Abstract
Estrogens are well known steroid hormones necessary to maintain bone health. In addition, mechanical loading, in which estrogen signaling may intersect with the Wnt/β-catenin pathway, is essential for bone maintenance. As osteocytes are known as the major mechanosensory cells embedded in mineralized bone matrix, osteocyte ERα deletion mice (ERαΔOcy/ΔOcy) were generated by mating ERα floxed mice with Dmp1-Cre mice to determine the role of ERα in osteocytes. Trabecular bone mineral density of female, but not male ERαΔOcy/ΔOcy mice was significantly decreased. Bone formation parameters in ERαΔOcy/ΔOcy were significantly decreased while osteoclast parameters were unchanged. This suggests that ERα in osteocytes exerts osteoprotective function by positively controlling bone formation. To identify potential targets of ERα, gene array analysis of Dmp1-GFP osteocytes sorted by FACS from ERαΔOcy/ΔOcy and control mice was performed. Gene expression microarray followed by gene ontology analyses revealed that osteocytes from ERαΔOcy/ΔOcy highly expressed genes categorized in ‘Secreted’ when compared to control osteocytes. Among them, expression of Mdk and Sostdc1, both of which are Wnt inhibitors, was significantly increased without alteration of expression of the mature osteocyte marker Sost or β-catenin. Moreover, hindlimb suspension experiments showed that trabecular bone loss due to unloading was greater in ERαΔOcy/ΔOcy mice with no loss of cortical bone. These data suggest that ERα in osteocytes has osteoprotective functions in trabecular bone formation through regulating expression of Wnt antagonists, but conversely plays a negative role in cortical bone loss due to unloading.
Keywords: Estrogen, Estrogen Receptor α, Osteocyte, Bone Formation, Wnt signaling
Introduction
Estrogens clearly maintain physiological homeostasis through the development of reproductive organs and the mammary gland, potentiation of muscles, and through osteoprotection. The osteoprotective actions of estrogens are clearly demonstrated by post-menopausal osteoporosis [1]. The effects of sex steroid hormones on bone tissue can be considered as the combination or sum of the direct effects on bone cells and the indirect effects on other tissues [2]. The indirect effects of estrogen on bone through other tissues have been well described, such as modulation of cytokine production by immune cells and the increased induction of pituitary gland hormones [3, 4]. However, the direct effect of estrogens on bone tissue is not fully understood.
Estrogens exert their effects by binding to their own nuclear receptors, such as Estrogen Receptor (ER) α and β, which also function as transcription factors. The conventional ERα null mouse model could not be used to address the direct functions of the receptor in bone due to hormonal imbalance and endocrine disturbances [5-7]. Therefore, the generation and analyses of bone cell type specific deletion is required to clarify the functions of ERα in bone.
Osteoclastic ERα null mice were generated showing that osteoclastic ERα shortens the life span of osteoclasts by promoting apoptosis [8, 9]. Ovariectomy can induce osteocyte apoptosis [10] and conventional ERαKO mice do not increase bone mass in response to anabolic mechanical loading [11]. Moreover, various groups reported murine skeletal phenotype due to ERα deletion in cells of the osteoblast lineage, suggesting ERα in osteoblastic lineage cells could play important roles in the maintenance of bone metabolism [12-15]. Recently, Windahl et al. [13] reported that ERα in osteocytes regulates trabecular bone formation and thus trabecular bone volume in male mice. These results are in contrast to our own findings showing that the precise molecular functions and target genes of ERα in osteocytes still remain elusive.
Osteocytes are embedded in the extracellular matrix of bone and represents more than 90% of the cells existing in bone. Osteocytes possess dendrites that extend throughout the bone and are used to communicate with each other and also with osteoblasts and osteoclasts on the surface of the bone. The function of osteocytes as mechanosensory cells is inferred from their shape and location [16]. In fact, mechanical loading and unloading change osteocyte gene expression in vivo, indicating that osteocyte function is affected by loading conditions [17-20]. In addition, they are known to be involved in mineral metabolism through expression of proteins such as FGF23, Phex, Mepe, and Dmp1 [21-24] (for review, see [25]). Recently, it has been postulated that osteocytes can orchestrate skeletal homeostasis through mineral metabolism as well as the regulation of osteoblastic bone formation and osteoclastic bone resorption by secretory proteins such as sclerostin and FGF23. Osteocytes are also reported to regulate osteoblastic bone formation through IGF-1, TGFβ, NO, PGE2 and sclerostin and to regulate osteoclastic bone resorption through TGFβ, NO, and PGE2, and RANKL/OPG [26].
Bone mass can be maintained by mechanical loading while unloading or immobilization decreases bone mass. In vivo unloading rodent models such as tail suspension can induce bone loss in hind limbs [27] and mechanical loading can increase bone mass in forelimbs [28]. The regulation of bone mass by mechanical loading is mediated, at least in part, through β-catenin signaling [29-31], and estrogen/ER signaling might also be involved in this mechanism [32].
In this study, we examined the functions of ERα in osteocytes by generating mice lacking ERα in osteocytes and analyzing osteocyte gene expression profiles and subjecting them to hindlimb unloading.
Materials and Methods
Animals
The ERα floxed mutant (ERαL2/L2) mice kindly provided by Dr. Chambon and null alleles with a C57BL/6J background have been previously described [5]. ERαL2/L2 mice were crossed with Dmp1Cre mice [33] to generate Dmp1Cre; ERαL2/+ mice, and Dmp1Cre; ERαL2/L2 (ERαΔOcy/ΔOcy) and ERαL2/L2 (ERαflox/flox) were obtained by crossing Dmp1Cre; ERαL2/+ and ERαL2/L2. Dmp1-GFP mice were kindly provided by Dr. Ivo Kalajzic [34]. All mice were housed in a specific-pathogen-free facility under climate-controlled conditions with a 12-hour light/dark cycle and were provided with water and standard diet (CE-2, CLEA, Japan) ad libitum. All animals were maintained and examined according to the protocol approved by the Animal Care and Use Committee of the University of Tokyo.
Genome DNA extraction and Cell culture
Various tissues (0.5 g) from ERαΔOcy/ΔOcy were harvested, washed with PBS and lysed in 2 ml of lysis buffer with proteinase K (150 μg/ml) overnight. Also, DNA of osteocytes was isolated from the calvariae of ERαΔOcy/ΔOcy in which cells on the surface of the bone such as osteoclasts and osteoblasts were removed by sequential enzymatic treatment. Primary osteoblasts obtained from the neonatal calvariae were cultured in αMEM (Life Technologies) containing 10% FBS (Cell Culture Bioscience), 50 μg/ml ascorbic acid (Sigma-Aldrich) and 10 nM β-glycerophosphate (Sigma-Aldrich) for 21 days. Cells were cultured with phenol red free media 24 hours before cells were treated with 17β-estradiol. Primary osteoclasts were differentiated from the bone marrow obtained from 6-week-old ERαΔOcy/ΔOcy mice using 10 ng/ml of M-CSF (R&D Systems) and 234 ng/ml of GST-RANKL (Oriental Yeast) for 5 days. The genomic DNA was extracted using phenol/chloroform and isopropanol precipitation.
ELISAs
Enzyme-linked Immunoassays, Elisas, were was performed following the protocols of the Estradiol EIA Kit (Cayman Chemical Company) for estradiol, Testosterone EIA Kit (Cayman Chemical Company) for testosterone, and Rodent Luteinizing Hormone (LH) ELISA TEST (Endocrine Technologies) for LH.
Bone analyses
The BMD of femurs and tibiae obtained from 12-week-old littermates were measured by DXA using a bone mineral analyzer (DCS-600EX: ALOKA). Micro Computed Tomography scanning of the tibiae and femurs was performed using a Scanco Medical μCT35 System (SCANCO Medical) with an isotropic voxel size of 6 μm for trabecular analyses and 12 μm for cortical analyses according to the manufacturer's instructions and the recent guidelines of the American Society for Bone and Mineral Research (ASBMR) [35]. For bone histomorphometry, the mice were double-labeled with subcutaneous injections of 16 mg/kg of calcein (Sigma) at 4 and 2 days before sacrifice. Lumbar vertebral bodies were removed from each mouse and fixed with 4% PFA in PBS overnight. Lumbar vertebrae were embedded with MMA after dehydration and the plastic sections were cut by a standard microtome (LEICA) into 7 μm for von Kossa staining and 4 μm for TRAP and Toluidine-blue staining. The region of interest was the secondary spongiosa of L3 and L4. Sections were used for analyses when the bases of the bilateral transverse processes were opened. The region of interest (ROI) in the lumbar vertebral body is the secondary spongiosa, which is separated from the primary spongiosa, cranial and caudal growth plate, according to the same protocol as previously performed [8, 36]. Histomorphometric analyses were performed using OsteoMeasure (OsteoMetrics, Inc., GA, USA) according to the ASBMR guideline [37].
Isolation of Dmp1-GFP positive osteocytes by FACS
A highly purified population of osteocytes was isolated from neonatal calvariae by FACS using a modified version of the protocol of Paic F et al [38]. Cells were isolated from 10-day-old fetal mice calvariae of ERαΔOcy/ΔOcy and ERαflox/flox also expressing Dmp1-GFP. After removal of the sutures, pooled calvarial tissue was subjected to six sequential, 30-minute digestions in a mixture containing 0.05%/0.2 mM trypsin/EDTA and 1.5 U/ml collagenase-P (Roche) at 37°C. Cell fractions 4 to 6 were collected, pooled, and re-suspended in Dulbecco's modified Eagle's medium (DMEM, Life Technologies) containing 10% FBS (Hyclone) and centrifuged. Cells were rinsed with PBS and re-suspended in PBS/2% FBS and filtered through a 70-μm filter. Cell sorting was performed using a BD FACS Aria cell sorter. The gate for collecting GFP + cells was set as GFP + population to represent 10% to 15% of the total cells in GFP+ mice and 0.8% to 1.0% of total cells in GFP− mice (negative control). GFP+ cells were collected in a tube with 500 μl of PBS/3% FBS.
Gene Expression Microarray
Gene expression microarray were generated using total RNA extracted from the isolated GFP+ osteocytes of ERαΔOcy/ΔOcy and ERαflox/flox as previously described [8] and RNA samples were evaluated using the Affymetrix Mouse Genome 430 2.0 Array following standard Affymetrix protocols (GEO: GSE41997). Gene ontology analyses were performed using DAVID Bioinformatics Resources 6.7 [39].
RNA extraction and RT-qPCR
Total RNA from the pulverized femurs or sorted cells was extracted using TRIZOL (Invitrogen) and RNeasy purification kit (QIAGEN). First-strand cDNA was synthesized from total RNA using PrimeScript RT Master Mix (TaKaRa) and subjected to RT-qPCR using SYBR Premix Ex Taq II (TaKaRa) or KAPA SYBR Fast qPCR Kits (KAPA Biosystems) with Thermal Cycler Dice (TaKaRa) according to the manufacturer's instructions. Primers were purchased from Takara Bio Inc. (Otsu, Japan) or Operon Biotechnologies (Tokyo, Japan) [8]. Gene expression levels were normalized by Gapdh or Rplp0. Primer sequences were as follow; Rplp0: F 5’-TTCCAGGCTTTGGGCATCA-3’ and R 5’-ATGTTCAGCATGTTCAGCAGTGTG-3’, Gapdh: F 5’-AAATGGTGAAGGTCGGTGTG-3’ and R 5’-TGAAGGGGTCGTTGATGG-3’, ERα: F 5’-CATGGTCATGGTAAGTGGCA-3’ and R 5’-TCTCTGGGCGACATTCTTCT-3’, Dmp1: F 5’-TGAAGAGAGGACGGGTGATT-3’ and R 5’-TCCGTGTGGTCACTATTTGC-3’, Kera: F 5’-TGGGATGTCCACGACGACTT-3’ and R 5’-AAGGCAGTAGGGAAACTGGGA-3’, Mdk: F 5’-TGGAGCCGACTGCAAATACAA-3’ and R 5’-GGCTTAGTCACGCGGATGG-3’, Sostdc1: F 5’-AAATGTATTTGGTGGACCGC-3’ and R 5’-GAATCAAGCCAGGAATGGAG-3’.
Tail Suspension
Tail suspension experiments were performed for female ERαΔOcy/ΔOcy and ERαflox/flox mice for 4 weeks starting at 8 weeks of age according to previous reports [40, 41]. Briefly, a stainless steel harness was superglued to the sides of the tail. Female ERαΔOcy/ΔOcy and ERαflox/flox mice were then suspended from an eye bolt which was secured into the bars of the top of the rat cage. The animal could rotate 360 degrees with the fish swivel and could also move backwards and forwards about 7.5 cm. Water was provided through a standard water bottle with an extra long angled sipper tube to allow the animals to reach the water. Control female ERαΔOcy/ΔOcy and ERαflox/flox mice were chained to the cage top during the same period of time, but were allowed to load their hindlimbs to minimize the difference in stress-related effects between the tail-suspended groups and the control groups (n=6 per group).
Statistical analysis
Data were analyzed by a two-tailed student's t-test or one-way analysis of variance (ANOVA) to initially determine whether an overall statistically significant change existed before using Tukey's post hoc test. For all graphs, data are represented as mean ± SEM. A p-value less than 0.05 was considered statistically significant.
Results
Generation of osteocytic ERα deletion mice
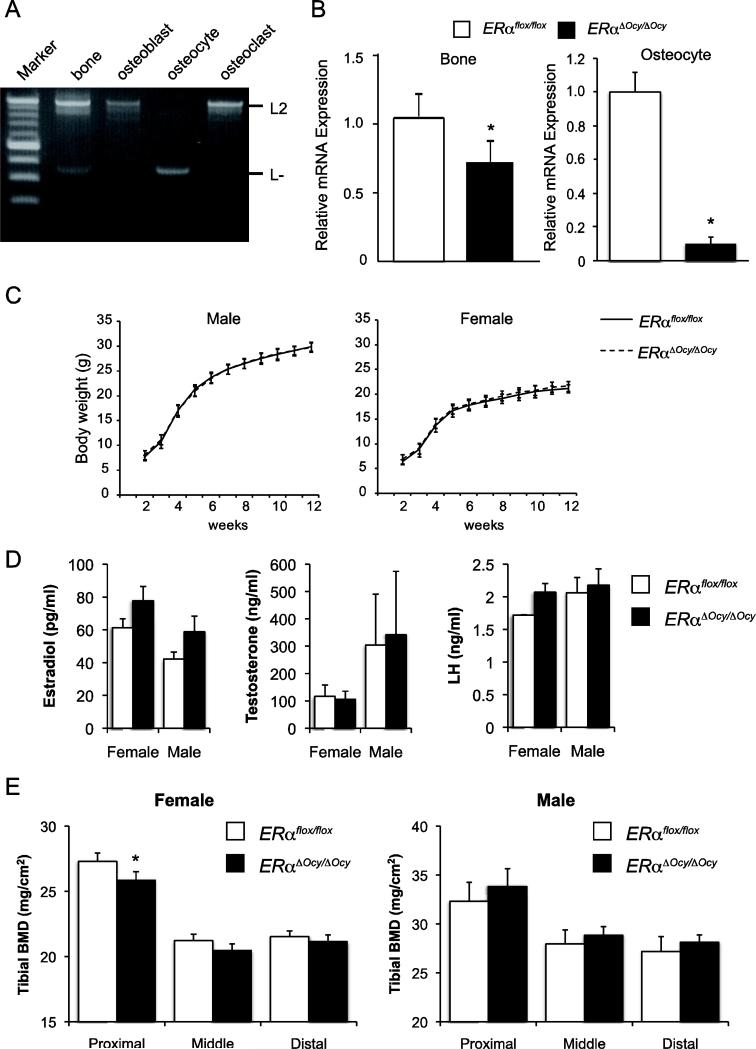
To investigate the function of ERα in osteocytes, we generated mice lacking ERα in late-osteoblasts/osteocytes by crossing ERα floxed mice with Dmp1-Cre mice, which express Cre recombinase driven by the Dmp1 promoter. The mice harboring the genotypes of Dmp1Cre; ERαL2/L2 and ERαL2/L2 were analyzed as ERαΔOcy/ΔOcy and ERαflox/flox, respectively. First, to assess cell type specificity of the deletion of the ERα gene locus by Dmp1 promoter-driven Cre recombinase, genomic PCR was performed using DNA extracted from ERαΔOcy/ΔOcy. As a result, a relatively specific deletion of ERα in osteocytes, which were isolated by sequential enzymatic digestion, was detected as an L-band, which was seen only in osteocytes and not in primary cultured osteoblasts or osteoclasts (Fig. 1A). In addition, the ERα mRNA level was examined by qPCR using RNA extracted from femoral bones and GFP-mediated FACS sorted osteocytes of ERαΔOcy/ΔOcy and ERαflox/flox mice. As a result, there was an approximately 30% and 90% reduction of ERα expression in whole bone and osteocytes, respectively, in ERαΔOcy/ΔOcy compared to ERαflox/flox mice (Fig. 1B). This significant but low percent deletion in whole bone might reflect ERα expression by other cell types, which are present in the intact femur even though the bone marrow was removed. Also, one group reported that clear deletion of the target gene was detected at the genome level but not the mRNA level when using the Dmp1-Cre mice [42]. Next, body weight was measured every other week from 3 to 12 weeks old. There was no significant difference in body weight between ERαΔOcy/ΔOcy and ERαflox/flox, whereas it was previously reported that ERα total KO mice exhibited a significant increase in body weight [43] (Fig. 1C). Next, we asked if these mice could be a suitable model for analyzing ERα function without the systemic influence of hormones (endocrine disturbances) as described in the conventional ERα null mouse, by examining the concentration of sex steroid hormones. Serum estradiol, testosterone and luteinizing hormone concentrations were measured by ELISA, showing that there were no significant differences between the 12-week-old ERαΔOcy/ΔOcy and ERαflox/flox, regardless of gender (Fig. 1D). Since ERαΔOcy/ΔOcy mice exhibited a relatively specific deletion of ERα in osteocytes and normal serum sex steroid hormone levels, we concluded that ERαΔOcy/ΔOcy could be used for analysis of ERα function in osteocytes without the complications of endocrine disturbances.
Fig. 1. Generation of mice with targeted deletion of ERα in osteocytes.
(A) Deletion of ERα gene locus in osteocyte was detected by genome PCR in ERαΔOcy/ΔOcy. (B) mRNA levels of ERα from whole femurs (left panel) and isolated osteocytes (right panel) of ERαflox/flox and ERαΔOcy/ΔOcy mice was evaluated by RT-qPCR. Data are represented as mean ± SEM (n=3). (C) The growth curves of ERαflox/flox and ERαΔOcy/ΔOcy mice. Data are represented as mean ± SEM (n=7-10). (D) Serum hormone levels of 12-week-old ERαflox/flox and ERαΔOcy/ΔOcy mice. Data are represented as mean ± SEM (n=4-7). (E) BMD of 1/3 portion of longitudinal divisions of tibiae from 12-week-old ERαflox/flox and ERαΔOcy/ΔOcy mice. Data are represented as mean ± SEM (Female n=8, Male n=7). * indicates p<0.05.
Osteocytic ERα deletion female mice exhibit an osteopenic phenotype
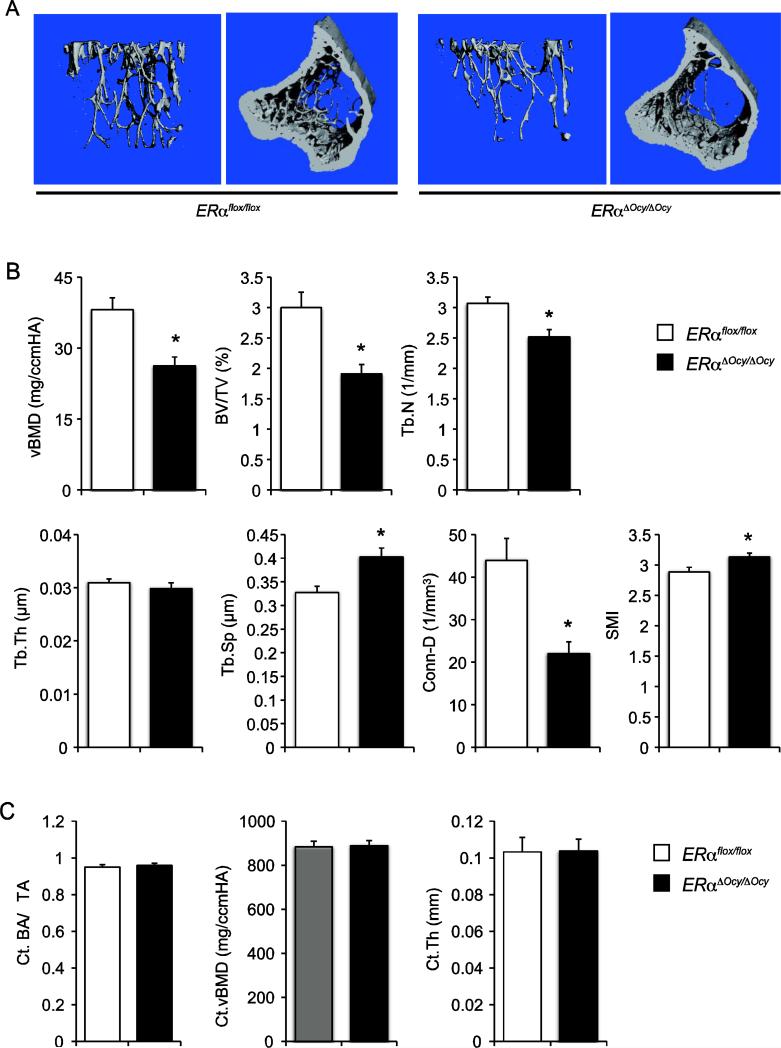
The BMD of 12-week-old ERαΔOcy/ΔOcy and ERαflox/flox were measured by DXA, showing that the BMD of female ERαΔOcy/ΔOcy was significantly decreased in the proximal, not in middle and distal, tibiae compared to that of female ERαflox/flox (Fig. 1E). However, the BMD of tibiae from male ERαΔOcy/ΔOcy were not significantly different from that of male ERαflox/flox (Fig. 1E). Next, to assess changes in bone structure between female ERαΔOcy/ΔOcy and ERαflox/flox mice, μCT analysis was performed. Decreased trabecular bone mass in ERαΔOcy/ΔOcy mice was observed by μCT analysis (Fig. 2A). Trabecular bone of female ERαΔOcy/ΔOcy exhibited a significant decrease in BV/TV, vBMD, Tb.N and Conn-D, and increase in Tb.Sp and SMI compared to those of female ERαflox/flox (Fig. 2B). The parameters in metaphyseal cortical bone of female ERαΔOcy/ΔOcy were not significantly different from that of female ERαflox/flox (Fig. 2C).
Fig. 2. μCT analyses of the mice lacking ERα in osteocytes.
(A) Representative μCT views. (B) 3D measurements of proximal tibiae from ERαflox/flox and ERαΔOcy/ΔOcy mice. Data are represented as mean ± SEM (n=10). * indicates p<0.05.
Osteocytic ERα regulates bone formation through control of osteoblasts
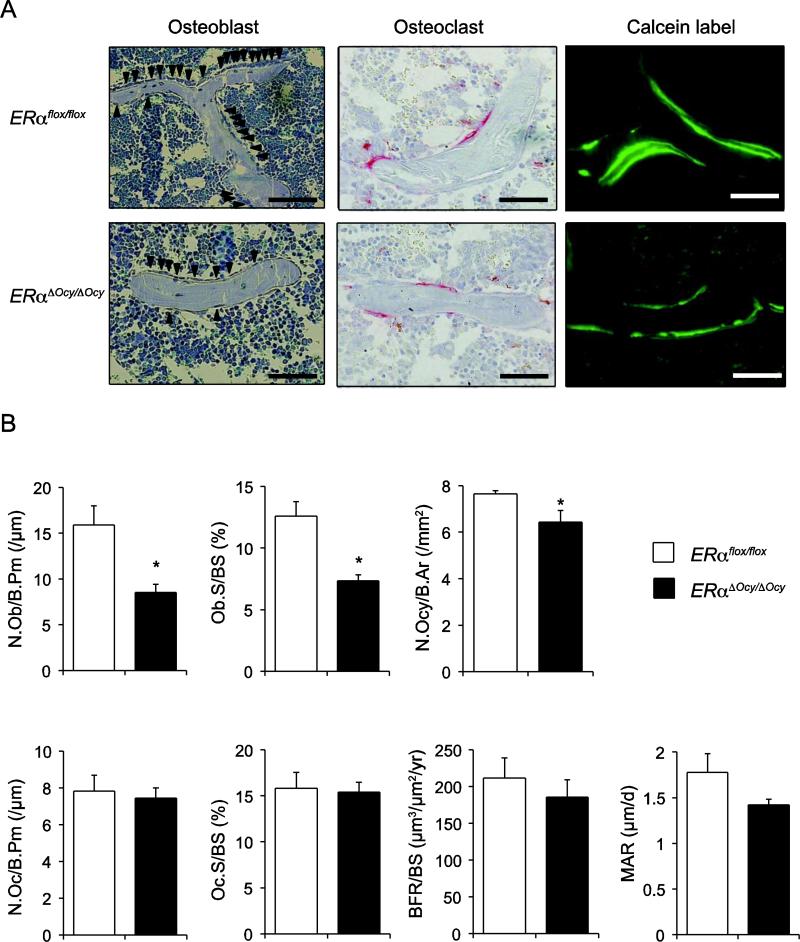
To examine whether the reduced bone phenotype of ERαΔOcy/ΔOcy could be caused by alterations in the potential interaction between osteocytes and either osteoblasts or osteoclasts, bone histomorphometry was performed. The number and/or activity of osteoblasts/osteoclasts were examined in ERαΔOcy/ΔOcy and ERαflox/flox, using lumbar vertebrae of 12-week-old female ERαΔOcy/ΔOcy and ERαflox/flox. Parameters related to osteoblastic bone formation, such as N.Ob/B.Pm and Ob.S/BS, were significantly decreased in ERαΔOcy/ΔOcy compared to ERαflox/flox (Fig. 3). In addition, N.Ocy/B.Ar was also decreased in ERαΔOcy/ΔOcy, which might be due to a decreased number of osteoblasts, which are precursors of osteocytes. Also, the reduction of BFR/BS and MAR in ERαΔOcy/ΔOcy tended to be significant (p=0.07), due to the reduction of osteoblastic parameters. On the other hand, parameters related to osteoclastic bone resorption, such as N.Oc/B.Pm and Oc.S/BS, were not altered in ERαΔOcy/ΔOcy when compared to ERαflox/flox (Fig. 3). These results suggested that deficiency of ERα in osteocytes could decrease the number of osteoblasts and consequently their bone forming activity, indicating that bone mass reduction in ERαΔOcy/ΔOcy could be caused by a reduction of osteoblastic bone formation, not a promotion of osteoclastic bone resorption. In addition, this result implies that osteocytic ERα might positively regulate osteoblastic bone formation by signaling from osteocytes, such as in a paracrine manner or by cell-cell contact.
Fig. 3. ERαΔOcy/ΔOcy mice exhibit decreased bone formation.
(A) Representative views of Toluidine blue staining for mononuclear cuboidal osteoblasts (arrowhead), TRAP staining for multinuclear TRAP-positive osteoclasts and calcein labeling for dynamic parameters are shown. Bars indicate 50 μm. (B) Data are represented as mean ± SEM (n=6). * indicates p<0.05.
Gene expression profiles of osteocytes lacking ERα
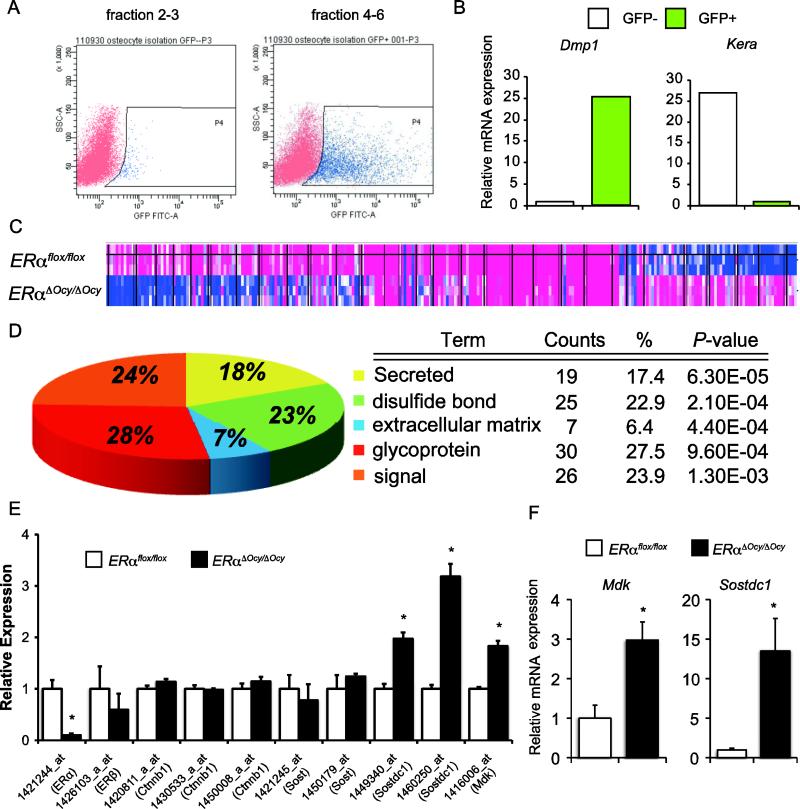
To determine what secretory proteins or signaling pathways ERα may utilize in osteocytes, a gene array analysis of Dmp1-GFP-positive cells from controls and mice with a targeted deletion of ERα in osteocytes was performed. Dmp1-GFP mice were crossed with Dmp1Tg/0; ERαL2/L2 mice to generate Dmp1-GFP+; Dmp1Tg/0; ERαL2/+ mice, and then Dmp1-GFP+; Dmp1Tg/0; ERαL2/L2 (Dmp1-GFP+; ERαΔOcy/ΔOcy) and Dmp1-GFP+; ERαL2/L2 (Dmp1-GFP+; ERαflox/flox) were generated by crossing Dmp1-GFP+; Dmp1Tg/0; ERαL2/+ and ERαL2/L2. Calvariae obtained from approximately 10-day-old female Dmp1-GFP+; ERαΔOcy/ΔOcy and Dmp1-GFP+; ERαflox/flox were treated with sequential enzymatic digestion and subjected to FACS. The percentage of GFP+ cells in fractions 4 to 6 was increased compared to that in fractions 2 to 4 (23.3% and 8.2%, respectively) (Fig. 4A). To determine if osteocytes were highly purified in this system, gene expression of cell-type specific marker genes in GFP+ cells (osteocytes) and GFP− cells (osteoblasts) was confirmed by RT-qPCR. As a result, the expression of Dmp1 (osteocyte marker gene) in GFP+ cells was about 25 times higher than in GFP− cells, while the expression of keratocan, Kera, (osteoblast marker gene) in GFP− cells was about 25 times higher than in GFP+ cells (Fig. 4B). Extracted total RNA from Dmp1-GFP+; ERαΔOcy/ΔOcy (n = 3) and Dmp1-GFP+; ERαflox/flox (n = 3) was subjected to a gene expression microarray analysis with GeneChip Mouse Genome 430 2.0 (Affymetrix). There were 276 genes found to be significantly differentially expressed between ERαΔOcy/ΔOcy and ERαflox/flox (p <0.01). Among them, 76 genes were significantly down-regulated and 200 genes were up-regulated (Fig. 4C). Gene ontology analyses revealed that ‘secreted’ was listed top in the Keyword analysis when sorted by p-value (Fig. 4D). Among these genes, Mdk (Midkine) and Sostdc1 (Sclerostin domain containing 1) were significantly up-regulated in ERαΔOcy/ΔOcy although there were no significant differences in Sost or β-catenin (Ctnnb1) gene expression (Fig. 4E). Up-regulation of mRNA of Mdk and Sostdc1 in ERαΔOcy/ΔOcy was also validated when determined by RT-qPCR (Fig. 4F). From the results of functional annotation in differentially expressed genes between ERαΔOcy/ΔOcy and ERαflox/flox, osteocytic ERα could regulate the expression of secretory protein genes such as Mdk and Sostdc1, which have been shown to be inhibitors of Wnt signaling-related bone formation [44-46]. However, the expression levels of Mdk and Sostdc1 were not significantly altered when late-stage primary cultured osteoblasts were treated with 17β-estradiol for 2 or 6 hours (Supplemental Fig.S1), indicating that Mdk and Sostdc1 might not be early responsive genes, but be indirect target genes.
Fig. 4. Osteocytes lacking ERα show increased Mdk and Sostdc1 expression.
(A) Two-dimensional dot plot of cells obtained from sequential enzymatic digestion of calvariae of mice expressing Dmp1-GFP. Left: fraction 2-3, Right: fraction 4-6. (B) Expression of osteocyte (Dmp1) and osteoblast (Kera) marker genes in the GFP− and GFP+ population of isolated cells. (C) Heat map of significantly regulated genes in the gene expression microarray using total RNA from isolated GFP+ cells of ERαflox/flox and ERαΔOcy/ΔOcy mice harboring Dmp1-GFP (n=3). Red: high expression. Blue: low expression. (D) Functional annotation clustering of Keywords by DAVID Bioinformatic Resources. (E) Relative microarray intensity of each probe for ERα, ERβ, Ctnnb1 (β-catenin), Sost, Sostdc1 and Mdk. Data are represented as mean ± SEM (n=3). (F) RT-qPCR for Mdk and Sostdc1 as same as panel E. * indicates p<0.05.
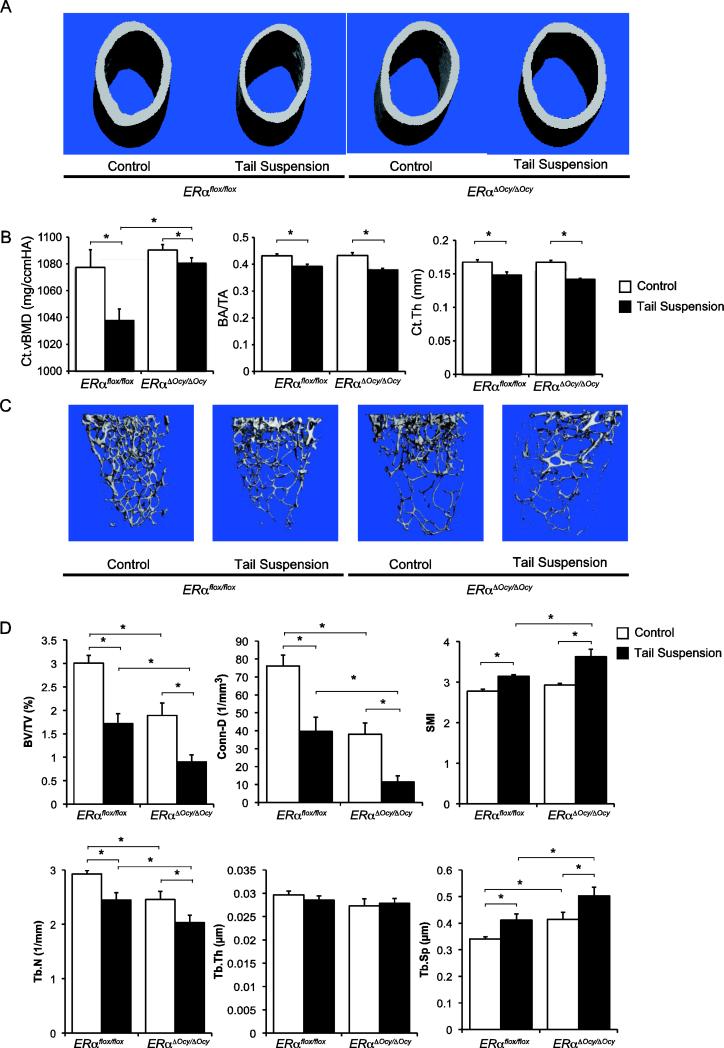
Trabecular bone loss is exacerbated in ERαΔOcy/ΔOcy in response to unloading while cortical bone is resistant to unloading-induced bone loss
ERα has been reported to be involved in mechanosensing and increasing cortical bone formation under overloading conditions [11]. The hindlimb tail suspension model is a well-known model for unloading (or immobilization) and it is also reported that tail suspension-induced bone loss is significantly enhanced by ovariectomy [47]. To determine whether osteocytic ERα plays any roles in unloading-induced bone loss, a hindlimb unloading tail suspension experiment was performed for female ERαΔOcy/ΔOcy and ERαflox/flox for 4 weeks starting at 8 weeks of age. Control mice were chained to the cage top during the same period but allowed to load their hindlimbs to control for stress related effects.
During the 4-week experimental period, the average body weight of the experimental group increased 1 g, whereas the control group increased 2 g (Supplemental Fig. S2). Although there was a significant difference in body weight increase over the four weeks between the experimental and control groups, there was no significant difference in body weight between ERαΔOcy/ΔOcy and ERαflox/flox within each group at the end of the experiment (Supplemental Fig. S2). Femoral diaphysis and distal metaphysis of the unloaded and loaded groups of both genotypes (ERαΔOcy/ΔOcy and ERαflox/flox) were measured using μCT. vBMD in the femoral diaphysis of tail suspended female ERαΔOcy/ΔOcy was significantly higher than that of ERαflox/flox (Fig. 5A and 5B), although there were no significant differences in bone area or cortical thickness between genotypes. Upon further analysis, it was found that the trabecular bone mass was decreased in unloaded mice regardless of genotypes, and tail suspension induced trabecular bone loss in ERαΔOcy/ΔOcy was greater than that in ERαflox/flox (Fig. 5C and 5D). These data indicate that osteocytic ERα is protective against trabecular bone loss due to unloading.
Fig. 5. Effects of unloading on trabecular and cortical bone in mice with targeted deletion of ERα in osteocytes.
(A and C) Representative μCT views. (B) 3D measurements of femoral distal trabecular area and (D) 3D measurements of femoral diaphyses from ERαflox/flox and ERαΔOcy/ΔOcy mice subjected or not subjected to tail suspension. Data are represented as mean ± SEM (n=6). * indicates p<0.05.
Discussion
Based on reports on the functions of ERα in bone, estrogens are osteoprotective by regulating the life span of osteoclasts through osteoclastic and osteoblastic ERα and also by inhibiting apoptosis of osteoblasts and osteocytes [8, 9, 48-50]. Recently, it was reported that osteoblastic ERα has an osteoprotective action [12, 14, 15], however, little is known about the role of osteocytes in the osteoprotective actions of estrogens in skeletal homeostasis. To decipher the direct functions of ERα in osteocytes, the most abundant bone cell type in the adult skeleton, mice lacking ERα in osteocytes were genetically generated and their bone phenotype were analyzed in this study. ERα in osteocytes was found to play a significant role in maintaining bone mass by regulating osteoblastic bone formation only in females. It was further revealed that ERα in osteocytes is supportive for maintaining trabecular bone mass not only under normal loading conditions but also under tail suspension-induced unloading, which can be considered as experimental recapitulation of immobilization or space flight. However, the absence of this receptor protected against cortical bone loss. These results are consistent with a previous report in which bone mass adaptation induced by mechanical loading was impaired in ERα total KO mice [11]. Together, these results indicate that osteocyte mechanosensations at least in part via osteocytic ERα.
Maatta et al. and Melville et al. suggested that ERα in mature osteoblasts plays a role in maintaining trabecular bone mass in females based on analyses of mice lacking ERα in mature osteoblasts using Osteocalcin-Cre mice [12, 15]. Almeida et al. suggested that ERα in osteoblast progenitors, but not in mature osteoblasts or osteocytes, is essential for regulation of female cortical bone [14]. As mentioned above, the functions of ERα in osteoblast lineage cells in vivo are still controversial and it is important to combine knowledge from various studies. All female mice exhibited an osteopenic phenotype in both the osteoblast-specific ERα knockout mice by Maatta et al. and Almeida et al., and in previous reports regarding osteoclast-specific ERα knockout mice [8, 9]. As would be predicted, androgen receptor knockout mice (ARKO), including both systemic ARKO [51] and osteocyte conditional ARKO [52], exhibited bone loss in male mice. These gender-specific phenotypes are probably caused by differences in concentration of circulating sex steroids, estrogens and androgens. In contrast to these studies and our present study, a recent report showed that mice lacking ERα using the same Dmp1-Cre mouse exhibited trabecular bone loss only in male mice, but not in female mice [13]. In this report, Windahl et al. proposed that the physiological trabecular bone-sparing effect of estrogen is mediated via ERα in osteocytes in males, but also via ERα in osteoclasts in females [13]. At present, it is difficult to provide a convincing explanation to describe the discrepancies between our current study and this report [13]. However, one possible reason may be differences in the genetic background of the mouse strain of the ERα-floxed mice since the Dmp1-Cre mice were identical. The ERα-floxed mice used in our study have been registered as Esr1tm1Mma and originated from 129S2/SvPas mixed background, and published in 2000 [5], then backcrossed with C57BL6 line for more than 10 times. On the other hand, the ERα-floxed mice used in the study by Windahl et al. have been registered as Esr1tm1Gust and originated from 129X1/SvJ mixed background, and published in 2012 [53]. These differences might be responsible for the discrepancies between the two studies. Regardless, the results of these two studies suggest that osteocytic ERα may have a role in maintenance of trabecular bone homeostasis regardless of gender.
To investigate the possible molecular basis underlying ERα function in osteocytes, we performed an osteocyte isolation technique using FACS analysis of Dmp1-GFP positive cells from conditional null mice and their controls. The results obtained from the Functional Annotation Clustering of differentially expressed genes suggested that osteocytic ERα might regulate transcription of the genes related to secretory proteins, which may regulate osteoblastic bone formation and contribute to maintenance of bone homeostasis. In fact, Sostdc1, an antagonist of the Wnt signaling [45, 54], was elevated as a down stream gene of osteocytic ERα. Sostdc1 is a gene also called Wise or Ectodin whose domain is similar to Sost (Sclerostin). Sost and Sostdc1 bind to Wnt co-receptors called Lrps and regulate the Wnt/β-catenin pathway negatively [55]. Wnt signal proteins are reported to modulate bone mass in vivo by acting directly on mesenchymal stem cells [56-59]. Genes involved in the Wnt signaling are known to regulate the cell proliferation, differentiation, and apoptosis of osteoblasts [60]. Interaction between β-catenin and ERα has been previously reported [61] and the expressions of some Wnt family genes are important for responding to mechanical stress and are reportedly regulated by ERα [32]. Conventional Sostdc1 KO mice are reported to exhibit abnormal tooth development, which has similar characteristics as bone [45, 54]. Also, it has been reported that estradiol regulates mRNA levels of Sostdc1 in U2OS cells [62]. In addition, a meta-analysis of BMD in a female Chinese population revealed that a mutation in the Sostdc1 cording region was correlated with BMD, suggesting that Sostdc1 might play a role in homeostasis of bone metabolism [46].
Also, Midkine, Mdk, was elevated as a downstream molecule of ERα in mice with this targeted deletion. Mdk is a member of a family of heparin-binding growth factors known primarily for their effects on neural cells [63]. Mdk expression is reported to increase during the course of primary osteoblast differentiation. Mdk has been shown to bind to a complex of protein tyrosine phosphatase zeta (Ptprx), low-density lipoprotein receptor-related protein-6 (Lrp6), and exert negative effects on Wnt signaling [64]. Conventional Mdk null mice exhibit increased bone formation, suggesting Mdk is a negative regulator of osteoblastic bone formation. Furthermore, Mdk KO mice are resistant to OVX-induced bone loss and sensitive to mechanical loading induced cortical bone increase [44]. In addition, the expression of ALP and the induction of canonical Wnt signaling in MC3T3E1, an osteoblastic cell line, were inhibited by Mdk treatments [64]. These reports and the results from our current study suggest that Sostdc1 and Mdk might be responsible for a component of estrogen's osteoprotective actions.
However, questions remain regarding how ERα negatively regulates the transcription of these genes because there are no reports of a negative transcriptional regulation of the estrogen receptor response element (negative ERE), although details of a negative glucocorticoid receptor response element (nGRE) have been reported [65]. Alternatively, it is possible that the expression of these factors might be regulated by an ERα-dependent miRNA. The precise molecular basis of transcriptional regulation or mRNA stabilization of these genes must be clarified in future studies. Neutralizing or deletion studies of these two proteins in this mouse model could provide possible answers for these questions.
In conclusion, osteocytic ERα might play a role in estrogen's osteoprotective action by controlling the expression of Wnt antagonists, which regulate osteoblastic bone formation in trabecular bone.
Supplementary Material
Highlights.
The mice lacking ERα in osteocytes (ERαΔOcy/ΔOcy) were generated using Dmp1-Cre and ERα flox mice.
Female ERαΔOcy/ΔOcy mice exhibited trabecular bone loss due to reduced bone formation.
Tail suspension induced bone loss was confirmed in trabecular bone, not in cortical bone, of female ERαΔOcy/ΔOcy mice.
Osteocytes obtained from ERαΔOcy/ΔOcy mice highly expressed Wnt antagonists, such as Mdk and Sostdc1.
Acknowledgments
The authors thank Dr. S. Kato for his general support and discussion, Ms. Noriko Moriyama for her technical support (microarray). This work was supported by Grant-in-Aids from Japan Society for the Promotion of Science (Research fellowship for young scientist to SK), NIH NIAMS PO1 AR046798 (to LFB) and JSPS KAKENHI (Grant number 23689066 and 23659712 to YI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors state that they have no conflicts of interest.
References
- 1.Nelson HD. Menopause. Lancet. 2008;371:760–70. doi: 10.1016/S0140-6736(08)60346-3. [DOI] [PubMed] [Google Scholar]
- 2.Imai Y, Kondoh S, Kouzmenko A, Kato S. Minireview: osteoprotective action of estrogens is mediated by osteoclastic estrogen receptor-alpha. Mol Endocrinol. 2010;24:877–85. doi: 10.1210/me.2009-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, Zallone A, Sairam MR, Kumar TR, Bo W, Braun J, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M. FSH directly regulates bone mass. Cell. 2006;125:247–60. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 4.Pacifici R. Estrogen deficiency, T cells and bone loss. Cell Immunol. 2008;252:68–80. doi: 10.1016/j.cellimm.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–91. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 6.Windahl SH, Andersson G, Gustafsson JA. Elucidation of estrogen receptor function in bone with the use of mouse models. Trends Endocrinol Metab. 2002;13:195–200. doi: 10.1016/s1043-2760(02)00594-5. [DOI] [PubMed] [Google Scholar]
- 7.Sims NA, Dupont S, Krust A, Clement-Lacroix P, Minet D, Resche-Rigon M, Gaillard-Kelly M, Baron R. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-beta in bone remodeling in females but not in males. Bone. 2002;30:18–25. doi: 10.1016/s8756-3282(01)00643-3. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130:811–23. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 9.Martin-Millan M, Almeida M, Ambrogini E, Han L, Zhao H, Weinstein RS, Jilka RL, O'Brien CA, Manolagas SC. The estrogen receptor-alpha in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol. 2010;24:323–34. doi: 10.1210/me.2009-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomkinson A, Reeve J, Shaw RW, Noble BS. The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J Clin Endocrinol Metab. 1997;82:3128–35. doi: 10.1210/jcem.82.9.4200. [DOI] [PubMed] [Google Scholar]
- 11.Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L. Endocrinology: bone adaptation requires oestrogen receptor-alpha. Nature. 2003;424:389. doi: 10.1038/424389a. [DOI] [PubMed] [Google Scholar]
- 12.Maatta JA, Buki KG, Gu G, Alanne MH, Vaaraniemi J, Liljenback H, Poutanen M, Harkonen P, Vaananen K. Inactivation of estrogen receptor alpha in bone-forming cells induces bone loss in female mice. FASEB J. 2012 doi: 10.1096/fj.12-213587. [DOI] [PubMed] [Google Scholar]
- 13.Windahl SH, Borjesson AE, Farman HH, Engdahl C, Moverare-Skrtic S, Sjogren K, Lagerquist MK, Kindblom JM, Koskela A, Tuukkanen J, Divieti Pajevic P, Feng JQ, Dahlman-Wright K, Antonson P, Gustafsson JA, Ohlsson C. Estrogen receptor-alpha in osteocytes is important for trabecular bone formation in male mice. Proc Natl Acad Sci U S A. 2013;110:2294–9. doi: 10.1073/pnas.1220811110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almeida M, Iyer S, Martin-Millan M, Bartell SM, Han L, Ambrogini E, Onal M, Xiong J, Weinstein RS, Jilka RL, O'Brien CA, Manolagas SC. Estrogen receptor-alpha signaling in osteoblast progenitors stimulates cortical bone accrual. J Clin Invest. 2013;123:394–404. doi: 10.1172/JCI65910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melville KM, Kelly NH, Khan SA, Schimenti JC, Ross FP, Main RP, van der Meulen MC. Female mice lacking estrogen receptor-alpha in osteoblasts have compromised bone mass and strength. J Bone Miner Res. 2013 doi: 10.1002/jbmr.2082. [DOI] [PubMed] [Google Scholar]
- 16.Burger EH, Klein-Nulend J. Mechanotransduction in bone--role of the lacuno-canalicular network. Faseb J. 1999;13(Suppl):S101–12. [PubMed] [Google Scholar]
- 17.Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–75. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 18.Skerry TM, Bitensky L, Chayen J, Lanyon LE. Early strain-related changes in enzyme activity in osteocytes following bone loading in vivo. J Bone Miner Res. 1989;4:783–8. doi: 10.1002/jbmr.5650040519. [DOI] [PubMed] [Google Scholar]
- 19.Gluhak-Heinrich J, Ye L, Bonewald LF, Feng JQ, MacDougall M, Harris SE, Pavlin D. Mechanical loading stimulates dentin matrix protein 1 (DMP1) expression in osteocytes in vivo. J Bone Miner Res. 2003;18:807–17. doi: 10.1359/jbmr.2003.18.5.807. [DOI] [PubMed] [Google Scholar]
- 20.Gluhak-Heinrich J, Pavlin D, Yang W, MacDougall M, Harris SE. MEPE expression in osteocytes during orthodontic tooth movement. Arch Oral Biol. 2007;52:684–90. doi: 10.1016/j.archoralbio.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson DL, Sabbagh Y, Tenenhouse HS, Roche PC, Drezner MK, Salisbury JL, Grande JP, Poeschla EM, Kumar R. Ontogeny of Phex/PHEX protein expression in mouse embryo and subcellular localization in osteoblasts. J Bone Miner Res. 2002;17:311–20. doi: 10.1359/jbmr.2002.17.2.311. [DOI] [PubMed] [Google Scholar]
- 22.Nampei A, Hashimoto J, Hayashida K, Tsuboi H, Shi K, Tsuji I, Miyashita H, Yamada T, Matsukawa N, Matsumoto M, Morimoto S, Ogihara T, Ochi T, Yoshikawa H. Matrix extracellular phosphoglycoprotein (MEPE) is highly expressed in osteocytes in human bone. J Bone Miner Metab. 2004;22:176–84. doi: 10.1007/s00774-003-0468-9. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab. 2006;291:E38–49. doi: 10.1152/ajpendo.00008.2006. [DOI] [PubMed] [Google Scholar]
- 24.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–5. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonewald LF. Osteocytes as dynamic multifunctional cells. Ann N Y Acad Sci. 2007;1116:281–90. doi: 10.1196/annals.1402.018. [DOI] [PubMed] [Google Scholar]
- 26.Bergmann P, Body JJ, Boonen S, Boutsen Y, Devogelaer JP, Goemaere S, Kaufman J, Reginster JY, Rozenberg S. Loading and skeletal development and maintenance. J Osteoporos. 2010;2011:786752. doi: 10.4061/2011/786752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lloyd SA, Bandstra ER, Willey JS, Riffle SE, Tirado-Lee L, Nelson GA, Pecaut MJ, Bateman TA. Effect of proton irradiation followed by hindlimb unloading on bone in mature mice: A model of long-duration spaceflight. Bone. 2012;51:756–64. doi: 10.1016/j.bone.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee KC, Maxwell A, Lanyon LE. Validation of a technique for studying functional adaptation of the mouse ulna in response to mechanical loading. Bone. 2002;31:407–12. doi: 10.1016/s8756-3282(02)00842-6. [DOI] [PubMed] [Google Scholar]
- 29.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–15. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, Bex FJ. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–8. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- 31.Javaheri B, Stern A, Lara N, Dallas M, Zhao H, Liu Y, Bonewald LF, Johnson ML. Deletion of a single beta-catenin allele in osteocytes abolishes the bone anabolic response to loading. J Bone Miner Res. 2013 doi: 10.1002/jbmr.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armstrong VJ, Muzylak M, Sunters A, Zaman G, Saxon LK, Price JS, Lanyon LE. Wnt/beta-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor alpha. J Biol Chem. 2007;282:20715–27. doi: 10.1074/jbc.M703224200. [DOI] [PubMed] [Google Scholar]
- 33.Lu Y, Xie Y, Zhang S, Dusevich V, Bonewald LF, Feng JQ. DMP1-targeted Cre expression in odontoblasts and osteocytes. J Dent Res. 2007;86:320–5. doi: 10.1177/154405910708600404. [DOI] [PubMed] [Google Scholar]
- 34.Kalajzic I, Braut A, Guo D, Jiang X, Kronenberg MS, Mina M, Harris MA, Harris SE, Rowe DW. Dentin matrix protein 1 expression during osteoblastic differentiation, generation of an osteocyte GFP-transgene. Bone. 2004;35:74–82. doi: 10.1016/j.bone.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 36.Sato S, Hanada R, Kimura A, Abe T, Matsumoto T, Iwasaki M, Inose H, Ida T, Mieda M, Takeuchi Y, Fukumoto S, Fujita T, Kato S, Kangawa K, Kojima M, Shinomiya K, Takeda S. Central control of bone remodeling by neuromedin U. Nat Med. 2007;13:1234–40. doi: 10.1038/nm1640. [DOI] [PubMed] [Google Scholar]
- 37.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 38.Paic F, Igwe JC, Nori R, Kronenberg MS, Franceschetti T, Harrington P, Kuo L, Shin DG, Rowe DW, Harris SE, Kalajzic I. Identification of differentially expressed genes between osteoblasts and osteocytes. Bone. 2009;45:682–92. doi: 10.1016/j.bone.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 40.McCarthy JJ, Fox AM, Tsika GL, Gao L, Tsika RW. beta-MHC transgene expression in suspended and mechanically overloaded/suspended soleus muscle of transgenic mice. Am J Physiol. 1997;272:R1552–61. doi: 10.1152/ajpregu.1997.272.5.R1552. [DOI] [PubMed] [Google Scholar]
- 41.Tsika G, Ji J, Tsika R. Sp3 proteins negatively regulate beta myosin heavy chain gene expression during skeletal muscle inactivity. Mol Cell Biol. 2004;24:10777–91. doi: 10.1128/MCB.24.24.10777-10791.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–41. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–34. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neunaber C, Catala-Lehnen P, Beil FT, Marshall RP, Kanbach V, Baranowsky A, Lehmann W, Streichert T, Ignatius A, Muramatsu T, Schinke T, Amling M. Increased trabecular bone formation in mice lacking the growth factor midkine. J Bone Miner Res. 2010;25:1724–35. doi: 10.1002/jbmr.75. [DOI] [PubMed] [Google Scholar]
- 45.Ahn Y, Sanderson BW, Klein OD, Krumlauf R. Inhibition of Wnt signaling by Wise (Sostdc1) and negative feedback from Shh controls tooth number and patterning. Development. 2010;137:3221–31. doi: 10.1242/dev.054668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He JW, Yue H, Hu WW, Hu YQ, Zhang ZL. Contribution of the sclerostin domain-containing protein 1 (SOSTDC1) gene to normal variation of peak bone mineral density in Chinese women and men. J Bone Miner Metab. 2011;29:571–81. doi: 10.1007/s00774-010-0253-5. [DOI] [PubMed] [Google Scholar]
- 47.Tou JC, Foley A, Yuan YV, Arnaud S, Wade CE, Brown M. The effect of ovariectomy combined with hindlimb unloading and reloading on the long bones of mature Sprague-Dawley rats. Menopause. 2008;15:494–502. doi: 10.1097/gme.0b013e318148bbad. [DOI] [PubMed] [Google Scholar]
- 48.Almeida M, Martin-Millan M, Ambrogini E, Bradsher R, 3rd, Han L, Chen XD, Roberson PK, Weinstein RS, O'Brien CA, Jilka RL, Manolagas SC. Estrogens attenuate oxidative stress and the differentiation and apoptosis of osteoblasts by DNA-binding-independent actions of the ERalpha. J Bone Miner Res. 2010;25:769–81. doi: 10.1359/jbmr.091017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomkinson A, Gevers EF, Wit JM, Reeve J, Noble BS. The role of estrogen in the control of rat osteocyte apoptosis. J Bone Miner Res. 1998;13:1243–50. doi: 10.1359/jbmr.1998.13.8.1243. [DOI] [PubMed] [Google Scholar]
- 50.Krum SA, Miranda-Carboni GA, Hauschka PV, Carroll JS, Lane TF, Freedman LP, Brown M. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J. 2008;27:535–45. doi: 10.1038/sj.emboj.7601984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawano H, Sato T, Yamada T, Matsumoto T, Sekine K, Watanabe T, Nakamura T, Fukuda T, Yoshimura K, Yoshizawa T, Aihara K, Yamamoto Y, Nakamichi Y, Metzger D, Chambon P, Nakamura K, Kawaguchi H, Kato S. Suppressive function of androgen receptor in bone resorption. Proc Natl Acad Sci U S A. 2003;100:9416–21. doi: 10.1073/pnas.1533500100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sinnesael M, Claessens F, Laurent M, Dubois V, Boonen S, Deboel L, Vanderschueren D. Androgen receptor (AR) in osteocytes is important for the maintenance of male skeletal integrity: evidence from targeted AR disruption in mouse osteocytes. J Bone Miner Res. 2012;27:2535–43. doi: 10.1002/jbmr.1713. [DOI] [PubMed] [Google Scholar]
- 53.Antonson P, Omoto Y, Humire P, Gustafsson JA. Generation of ERalpha-floxed and knockout mice using the Cre/LoxP system. Biochem Biophys Res Commun. 2012;424:710–6. doi: 10.1016/j.bbrc.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 54.Cho SW, Kwak S, Woolley TE, Lee MJ, Kim EJ, Baker RE, Kim HJ, Shin JS, Tickle C, Maini PK, Jung HS. Interactions between Shh, Sostdc1 and Wnt signaling and a new feedback loop for spatial patterning of the teeth. Development. 2011;138:1807–16. doi: 10.1242/dev.056051. [DOI] [PubMed] [Google Scholar]
- 55.Itasaki N, Jones CM, Mercurio S, Rowe A, Domingos PM, Smith JC, Krumlauf R. Wise, a context-dependent activator and inhibitor of Wnt signalling. Development. 2003;130:4295–305. doi: 10.1242/dev.00674. [DOI] [PubMed] [Google Scholar]
- 56.Liu G, Vijayakumar S, Grumolato L, Arroyave R, Qiao H, Akiri G, Aaronson SA. Canonical Wnts function as potent regulators of osteogenesis by human mesenchymal stem cells. J Cell Biol. 2009;185:67–75. doi: 10.1083/jcb.200810137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takada I, Kouzmenko AP, Kato S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol. 2009;5:442–7. doi: 10.1038/nrrheum.2009.137. [DOI] [PubMed] [Google Scholar]
- 58.Augello A, De Bari C. The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther. 2010;21:1226–38. doi: 10.1089/hum.2010.173. [DOI] [PubMed] [Google Scholar]
- 59.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19:179–92. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 60.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 61.Kouzmenko AP, Takeyama K, Ito S, Furutani T, Sawatsubashi S, Maki A, Suzuki E, Kawasaki Y, Akiyama T, Tabata T, Kato S. Wnt/beta-catenin and estrogen signaling converge in vivo. J Biol Chem. 2004;279:40255–8. doi: 10.1074/jbc.C400331200. [DOI] [PubMed] [Google Scholar]
- 62.Tee MK, Rogatsky I, Tzagarakis-Foster C, Cvoro A, An J, Christy RJ, Yamamoto KR, Leitman DC. Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors alpha and beta. Mol Biol Cell. 2004;15:1262–72. doi: 10.1091/mbc.E03-06-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakamura E, Kadomatsu K, Yuasa S, Muramatsu H, Mamiya T, Nabeshima T, Fan QW, Ishiguro K, Igakura T, Matsubara S, Kaname T, Horiba M, Saito H, Muramatsu T. Disruption of the midkine gene (Mdk) resulted in altered expression of a calcium binding protein in the hippocampus of infant mice and their abnormal behaviour. Genes Cells. 1998;3:811–22. doi: 10.1046/j.1365-2443.1998.00231.x. [DOI] [PubMed] [Google Scholar]
- 64.Liedert A, Mattausch L, Rontgen V, Blakytny R, Vogele D, Pahl M, Bindl R, Neunaber C, Schinke T, Harroch S, Amling M, Ignatius A. Midkine-deficiency increases the anabolic response of cortical bone to mechanical loading. Bone. 2011;48:945–51. doi: 10.1016/j.bone.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 65.Surjit M, Ganti KP, Mukherji A, Ye T, Hua G, Metzger D, Li M, Chambon P. Widespread negative response elements mediate direct repression by agonist-liganded glucocorticoid receptor. Cell. 2011;145:224–41. doi: 10.1016/j.cell.2011.03.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.