Abstract
BACKGROUND CONTEXT
Tobacco smoking is a key risk factor for spine degeneration. However, the underlying mechanism by which smoking induces degeneration is not known. Recent studies implicate DNA damage as a cause of spine and intervertebral disc degeneration. Because tobacco smoke contains many genotoxins, we hypothesized that tobacco smoking promotes spine degeneration by inducing cellular DNA damage.
PURPOSE
To determine if DNA damage plays a causal role in smoking-induced spine degeneration.
STUDY DESIGN
To compare the effect of chronic tobacco smoke inhalation on intervertebral disc and vertebral bone in normal and DNA repair-deficient mice to determine the contribution of DNA damage to degenerative changes.
METHODS
Two month-old wild-type (C57BL/6) and DNA repair-deficient Ercc1−/Δ mice were exposed to tobacco smoke by direct inhalation (4 cigarettes/day, 5 days/week for 7 weeks) to model first-hand smoking in humans. Total disc proteoglycan (PG) content (1,9-dimethylmethylene blue assay), PG synthesis (35S-sulfate incorporation assay), aggrecan proteolysis (immunoblotting analysis) and vertebral bone morphology (micro-computed tomography) were measured.
RESULTS
Exposure of wild-type mice to tobacco smoke led to a 19% increase in vertebral porosity and a 61% decrease in trabecular bone volume. Intervertebral discs of smoke-exposed animals also showed a 2.6-fold decrease in GAG content and a 8.1-fold decrease in new PG synthesis. These smoking-induced degenerative changes were similar but not worse in Ercc1−/Δ mice.
CONCLUSIONS
Short-term exposure to high levels of primary tobacco smoke inhalation promotes degeneration of vertebral bone and discs. Disc degeneration is primarily driven by reduced synthesis of proteoglycans needed for vertebral cushioning. Degeneration was not exacerbated in congenic DNA repair-deficient mice indicating that DNA damage per se does not have a significant causal role in driving smoke-induced spine degeneration.
Keywords: Tobacco smoking, Intervertebral disc degeneration, Matrix proteoglycans, Aggrecan, Matrix metalloproteinases, DNA damage repair
INTRODUCTION
Low back pain (LBP) is the most common cause of job-related disability in U.S. and affects almost 80% of the adult population in Western countries at least once in their life 1, 2. Association between intervertebral disc degeneration (IDD) and LBP is well-established 3-5 This has fostered much research aimed at identifying the mechanisms underlying disc degeneration and rationale strategies for therapeutic interventions to treat LBP 6-8. Nevertheless, the exact etiology of IDD is extremely complex and still poorly understood. Multiple risk factors have been hypothesized as the underlying causes of IDD, including aging 9, genetic predisposition 10, 11, and environmental factors 12, 13. Among environmental factors implicated in IDD, heavy and repetitive mechanical loading, 12 obesity, 14 and cigarette smoking 13 have been reported.
Approximately 1 in 5 Americans smoke cigarettes 15 and at least 1 in 10 nonsmokers is exposed to second-hand smoke at home 16. Tobacco smoking is associated with many diseases, including cancer, respiratory diseases, and cardiovascular disease. Tobacco smoking is also associated with higher incidence of LBP 13. An early cross-sectional study by Frymoyer and coworkers demonstrated higher frequency of back pain in smokers than nonsmokers 17. Likewise, a longitudinal study of 5180 Finnish forest industry workers revealed smoking as a key contributing factor to radiating neck pain and sciatic pain 18. Moreover, a U.S. National Survey on 10,404 men and women also showed higher incidence of back pain in heavy smokers, suggesting a dose-dependent relationship between LBP and tobacco smoking 14. Finally, Battié and coworkers reported 20% greater disc degeneration in smokers compared to nonsmokers measured by magnetic resonance imaging using a cohort of identical twins 13.
Tobacco smoking promotes multiple degenerative changes in the spine. We recently demonstrated increased vertebral bone porosity and reduced trabecular bone thickness in mice chronically exposed to tobacco smoke. 19 The adverse effect of smoking is not limited to vertebral bone 20, 21. Torsional strength of the tibia is decreased significantly in rabbits exposed to smoke inhalation compared with the non-smoke group. In the same study, granulation tissue resorption, bone formation and remodeling are delayed in smoke-exposed rabbits21. Intervertebral discs (IVDs) of rats exposed to passive tobacco smoke for 8 weeks exhibit decreased collagen expression, increased interleukin (IL)-1β expression and annular disorganization 22. Likewise chronic exposure of wild-type mice to direct smoke inhalation leads to decreased IVD matrix proteoglycan content and synthesis, and an increase of ADAMTS- and MMP- mediated aggrecan breakdown in the IVD 19.
Previously we demonstrated that long term (6 months) exposure of wild-type (Wt) mice to tobacco smoke induced spine degeneration, both in the IVDs and the bony vertebrae 23. Tobacco smoke contains more than 5,000 chemicals, many of which are genotoxins that covalently modify DNA 24. DNA damage is a major risk factor in disc aging and the development of age-dependent IDD 25. This raises an important question as to whether tobacco smoking induces IDD through DNA damage. To answer this question, we exposed Wt mice and their DNA repair-deficient Ercc1−/Δ littermates to direct tobacco smoke inhalation for seven weeks to model short-term heavy smoking in humans. Analyses of exposed Wt mice revealed premature onset of spine degenerative changes including IVD proteoglycan loss and enhanced osteoporosis in vertebral bone. These outcomes were similar but not exacerbated in the DNA repair-deficient Ercc1−/Δ mice demonstrating that DNA damage does not play a primary causal role in mediating smoking-induced spine degeneration.
METHODS
Exposing mice to tobacco smoke
Two month-old C57BL/6 mice (n = 4) and Ercc1−/Δ mice (n = 4) were exposed to tobacco smoke by direct inhalation (4 unfiltered cigarettes/day × 5 days/week for 7 weeks) using a smoking apparatus as previously described 19. University of Kentucky 3R4F research referenced cigarettes were used in this study 26. Unexposed age- and sex-matched littermates of both wild-type C57BL/6 (n = 3) and Ercc1−/Δ (n = 3) kept in the same facility were used as controls. Exposure to four unfiltered cigarettes per day in mice corresponds to a smoking regimen of more than one pack per day in humans 27, 28.
Isolation of nucleus pulposus (NP), annulus fibrosus, and whole mouse IVDs
The experiments involving mice were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Mice were euthanized at the end of the exposure study and the spines were isolated and dissected with the aid of a 5× magnifier. Entire intervertebral discs (IVDs) were removed en bloc from the surrounding vertebral bodies through an incision along the endplates using a surgical no. 11 blade. To harvest NP tissue, an axial cut was made on the disc side of the endplate to expose the disc center, followed by gentle aspiration of the NP tissue using a sterile P-10 pipette tip under a dissecting microscope (20-40 × magnification, Nikon SMZ645) 29.
Histological staining
Isolated spines were decalcified and embedded in paraffin (Tissue Tek processor and Leica embedder). Seven-micrometer sections were stained with safranin O and fast green dyes (Fisher Scientific) by standard procedures and photographed under 40-200× magnification (Nikon Eclipse Ts100).
1,9-dimethylmethylene blue (DMMB) colorimetric assay for sulfated GAGs
For each mouse NP tissues isolated from four lumbar IVDs of each mouse were pooled and digested using papain at 60°C for 2 h. GAG content was measured in duplicates by the DMMB procedure using chondroitin-6-sulfate (Sigma C-8529) as a standard 30. The DNA concentration of each sample was measured using the PicoGreen assay (Molecular Probes) and used to normalize the GAG values.
Quantitation of matrix synthesis
Disc organ cultures of isolated functional spine units (FSUs), each consisting of vertebra-disc-vertebra, were established as previously described 31. For each mouse, two separate incubations each containing two thoracic FSUs (i.e., duplicate reactions) were cultured in complete growth medium (F-12/D-MEM containing 10% FBS, 1% PS, and 25 g/ml L-ascorbic acid) for two days to equilibrate after the trauma of surgical dissection, followed by 12 hour labeling incubation with 35S-sulfate (20 μCi/ml). PG synthesis was measured by 35S-sulfate incorporation, as described previously 32. The amount of PG synthesis was calculated as the fmoles of sulfate incorporated per μg of DNA. Average values from three mice, each measured in duplicate, were calculated and reported ± standard error.
Immunoblots
Five to ten thoracic discs from each mouse were used to extract proteins at 4°C under continuous agitation for 48 h using 30 volumes (volume to weight of disc tissue) of 4 M guanidinium chloride, 50 mmol/L sodium acetate, pH 5.8, 10 mmol/L ethylenediaminetetraacetate (EDTA), and COMPLEAT proteinase inhibitor cocktail (Roche). Aliquots of disc extracts (100 μL) were precipitated with 9 volumes of ethanol and recovered by centrifugation. Pellets were washed twice in 75% ethanol, lyophilized and redissolved in 100 μL, 50 mM sodium acetate (pH 6.0), and digested overnight with 1 mU keratanase II. The solution was then adjusted to 100 mM Tris, 100 mM sodium acetate (pH 7.3), and digested for 6 h with 10 mU chondroitinase ABC. Digested protein extracts were resolved by sodium dodecyl sulfate (SDS)/polyacrylamide gel electrophoresis (PAGE) (4-12% gradient gel, Invitrogen). To control for loading, the same amount of tissue wet weight (1 mg) was loaded per well 33, i.e., 33 μL of the 100 μL containing the protein extracted from 3 mg tissue was used for SDS/PAGE. Samples were analyzed by immunoblot as previously described 33 using antibodies (1:1000 dilution 1° Ab, 1:5000 dilution 2° Ab) raised against the ADAMTS-generated neoepitope NITEGE (Ab1320) 34 and MMP-generated neoepitope VDIPEN (Ab1319) 35. The anti-NITEGE neoepitope antibody cross-reacts with the NVTEGE neoepitope generated from mouse aggrecan.
Micro-computed tomography (μCT)
μCT scans of the lumbar spines isolated from mice were acquired using a VivaCT 40 (Scanco Medical) with 15 μm isotropic voxel size resolution, 70 kVp of energy and 114 μA of current. Three-dimensional (3D) reconstruction of the lumbar vertebrae was performed using a constant threshold value which was selected manually for the bone voxels by visually matching the threshold areas to the gray-scale images. Trabecular bone was evaluated in the region approximately 150 slices below the cranial and above the caudal growth plates, as described 36. Trabecular bone parameters were assessed using Scanco evaluation software provided by the manufacturer, which calculates bone volume (BV) fraction. Porosity was calculated using the formula 1-BV/TV.
Statistical analysis
Values represent the averages from different mice with 95% confidence intervals calculated to determine statistical significance at p=0.05. The confidence intervals were calculated based on the t-distribution because of the small sample size.
RESULTS
Mice exposed to tobacco smoke display loss of matrix PG in their intervertebral discs
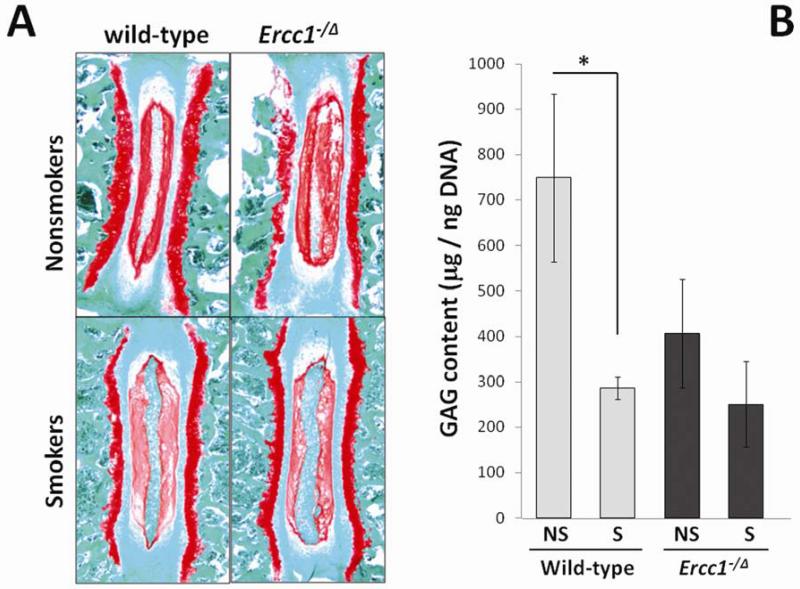
Wild-type and Ercc1−/Δ mice chronically exposed to tobacco smoke showed loss of cellularity in the vertebral endplates and dramatic loss of matrix PG in the intervertebral discs (Fig. 1A). Safranin O staining of sulfated PGs revealed a substantial reduction of staining in the nucleus pulposus of smokers compared to that of nonsmoking controls (Fig. 1A). This was confirmed by the quantitative DMMB assay measuring sulfated GAGs in NP tissues (Fig. 1B). The GAG content of exposed wild-type mice (286 ± 25 μg GAG/ng DNA in NP) was significantly reduced compared to unexposed mice (749 ± 184 μg GAG/ng DNA in NP), accounting for a 62% reduction in GAG content in age-matched treated mice compared to nontreated controls (p<0.05). GAG content of unexposed Ercc1−/Δ mice (406 ± 120 μg GAG/ng DNA in NP) was much lower (54%) than unexposed age-matched wild-type controls due to accelerated aging in the mutant mice, as previously reported 25. Exposure of Ercc1−/Δ mice to tobacco smoke resulted in a further decrease (39) in GAG content (250 ± 94 μg GAG/ng DNA in NP), although the difference was not statistically significant (p<0.18).
Fig 1. Cigarette smoke exposure decreased disc matrix proteoglycan.
A, safranin O histological staining of disc for matrix proteoglycan in smoke-exposed mice and unexposed controls. Decreased safranin O staining of PG (red stain) in the NP was observed in smoke-exposed mice compared to unexposed mice. B, DMMB assay for total GAG content in NP tissue of smoke-exposed mice and untreated controls. NS, nonsmokers. S, smokers. * p<0.05. Average values from three mice each measured in duplicate are shown with one standard deviation.
Chronic tobacco smoke exposure reduced disc matrix protein synthesis
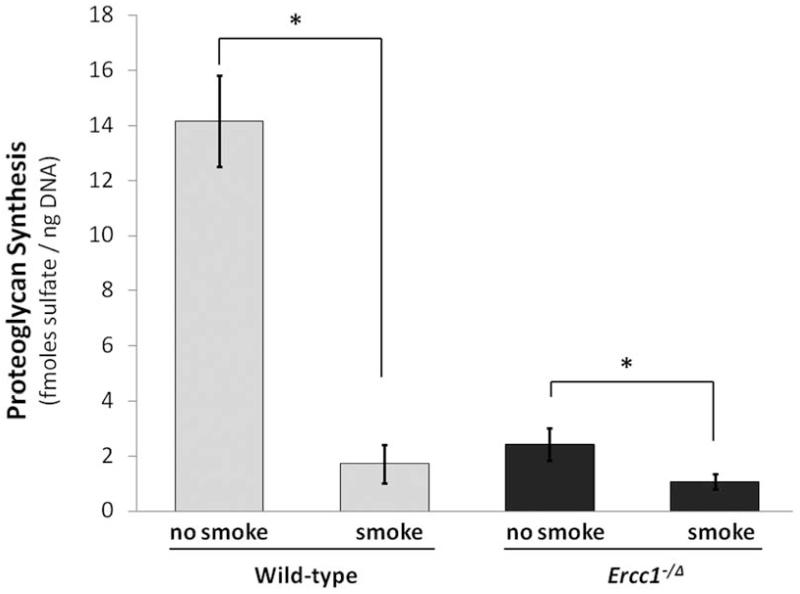
New matrix PG synthesis was measured ex vivo by 35S-sulfate incorporation in disc organotypic culture to determine if loss of disc PG is due to down-regulation of PG synthesis in disc cells by tobacco smoking. Smoke-exposed Wt mice had 1.7 ± 0.6 fmoles sulfate/ng DNA, an 88% reduction compared to that of unexposed controls (14.1 ± 1.6 fmoles sulfate/ng DNA) (p<0.001) (Fig. 2). Unexposed Ercc1−/Δ mice had significantly lower sulfate incorporation than unexposed wild-type controls due to the progeroid phenotype of mutant mice, consistent with our earlier report 25. However, smoke-exposed Ercc1−/Δ mice showed a further 60% decrease in PG synthesis (1.0 ± 0.3 fmoles sulfate/ng DNA) compared to unexposed controls (2.4 ± 0.7 fmoles sulfate/ng DNA) (p<0.05) (Fig. 2).
Fig 2. Tobacco smoke exposure decreased new matrix protein synthesis in intervertebral disc.
PG synthesis was measured by 35S-sulfate incorporation. Average values from three mice each measured in duplicate are shown with one standard deviation. * p<0.05.
Chronic exposure to cigarette smoke increased proteolytic cleavage of disc aggrecan in Ercc1−/Δ mice
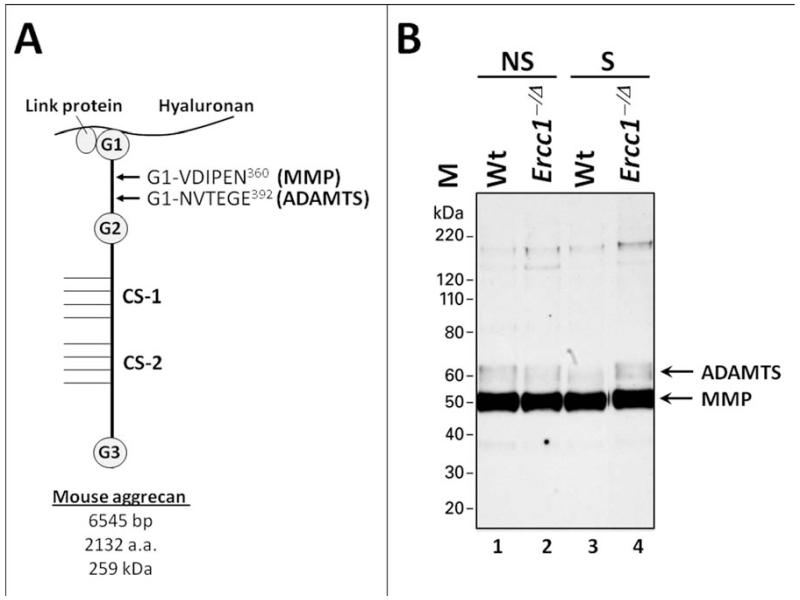
Matrix PG breakdown is a major feature of disc degeneration. To assess the contribution of PG breakdown to loss of matrix PG in smoke-exposed mice we performed immunoblot analysis using antibodies raised against the VDIPEN and NVTEGE epitopes. These are neo-epitopes of aggrecan resulting from proteolysis of aggrecan by matrix-metalloproteinases (MMP−) and disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS), respectively 34, 35 (Fig. 3A). Aggrecan is the primary extracellular proteoglycan of disc matrix and the main constituent responsible for the osmotic turgidity of the disc. Enzymatic cleavage of the aggrecan molecule within its interglobular domain (IGD), is considered pathological because it leads to loss of the entire GAG-attachment region essential for the biomechanical properties of aggrecan 37.
Fig. 3. Effects of cigarette smoke exposure on disc aggrecan proteolysis.
(A) A schematic representation of mouse PG aggregate consisting of the core aggrecan protein bound to hyaluronan via a linker protein. The MMP-mediated cleavage site (yielding VDIPEN neo-epitope) and ADAMTS-mediated cleavage site (yielding NVTEGE neo-epitope) within the IGD residing between the G1 and G2 domain of aggrecan are indicated. (B) A representative immunoblot of G1 fragments bearing the NVTEGE and VDIPEN neo-epitopes. Protein size marker (M),unexposed controls (U), smoke-exposed (S). To control for loading, proteins extracted from 1 mg of disc tissue wet weight were loaded per well. The anti-NITEGE neoepitope antibody crossreacts with the NVTEGE neoepitope generated from mouse aggrecan.
ADAMTS-generated aggrecan G1 fragment terminating in NVTEGE−392 demonstrated no significant differences in the discs of smoke-exposed Ercc1−/Δ mice compared to non-exposed Ercc1−/Δ controls (Fig. 3B, lanes 2-4). Similarly, no difference in NVTEGE−392 levels was observed between exposed and unexposed Wt mice (Fig. 3B, lanes 1-3). The levels of MMP-generated aggrecan G1 fragment terminating in VDIPEN−360 were similar across the treatment groups both in Wt and Ercc1−/Δ mice (Fig. 3B, lanes 1-4). These results suggest that disc PG loss in smoke-exposed mice is due primarily to reduced PG synthesis and not increased matrix breakdown.
Smoking-induced vertebral bone loss
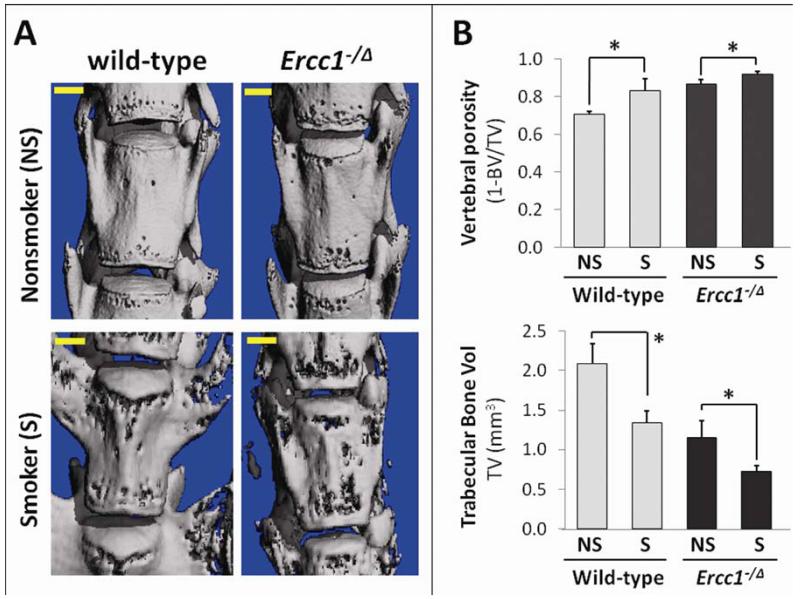
IDD is associated with degenerative changes in many spine tissues, including the vertebral endplate and the vertebral bodies 38. To determine whether these changes also occur in our tobacco smoke-exposed mice, we performed a μCT analysis of bone microstructure of the vertebral bodies (Fig. 4A). Wt mice exposed to tobacco smoke showed degenerative changes in their vertebrae, including increased bone porosity and reduced trabecular bone volume (Fig. 4B). Vertebral bone porosity of exposed Wt mice was 0.83 ± 0.06 (+18.5%) compared to 0.70 ± 0.01 for unexposed controls (p<0.05). Trabecular bone volume decreased from 2.09 ± 0.26 mm3 in non smoke-exposed mice to 1.34 ± 0.145 mm3 in smoke exposed mice (−35.9%) (p<0.05). Similar degenerative changes were observed in Ercc1−/Δ mice for vertebral bone porosity (0.92 ± 0.014 in smoke exposed mice vs 0.86 ± 0.026 in non smoke exposed mice, +7%) and trabecular bone volume (1.15 ± 0.21 mm3 in non smoke exposed mice vs 0.73 ± 0.065 in smoke exposed mice, −36.5%).
Fig 4. Smoking exposure effects on vertebral bone.
A, Representative 3D reconstruction of the micro-computed tomographical images of the spine. B, Smoking-induced changes in vertebral bone porosity and trabecular bone volume. Quantitative bone parameters (TV, BV). Average values from three mice each measured in duplicates are shown with one standard deviation. * p<0.05.
DISCUSSION
The goal of this study is to determine the short-term effects of smoking on spinal health and whether or not the effects are exacerbated in the genetic background of DNA repair deficiency. Our previous work showed that long term (6 month exposure) direct inhalation of tobacco smoke induced significant degeneration of IVD and vertebral bone in Wt mice 23. In this study we exposed two months old wild-type and DNA repair-deficient Ercc1−/Δ mice to tobacco smoke for seven weeks. Since Ercc1−/Δ mice exhibit an accelerated aging phenotype and short lifespan, smoke treatment was started at two months of age for both Wt and Ercc1−/Δ mice, as opposed to three month of age of the Wt mice in our previous study 16. This time point was chosen because Ercc1−/Δ mice at two months of age exhibit initial aging symptoms. Moreover, short-term smoke exposure (7 weeks) was carried out because Ercc1−/Δ mice were neared the end of their lifespan due to severe smoke-induced degenerative effects.
We found degenerative changes in both discs and vertebrae in mice after 7 weeks of exposure to direct inhalation of a high level of tobacco smoke. Seven week exposure in mice translates to about 3.5 years of smoking in humans, a relatively short duration. The major detrimental effects include vertebral bone loss and a reduction in both disc GAG content and new PG synthesis. The effect was slightly exaggerated in the DNA repair-deficient Ercc1−/Δ mice relative to Wt mice, suggesting that nuclear DNA damage is only in part directly implicated in disc degeneration brought on by exposure to tobacco smoke.
Our study clearly established a direct cause and effect relationship between smoking and spine degeneration in mice. In addition, our findings suggest that smoking adversely affect spine health only in part through DNA damage. This is evident from the similar changes observed in both disc and vertebrae in the DNA repair-deficient Ercc1−/Δ mice in comparison to Wt mice. However, although not strongly supported by present data it is still conceivable that direct contact of disc cells with the vascular system containing soluble tobacco smoking genotoxins could perturb normal metabolic activities, such as cells in the outermost annulus and those present along fissures in degenerating discs 39, 40. Cells of nucleus pulposus, the tissue buried deep within the disc structure that is most hypoxic, could also come in direct contact with the water-soluble constituents of tobacco smoke diffusing through the vertebral endplate.
Tobacco smoking clearly had detrimental effects on the spine health of Ercc1−/Δ mice, although, surprisingly, the relative percent change of damage in Ercc1−/Δ was lower than Wt mice. For example, smoke exposure induced a 2.6 fold decrease in disc GAG in Wt mice (286 μg GAG/ng DNA in smokers vs. 749 GAG/ng DNA in nonsmokers), but only 1.6 fold decrease in Ercc1−/Δ mice (250 μg GAG/ng DNA in smokers vs. 406 μg GAG/ng DNA in nonsmokers) (Fig. 1B). Similarly, smoking caused an 8 fold drop in disc PG synthesis in Wt mice (1.7 fmoles sulfate/ng DNA smokers vs. 14.1 fmoles sulfate/ng DNA nonsmokers) but only 2.4 fold decrease in Ercc1−/Δ mice. A similar trend was seen in vertebrae, where smoking caused a 14% increase in vertebral porosity in Wt mice (0.83 in smokers vs. 0.7 nonsmokers) but only a 7% increase in porosity in Ercc1−/Δ mice (0.92 in smokers vs. 0.86 nonsmokers). These findings may be indicative of a plateau effect in damage accumulation, where detrimental effects of smoking reached their lower limit with a combination of both smoke exposure and DNA repair deficiency in smoke-treated Ercc1−/Δ mice.
Previously we demonstrated that long term (6 months) exposure of Wt mice to tobacco smoke induced aggrecanolysis in disc tissue 23. In the current study, we did not observe any detectable increase in proteolysis of disc aggrecan in Wt mice following seven week exposure. This is an important observation as it suggests that long-term exposure is required for ADAMTS-mediated cleavage of the aggrecan IGD. It is possible in theory that longer exposure (>2 months) would dramatically increase aggrecanolysis in these animals. Importantly, these data demonstrating fewer catabolic effects after short term exposure are reassuring, and suggest that the negative effects of tobacco on matrix homeostasis may require chronic exposure, underscoring the importance of efforts supporting smoking cessation. However, it remains unclear how the exposures modeled in this study relate to the clinical scenario. In addition, while it is possible that humans with DNA repair deficiencies may have additional negative effects from smoking, these data suggest that the negative effects on matrix will be no worse than in humans without such deficiencies. Further investigation is required to demonstrate if the negative effects of DNA repair defects and tobacco expsoure on disc matrix will be synergistic in humans.
The mice in this study were started on tobacco smoke when they just reached skeletal maturity (8 weeks) as compared to those fully matured (3 months) exposed in our previous study 19. This might explain the greater effects on vertebral bone porosity and PG synthesis by smoking on these young mice. For instance, in our previous study we observed that disc total PG content and new PG synthesis of smoke-exposed mice was about 60% of unexposed control. In the current study, disc total PG content and new PG synthesis of smoke-exposed Wt mice was about 39% and 14%, respectively of unexposed Wt control. Moreover, smoking increased vertebral porosity by 19% in the exposed Wt mice in the current study as compared to a 5% increase in exposed mice in our previous study. It is possible that the larger effects were due to the fact that mice in the current study were exposed to smoking at a younger age (2 months) when they actively produce and lay down disc and bone matrix. If confirmed, smoking would be especially harmful for younger human smokers when they are still growing, i.e., teenagers who begin to smoke potentially have a much greater likelihood of having disc matrix alterations compared to those who begin to smoke later in their adult life. In fact, a similar phenomenon has been described in the lungs, where studies have shown that early smoking has a more detrimental effect on lungs than late onset smoking 41. The significance of these on youth health is evident in that about 90% of smokers started smoking by the age of 18 and currently more than 600,000 middle school students and 3 million high school students smoke cigarettes 42.
Summary statement
The findings in the present study suggest that smoking, which contains many strong oxidants, inflammatory compounds, and genotoxins induces degenerative changes in the spine only in part through the mechanism of cellular DNA damage. Moreover, exposure to tobacco smoking before skeletal maturity has a very strong detrimental effect on disc matrix homeostasis. With the staggering number of smokers in the world, these findings will be important in contributing to our knowledge of how spine degeneration occurs with smoking and will help guide future research to further elucidate this process to prevent or delay the onset of smoke-induced spine degeneration and related diseases.
ACKNOWLEDGEMENTS
This work was supported by the 2010 ORS Collaborative Exchange Award to Nam Vo and Peter Roughley, and NIH grants AG033046 to Nam Vo, ES016114 to Laura Niedernhofer. The authors would like to thank Yeqing Geng (Western analysis), and Robbins Flint (exposure of mice to tobacco smoke) for their technical assistance, and Johnny Huard for the use of ⍰CT facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Borenstein D. Epidemiology, etiology, diagnostic evaluation, and treatment of low back pain. Curr Opin Rheumatol. 1992;4:226–32. doi: 10.1097/00002281-199204000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Hart LG, Deyo RA, Cherkin DC. Physician office visits for low back pain. Frequency, clinical evaluation, and treatment patterns from a U.S. national survey. Spine (Phila Pa 1976) 1995;20:11–9. doi: 10.1097/00007632-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Luoma K, et al. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976) 2000;25:487–92. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 4.Cheung KM, Samartzis D, Karppinen J, Luk KD. Are “patterns” of lumbar disc degeneration associated with low back pain?: new insights based on skipped level disc pathology. Spine (Phila Pa 1976) 37:E430–8. doi: 10.1097/BRS.0b013e3182304dfc. [DOI] [PubMed] [Google Scholar]
- 5.de Schepper EI, et al. The association between lumbar disc degeneration and low back pain: the influence of age, gender, and individual radiographic features. Spine (Phila Pa 1976) 35:531–6. doi: 10.1097/BRS.0b013e3181aa5b33. [DOI] [PubMed] [Google Scholar]
- 6.Erkintalo MO, Salminen JJ, Alanen AM, Paajanen HE, Kormano MJ. Development of degenerative changes in the lumbar intervertebral disk: results of a prospective MR imaging study in adolescents with and without low-back pain. Radiology. 1995;196:529–33. doi: 10.1148/radiology.196.2.7617872. [DOI] [PubMed] [Google Scholar]
- 7.Paajanen H, Erkintalo M, Kuusela T, Dahlstrom S, Kormano M. Magnetic resonance study of disc degeneration in young low-back pain patients. Spine (Phila Pa 1976) 1989;14:982–5. doi: 10.1097/00007632-198909000-00012. [DOI] [PubMed] [Google Scholar]
- 8.van Tulder MW, Assendelft WJ, Koes BW, Bouter LM. Spinal radiographic findings and nonspecific low back pain. A systematic review of observational studies. Spine (Phila Pa 1976) 1997;22:427–34. doi: 10.1097/00007632-199702150-00015. [DOI] [PubMed] [Google Scholar]
- 9.Videman T, et al. Magnetic resonance imaging findings and their relationships in the thoracic and lumbar spine. Insights into the etiopathogenesis of spinal degeneration. Spine (Phila Pa 1976) 1995;20:928–35. doi: 10.1097/00007632-199504150-00009. [DOI] [PubMed] [Google Scholar]
- 10.Battie MC, Videman T, Parent E. Lumbar disc degeneration: epidemiology and genetic influences. Spine (Phila Pa 1976) 2004;29:2679–90. doi: 10.1097/01.brs.0000146457.83240.eb. [DOI] [PubMed] [Google Scholar]
- 11.Ala-Kokko L. Genetic risk factors for lumbar disc disease. Ann Med. 2002;34:42–7. doi: 10.1080/078538902317338634. [DOI] [PubMed] [Google Scholar]
- 12.Videman T, et al. The long-term effects of physical loading and exercise lifestyles on back-related symptoms, disability, and spinal pathology among men. Spine (Phila Pa 1976) 1995;20:699–709. doi: 10.1097/00007632-199503150-00011. [DOI] [PubMed] [Google Scholar]
- 13.Battie MC, et al. Volvo Award in clinical sciences. Smoking and lumbar intervertebral disc degeneration: an MRI study of identical twins. Spine (Phila Pa 1976) 1991;16:1015–21. 1991. [PubMed] [Google Scholar]
- 14.Deyo RA, Bass JE. Lifestyle and low-back pain. The influence of smoking and obesity. Spine (Phila Pa 1976) 1989;14:501–6. doi: 10.1097/00007632-198905000-00005. [DOI] [PubMed] [Google Scholar]
- 15.CDC. C.f.D.C.a.P. Cigarette smoking among adults - United States, 2007. MMWR Morb Mortal Wkly Rep. 2008:1221–6. [PubMed] [Google Scholar]
- 16.CDC. C.f.D.C.a.P. Disparities in secondhand smoke exposure - United States, 1988-1994 and 1999-2004. MMWR Morb Mortal Wkly Rep. 2008:744–7. [PubMed] [Google Scholar]
- 17.Frymoyer JW, et al. Epidemiologic studies of low-back pain. Spine (Phila Pa 1976) 1980;5:419–23. doi: 10.1097/00007632-198009000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Viikari-Juntura E, et al. Longitudinal study on work related and individual risk factors affecting radiating neck pain. Occup Environ Med. 2001;58:345–52. doi: 10.1136/oem.58.5.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D, et al. Spine degeneration in a murine model of chronic human tobacco smokers. Osteoarthritis Cartilage. 2012;20:896–905. doi: 10.1016/j.joca.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Zawawy HB, Gill CS, Wright RW, Sandell LJ. Smoking delays chondrogenesis in a mouse model of closed tibial fracture healing. J Orthop Res. 2006;24:2150–8. doi: 10.1002/jor.20263. [DOI] [PubMed] [Google Scholar]
- 21.Ueng SW, et al. Hyperbaric oxygen therapy mitigates the adverse effect of cigarette smoking on the bone healing of tibial lengthening: an experimental study on rabbits. J Trauma. 1999;47:752–9. doi: 10.1097/00005373-199910000-00023. [DOI] [PubMed] [Google Scholar]
- 22.Ogawa T, et al. Alteration of gene expression in intervertebral disc degeneration of passive cigarette-smoking rats: separate quantitation in separated nucleus pulposus and annulus fibrosus. Pathobiology. 2005;72:146–51. doi: 10.1159/000084118. [DOI] [PubMed] [Google Scholar]
- 23.Wang D, et al. Spine degeneration in a murine model of chronic human tobacco smokers. Osteoarthritis Cartilage. 2012 doi: 10.1016/j.joca.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramos KS, Moorthy B. Bioactivation of polycyclic aromatic hydrocarbon carcinogens within the vascular wall: implications for human atherogenesis. Drug Metab Rev. 2005;37:595–610. doi: 10.1080/03602530500251253. [DOI] [PubMed] [Google Scholar]
- 25.Vo N, et al. Accelerated aging of intervertebral discs in a mouse model of progeria. J Orthop Res. 2010;28:1600–7. doi: 10.1002/jor.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–4. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 27.Houghton AM, et al. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest. 2006;116:753–9. doi: 10.1172/JCI25617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindblad SS, et al. Smoking and nicotine exposure delay development of collagen-induced arthritis in mice. Arthritis Res Ther. 2009;11:R88. doi: 10.1186/ar2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nasto LA, et al. Issls Prize Winner: Inhibition of Nf-kb Activity Ameliorates Age-associated Disc Degeneration in a Mouse Model of Accelerated Aging. Spine (Phila Pa 1976) 2012 doi: 10.1097/BRS.0b013e31824ee8f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 31.Wang D, et al. Bupivacaine decreases cell viability and matrix protein synthesis in an intervertebral disc organ model system. Spine J. 2011;11:139–46. doi: 10.1016/j.spinee.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilbertson L, et al. The effects of recombinant human bone morphogenetic protein-2, recombinant human bone morphogenetic protein-12, and adenoviral bone morphogenetic protein-12 on matrix synthesis in human annulus fibrosis and nucleus pulposus cells. Spine J. 2008;8:449–56. doi: 10.1016/j.spinee.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Haglund L, Ouellet J, Roughley P. Variation in chondroadherin abundance and fragmentation in the human scoliotic disc. Spine (Phila Pa 1976) 2009;34:1513–8. doi: 10.1097/BRS.0b013e3181a8d001. [DOI] [PubMed] [Google Scholar]
- 34.Lee ER, et al. Enzymes active in the areas undergoing cartilage resorption during the development of the secondary ossification center in the tibiae of rats ages 0-21 days: I. Two groups of proteinases cleave the core protein of aggrecan. Dev Dyn. 2001;222:52–70. doi: 10.1002/dvdy.1168. [DOI] [PubMed] [Google Scholar]
- 35.Lee ER, et al. Immunolocalization of the cleavage of the aggrecan core protein at the Asn341-Phe342 bond, as an indicator of the location of the metalloproteinases active in the lysis of the rat growth plate. Anat Rec. 1998;252:117–32. doi: 10.1002/(SICI)1097-0185(199809)252:1<117::AID-AR10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 36.Bouxsein ML, et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 37.Roughley PJ, Alini M, Antoniou J. The role of proteoglycans in aging, degeneration and repair of the intervertebral disc. Biochem Soc Trans. 2002;30:869–74. doi: 10.1042/bst0300869. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez AG, et al. Morphology of the human vertebral endplate. J Orthop Res. 2012;30:280–7. doi: 10.1002/jor.21513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kauppila LI. Ingrowth of blood vessels in disc degeneration. Angiographic and histological studies of cadaveric spines. J Bone Joint Surg Am. 1995;77:26–31. doi: 10.2106/00004623-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Ali R, Le Maitre CL, Richardson SM, Hoyland JA, Freemont AJ. Connective tissue growth factor expression in human intervertebral disc: implications for angiogenesis in intervertebral disc degeneration. Biotech Histochem. 2008;83:239–45. doi: 10.1080/10520290802539186. [DOI] [PubMed] [Google Scholar]
- 41.Xu X, Li B. Exposure-response relationship between passive smoking and adult pulmonary function. Am J Respir Crit Care Med. 1995;151:41–6. doi: 10.1164/ajrccm.151.1.7812570. [DOI] [PubMed] [Google Scholar]
- 42.2012 Surgeon General’s Report—Preventing Tobacco Use Among Youth and Young Adults. Center for Disease Control. 2012 [PubMed] [Google Scholar]