National Cancer Institute (grants R21 CA164568-01A1); and the U.S. Department of Defense (grants PR101053 and LC110202).
We thank Irina Linkov, for her technical assistance and tireless efforts. We are grateful for Daniel Ngai’s assistance in the experiments. We also thank David Sewell, for his help with manuscript preparation.
Footnotes
COI statement
All authors have no potential conflicts of interest.
References
- 1.Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: Male:Female differences diminishing and adenocarcinoma rates rising. International Journal of Cancer. 2005;117:294–9. doi: 10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- 2.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. The New England journal of medicine. 2004;350:351–60. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 3.Marks JL, Broderick S, Zhou Q, Chitale D, Li AR, Zakowski MF, et al. Prognostic and therapeutic implications of EGFR and KRAS mutations in resected lung adenocarcinoma. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2008;3:111–6. doi: 10.1097/JTO.0b013e318160c607. [DOI] [PubMed] [Google Scholar]
- 4.Marchetti A, Martella C, Felicioni L, Barassi F, Salvatore S, Chella A, et al. EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:857–65. doi: 10.1200/JCO.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 5.D’Angelo SP, Pietanza MC, Johnson ML, Riely GJ, Miller VA, Sima CS, et al. Incidence of EGFR exon 19 deletions and L858R in tumor specimens from men and cigarette smokers with lung adenocarcinomas. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:2066–70. doi: 10.1200/JCO.2010.32.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brentjens RJ, Rivière I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011 doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassan R, Bera T, Pastan I. Mesothelin. Clinical Cancer Research. 2004;10:3937–42. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 8.Cheng WF, Huang CY, Chang MC, Hu YH, Chiang YC, Chen YL, et al. High mesothelin correlates with chemoresistance and poor survival in epithelial ovarian carcinoma. Br J Cancer. 2009;100:1144–53. doi: 10.1038/sj.bjc.6604964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cristaudo A, Foddis R, Vivaldi A, Guglielmi G, Dipalma N, Filiberti R, et al. Clinical Significance of Serum Mesothelin in Patients with Mesothelioma and Lung Cancer. Clinical Cancer Research. 2007;13:5076–81. doi: 10.1158/1078-0432.CCR-07-0629. [DOI] [PubMed] [Google Scholar]
- 10.Gubbels J, Belisle J, Onda M, Rancourt C, Migneault M, Ho M, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Molecular Cancer. 2006;5:50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang T, Kajino K, Abe M, Tan K, Maruo M, Sun G, et al. Suppression of cell death by the secretory form of N-terminal ERC/mesothelin. International journal of molecular medicine. 2010;26:185–91. doi: 10.3892/ijmm_00000451. [DOI] [PubMed] [Google Scholar]
- 12.Rizk NP, Servais EL, Tang LH, Sima CS, Gerdes H, Fleisher M, et al. Tissue and Serum Mesothelin Are Potential Markers of Neoplastic Progression in Barrett’s Associated Esophageal Adenocarcinoma. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012 doi: 10.1158/1055-9965.EPI-11-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tchou J, Wang LC, Selven B, Zhang H, Conejo-Garcia J, Borghaei H, et al. Mesothelin, a novel immunotherapy target for triple negative breast cancer. Breast cancer research and treatment. 2012;133:799–804. doi: 10.1007/s10549-012-2018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho M, Bera TK, Willingham MC, Onda M, Hassan R, FitzGerald D, et al. Mesothelin Expression in Human Lung Cancer. Clinical Cancer Research. 2007;13:1571–5. doi: 10.1158/1078-0432.CCR-06-2161. [DOI] [PubMed] [Google Scholar]
- 15.Servais EL, Colovos C, Rodriguez L, Bograd AJ, Nitadori J, Sima C, et al. Mesothelin overexpression promotes mesothelioma cell invasion and MMP-9 secretion in an orthotopic mouse model and in epithelioid pleural mesothelioma patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:2478–89. doi: 10.1158/1078-0432.CCR-11-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rekhtman N, Ang DC, Sima CS, Travis WD, Moreira AL. Immunohistochemical algorithm for differentiation of lung adenocarcinoma and squamous cell carcinoma based on large series of whole-tissue sections with validation in small specimens. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2011;24:1348–59. doi: 10.1038/modpathol.2011.92. [DOI] [PubMed] [Google Scholar]
- 17.Pan Q, Pao W, Ladanyi M. Rapid polymerase chain reaction-based detection of epidermal growth factor receptor gene mutations in lung adenocarcinomas. The Journal of molecular diagnostics : JMD. 2005;7:396–403. doi: 10.1016/S1525-1578(10)60569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pao W, Wang TY, Riely GJ, Miller VA, Pan Q, Ladanyi M, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS medicine. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Servais EL, Suzuki K, Colovos C, Rodriguez L, Sima C, Fleisher M, et al. An in vivo platform for tumor biomarker assessment. PloS one. 2011;6:e26722. doi: 10.1371/journal.pone.0026722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazumdar M, Glassman JR. Categorizing a prognostic variable: review of methods, code for easy implementation and applications to decision-making about cancer treatments. Statistics in medicine. 2000;19:113–32. doi: 10.1002/(sici)1097-0258(20000115)19:1<113::aid-sim245>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 21.Miettinen M, Sarlomo-Rikala M. Expression of calretinin, thrombomodulin, keratin 5, and mesothelin in lung carcinomas of different types: an immunohistochemical analysis of 596 tumors in comparison with epithelioid mesotheliomas of the pleura. The American journal of surgical pathology. 2003;27:150–8. doi: 10.1097/00000478-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Thomas AM, Santarsiero LM, Lutz ER, Armstrong TD, Chen Y-C, Huang L-Q, et al. Mesothelin-specific CD8+ T Cell Responses Provide Evidence of In Vivo Cross-Priming by Antigen-Presenting Cells in Vaccinated Pancreatic Cancer Patients. The Journal of Experimental Medicine. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proceedings of the National Academy of Sciences. 2009;106:3360–5. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:1455–63. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Servais E, Colovos C, Rodriguez L, Bograd A, Nitadori J, Sima C, et al. Mesothelin overexpression promotes mesothelioma cell invasion and MMP-9 secretion in an orthotopic mouse model and in epithelioid pleural mesothelioma patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012 doi: 10.1158/1078-0432.CCR-11-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassan R, Viner JL, Wang QC, Margulies I, Kreitman RJ, Pastan I. Anti-tumor activity of K1-LysPE38QQR, an immunotoxin targeting mesothelin, a cell-surface antigen overexpressed in ovarian cancer and malignant mesothelioma. J Immunother. 2000;23:473–9. doi: 10.1097/00002371-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham MC, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:5144–9. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 28.Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur J Cancer. 2008;44:46–53. doi: 10.1016/j.ejca.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas A, Hassan R. Immunotherapies for non-small-cell lung cancer and mesothelioma. The lancet oncology. 2012;13:e301–10. doi: 10.1016/S1470-2045(12)70126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanitis E, Poussin M, Hagemann IS, Coukos G, Sandaltzopoulos R, Scholler N, et al. Redirected Antitumor Activity of Primary Human Lymphocytes Transduced With a Fully Human Anti-mesothelin Chimeric Receptor. Molecular therapy : the journal of the American Society of Gene Therapy. 2011 doi: 10.1038/mt.2011.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.