Immunity protein TsiV3 from Vibrio cholera has been expressed, purified and crystallized. The crystal belonged to space group P212121, with unit-cell parameters a = 73.3, b = 78.12, c = 106.18 Å and diffract to 2.55 Å resolution.
Keywords: Vibrio cholerae, TsiV3
Abstract
The bacterial type VI secretion system (T6SS), a dynamic organelle, participates in microbial competition by transporting toxic effector molecules to neighbouring cells to kill competitors. TsiV3, a recently defined T6SS immunity protein in Vibrio cholerae, possesses self-protection against killing by T6SS predatory cells by directly binding to and inhibiting their effector protein VgrG-3. Structural information about TsiV3 could help to illuminate its specific mechanism. In this study, TsiV3 from V. cholerae was cloned, expressed and crystallized and single-crystal X-ray diffraction data sets were collected to a resolution of 2.55 Å. Specifically, the crystal belonged to space group P212121, with unit-cell parameters a = 73.3, b = 78.12, c = 106.18 Å. Matthews coefficient calculations indicated that the crystal may contain six TsiV3 molecules in one asymmetric unit, with a V M value of 2.25 Å3 Da−1 and a solvent content of 45.42%.
1. Introduction
The recently discovered bacterial type VI secretion system (T6SS) is present in Vibrio cholerae, which can cause diarrhoeal diseases such as cholera and mild gastroenteritis (Pukatzki et al., 2006 ▶). T6SS allows V. cholerae to kill target cells through the translocation of toxic effector molecules in a cell–cell contact-dependent antagonistic way during various competition scenarios (Russell et al., 2011 ▶). A typical T6SS gene cluster generally contains 15–25 genes and is highly conserved (Basler et al., 2012 ▶). Surprisingly, both the conserved haemolysin coregulated protein (Hcp) that forms a membrane-spanning nanotube with an internal diameter of 4 nm (Ballister et al., 2008 ▶) and valine–glycine repeat G (VgrG) proteins (VgrG-1–VgrG-3) are secreted in the products of these T6SS genes and are required for the function of the T6SS machine; they represent two hallmark proteins of T6SS (Pukatzki et al., 2007 ▶, 2009 ▶). Bioinformatic and structural analyses have suggested that VgrG-related proteins can assemble into a trimeric complex that is a structural homologue of the bacteriophage T4 needle or spike complex (Leiman et al., 2009 ▶; Pukatzki et al., 2007 ▶). This trimeric VgrG complex is located at the tip of the transmembrane nanotube comprised of hexameric rings formed by Hcp (Ballister et al., 2008 ▶), and it can pierce the adjacent cell using its spike-shaped carboxy-terminal β-helical domain to kill prey cells (Shneider et al., 2013 ▶). VgrG proteins possess additional C-terminal domains that act as effectors to attack prey cells, as found in VgrG-1 and VgrG-3 of the three VgrG proteins (VgrG-1, VgrG-2 and VgrG-3) encoded by the T6SS genes of V. cholerae (Pukatzki et al., 2007 ▶). Previous studies have shown that VgrG-3 from V. cholerae contains a peptidoglycan-binding domain (PG1) at the C-terminus (Pukatzki et al., 2009 ▶) and that this domain plays an important role in hydrolyzing the cell wall of Gram-negative bacteria, thereby granting V. cholerae a competitive advantage against adjacent cells (Brooks et al., 2013 ▶).
Recently, the TsiV3 gene from V. cholerae that encodes a novel immunity protein required for self-protection to avoid T6SS-mediated self-intoxication was identified and the upstream gene product of TsiV3, VgrG-3, was identified as its corresponding effector protein (Dong, Ho et al., 2013 ▶). Subsequently, it was reported that TsiV3 (TsaB) directly binds to and inhibits effector protein VgrG-3 in a type II toxin–antitoxin (TA) manner and thus presents immunity to the toxic activity of VgrG-3 (Brooks et al., 2013 ▶). As a novel T6SS immunity protein, TsiV3 does not share high sequence identity with other immunity proteins of known structure (Q9I2Q0, C6BHF3, Q8ZRL5, Q9HYC4, Q4KC91 and Q9I0D9; Shang et al., 2012 ▶; Dong, Zhang et al., 2013 ▶; Zhang et al., 2013 ▶; Wang et al., 2013 ▶; Whitney et al., 2013 ▶; Li et al., 2012 ▶). Moreover, little detail is known of how TsiV3 inhibits VgrG-3. However, it is believed that TsiV3 possesses the essential function of self-defence by resisting attack by other T6SSs. Structural characterization of TsiV3 may be the key to elucidating its inhibitory mechanism and may help in finding structural homologues to further classify immunity proteins. In an effort to determine the three-dimensional structure of TsiV3 by X-ray crystallography, this immunity protein TsiV3 was cloned, expressed, purified and crystallized. We obtained single crystals of TsiV3 in different conditions and report their preliminary crystallographic analyses.
2. Materials and methods
2.1. Cloning and expression
The coding sequence for TsiV3 (amino acids 25–122) was PCR-amplified from the genomic DNA of V. cholerae using the primers 5′-CATGCCATGGGTGAAAACTGCAATGATACATCTGG-3′ and 5′-CCGCTCGAGCAAACTATTATCAACATCCTCTA-3′; bold sequences represent NcoI and XhoI restriction sites, respectively. The product was cloned between the NcoI and XhoI restriction sites of the expression vector pET-28a (Novagen) with a hexahistidine tag at the C-terminus as described previously (Brooks et al., 2013 ▶). After sequencing, the plasmid was transformed into Escherichia coli BL21 (DE3) competent cells (Novagen, USA). The cells were incubated at 310 K in 2.0 l LB medium containing 50 mg l−1 kanamycin. When the OD600 reached about 0.8, the cells were incubated at 289 K for 20 min, and 1 mM isopropyl β-d-1-thiogalactopyranoside (final concentration) was then added for induction at 289 K. Growth of the cells was continued at 289 K for 18 h. Finally, the cultured cells were harvested by centrifugation at 4500g for 30 min at 277 K.
2.2. Purification
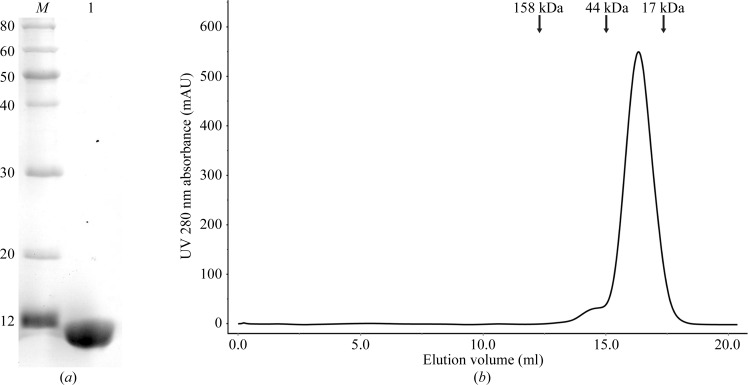
The harvested cells were resuspended in 80 ml lysis buffer (20 mM Tris–HCl pH 8.0, 400 mM sodium chloride) and lysed by sonication on ice. The lysate was then centrifuged at 15 000g for 40 min at 277 K. Subsequent purification steps were all performed at 277 K. The supernatant was applied onto an Ni–NTA affinity column (GE Healthcare) pre-equilibrated with lysis buffer. The nonspecifically bound protein was removed by washing the column with 150 ml washing buffer (20 mM Tris–HCl pH 8.0, 150 mM sodium chloride, 40 mM imidazole). The affinity-bound protein was eluted with 40 ml elution buffer (20 mM Tris–HCl pH 8.0, 100 mM sodium chloride, 500 mM imidazole). Subsequently, the eluted protein was concentrated to 5 ml with a Millipore 10 kDa centrifugal device and purified by ion-exchange chromatography on a HiTrap Q FF column (GE Healthcare) pre-equilibrated with buffer A (20 mM Tris–HCl pH 8.0, 100 mM sodium chloride). The sample was eluted with a linear gradient from 0 to 45% buffer B (20 mM Tris–HCl pH 8.0, 1 M sodium chloride) over 12 min. The relevant fractions were concentrated by centrifugal ultrafiltration (Millipore, 10 kDa) to a volume of about 2 ml. The sample was further loaded onto a HiLoad 16/60 Superdex 200 (GE Healthcare) size-exclusion column previously equilibrated with 20 mM Tris–HCl pH 8.0, 150 mM sodium chloride. The relevant fractions were pooled and concentrated to 16 mg ml−1 using a Millipore 10 kDa centrifugal ultrafiltration device without changing the buffer. The purity of TsiV3 was analyzed by 12%(w/v) SDS–PAGE (Fig. 1 ▶ a). The oligomerization state of TsiV3 from size-exclusion chromatography was estimated as a dimer (Fig. 1 ▶ b).
Figure 1.
(a) 15% SDS–PAGE analysis of TsiV3. Lane M, molecular-mass standards (labelled in kDa); lane 1, purified TsiV3 protein (11 kDa). (b) Size-exclusion chromatography of TsiV3. The elution volume of TsiV3 is 16.3 ml, which is consistent with a homodimeric state.
2.3. Crystallization
The concentrated protein was centrifuged at 13 000g for 35 min at 277 K before use in crystallization trials. Initially, crystallization conditions of TsiV3 were screened by the sitting-drop vapour-diffusion method by mixing 1 µl protein solution and 1 µl reservoir solution and equilibrating against 100 µl reservoir solution using the Crystal Screen, Crystal Screen 2, Index, PEG/Ion, PEG/Ion 2, PEGRx and PEGRx 2 reagent kits (Hampton Research) at 277 and 293 K. Crystals appeared after 10 d in several conditions. The single crystals that were used for X-ray diffraction measurements were obtained at 277 K in a condition consisting of 0.2 M ammonium citrate tribasic pH 7.0, 20%(w/v) polyethylene glycol monomethyl ether 2000, 0.1 M imidazole pH 7.0.
2.4. Data collection and analysis
For data collection, the crystals were mounted in a nylon-fibre loop, transferred into solution corresponding to the reservoir solution supplemented with cryoprotectant solution [20%(v/v) glycerol] and flash-cooled in liquid nitrogen. X-ray diffraction data were collected using a MAR CCD detector (crystal-to-detector distance of 200 mm, 1° oscillation per image, total rotation angle of 180°) on beamline 3W1A of the Beijing Synchrotron Radiation Facility (BSRF), China. Subsequently, the data were processed using the HKL-2000 program package (Otwinowski & Minor, 1997 ▶). Detailed data-collection statistics are given in Table 1 ▶.
Table 1. Data-collection statistics for TsiV3.
Values in parentheses are for the highest resolution shell.
| Source | Beamline 3W1A, BSRF |
| Space group | P212121 |
| Unit-cell parameters (Å) | a = 73.3, b = 78.12, c = 106.18 |
| Wavelength (Å) | 1.0000 |
| Resolution range (Å) | 50.00–2.55 (2.59–2.55) |
| Total/unique reflections | 144769/20390 |
| Completeness (%) | 99.94 (99.9) |
| R merge † (%) | 3.9 (39.9) |
| 〈I/σ(I)〉 | 31.7 (2.3) |
R
merge = 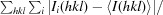
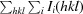 , where Ii(hkl) is the intensity of the ith measurement of reflection hkl and 〈I(hkl)〉 is the weighted average intensity for all observations i of reflection hkl.
, where Ii(hkl) is the intensity of the ith measurement of reflection hkl and 〈I(hkl)〉 is the weighted average intensity for all observations i of reflection hkl.
3. Results and discussion
TsiV3 was predicted to contain a 24-amino-acid signal peptide at its N-terminus by Phobius prediction (http://phobius.sbc.su.se). TsiV3 (amino acids 25–122) was cloned and subsequently expressed with a fused C-terminal hexahistidine tag in E. coli BL21 cells. Sufficient and highly pure TsiV3 protein could be obtained after purification by Ni–NTA chromatography. When this protein was further purified by ion-exchange chromatography on a HiTrap Q FF column connected to an ÄKTApurifier 100 system, two separated peaks appeared (data not shown). The collected protein from each peak was purified by size-exclusion chromatography. The result suggested that the TsiV3 protein in the first peak existed in a homodimeric state (Fig. 1 ▶ b) and the TsiV3 protein in the second peak was highly oligomeric or aggregated (data not shown). Purified TsiV3 was observed upon SDS–PAGE analysis with an apparent molecular weight of 11.0 kDa (Fig. 1 ▶ a).
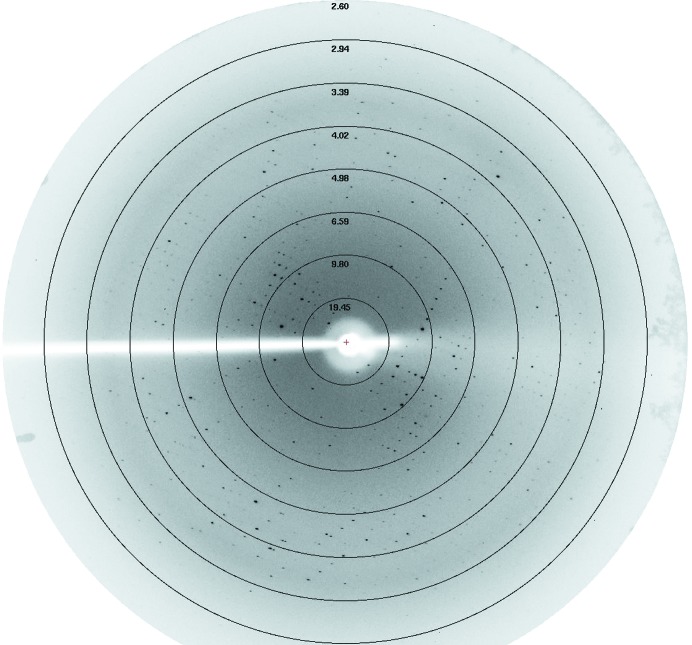
Crystals could be obtained in different conditions using the dimeric protein for screening (Fig. 2 ▶). We collected a complete diffraction data set (Fig. 3 ▶) from a single crystal which displayed a good-quality diffraction pattern. This crystal diffracted to 2.55 Å resolution and belonged to space group P212121, with unit-cell parameters a = 73.3, b = 78.12, c = 106.18 Å. The data-collection statistics are reported in Table 1 ▶. The CCP4 suite (Winn et al., 2011 ▶) was used to calculate the Matthews coefficient as 2.25 Å3 Da−1 (Matthews, 1968 ▶). The asymmetric unit is most likely to contain six molecules, with a corresponding solvent content of 45.42%. The molecular-replacement phasing method (MR) was not used to attempt to solve the TsiV3 crystal structure owing to the lack of an appropriate search model. A selenomethionine-substituted protein for single-wavelength anomalous diffraction (SAD) phasing has now been prepared in an effort to determine the structure of TsiV3.
Figure 2.
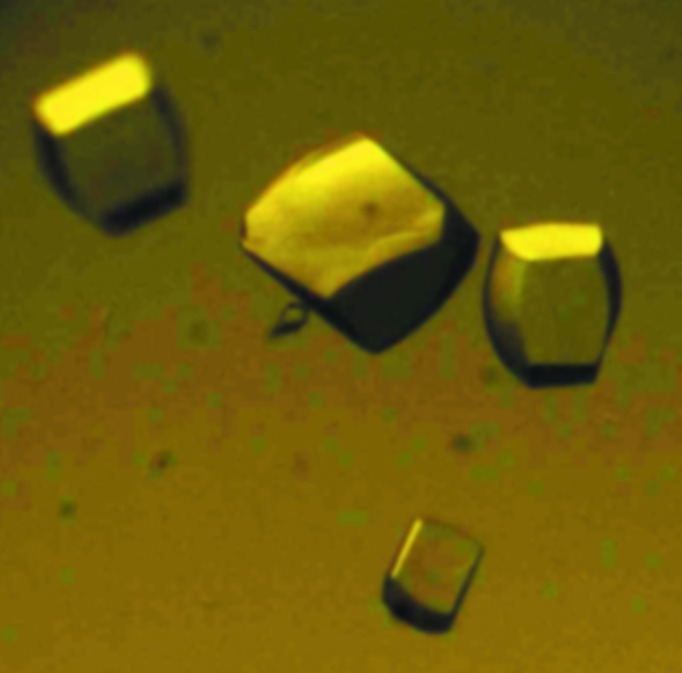
Crystals of TsiV3 grown under the condition 0.2 M ammonium citrate tribasic pH 7.0, 20%(w/v) polyethylene glycol monomethyl ether 2000, 0.1 M imidazole pH 7.0.
Figure 3.
An X-ray diffraction image (labelled in Å) of the TsiV3 crystal obtained at the Beijing Synchrotron Radiation Facility (BSRF), China.
Acknowledgments
The authors thank Zengqiang Gao and Haidai Hu for helpful discussions and analyses. We would like to acknowledge the Beijing Synchrotron Radiation Facility (BSRF) for crystal diffraction data collection. This project was financially supported by grants from the National Basic Research Program of China (2012CB917203) and the National Natural Science Foundation of China (10979005).
References
- Ballister, E. R., Lai, A. H., Zuckermann, R. N., Cheng, Y. & Mougous, J. D. (2008). Proc. Natl Acad. Sci. USA, 105, 3733–3738. [DOI] [PMC free article] [PubMed]
- Basler, M., Pilhofer, M., Henderson, G. P., Jensen, G. J. & Mekalanos, J. J. (2012). Nature (London), 483, 182–186. [DOI] [PMC free article] [PubMed]
- Brooks, T. M., Unterweger, D., Bachmann, V., Kostiuk, B. & Pukatzki, S. (2013). J. Biol. Chem. 288, 7618–7625. [DOI] [PMC free article] [PubMed]
- Dong, C., Zhang, H., Gao, Z.-Q., Wang, W.-J., She, Z., Liu, G.-F., Shen, Y.-Q., Su, X.-D. & Dong, Y.-H. (2013). Biochem. J. 454, 59–68. [DOI] [PubMed]
- Dong, T. G., Ho, B. T., Yoder-Himes, D. R. & Mekalanos, J. J. (2013). Proc. Natl Acad. Sci. USA, 110, 2623–2628. [DOI] [PMC free article] [PubMed]
- Leiman, P. G., Basler, M., Ramagopal, U. A., Bonanno, J. B., Sauder, J. M., Pukatzki, S., Burley, S. K., Almo, S. C. & Mekalanos, J. J. (2009). Proc. Natl Acad. Sci. USA, 106, 4154–4159. [DOI] [PMC free article] [PubMed]
- Li, M., Le Trong, I., Carl, M. A., Larson, E. T., Chou, S., De Leon, J. A., Dove, S. L., Stenkamp, R. E. & Mougous, J. D. (2012). PLoS Pathog. 8, e1002613. [DOI] [PMC free article] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Pukatzki, S., Ma, A. T., Revel, A. T., Sturtevant, D. & Mekalanos, J. J. (2007). Proc. Natl Acad. Sci. USA, 104, 15508–15513. [DOI] [PMC free article] [PubMed]
- Pukatzki, S., Ma, A. T., Sturtevant, D., Krastins, B., Sarracino, D., Nelson, W. C., Heidelberg, J. F. & Mekalanos, J. J. (2006). Proc. Natl Acad. Sci. USA, 103, 1528–1533. [DOI] [PMC free article] [PubMed]
- Pukatzki, S., McAuley, S. B. & Miyata, S. T. (2009). Curr. Opin. Microbiol. 12, 11–17. [DOI] [PubMed]
- Russell, A. B., Hood, R. D., Bui, N. K., LeRoux, M., Vollmer, W. & Mougous, J. D. (2011). Nature (London), 475, 343–347. [DOI] [PMC free article] [PubMed]
- Shang, G., Liu, X., Lu, D., Zhang, J., Li, N., Zhu, C., Liu, S., Yu, Q., Zhao, Y., Zhang, H., Hu, J., Cang, H., Xu, S. & Gu, L. (2012). Biochem. J. 448, 201–211. [DOI] [PubMed]
- Shneider, M. M., Buth, S. A., Ho, B. T., Basler, M., Mekalanos, J. J. & Leiman, P. G. (2013). Nature (London), 500, 350–353. [DOI] [PMC free article] [PubMed]
- Wang, T., Ding, J., Zhang, Y., Wang, D.-C. & Liu, W. (2013). Acta Cryst. D69, 1889–1900. [DOI] [PMC free article] [PubMed]
- Whitney, J. C., Chou, S., Russell, A. B., Biboy, J., Gardiner, T. E., Ferrin, M. A., Brittnacher, M., Vollmer, W. & Mougous, J. D. (2013). J. Biol. Chem. 288, 26616–26624. [DOI] [PMC free article] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.
- Zhang, H., Zhang, H., Gao, Z.-Q., Wang, W.-J., Liu, G.-F., Xu, J.-H., Su, X.-D. & Dong, Y.-H. (2013). J. Biol. Chem. 288, 5928–5939. [DOI] [PMC free article] [PubMed]