Abstract
Background
Studies support efficacy of ultraviolet A1 (UVA1) phototherapy, but little is known about recurrence after successful UVA1 treatment.
Objective
Determine the frequency of recurrent activity after UVA1 phototherapy and variables associated with recurrence.
Methods
Case series and prospective cohort study of patients treated with UVA1 phototherapy with minimum 6 months follow-up. Demographics, clinical features, and cumulative UVA1 dose were analyzed for association with recurrence.
Results
Of 37 patients, 46% (n=17) had recurrence of active morphea lesions after successful UVA1 phototherapy. Two-year and three-year (after the last UVA1 phototherapy treatment) recurrence rates were 44.5% (95% CI: 30.1% – 62.2%) and 48.4% (95% CI: 33.2% – 66.1%). The only variable associated with recurrence was duration of morphea prior to UVA1 (p-value=0.02, HR=1.15, 95% CI=(1.06–1.27)).
Limitations
Sample size limits conclusions.
Conclusion
With the exception of increased duration of morphea, risk of recurrence is no different in adults and children, between morphea subtypes, skin type, and medium to high dose regimens. This indicates treatment doses in the medium-high UVA1 range are adequate with respect to frequency of recurrence.
INTRODUCTION
Morphea, also known as localized scleroderma, is an inflammatory skin disorder that affects the dermis and sometimes subcutaneous tissues. Lesions are marked by inflammation and excessive collagen and extracellular matrix deposition which may produce significant morbidity, including functional and cosmetic impairment.1, 2
Recently, methotrexate with systemic corticosteroids has shown promise in the treatment of morphea in both adult and pediatric patients.2–5 In addition, methotrexate with or without systemic steroids has been associated with long-term remission in disease activity.6–9 However, some patients are unable to tolerate immunosuppressives or have contraindications to them. In these cases, phototherapy, especially UVA1 phototherapy, is an attractive therapeutic option.9–12
Several clinical studies support the use of UVA1 phototherapy in the treatment of morphea.10–15 However, little is known about the frequency of recurrence of disease activity after UVA1 treatment or demographic and clinical variables associated with recurrence. The Morphea in Adults and Children (MAC) cohort is a disease Registry with a UVA1 phototherapy center. Within the MAC cohort, all patients are examined and photographed by one examiner with expertise in morphea, and treated with a standardized UVA1 treatment protocol allowing for the evaluation of UVA1 phototherapy and its long-term efficacy. The aim of this study was to determine the frequency of recurrent morphea activity after successful UVA1 phototherapy and whether specific clinical and treatment variables were associated with recurrence.
METHODS
Study Patients
Table I and Figure 1 provide details of patient eligibility for UVA1 phototherapy in the MAC cohort and identification of study patients. The MAC cohort is designed to examine demographic, clinical, immunogenetic, and autoimmune features of adults and children with morphea. The autoimmune features of the cohort were described elsewhere.16 For this study, patients from the MAC cohort were included if they received a minimum of five UVA1 phototherapy treatments and had ≥ 6 months of follow-up after completing UVA1 phototherapy. Only patients with linear, plaque, generalized, and mixed subtypes17 of morphea were treated with UVA1 phototherapy per the protocol of the MAC cohort. Patients with eosinophilic fasciitis, morphea profunda, hemifacial atrophy, or scalp lesions were not treated with UVA1 phototherapy per the registry protocol and were therefore excluded from the present study. Patients who were on systemic therapy upon entry into the MAC cohort were also excluded from the present study. Topical treatments were allowed throughout the study. Morphea subtypes were assigned by HJ and are defined by the criteria of Zulian and Laxer.18 Of 252 patients enrolled in the MAC cohort between July 2007 and October 2011, 62 patients received UVA1 phototherapy. Of the 62 patients who received UVA1, 37 (60%) met inclusion criteria. The following data were collected for each patient: demographics (age at enrollment, sex, and race), clinical features (age of onset, duration of disease starting from date of diagnosis by dermatologist or rheumatologist, disease subtype, and Fitzpatrick skin type), cumulative UVA1 dose, number and duration of UVA1 treatments, and missed number of UVA1 treatments using predetermined standardized case report forms.
Table 1.
MAC Cohort Protocol for Eligibility of UVA1 Phototherapy*
| 1. Diagnosis of Morphea : |
| - linear, plaque, generalized, or mixed16 |
| 2. Presence of at least one active morphea lesion. Active morphea is defined as follows: |
| Active disease criteria |
| Presence of one of these features qualifies as active disease: |
| 1. New lesion developed within preceding 3 months, documented by clinician |
| 2. Extension of existing lesion within prior 3 months, documented by clinician |
| 3. Erythema of moderate or marked level in lesion or an erythematous lesion border, + documented by clinician |
| 4. Violaceous lesion or border color, documented by clinician |
| Presence of 2 or more of these features qualifies as active disease: |
| 1. Patient or parent report of new lesion OR extension of existing lesion occurring within prior 3 months |
| a. This criteria ONLY allowed for new patients (i.e., first visit) |
| 2. Lesion warmth |
| 3. Mild erythema of lesion |
| 4. Marked or moderate induration of lesion border |
| 5. Worsening hair loss in scalp, eyebrow, or eyelashes; must be documented by clinician |
| 6. Lesion biopsy showing active disease. Pathologist reading biopsy would specify if there was activity defined as presence of inflammatory cell infiltrate. |
This is not a proposed therapeutic guideline. This is current practice in the MAC cohort protocol and derived from a previous study.20
Figure 1.
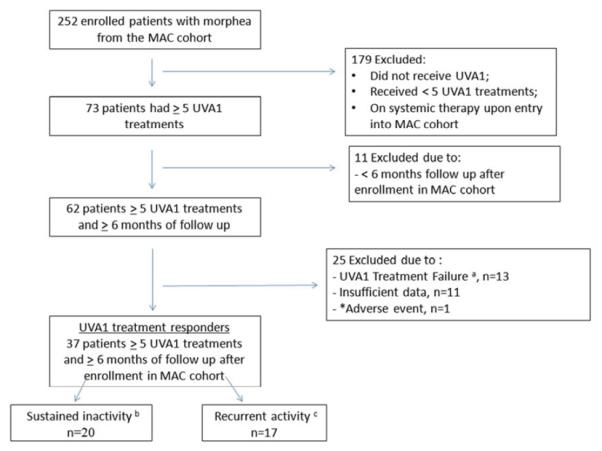
Identification of the 37 study patients. a) Treatment failure defined as need for addition of another treatment (i.e. systemic treatment) due to development of new lesions, extension of existing lesions, or persistent activity during UVA1 phototherapy or within 1 month of discontinuation. b) Sustained inactivity defined as no new lesions or extension of existing lesions (using serial photographs) and no signs of active disease (see Table I) at the end of their treatment course. c) Recurrent activity defined as the development of a new lesion, extension of an existing lesion (using serial photographs), or new signs of disease activity >1 month after completion of UVA1 phototherapy.
*1 patient developed in a skin cancer during ultraviolet A1 phototherapy. Of note, the patient had a history of prior NMSC but was treated with UVA1 phototherapy due to morphea severity and contraindications to immunosuppressive making an association with UVA1 PT unlikely.
Treatment protocol
Indications for UVA1 phototherapy in the MAC cohort are listed in Table I. Details of the treatment protocol are provided in Table II. Patients were treated with a Sellamed 24000 UVA-1 bed manufactured by Sellas Medizinische Geräte and imported by Daavlin. Output is 5 J/cm2 per minute. The metal halide bulbs allow for the emission of UV light between 340–400 nm (UVA1 wavelength) at very high output, making all doses of UVA1 phototherapy feasible.
Table II.
UVA1 phototherapy protocol and dosing regimen
| 1. Patients should be treated 2–3 limes per week. |
| 2. Treat whole body except for face and genitals. |
| 3. Goggles should be worn at all times by the patient and the phototherapist. |
| 4. For skin phototypes ≤ Fitzpatrick III. start at 40 J/cm2. For skin phototypes ≥ IV, start at 70 J/cm2.. Increase by 10–20 J/cm2 per treatment to a maximum of 120 J/cm2. No need to apply emollient. |
| 5. Maintain dose as long as no erythema occurs. |
| If sunburn erythema should occur: |
| • Mild to moderate- decrease dose by 10%. Once the patient tolerates the reduced dose without erythema, increase as tolerated per above protocol. |
| • Severe- notify the PI who will evaluate the patient. |
| • If erythema is a repeated problem, notify physician. Erythema is unusual with UVA1 light treatments. A recurrent problem warrants a physician evaluation. |
| 6. Assess after 10–15 treatments* |
| • Good response- maintain dose (no extension or new lesions and erythema and signs of disease activity improved |
| • Partial response – to maximal dose (120 J/cm2) per protocol, no new lesions, minimal extension of exisiting lesions, and/or persistent erythema persistent/worsening erythema |
| • No response- worsening (new lesions or lesion extension, persistent/worsening erythema); stop UVA1 phototherapy and consider alternative therapy |
| 7. Reassess after another 10–15 treatments (for a total of 20–30 treatments or 6 weeks of treatment)** |
| • Good response or maximal improvement (no new lesions or extension of existing lesions; complete resolution of erythema and signs of activity, i.e. erythema) - stop UVA1 |
| • Partial or no response- discontinue UVA1 and consider alternative therapy |
Treatment response
Patients were assessed every 10–15 treatments, 4 weeks after discontinuation of treatment, and every 2–4 months thereafter. After 1 year, patients who had no signs of active disease were followed annually. All patients were evaluated by a single investigator (HJ). UVA1 treatment responders were defined as those with: no new lesions or extension of existing lesions as documented by photography and complete resolution of signs of disease activity for at least 10 UVA1 treatments. UVA1 treatment responders were divided into recurrent activity and sustained inactivity groups. (Figure 1). The definitions of sustained inactivity and recurrent activity were established at the study outset and determined by a single investigator (HJ) (also see Fig. 1). Patients were categorized as sustained inactivity if they did not have new lesions or extension of existing lesions (accomplished by comparing serial patient photographs) and if they did not have signs of active disease (Table I) at the end of their treatment course and for >1 month after completion of phototherapy. Recurrence was defined as the development of a new lesion, extension of an existing lesion (using serial photographs), or new signs of disease activity >1 month after completion of UVA1 phototherapy. If a patient developed a new lesion(s), extension of an existing lesion or new signs of disease activity <1 month after discontinuation of phototherapy, they were defined as a treatment failure and excluded from the analysis. At the inception of this study there were no widely accepted and validated outcome measures to define sustained inactivity or recurrent activity (although various measures of skin thickness had been reported). Therefore, the parameters for this study were selected based on published criteria for clinical morphea activity19, 20 and sustained inactivity and recurrent activity morphea were defined according to sustained resolution or recurrence of these features.
Statistical Analysis
Mean, standard deviation, median and range were calculated for continuous variables. Categorical variables were described via frequency counts and percents. UVA1 treatment responders were divided into recurrent activity and sustained inactivity groups and were compared with respect to baseline demographic, clinical, and disease characteristics using univariate Cox proportional hazards regression and Wilcoxon rank-sum test. The recurrent activity group was censored at the time of recurrent disease. Cox proportional hazard assumption was checked using Schoenfeld residuals with SAS software, and no violation of assumptions was observed with p-value>0.05. The Kaplan-Meier survival curve was used to estimate the time to recurrence. All time to recurrence is reported as months after discontinuation of therapy. Independent variables with p-values ≤ 0.2 were entered as candidate variables for a stepwise Cox proportional hazards regression. The stepwise Cox proportional-hazards regression was used to identify significant independent factors associated with the time to recurrence. P-values less than or equal to 0.05 were considered significant. Statistical Analysis System software, version 9.2 (SAS Institute Inc, Cary, North Carolina), was used for data analysis.
RESULTS
Patient Characteristics
Characteristics of UVA1 treatment responders are available in Table III. Overall, there was an initial response rate of 60% (n=37) and all responders were analyzed and included in the following results. Median follow-up times were 37 (range 18.3–52.8) months and 10 (range 4.3–25.3) months after discontinuation of UVA1 phototherapy for sustained inactivity and recurrent activity patients, respectively (patients were censored at the time of recurrent activity).
Table III.
Univariate analysis of patient and treatment variables on risk of recurrent morphea in UVA1 treatment responders
| Patient Characteristics | UVA1 Responders (n=37) | |||
|---|---|---|---|---|
| Sustained inactivity(N=20) | Recurrent activity (N=17) | Hazard Ratio (95% CI) | p-value | |
| Follow-up (month), Median (range) | 37.0 (18.3–52.8) | 10.3 (4.3–25.3) | - | |
| Age of onset (year) Mean±SD | 35.9±24.1 | 33.2±26.7 | 0.998(0.978–1.019) | 0.87 |
| Duration of disease (year) | 1.2±1.4 | 5.2±8.6 | 1.154(1.062–1.268) | 0.002 |
| Cumulative UVA1 dose J/cm2 | 3781.2±1259.4 | 3622.6±1008.4 | 1.000(0.999–1.000) | 0.56 |
| Number of treatments Mean±SD | 37.7±11.0 | 40.0±6.8 | 1.015(0.966–1.067) | 0.56 |
| Duration of treatment | 20.1±10.2 | 18.5±10.3 | 0.980(0.929–1.034) | 0.46 |
| Frequency (%) | ||||
| Sex | 0.31 | |||
| Male | 7(35%) | 3(18%) | ||
| Female | 13(65%) | 14(82%) | 1.900(0.546–6.619) | |
| Race | 0.21 | |||
| Caucasian | 18(90%) | 13(76%) | - | |
| Other | 2(10%) | 4(24%) | 2.021(0.656–6.227) | |
| Age at Enrollment | 0.75 | |||
| ≤17 | 7(35%) | 6(35%) | - | |
| >17 | 13(65%) | 11(65%) | 1.173(0.433–3.177) | |
| Subtype | 0.28 | |||
| Plaque | 4(20%) | 3(19%) | - | |
| Linear | 10(50%) | 4(25%) | 0.740(0.165–3.307) | |
| Generalized | 6(30%) | 7(44%) | 1.891(0.487–7.349) | |
| Mixed | 0(0%) | 2(13%) | 2.803(0.463–16.97) | |
| Skin Type | 0.04 | |||
| I–II | 11(55%) | 15( 88%) | - | |
| III–V | 9(45%) | 2( 12%) | 0.235(0.054–1.035) | |
The p-value is from two-sample t-test. All the other p-values and hazard ratios are from univariate Cox regression.
Variables Associated with Recurrence
The variables associated with recurrence are illustrated in Table III and Figure 1 outlines definitions of UVA1 treatment responders. Of 37 UVA1 treatment responders, 46% (n=17) had recurrent activity. The demographic features and morphea subtype were not significantly different between those with recurrent activity and sustained inactivity. Mean cumulative UVA1 doses (including mean number and duration of treatments) were no different among recurrent activity and sustained inactivity morphea patients. With respect to those with recurrent activity (n=17), mean duration of disease prior to UVA1 therapy was longer (5.2 years versus 1.2 years, p-value=0.002) than those with sustained inactivity.
Figure 2 shows the time to recurrent morphea activity for all UVA1 treatment responders (n=37). Two-year and three-year recurrence rates were 44.5% (95% CI: 30.1% – 62.2%) and 48.4% (95% CI: 33.2% – 66.1%) after the last UVA1 phototherapy treatment. After 3-years, recurrence rates remained near 50%. Cumulative UVA1 doses (equivalent to a range of 66–115 J/cm2 per session, which is in the medium to high dose UVA1 range) were not associated with the development of recurrent activity (p-value=0.56). Duration of morphea prior to UVA1 phototherapy was associated with increased risk of recurrence and was identified as a predictor of morphea recurrence [p-value=0.002, hazard ratio (HR) =1.15 95% CI (1.06–1.27)]. There was a 1.15 times higher chance of disease recurrence for an increment of 1 year in duration of morphea prior to UVA1 treatment. Higher Fitzpatrick skin type (III–V) was protective for risk of recurrent morphea activity [p-value=0.04, hazard ratio (HR)=0.235 (0.054–1.035)]. Age at morphea onset (including adult versus pediatric onset) and disease subtype did not increase the risk of recurrent morphea activity. Stepwise Cox regression analysis showed that only duration of morphea was significantly associated with the time to recurrent morphea activity.
Figure 2.
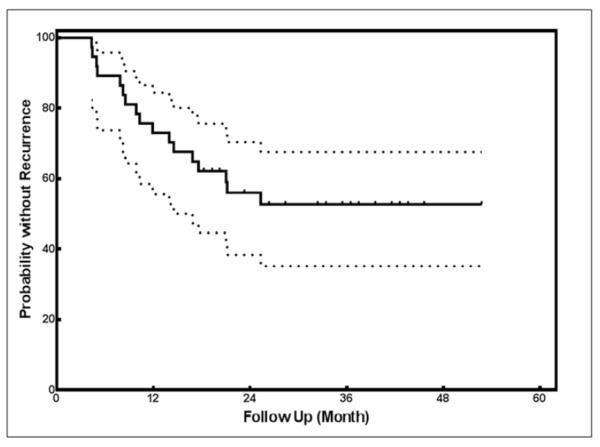
Kaplan-Meier survival curve. Kaplan-Meier survival curve for the time to recurrence in 37 UVA1-treated morphea patients. All patients (n=37) remained in cohort through time points listed.
DISCUSSION
In this study, we examined the frequency of recurrent morphea activity in patients who were UVA1 treatment responders in the MAC cohort. Forty-six percent (n=17) of UVA1 treatment responders had recurrent morphea activity after successful UVA1 phototherapy with two-year and three-year recurrence rates of 44.5% (95% CI: 30.1% – 62.2%) and 48.4% (95% CI: 33.2% – 66.1%) after the last UVA1 phototherapy treatment.
Our initial UVA1 treatment response rate of 60% confirms prior reports indicating a beneficial effect of UVA1 phototherapy in morphea. Although our response rate was lower than prior observations using similar UVA1 regimens in which 100% response rates were reported10, 12, 13 and in comparison to immunosuppressive therapy21–23 (81–100% response), further conclusions are limited due to variation in outcome measures, duration of follow-up, and definitions of therapeutic response between studies. Interestingly, the present study is the first to suggest no difference between medium (60–90 J/cm2) and high dose (>90 J/cm2) UVA1 with respect to likelihood of recurrence. This indicates that doses in the medium dose UVA1 range (40–70 J/cm2) delivered over 40 treatments are adequate for most patients with respect to the likelihood of recurrent morphea activity, and may theoretically decrease risk of short- (e.g. marked hyperpigmentation, erythema, pruritus) and long-term adverse events (including photoaging and carcinogenesis). Further studies are needed to determine whether initial response rates differ between medium and high dose UVA1 phototherapy.21
Frequency of and time to recurrence of active morphea after completion of UVA1 phototherapy are largely unaddressed in the literature. Studies examining recurrence rates after treatment with methotrexate and/or systemic steroids report recurrence 6–19 months after discontinuation of therapy in 10–44% of patients.6–8, 22 Comparison between recurrence in the present study versus reports of those treated with methotrexate is difficult due to large variations in study methods and duration of follow up. However, with a recurrence rate of 46%, our results indicate recurrence with UVA1 phototherapy may be more frequent than with methotrexate. In addition, the reported duration of treatment with immunosuppressives often exceeds 12 months, while the mean treatment duration in this study was 4 months.4, 7, 8 It is possible that the long duration of therapy with immunosuppressives may suppress initial episodes of recurrence while patients treated with UVA1 phototherapy who received treatment of much shorter duration do not benefit from this effect. Further, mean and/or median follow up duration in studies with immunosuppressives were 25–58 months.6, 22, 23 Our median follow-up duration (42 months) is longer than many studies which may account for the higher rate of recurrence in this study. This raises the question of whether maintenance therapy might be of benefit in UVA1 phototherapy and whether this offers an acceptable safety profile.
We also examined demographic and clinical features associated with recurrent morphea activity. Increased duration of disease prior to treatment was the only variable that significantly increased the probability of recurrent morphea activity after UVA1 phototherapy and was identified as a risk for recurrent activity. These patients may represent a subgroup of morphea patients with prolonged periods of disease activity over time (as our group previously reported) or with difficult to treat morphea.6, 24 Contrary to a study by Mirsky et al 7 which investigated recurrence after treatment with methotrexate in pediatric patients, we did not find an association with morphea subtype or older age of onset. Lack of an association between risk of recurrence and other clinical factors (Table III) implies children and adults have no difference in risk of recurrent morphea activity. Similarly, other clinical factors including disease subtype, Fitzpatrick skin type, and differences in number and duration of UVA1 treatment (in the medium to high dose range) did not alter risk for recurrent morphea activity. This is similar to our group's prior report in which UVA1 phototherapy efficacy was similar across Fitzpatrick skin type.25
There are several limitations to this study. Patients were exclusively treated with medium to high dose UVA1 phototherapy (66–118 J/cm2), so comparisons cannot be made with low-dose UVA1 (30–60 J/cm2). Although Fitzpatrick skin types I–III were well-represented, there were few patients with IV–V, therefore conclusions regarding decreased risk of recurrent activity in higher Fitzpatrick skin types implicated in this study are limited. This may reflect a predisposition of the Caucasian population to morphea and was not the result of exclusion of these patients from our study.26, 27 Recurrence may be over-estimated in this cohort from a specialty center, which may represent more severe or recalcitrant morphea. The majority of participants were >17 years of age which may limit generalizability to pediatric population. Although the present study is the largest to date, the number of patients with recurrent morphea activity was limited, therefore there are likely more variables associated with recurrence than those we were able to detect.
In summary, this prospective study from the MAC cohort examined recurrent morphea activity after successful UVA1 phototherapy. Sixty percent of patients were UVA1 treatment responders indicating that UVA1 phototherapy remains an acceptable therapeutic option for patients who are not candidates for systemic immunosuppressive therapy. However, relatively high rates of recurrence after successful UVA1 phototherapy suggest studies examining the safety and efficacy of maintenance UVA1 therapy are indicated. Identification of longer disease duration prior to UVA1 therapy as a risk factor for recurrent activity indicates these patients may benefit from frequent follow-up.
CAPSULE SUMMARY
Little is known about duration of remission after ultraviolet A1 (UVA1) phototherapy for patients with active inflammatory morphea.
In our series, 46% of responders had disease recurrence after a median of 10.8 months. Duration of morphea was a significant predictor of recurrence, which was not significantly different after medium or high dose UVA1.
Patients with longstanding morphea may benefit from frequent follow-up and maintenance therapy.
Acknowledgments
Funding Sources:
Research for this manuscript was supported in part by NIH Grant No. K23AR056303-4.
This work was conducted with support from UT-STAR, NIH/NCATS Grant Number UL1TR0000451. The content is solely the responsibility of the authors and does not necessarily represent the official views of UT-STAR, The University of Texas Southwestern Medical Center at Dallas and its affiliated academic and health care centers, the National Center for Advancing Translational Sciences, or the National Institutes of Health.
ABBREVIATIONS AND ACRONYMS
- MAC
Morphea in Adults and Children
- UVA1
ultraviolet A1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure: None declared
Author Contributions: Concept and Design: Vasquez, Jabbar, Khan, Buethe, Ahn, and Jacobe Analysis: Jacobe, Vasquez, and Ahn
Drafting of manuscript: Vasquez, Jabbar, Khan, Buethe, Ahn, and Jacobe
This study has not been presented or published previously.
REFERENCES
- 1.Zulian F, Vallongo C, Woo P, Russo R, Ruperto N, Harper J, et al. Localized scleroderma in childhood is not just a skin disease. Arthritis Rheum. 2005;52(9):2873–81. doi: 10.1002/art.21264. [DOI] [PubMed] [Google Scholar]
- 2.Zulian F, Athreya BH, Laxer R, Nelson AM, Feitosa de Oliveira SK, Punaro MG, et al. Juvenile localized scleroderma: clinical and epidemiological features in 750 children. An international study. Rheumatology (Oxford) 2006;45(5):614–20. doi: 10.1093/rheumatology/kei251. [DOI] [PubMed] [Google Scholar]
- 3.Zulian F, Martini G, Vallongo C, Vittadello F, Falcini F, Patrizi A, et al. Methotrexate treatment in juvenile localized scleroderma: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2011;63(7):1998–2006. doi: 10.1002/art.30264. [DOI] [PubMed] [Google Scholar]
- 4.Seyger MM, van den Hoogen FH, de Boo T, de Jong EM. Low-dose methotrexate in the treatment of widespread morphea. J Am Acad Dermatol. 1998;39(2 Pt 1):220–5. doi: 10.1016/s0190-9622(98)70079-9. [DOI] [PubMed] [Google Scholar]
- 5.Kreuter A, Gambichler T, Breuckmann F, Rotterdam S, Freitag M, Stuecker M, et al. Pulsed high-dose corticosteroids combined with low-dose methotrexate in severe localized scleroderma. Arch Dermatol. 2005;141(7):847–52. doi: 10.1001/archderm.141.7.847. [DOI] [PubMed] [Google Scholar]
- 6.Kroft EB, Creemers MC, van den Hoogen FH, Boezeman JB, de Jong EM. Effectiveness, side-effects and period of remission after treatment with methotrexate in localized scleroderma and related sclerotic skin diseases: an inception cohort study. Br J Dermatol. 2009;160(5):1075–82. doi: 10.1111/j.1365-2133.2008.09017.x. [DOI] [PubMed] [Google Scholar]
- 7.Mirsky L, Chakkittakandiyil A, Laxer RM, O'Brien C, Pope E. Relapse after systemic treatment in paediatric morphoea. Br J Dermatol. 2012;166(2):443–5. doi: 10.1111/j.1365-2133.2011.10535.x. [DOI] [PubMed] [Google Scholar]
- 8.Cox D, G OR, Collins S, Byrne A, Irvine A, Watson R. Juvenile localised scleroderma: a retrospective review of response to systemic treatment. Ir J Med Sci. 2008;177(4):343–6. doi: 10.1007/s11845-008-0217-0. [DOI] [PubMed] [Google Scholar]
- 9.Zwischenberger BA, Jacobe HT. A systematic review of morphea treatments and therapeutic algorithm. J Am Acad Dermatol. 2011;65(5):925–41. doi: 10.1016/j.jaad.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Kreuter A, Hyun J, Stucker M, Sommer A, Altmeyer P, Gambichler T. A randomized controlled study of low-dose UVA1, medium-dose UVA1, and narrowband UVB phototherapy in the treatment of localized scleroderma. J Am Acad Dermatol. 2006;54(3):440–7. doi: 10.1016/j.jaad.2005.11.1063. [DOI] [PubMed] [Google Scholar]
- 11.Tuchinda C, Kerr HA, Taylor CR, Jacobe H, Bergamo BM, Elmets C, et al. UVA1 phototherapy for cutaneous diseases: an experience of 92 cases in the United States. Photodermatol Photoimmunol Photomed. 2006;22(5):247–53. doi: 10.1111/j.1600-0781.2006.00245.x. [DOI] [PubMed] [Google Scholar]
- 12.Sator PG, Radakovic S, Schulmeister K, Honigsmann H, Tanew A. Medium-dose is more effective than low-dose ultraviolet A1 phototherapy for localized scleroderma as shown by 20-MHz ultrasound assessment. J Am Acad Dermatol. 2009;60(5):786–91. doi: 10.1016/j.jaad.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Stege H, Berneburg M, Humke S, Klammer M, Grewe M, Grether-Beck S, et al. High-dose UVA1 radiation therapy for localized scleroderma. J Am Acad Dermatol. 1997;36(6 Pt 1):938–44. doi: 10.1016/s0190-9622(97)80277-0. [DOI] [PubMed] [Google Scholar]
- 14.Camacho NR, Sanchez JE, Martin RF, Gonzalez JR, Sanchez JL. Medium-dose UVA1 phototherapy in localized scleroderma and its effect in CD34-positive dendritic cells. J Am Acad Dermatol. 2001;45(5):697–9. doi: 10.1067/mjd.2001.117735. [DOI] [PubMed] [Google Scholar]
- 15.Andres C, Kollmar A, Mempel M, Hein R, Ring J, Eberlein B. Successful ultraviolet A1 phototherapy in the treatment of localized scleroderma: a retrospective and prospective study. Br J Dermatol. 2010;162(2):445–7. doi: 10.1111/j.1365-2133.2009.09438.x. [DOI] [PubMed] [Google Scholar]
- 16.Dharamsi JW, Victor S, Aguwa N, Ahn C, Arnett F, Mayes MD, et al. Morphea in adults and children cohort III: Nested case-control study: the clinical significance of autoantibodies in morphea. JAMA Dermatol. 2013 doi: 10.1001/jamadermatol.2013.4207. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zulian F, Woo P, Athreya BH, Laxer RM, Medsger TA, Jr., Lehman TJ, et al. The Pediatric Rheumatology European Society/American College of Rheumatology/European League against Rheumatism provisional classification criteria for juvenile systemic sclerosis. Arthritis Rheum. 2007;57(2):203–12. doi: 10.1002/art.22551. [DOI] [PubMed] [Google Scholar]
- 18.Laxer RM, Zulian F. Localized scleroderma. Curr Opin Rheumatol. 2006;18(6):606–13. doi: 10.1097/01.bor.0000245727.40630.c3. [DOI] [PubMed] [Google Scholar]
- 19.Arkachaisri T, Fertig N, Pino S, Medsger TA., Jr. Serum autoantibodies and their clinical associations in patients with childhood- and adult-onset linear scleroderma. A single-center study. J Rheumatol. 2008;35(12):2439–44. doi: 10.3899/jrheum.080098. [DOI] [PubMed] [Google Scholar]
- 20.Li SC, Liebling MS, Haines KA. Ultrasonography is a sensitive tool for monitoring localized scleroderma. Rheumatology (Oxford) 2007;46(8):1316–9. doi: 10.1093/rheumatology/kem120. [DOI] [PubMed] [Google Scholar]
- 21.Vasquez R, Jacobe H. Something new under the sun: Phototherapy for sclerosing conditions. Expert Rev Dermatol. 2011;6(6):595–612. [Google Scholar]
- 22.Weibel L, Sampaio MC, Visentin MT, Howell KJ, Woo P, Harper JI. Evaluation of methotrexate and corticosteroids for the treatment of localized scleroderma (morphoea) in children. Br J Dermatol. 2006;155(5):1013–20. doi: 10.1111/j.1365-2133.2006.07497.x. [DOI] [PubMed] [Google Scholar]
- 23.Uziel Y, Feldman BM, Krafchik BR, Yeung RS, Laxer RM. Methotrexate and corticosteroid therapy for pediatric localized scleroderma. J Pediatr. 2000;136(1):91–5. doi: 10.1016/s0022-3476(00)90056-8. [DOI] [PubMed] [Google Scholar]
- 24.Saxton-Daniels S, Jacobe HT. An evaluation of long-term outcomes in adults with pediatric-onset morphea. Arch Dermatol. 2010;146(9):1044–5. doi: 10.1001/archdermatol.2010.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobe HTCR, Nguyen J. UVA1 phototherapy is effective in darker skin: a review of 101 patients of Fitzpatrick skin types I–V. Br J Dermatol. 2008;159(3):691–6. doi: 10.1111/j.1365-2133.2008.08672.x. [DOI] [PubMed] [Google Scholar]
- 26.Leitenberger JJ, Cayce RL, Haley RW, Adams-Huet B, Bergstresser PR, Jacobe HT. Distinct autoimmune syndromes in morphea: a review of 245 adult and pediatric cases. Arch Dermatol. 2009;145(5):545–50. doi: 10.1001/archdermatol.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christen-Zaech S, Hakim MD, Afsar FS, Paller AS. Pediatric morphea (localized scleroderma): review of 136 patients. J Am Acad Dermatol. 2008;59(3):385–96. doi: 10.1016/j.jaad.2008.05.005. [DOI] [PubMed] [Google Scholar]


