Abstract
Yeast-CEA (GI-6207) is a therapeutic cancer vaccine genetically modified to express recombinant carcinoembryonic antigen (CEA) protein, using heat-killed yeast (Saccharomyces cerevisiae) as a vector. In preclinical studies, yeast-CEA induced a strong immune response to CEA and antitumor responses. Patients received subcutaneous vaccines every 2 weeks for 3 months and then monthly. Patients were enrolled at 3 sequential dose levels: 4, 16, and 40 yeast units (107 yeast particles/unit). Eligible patients were required to have serum CEA > 5 ng/mL or > 20 % CEA+ tumor block, ECOG PS 0–2, and no history of autoimmunity. Restaging scans were performed at 3 months and then bimonthly. Peripheral blood was collected for the analysis of immune response (e.g., by ELISPOT assay). Twenty-five patients with metastatic CEA-expressing carcinomas were enrolled. Median patient age was 52 (range 39–81). A total of 135 vaccines were administered. The vaccine was well tolerated, and the most common adverse event was grade 1/2 injection-site reaction. Five patients had stable disease beyond 3 months (range 3.5–18 months), and each had CEA stabilization while on-study. Some patients showed evidence post-vaccination of increases in antigen-specific CD8+ T cells and CD4+ T lymphocytes and decreases in regulatory T cells. Of note, a patient with medullary thyroid cancer had substantial T cell responses and a vigorous inflammatory reaction at sites of metastatic disease. Yeast-CEA vaccination had minimal toxicity and induced some antigen-specific T cell responses and CEA stabilization in a heterogeneous, heavily pre-treated patient population. Further studies are required to determine the clinical benefit of yeast-CEA vaccination.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1505-8) contains supplementary material, which is available to authorized users.
Keywords: Yeast-CEA vaccine, Immunity, Medullary thyroid cancer, ELISPOT, Immunotherapy
Introduction
Therapeutic cancer vaccines are designed to stimulate the immune system’s ability to detect and specifically lyse cancer cells. One immunotherapeutic strategy employs “off-the-shelf” vector-based vaccines that target tumor-associated antigens (TAAs) that are overexpressed on malignant cells [1]. Carcinoembryonic antigen (CEA) is a 180,000-dalton glycoprotein that is overexpressed on most adenocarcinomas of the colon, rectum, stomach, and pancreas, as well as on breast cancers and non-small cell lung cancer (NSCLC) [2–4]. CEA has also been implicated in the metastatic process [5–10]. The CEA gene family belongs to the immunoglobulin superfamily. CEA, which resides on the long arm of chromosome 19, has been identified in the fetal gut and in lesser levels in normal adult colonic mucosa. There appears to be a degree of immune tolerance to CEA, as demonstrated by the lack of immune responses to CEA in patients bearing CEA-expressing tumors. The ability to generate CEA-specific T cell responses in humans has been demonstrated in several clinical trials [11–15]. T cells capable of killing CEA-expressing cancer cells were successfully generated post-vaccination both in CEA-transgenic (CEA-Tg) animal models and in humans [16, 17]. Furthermore, the degree of CEA-specific T cell responses post-vaccination with a poxviral-based vaccine has been associated with improved survival in patients with CEA+ tumors [14].
Numerous characteristics of Saccharomyces cerevisiae, a nonpathogenic yeast species, make it a desirable vector for vaccines. Yeast can be readily engineered to express antigens for infectious diseases or cancer. Heat-killed yeast is extremely stable and therefore easy to transport and store, and is generally easy to administer [18–20]. Recombinant heat-killed yeast has been shown to induce maturation of murine dendritic cells (DCs), and the yeast-CEA vaccine can efficiently activate murine CEA-specific T cells in vitro [21, 22]. Previous studies have also shown that yeast-CEA can efficiently activate human DCs, resulting in increased surface expression of costimulatory molecules, MHC class I and II molecules, and increased production by DCs of cytokines and chemokines such as IL-12p70, TNF-α, IFN-γ, and IL-8 [23]. Human DCs treated with yeast-CEA can efficiently generate and activate CEA-specific T cell lines in vitro that are capable of lysing CEA+ human tumor cells. Gene profiles of human DCs treated with yeast-CEA showed increased expression of numerous genes involved in the production of chemokines and cytokines and their receptors, as well as genes related to antigen uptake, antigen presentation, and signal transduction [23]. Furthermore, repeat administration of the vaccine in CEA-Tg mice demonstrated enhanced T cell responses after each vaccination, indicating that repeated vaccination could boost the immune response [23]. In addition, since this is a killed vector and does not actively infect cells, it is possible that an anti-vector antibody response could facilitate uptake by antigen-presenting cells rather than a neutralizing response as may be seen with live vectors. In sum, recombinant heat-killed yeast serves as a delivery vehicle for a tumor antigen that is efficiently taken up by DCs, resulting in the release of protein in the cytoplasm for processing and MHC loading for T cell activation [20, 24]. Heat-killed yeast vaccines have demonstrated a good safety and tolerability profile in multicenter trials in patients with hepatitis C [18, 19] and in pancreatic cancer patients treated with yeast-ras (mutated) [25, 26] in combination with chemotherapy. An added benefit is that heat-killed yeast can be administered repeatedly without inducing host neutralizing activity [22].
In preclinical studies, vaccination of CEA-Tg mice bearing CEA+ carcinomas with yeast-CEA vaccine resulted in CEA-specific T cell responses, decreased tumor growth, and increased survival with no evidence of autoimmunity [22].
This phase I study evaluated the safety, tolerability, and potential for clinical benefit of yeast-CEA vaccine and analyzed CEA-specific immune responses in patients with metastatic CEA-expressing carcinomas who had failed standard treatments.
Patients and Methods
Eligibility
Eligible patients had histologically confirmed carcinomas whose CEA positivity was evidenced by either a serum CEA level > 5 μg/L or positive staining for CEA in > 20 % of tumor cells. This level of CEA expression was selected based on a previous vaccine trial targeting CEA antigen [14]. All patients had completed, or had disease progression on, at least one prior disease-appropriate therapy for metastatic cancer, or were not candidates for therapy of proven efficacy for their disease. Patients were ≥ 18 years of age, had ECOG performance status of 0–2, had a negative yeast allergy skin test, and estimated life expectancy of ≥ 3 months. Any prior chemotherapy, radiation therapy, or surgeries must have been completed ≥ 4 weeks prior to starting the study. Stable and treated brain metastases were acceptable as long as the patient did not require systemic steroids. Concomitant radiotherapy, chemotherapy, or hormonal therapy was not permitted. Patients could have no history of autoimmune disease. The study protocol was approved by the National Cancer Institute’s Institutional Review Board, and all patients gave written informed consent according to the institutional and federal guidelines.
Vaccine administration
Yeast-CEA (GI-6207) vaccine was supplied by GlobeImmune, Inc. (Louisville, CO), under a Cooperative Research and Development Agreement with the Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute. Each vial of vaccine contained 12 yeast units (YU; 1 YU = 1 × 107 heat-killed yeast cells). The total dose, based on assigned dose level, was equally divided and administered subcutaneously at 4 injection sites: right and left inguinal area and right and left axillary chest wall. This strategy was based on preclinical data demonstrating that multiple-site vaccination more effectively induces T cell immunity and antitumor responses than single-site vaccination [22]. Yeast-CEA was administered biweekly 7 times (days 1, 15, 29, 43, 57, 71, and 85) and then monthly until patients progressed clinically or by RECIST.
Assessment of toxicities
Toxicities were graded using the National Cancer Institute’s cancer clinical trials common toxicity criteria (initially CTCAE 3.0; CTCAE 4.0 after November 2010). Toxicities were identified by medical history, physical examination, and review of laboratory studies. A dose-limiting toxicity was defined as any grade 3, 4, or 5 hematologic or non-hematologic toxicity that was definitely, probably, or possibly related to the administration of vaccine.
Study design
This dose-escalation trial evaluated the maximum safely tolerated dose of yeast-CEA. Standard dose escalation was employed, whereby 3 patients on each study arm were treated at each dose level and escalated to the next higher dose if < 2 patients experienced a dose-limiting toxicity. Patients were given 4 YU at dose level 1, 16 YU at dose level 2, and 40 YU at dose level 3. Tumors were assessed by CT scan of the chest, abdomen, and pelvis at baseline and 3 months, and then every 2 months until disease progression. Tumor responses were assessed by RECIST 1.0.
Collection of PBMCs and immunoassays
Apheresis was performed at baseline and approximately 3 months after enrollment. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll (Amersham Biosciences, Piscataway, NJ) density gradient separation, washed 3 times, and cryopreserved in liquid nitrogen at a concentration of 1 to 2 × 107 cells/mL until assayed.
A modification of a previously described procedure [27, 28] was used to detect IFN-γ production in response to CEA peptide and/or tumor-associated antigen peptides MUC-1 [29], brachyury [30], and HIV (as control peptide). The assay was performed using K562/A*0201 as antigen-presenting cells (APCs).
Cryopreserved PBMCs were analyzed by 5-color flow cytometry for phenotypic characterization of CD4, CD8, and regulatory T cells (Tregs), as previously described [31]. For a given immune assay parameter, the actual change and/or percent change from baseline was noted and evaluated to determine whether it differed from zero using a Wilcoxon signed rank test. A 95 % confidence interval was formed about the fraction of patients with an immune response. Antibodies to yeast in sera (1:50 dilution) were analyzed using the Quanta Lite (S. cerevisiae) ASCAIgG ELISA kit from INOVA Diagnostics, Inc. (San Diego, CA).
Results
Of the 25 patients enrolled, 20 had colon adenocarcinoma, 2 had rectal adenocarcinoma, one had pancreatic adenocarcinoma, one had NSCLC, and one had medullary thyroid carcinoma (Supplemental Table 1). Median patient age was 52 (range 39–81; 10 males and 15 females). All patients were ECOG status 0 (n = 9) or 1 (n = 16). All patients had progressive treatment-refractory disease and had undergone a median of 4 prior chemotherapy regimens (range 1–6). Median baseline CEA was 107 ng/mL (5.7–10,982 ng/mL). Baseline CEA levels were < 20 ng/mL (n = 6), 20–100 ng/mL (n = 5), 101–1,000 ng/mL (n = 8), and > 1,000 ng/mL (n = 6). Patients were enrolled at 4 YU (n = 4), 16 YU (n = 3), and 40 YU (n = 18, which included the expansion phase) (Supplemental Table 1).
Table 1.
Toxicities seen with GI-6207
| Adverse event | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|
| Abdominal pain | 0 | 1 (< 1 %) | 0 |
| Anemia | 0 | 0 | 0 |
| Back pain | 0 | 1 (< 1 %) | 0 |
| Bruising | 0 | 0 | 0 |
| Chest wall pain | 0 | 0 | 0 |
| Chills | 0 | 0 | 0 |
| Dyspnea | 0 | 1 (< 1 %) | 0 |
| Edema | 0 | 0 | 0 |
| Elevated AST | 1 (< 1 %) | 0 | 0 |
| Fatigue | 0 | 0 | 0 |
| Fever | 1 (< 1 %) | 1 (< 1 %) | 0 |
| Flu-like syndrome | 1 (< 1 %) | 0 | 0 |
| Flushing | 0 | 0 | 0 |
| Headache | 1 (< 1 %) | 0 | 0 |
| Hypoxia | 0 | 1 (< 1 %) | 0 |
| Injection-site reaction | 1 (< 1 %) | 1 (< 1 %) | 0 |
| Myalgia | 1 (< 1 %) | 0 | 0 |
| Nausea | 0 | 0 | 0 |
| Pain | 0 | 1 (< 1 %) | 0 |
| Thrombocytopenia | 0 | 0 | 0 |
| Pleural effusion | 1 (< 1 %) | 1 (< 1 %) | 0 |
| Proteinuria | 0 | 0 | 0 |
| Pruritis | 0 | 0 | 0 |
| Rash | 1 (< 1 %) | 0 | 0 |
| Pneumonitis | 0 | 1 (< 1 %) | 0 |
All toxicities possibly, likely, or definitely attributable to vaccine, based on CTCAE 3.0/4.0
Percentages are based on total number of events from 135 vaccine administrations
Toxicity
The treatment was well tolerated, with no dose-limiting toxicities (Table 1). The most common side effect was a grade 1 or 2 injection-site reaction, typically manifested as erythema and induration. These reactions were self-limiting, rarely lasting > 2–3 days after vaccination. One grade 3 injection-site reaction was reported but rapidly resolved. One patient with medullary thyroid carcinoma (patient 21, dose level 3) developed grade 3 pneumonitis with hypoxemia plus pleural and pericardial effusions after 7 doses of vaccine. Treatment for this patient was halted as a precaution; however, the toxicities responded rapidly to high-dose steroids, suggesting that they may have been an immune response to pleural and/or pericardial tumors (see further discussion of this patient below). A patient with colorectal cancer developed grade 3 abdominal and back pain after the second dose of vaccine (day 15). Given the advanced stage of his disease, it was not immediately clear whether the pain represented symptomatic progression or was associated with the vaccine. The patient was taken off study after one month for disease progression. The recommended phase II dose is 40 YU, administered in divided doses of 10 YU injections at 4 sites.
Clinical outcomes and tumor markers
Serum CEA levels were monitored at baseline (median 107 ng/mL; range, 5.7–10,982 ng/mL) and then monthly. Median time to progression was 70 days (range 27–529 days) in this population with advanced cancer. With limited follow-up in 19 of 25 patients, median survival after enrollment was approximately 7 months. Of 24 patients evaluable for CEA declines, 7 (4 colon cancer, one rectal cancer, one medullary thyroid carcinoma, and one NSCLC) had declines in serum CEA after treatment (Table 2). For example, patient 16 (metastatic colorectal cancer) had stable CEA and remained on-study for 17.4 months (Fig. 1). (The CEA decline in one other patient is difficult to interpret because it coincided with ascites drainage. This patient was thus considered unevaluable for CEA response.) The 5 patients with stable disease beyond 3 months all had CEA declines while on-study and remained on trial for 3.5, 4.6, 8.2, 8.2, and 18.1 months, respectively (Table 2). The medullary thyroid carcinoma patient was taken off study at 3.5 months for potential toxicity that was possibly a manifestation of strong immune response and not for disease progression (see below).
Table 2.
Clinical outcomes
| Disease progression | Number of patients | Dose level (patients) |
|---|---|---|
| Disease progression within 3 months | 13 of 25 |
DL 1 (2 of 4) DL 2 (2 of 3) DL 3 (9 of 18) |
| Disease progression at 3-month restaging | 7 of 25 |
DL 1 (1 of 4) DL 2 (1 of 3) DL 3 (5 of 18) |
| Stable disease at beyond 3 months (patients had stable disease for 4.6, 8.2, 8.2, and 18.1 months, respectively. One patient was removed at 3.5 months for potential toxicity) | 5 of 25 |
DL 1 (1 of 4) DL 3 (4 of 18) |
| Patients with on-study CEA declines after treatment | 7 of 24a |
DL 1 (1 of 4) DL 3 (6 of 18) |
aOne patient was considered unevaluable for CEA response due to the removal of ascites
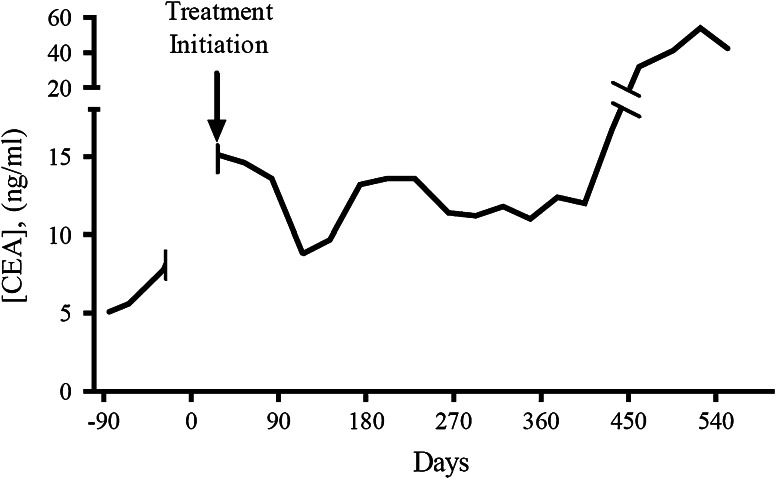
Fig. 1.
A patient (#16) with colon cancer with CEA decline/stabilization after treatment with yeast-CEA vaccine GI-6207. The figure shows the log of CEA values for a 67-year-old man initially diagnosed with stage IIIC colon cancer in January 2007 who was treated with surgery and adjuvant FOLFOX. When he developed metastatic disease, treatments included metastasectomy, FOLFIRI with bevacizumab, then cetuximab, and lastly another regimen of FOLFIRI with bevacizumab. His disease progressed and CEA continued to rise through the last 2 chemotherapy regimens prior to enrollment. At the time of enrollment, he had relatively small disease burden (4 mesenteric lymph nodes measuring ≤ 2.5 cm). He remained on-study at dose level 3 for 17.4 months with stable disease and CEA levels, and tolerated treatment without any adverse effects
Immune analyses
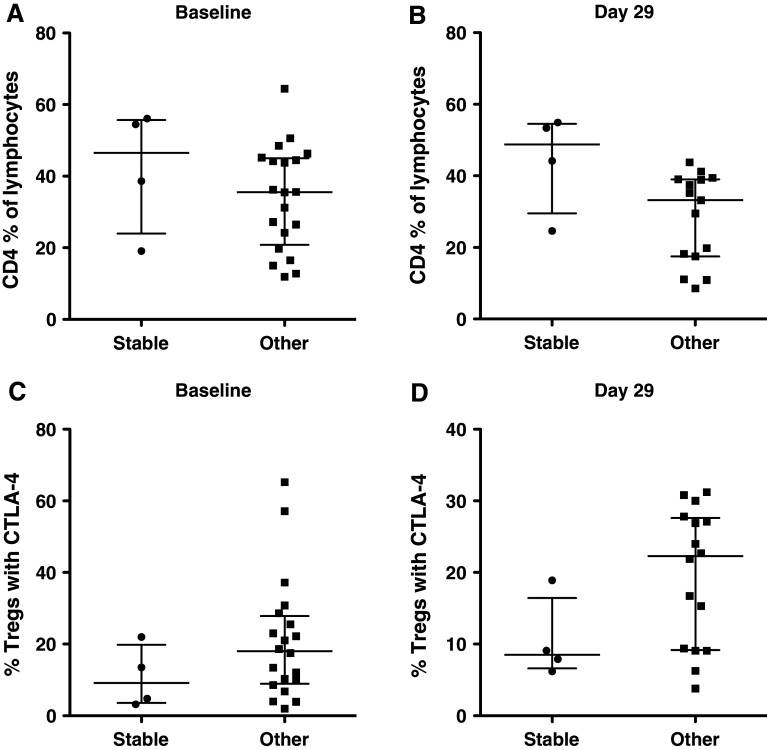
An ELISA performed before and after vaccination detected no antibodies to CEA. Antibodies to S. cerevisiae were evaluated in sera pre-treatment and around day 29 (after 2 vaccinations) and after subsequent vaccinations when sera were available. Twenty-two of 25 patients showed no increases in anti-yeast IgG > 1.5-fold over pre-treatment values at any time points. One patient each who received 4 YU, 16 YU, and 40 YU showed titers 1.9-, 2.3-, and 1.6-fold, respectively, over pre-treatment levels, suggesting modest post-vaccine increases. Venous access difficulties and progressive disease limited the ability to perform apheresis in some patients. IFN-γ ELISPOT assays directed against 9 mer peptides were performed in 11 HLA-A2+ and -A3+ patients pre- and post-vaccination. Antigen-specific T cell responses of ≥ twofold over baseline to either CEA or the cascade TAAs MUC-1 and brachyury were detected in 5 of 11 patients (Table 3). The medullary thyroid carcinoma patient with the possible inflammatory response (discussed below) had a > 20-fold increase over baseline in antigen-specific T cell responses to CEA, MUC-1, and brachyury. All samples were negative to HIV peptide by ELISPOT pre- and post-vaccine. As mentioned above, 5 patients had stable disease beyond the 3-month restaging. Immune-cell subsets of PBMCs from 4 of these patients were available for comparison to the other 20 patients receiving vaccine. CD4, CD8, and Tregs (CD4+/CD25high/CD127−/FOXP3+) were evaluated pre-vaccination and at day 29 (post 2 vaccinations). We also evaluated CTLA-4+ Tregs (CD4+/CD25high/CD127−/FOXP3+/CTLA4+), which have previously been shown to have more suppressive activity than CTLA-4− Tregs [31]. There was no difference between the 2 groups at baseline in CD4+ T cells as percent of lymphocytes (P = 0.29), with a trend (P = 0.04) of a higher percent of CD4 in the 4 patients with stable disease versus others at day 29 (Fig. 2a, b). Nor was there a difference in the 2 groups at baseline in the percent of Tregs expressing CTLA-4 (P = 0.26). There was, however, a weak trend (P = 0.098) in the stable disease group having a lower percent of CTLA-4+ Tregs at day 20 (Fig. 2c, d). There was also a slight trend of a higher CD8/Treg ratio in the stable disease group at baseline and at day 29, which did not reach significance. It must be emphasized that not only are these numbers small, but also that this is a heterogeneous, heavily pre-treated patient population.
Table 3.
Antigen-specific T cell response in evaluable patients after treatment with GI-6207
| Patient # | Days after treatment initiation | CEA response | MUC-1 response | Brachyury response | HIV |
|---|---|---|---|---|---|
| 2 (dose level 1) | Baseline | <1/200,000 | <1/200,000 | n/a | <1/200,000 |
| 14 | 1/100,000 | <1/200,000 | n/a | <1/200,000 | |
| 28 | <1/200,000 | <1/200,000 | n/a | <1/200,000 | |
| 7 (dose level 2) | Baseline | <1/200,000 | <1/200,000 | <1/200,000 | <1/200,000 |
| 15 | <1/200,000 | <1/200,000 | <1/200,000 | <1/200,000 | |
| 29 | <1/200,000 | 1/27,273 | <1/200,000 | <1/200,000 | |
| 84 | <1/200,000 | 1/13,636 | 1/60,000 | <1/200,000 | |
| 9 (dose level 3) | Baseline | <1/200,000 | <1/200,000 | <1/200,000 | <1/200,000 |
| 15 | 1/41,667 | <1/200,000 | <1/200,000 | <1/200,000 | |
| 57 | <1/200,000 | 1/75,000 | <1/200,000 | <1/200,000 | |
| 16 (dose level 3) | Baseline | <1/200,000 | <1/200,000 | n/a | <1/200,000 |
| 83 | <1/200,000 | <1/200,000 | n/a | <1/200,000 | |
| 229 | <1/200,000 | 1/100,000 | n/a | <1/200,000 | |
| 321 | <1/200,000 | <1/200,000 | n/a | <1/200,000 | |
| 21 (dose level 3) | Baseline | <1/200,000 | <1/200,000 | <1/200,000 | <1/200,000 |
| 69 | 1/9,677 | 1/17,143 | 1/12,766 | <1/200,000 | |
| 84 | 1/15,152 | 1/35,294 | 1/20,000 | <1/200,000 | |
| 163a | 1/20,000 | 1/26,316 | <1/200,000 | <1/200,000 |
T cell responses to flu and HIV served as positive and negative controls, respectively
Brachyury is a transcription factor that has been implicated in epithelial–mesenchymal transition and the metastatic process
Bold signifies a two fold or greater increase in antigen-specific T cells compared to baseline
n/a data not available because response to this antigen was not evaluated
a This analysis was performed after the patient received a course of high-dose steroids to treat an intense inflammatory reaction that was potentially related to an intense immune response, as depicted in Fig. 3
Fig. 2.
Five patients on study were stable beyond the 3-month staging. PBMCs from 4 of these 5 patients were available for the comparison of immune-cell subsets to the other 20 patients receiving vaccine at baseline and on day 29. a Frequency of CD4+ T cells at baseline for the 4 stable patients compared to the other 20 patients. There was no difference between the 2 groups at baseline. b Frequency of CD4+ T cells on day 29 for the 4 stable patients compared to the other 20 patients. There was a trend (P > 0.04) in the frequency of CD4+ T cells in the stable patients on day 29 after vaccine therapy. c Frequency of CTLA-4+ Tregs at baseline for the 4 stable patients compared to the other 20 patients. There was no difference between the 2 groups at baseline. d Frequency of CTLA-4+ Tregs on day 29 for the 4 stable patients compared to the other 20 patients. There was a trend (P = 0.098) toward a lower frequency of highly suppressive Tregs in the stable patients on day 29. Dot plots with the median and interquartile range are shown
Individual case report
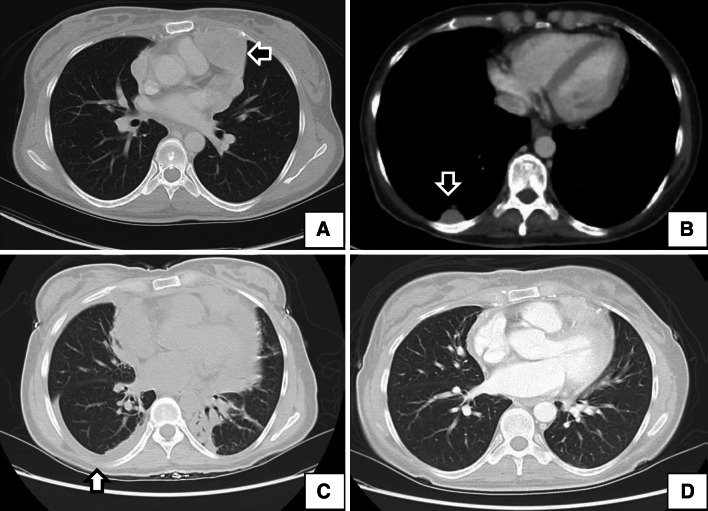
An index patient with a particularly interesting clinical course was a 39-year-old woman diagnosed in July 2002 with metastatic medullary thyroid carcinoma in the thorax and pericardial space (Fig. 3a, b). She underwent several surgeries and participated in a vandetanib clinical trial prior to starting this protocol. She went on protocol with progressive disease and was treated with yeast-CEA vaccine at dose level 3 and tolerated the treatment well for 3 months for a total of 7 vaccinations. The last vaccine dose occurred after her 3-month restaging CT, which demonstrated stable disease. Within this time period, the patient’s CEA and calcitonin, which had been rising prior to enrollment, had stabilized. Six days later, the patient was admitted with shortness of breath. A subsequent CT scan showed new right-sided pleural and pericardial effusions (Fig. 3c). The patient was treated with supportive measures and empiric antibiotics for presumed infection. A bronchoscopy with lavage and biopsy did not reveal any evidence of infection or lymphangitic spread of disease. When the symptoms continued, empiric treatment with high-dose steroids was administered and the patient had rapid resolution of her symptoms within 2 days and subsequent improvement on CT scan (Fig. 3d). It is important to note that this patient was re-evaluated for yeast allergies and found to be negative.
Fig. 3.
Inflammatory response in a patient with medullary thyroid cancer who was treated with vaccine. a, b Baseline computed tomography (CT) of a patient with medullary thyroid cancer who had a pericardial-based lesion (a, arrow) and a right-sided pleural-based lesion (b, arrow). c Six days after the seventh vaccine (approximately 13 weeks after initiating therapy), the patient developed shortness of breath and was admitted to a local hospital. CT showed a right-sided pleural effusion and a pericardial effusion. A biopsy evaluating lymphangitic spread, bronchoscopy with lavage, and empiric antibiotic therapy yielded no clear etiology. With no clear diagnosis, high-dose steroids were started to treat a potential immune-related reaction. The patient’s symptoms resolved within 48 h. d CT image obtained 18 days after the image in (c) and approximately 2 weeks after starting steroids. Notably, this patient was re-evaluated for yeast allergies and found to be negative, and the magnitude of antigen-specific T cell response to multiple tumor antigens increased 10- to 20-fold after vaccine. Based on the clinical course and absence of an alternative diagnosis, it is possible that this was a vigorous immune-mediated antitumor response. Interestingly, in image (c), the effusion was only on the right side (arrow), the same location of the pleural-based lesion seen in (b)
The exact etiology of this patient’s pneumonitis is not clear. It is possible that the fluid buildup was secondary to a vigorous vaccine-induced immune response generated against the tumor. Large tumor masses in the thorax and pericardial space could also explain the fluid buildup in these areas; however, the prompt resolution with corticosteroids is more consistent with an inflammatory response than a steroid-unresponsive tumor. It is interesting to note that the patient’s only pleural-based lesion was on the right side, which was the only side to have a pleural effusion. Alternatively, the patient could have had a slowly resolving uncultured infection that rapidly resolved with steroids, or a vigorous response to an undefined environmental allergen. This was the only patient on the study with large tumors in the thorax and pericardial space. As a precaution, the patient received no more vaccine. Notably, an immune analysis of this patient performed 5 months after enrollment and about 6 weeks after administration of high-dose steroids continued to show significant T cell responses compared to baseline (Table 3, patient 21, day 163). The patient was subsequently treated with sunitinib. She had significant declines in CEA and calcitonin, along with extended stable disease.
Discussion
In April 2010, the United States Food and Drug Administration (FDA) approved sipuleucel-T, the first therapeutic cancer vaccine, for the treatment of asymptomatic or minimally symptomatic castration-resistant prostate cancer, based on studies showing improved overall survival [32]. Sipuleucel-T production requires obtaining a patient’s PBMCs via apheresis, processing these cells at a central facility where they are pulsed with an antigen and then infusing the antigen-pulsed PBMCs back into the patient biweekly 3 times over 1 month [32]. While sipuleucel-T marks an important advance in the field of immunotherapy, the expense and logistical complications of ex vivo cellular processing make off-the-shelf vaccines of proven efficacy more appealing [33]. In this phase I study, the “off-the-shelf” recombinant yeast-CEA vaccine GI-6207 was shown to be safe and well tolerated. The most common toxicity was grade 1/2 injection-site reaction. Only 3 grade 3 toxicities were reported: an injection-site reaction, pain possibly confounded with progressing disease, and a possibly vigorous immune response that resolved with high-dose steroids.
This typical phase I patient population was heavily pre-treated with a median of 4 previous chemotherapy regimens. They also had rapidly progressing disease (13 of 25 had disease progression before the first restaging at 3 months). Furthermore, median survival after enrollment was approximately 7 months. Each of these factors could have significantly limited patients’ ability to generate an immune response to vaccine. A previous study with a poxvirus-based CEA-targeting vaccine demonstrated an inverse relationship between vaccine-specific immune response and number of previous chemotherapy regimens [34]. Additionally, several studies, including those with the FDA-approved sipuleucel-T and ipilimumab, have suggested delayed effects for immunotherapeutics, based on their lack of impact on short-term disease progression, but ultimate ability to extend survival [35, 36].
The majority of patients in this study were in the final months of a long disease course. They likely had heavy tumor burden, which has been associated with increased levels of immunosuppressive cytokines such as TGF-β and IL-10 and increased numbers of immunosuppressive Tregs [37, 38]. In spite of these factors, some patients demonstrated evidence of immunologic response (Table 3; Fig. 2). T cell responses seen only post-vaccination to MUC-1, a tumor antigen, and brachyury, a transcription factor associated with epithelial–mesenchymal transition and the metastatic process, suggest that the vaccine was able to break tolerance and induced antigen spreading, a process whereby a dynamic immune response leads to the targeting of tumor antigens not initially targeted by the vaccine [30, 39–41]. In other trials, an association has been established between antigen spreading and antitumor responses [42, 43]. Subsequent clinical trials with this vaccine will evaluate the extent of antigen spreading/T cell response, and any associations with antitumor responses or changes in tumor growth rates will be evaluated.
Carcinoembryonic antigen level is a common marker of overall disease burden in CEA-expressing adenocarcinoma and can be used to evaluate response to therapy [44]. Thus, all patients in this phase I trial had serial CEA measurements during the course of treatment. Although there were no complete or partial responses based on RECIST, stable disease was observed in 5 of 25 (20 %) patients at 3 months, including 3 with stable disease lasting > 8 months (2 with metastatic colon cancer and one with NSCLC). Notably, all 5 patients with stable disease also showed stabilization of CEA levels. Responses did not seem to be dose-related, although this was difficult to determine since the majority of patients (n = 18) were treated at the highest dose level.
The most intriguing results were seen in the patient with medullary thyroid carcinoma. The development of a possible inflammatory process in the area of large tumors that resolved only with high doses of steroids is suggestive of an antitumor immune response. The patient also had stabilization of CEA and calcitonin after treatment and 20-fold increases in antigen-specific T cell responses to all antigens evaluated. This patient, who had an indolent tumor and was far removed from her only cytotoxic therapy, may represent the type of patient who should be evaluated in future vaccine trials.
A study of yeast-CEA GI-6207 in minimally symptomatic medullary thyroid carcinoma patients (who are thus less ideal candidates for toxic therapies such as vandetanib or cabozantinib) is currently enrolling at the National Cancer Institute (NCI-13-C-0095). Additional trials are also being contemplated in the adjuvant setting for colorectal cancer patients. Since this heat-killed agent can be safely given with chemotherapy, combination trials with standard chemotherapy regimens are also being considered. The findings in these and other studies will help define the clinical potential of yeast-CEA vaccine GI-6207 in this and other CEA-expressing malignancies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. The authors thank Diane J. Poole for her technical assistance and Zhimin Guo, who was involved in early preclinical development of the vaccine. The authors also thank Bonnie L. Casey and Debra Weingarten for their editorial assistance in the preparation of this manuscript.
Conflict of interest
The authors have no conflicts of interest.
Footnotes
James L. Gulley and Ravi A. Madan have contributed equally to this manuscript.
References
- 1.Madan RA, Bilusic M, Heery C, Schlom J, Gulley JL. Clinical evaluation of TRICOM vector therapeutic cancer vaccines. Semin Oncol. 2012;39(3):296–304. doi: 10.1053/j.seminoncol.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guadagni F, Roselli M, Cosimelli M, Spila A, Cavaliere F, Arcuri R, D’Alessandro R, Fracasso PL, Casale V, Vecchione A, Casciani CU, Greiner JW, Schlom J. Quantitative analysis of CEA expression in colorectal adenocarcinoma and serum: lack of correlation. Int J Cancer. 1997;72(6):949–954. doi: 10.1002/(SICI)1097-0215(19970917)72:6<949::AID-IJC5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 3.Kass ES, Greiner JW, Kantor JA, Tsang KY, Guadagni F, Chen Z, Clark B, De Pascalis R, Schlom J, Van Waes C. Carcinoembryonic antigen as a target for specific antitumor immunotherapy of head and neck cancer. Cancer Res. 2002;62(17):5049–5057. [PubMed] [Google Scholar]
- 4.Robbins PF, Eggensperger D, Qi CF, Schlom J. Definition of the expression of the human carcinoembryonic antigen and non-specific cross-reacting antigen in human breast and lung carcinomas. Int J Cancer. 1993;53(6):892–897. doi: 10.1002/ijc.2910530604. [DOI] [PubMed] [Google Scholar]
- 5.Abdul-Wahid A, Huang EH, Lu H, Flanagan J, Mallick AI, Gariepy J. A focused immune response targeting the homotypic binding domain of the carcinoembryonic antigen blocks the establishment of tumor foci in vivo. Int J Cancer. 2012;131(12):2839–2851. doi: 10.1002/ijc.27582. [DOI] [PubMed] [Google Scholar]
- 6.Lee YJ, Han SR, Kim NY, Lee SH, Jeong JS, Lee SW. An RNA aptamer that binds carcinoembryonic antigen inhibits hepatic metastasis of colon cancer cells in mice. Gastroenterology. 2012;143(1):155–165. e8. doi: 10.1053/j.gastro.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 7.Li D, Hua S, Fan Y, Xu S, Duan X, Liu L, Che Y, Li S, Tan Y. DNA vaccine expressing repeated carcinoembryonic antigen (CEA)(625–667) induces strong immunity in mice. Immunol Lett. 2011;135(1–2):124–128. doi: 10.1016/j.imlet.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Taheri M, Saragovi U, Fuks A, Makkerh J, Mort J, Stanners CP. Self recognition in the Ig superfamily. Identification of precise subdomains in carcinoembryonic antigen required for intercellular adhesion. J Biol Chem. 2000;275(35):26935–26943. doi: 10.1074/jbc.M909242199. [DOI] [PubMed] [Google Scholar]
- 9.Thompson JA, Grunert F, Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal. 1991;5(5):344–366. doi: 10.1002/jcla.1860050510. [DOI] [PubMed] [Google Scholar]
- 10.Zheng C, Feng J, Lu D, Wang P, Xing S, Coll JL, Yang D, Yan X. A novel anti-CEACAM5 monoclonal antibody, CC4, suppresses colorectal tumor growth and enhances NK cells-mediated tumor immunity. PLoS ONE. 2011;6(6):e21146. doi: 10.1371/journal.pone.0021146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fong L, Hou Y, Rivas A, Benike C, Yuen A, Fisher GA, Davis MM, Engleman EG. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci U S A. 2001;98(15):8809–8814. doi: 10.1073/pnas.141226398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulley JL, Arlen PM, Tsang KY, Yokokawa J, Palena C, Poole DJ, Remondo C, Cereda V, Jones JL, Pazdur MP, Higgins JP, Hodge JW, Steinberg SM, Kotz H, Dahut WL, Schlom J. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin Cancer Res. 2008;14(10):3060–3069. doi: 10.1158/1078-0432.CCR-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lesterhuis WJ, De Vries IJ, Schreibelt G, Schuurhuis DH, Aarntzen EH, De Boer A, Scharenborg NM, Van De Rakt M, Hesselink EJ, Figdor CG, Adema GJ, Punt CJ. Immunogenicity of dendritic cells pulsed with CEA peptide or transfected with CEA mRNA for vaccination of colorectal cancer patients. Anticancer Res. 2010;30(12):5091–5097. [PubMed] [Google Scholar]
- 14.Marshall JL, Gulley JL, Arlen PM, Beetham PK, Tsang KY, Slack R, Hodge JW, Doren S, Grosenbach DW, Hwang J, Fox E, Odogwu L, Park S, Panicali D, Schlom J. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005;23(4):720–731. doi: 10.1200/JCO.2005.10.206. [DOI] [PubMed] [Google Scholar]
- 15.Mohebtash M, Tsang KY, Madan RA, Huen NY, Poole DJ, Jochems C, Jones J, Ferrara T, Heery CR, Arlen PM, Steinberg SM, Pazdur M, Rauckhorst M, Jones EC, Dahut WL, Schlom J, Gulley JL. A pilot study of MUC-1/CEA/TRICOM poxviral-based vaccine in patients with metastatic breast and ovarian cancer. Clin Cancer Res. 2011;17(22):7164–7173. doi: 10.1158/1078-0432.CCR-11-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodge JW, Sabzevari H, Yafal AG, Gritz L, Lorenz MG, Schlom J. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59(22):5800–5807. [PubMed] [Google Scholar]
- 17.Tsang KY, Zaremba S, Nieroda CA, Zhu MZ, Hamilton JM, Schlom J. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst. 1995;87(13):982–990. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- 18.Franzusoff A, Duke RC, King TH, Lu Y, Rodell TC. Yeasts encoding tumour antigens in cancer immunotherapy. Expert Opin Biol Ther. 2005;5(4):565–575. doi: 10.1517/14712598.5.4.565. [DOI] [PubMed] [Google Scholar]
- 19.Haller AA, Lauer GM, King TH, Kemmler C, Fiolkoski V, Lu Y, Bellgrau D, Rodell TC, Apelian D, Franzusoff A, Duke RC. Whole recombinant yeast-based immunotherapy induces potent T cell responses targeting HCV NS3 and Core proteins. Vaccine. 2007;25(8):1452–1463. doi: 10.1016/j.vaccine.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 20.Stubbs AC, Wilson CC. Recombinant yeast as a vaccine vector for the induction of cytotoxic T-lymphocyte responses. Curr Opin Mol Ther. 2002;4(1):35–40. [PubMed] [Google Scholar]
- 21.Bernstein MB, Chakraborty M, Wansley EK, Guo Z, Franzusoff A, Mostbock S, Sabzevari H, Schlom J, Hodge JW. Recombinant Saccharomyces cerevisiae (yeast-CEA) as a potent activator of murine dendritic cells. Vaccine. 2008;26(4):509–521. doi: 10.1016/j.vaccine.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 22.Wansley EK, Chakraborty M, Hance KW, Bernstein MB, Boehm AL, Guo Z, Quick D, Franzusoff A, Greiner JW, Schlom J, Hodge JW. Vaccination with a recombinant Saccharomyces cerevisiae expressing a tumor antigen breaks immune tolerance and elicits therapeutic antitumor responses. Clin Cancer Res. 2008;14(13):4316–4325. doi: 10.1158/1078-0432.CCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Remondo C, Cereda V, Mostbock S, Sabzevari H, Franzusoff A, Schlom J, Tsang KY. Human dendritic cell maturation and activation by a heat-killed recombinant yeast (Saccharomyces cerevisiae) vector encoding carcinoembryonic antigen. Vaccine. 2009;27(7):987–994. doi: 10.1016/j.vaccine.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stubbs AC, Martin KS, Coeshott C, Skaates SV, Kuritzkes DR, Bellgrau D, Franzusoff A, Duke RC, Wilson CC. Whole recombinant yeast vaccine activates dendritic cells and elicits protective cell-mediated immunity. Nat Med. 2001;7(5):625–629. doi: 10.1038/87974. [DOI] [PubMed] [Google Scholar]
- 25.Richards D, Muscarella P, Ritch P, Fisher W, Flynn P, Whiting S, Mathisen A, Ferrero J, Speyer S, Cohn A (2010) A randomized phase II adjuvant trial of resected patients with ras mutation bearing pancreas cancer treated with GI-4000 and gemcitabine or gemcitabine alone: a safety analysis of the first 100 treated patients. ASCO Gastrointestinal Cancers Symposium abstr 229
- 26.Richards D, Muscarella P, Bekaii-Saab T, Wilfong L, Rosemurgy A, Ross S, Raynov J, Flynn P, Fisher W, Whiting S, Timcheva C, Harrell F, Mercaldo N, Kosten S, Speyer S, Richman J, Coeshott C, Cohn A, Ferraro J, Rodell TC, Apelian D. A phase II adjuvant trial of GI-4000 plus gemcitabine versus gemcitabine alone in ras + patients with resected pancreas cancer: R1 subgroup analysis. Ann Oncol. 2012;23:0002. [Google Scholar]
- 27.Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, Panicali D, Beetham P, Tsang KY, Grosenbach DW, Feldman J, Steinberg SM, Jones E, Chen C, Marte J, Schlom J, Dahut W. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12(4):1260–1269. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Britten CM, Meyer RG, Kreer T, Drexler I, Wolfel T, Herr W. The use of HLA-A*0201-transfected K562 as standard antigen-presenting cells for CD8(+) T lymphocytes in IFN-gamma ELISPOT assays. J Immunol Methods. 2002;259(1–2):95–110. doi: 10.1016/S0022-1759(01)00499-9. [DOI] [PubMed] [Google Scholar]
- 29.Barnd DL, Lan MS, Metzgar RS, Finn OJ. Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic T cells. Proc Natl Acad Sci U S A. 1989;86(18):7159–7163. doi: 10.1073/pnas.86.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palena C, Polev DE, Tsang KY, Fernando RI, Litzinger M, Krukovskaya LL, Baranova AV, Kozlov AP, Schlom J. The human T-box mesodermal transcription factor Brachyury is a candidate target for T-cell-mediated cancer immunotherapy. Clin Cancer Res. 2007;13(8):2471–2478. doi: 10.1158/1078-0432.CCR-06-2353. [DOI] [PubMed] [Google Scholar]
- 31.Vergati M, Cereda V, Madan RA, Gulley JL, Huen NY, Rogers CJ, Hance KW, Arlen PM, Schlom J, Tsang KY. Analysis of circulating regulatory T cells in patients with metastatic prostate cancer pre- versus post-vaccination. Cancer Immunol Immunother. 2011;60(2):197–206. doi: 10.1007/s00262-010-0927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel PH, Kockler DR. Sipuleucel-T: a vaccine for metastatic, asymptomatic, androgen-independent prostate cancer. Ann Pharmacother. 2008;42(1):91–98. doi: 10.1345/aph.1K429. [DOI] [PubMed] [Google Scholar]
- 33.Longo DL. New therapies for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):479–481. doi: 10.1056/NEJMe1006300. [DOI] [PubMed] [Google Scholar]
- 34.von Mehren M, Arlen P, Gulley J, Rogatko A, Cooper HS, Meropol NJ, Alpaugh RK, Davey M, McLaughlin S, Beard MT, Tsang KY, Schlom J, Weiner LM. The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine (ALVAC-CEA B7.1) in patients with metastatic carcinoma. Clin Cancer Res. 2001;7(5):1181–1191. [PubMed] [Google Scholar]
- 35.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 37.Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in immune suppression and cancer. Curr Cancer Drug Targets. 2007;7(1):31–40. doi: 10.2174/156800907780006896. [DOI] [PubMed] [Google Scholar]
- 38.Teicher BA. Transforming growth factor-beta and the immune response to malignant disease. Clin Cancer Res. 2007;13(21):6247–6251. doi: 10.1158/1078-0432.CCR-07-1654. [DOI] [PubMed] [Google Scholar]
- 39.Hattrup CL, Gendler SJ. MUC1 alters oncogenic events and transcription in human breast cancer cells. Breast Cancer Res. 2006;8(4):R37. doi: 10.1186/bcr1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Disis ML, Goodell V, Schiffman K, Knutson KL. Humoral epitope-spreading following immunization with a HER-2/neu peptide based vaccine in cancer patients. J Clin Immunol. 2004;24(5):571–578. doi: 10.1023/B:JOCI.0000040928.67495.52. [DOI] [PubMed] [Google Scholar]
- 41.Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, Tsang KY, Yokokawa J, Hodge JW, Menard C, Camphausen K, Coleman CN, Sullivan F, Steinberg SM, Schlom J, Dahut W. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11(9):3353–3362. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 42.Kudo-Saito C, Schlom J, Hodge JW. Induction of an antigen cascade by diversified subcutaneous/intratumoral vaccination is associated with antitumor responses. Clin Cancer Res. 2005;11(6):2416–2426. doi: 10.1158/1078-0432.CCR-04-1380. [DOI] [PubMed] [Google Scholar]
- 43.Momita S, Ikeda S, Amagasaki T, Soda H, Yamada Y, Kamihira S, Tomonaga M, Kinoshita K, Ichimaru M. Survey of anti-human T-cell leukemia virus type I antibody in family members of patients with adult T-cell leukemia. Jpn J Cancer Res. 1990;81(9):884–889. doi: 10.1111/j.1349-7006.1990.tb02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldstein MJ, Mitchell EP. Carcinoembryonic antigen in the staging and follow-up of patients with colorectal cancer. Cancer Invest. 2005;23(4):338–351. doi: 10.1081/CNV-58878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.