Abstract
Idiopathic pulmonary fibrosis (IPF) is a progressive and invariably fatal disease with a median survival of less than three years from diagnosis. The last decade has seen an exponential increase in clinical trial activity in IPF and this in turn has led to important developments in the treatment of this terrible disease. Previous therapeutic approaches based around regimens including corticosteroids and azathioprine have, when tested in randomized clinical trials, been shown to be harmful in IPF. By contrast, compounds with anti-fibrotic actions have been shown to be beneficial. Subsequently, the novel anti-fibrotic agent pirfenidone has, in many parts of the world, become the first treatment ever to be licensed for use in IPF. This exciting development, coupled with ongoing clinical trials of a range of other novel compounds, is bringing hope to patients and their clinicians and raises the prospect that, in the future, it may become possible to successfully arrest the development of progressive scarring in IPF.
Introduction
IPF is a disease characterized by progressive scarring of the lung and carries an appalling prognosis [1]. The etiology of IPF remains unknown, thus the development of successful treatments has lagged behind other fields in medicine. Nevertheless, with the combination of increased understanding of IPF disease pathogenesis and intensified efforts from the pharmaceutical industry, all this is beginning to change. This short commentary will, in light of recent clinical trial developments, provide an overview of the changing landscape of IPF treatments.
Idiopathic pulmonary fibrosis
IPF is a disease that occurs more commonly in men and typically occurs in individuals aged between 40 and 70 years. The incidence of IPF is 6.8 to 16.3 per 100,000 and this figure is on the increase [2,3]. In the UK, IPF accounts for 5000 deaths per year, a figure far in excess of many cancers, including myeloma, lymphoma, and stomach cancer [3]. Individuals with IPF present with progressive breathlessness and cough, which, although usually mild at the outset, inevitably becomes disabling with time. Almost all those with IPF eventually die from respiratory failure, with median survival from diagnosis being less than 3 years. The 5-year survival for IPF, which stands at 20%, is worse than that of adenocarcinoma of the lung.
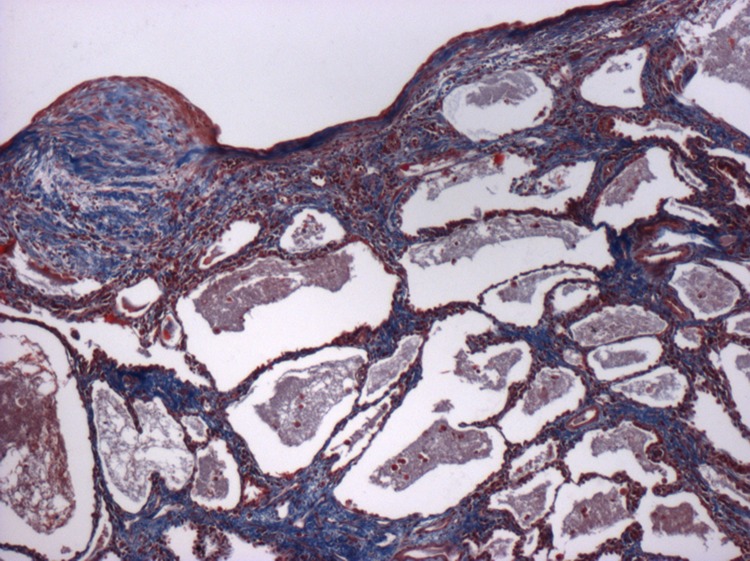
The pathogenesis of IPF remains incompletely understood. It was previously considered that inflammation preceded fibrosis, but the lack of efficacy and, moreover, deleterious effects of immunosuppressive therapy have led to a shift in thinking. Available evidence points to IPF developing in genetically susceptible individuals as a consequence of an aberrant wound-healing response arising following repetitive alveolar injury [1,4]. It appears that alveolar epithelial injury drives fibroblast proliferation, myofibroblast differentiation, and collagen deposition, leading to the formation of fibrotic foci, the hallmark histological feature of IPF. This laying down of collagen and extracellular matrix stiffens the lungs and destroys the delicate lace-like structure of the alveolar spaces (Figure 1). Ultimately, gas exchange is impaired, leading to respiratory failure and death.
Figure 1. Low-power photomicrograph illustrating the characteristic appearances of usual interstitial pneumonia, the histological lesion of idiopathic pulmonary fibrosis (IPF).
The image shows fibroblastic foci overlying a region of micro-cystic honeycomb change. The sections have been stained with a Trichrome stain. This clearly highlights the extensive collagen (stained blue) and extracellular matrix deposition that occurs in IPF.
None of the compounds currently under evaluation in clinical trials are able to reverse the scarring which characterizes IPF. This has necessitated a paradigm shift when it comes to evaluating treatment success in IPF. The goal of treatment, in this otherwise devastating and fatal disease, is not to improve lung function but instead simply to prevent progression of disease (and thus preserve functional status and quality of life). Stability in lung function, which has hitherto frequently been considered a failure of treatment by physicians and patients, should be regarded as a successful outcome.
This article will summarize the IPF trials that have influenced treatment together with the key negative trials that have informed current clinical trial activity. Promising compounds now in early-phase trials will be very briefly reviewed.
Trials that have shaped the idiopathic pulmonary fibrosis treatment landscape
The first true randomized placebo-controlled trial (RCT) in IPF was published as recently as 2004 [5]. This trial of the TH1 cytokine interferon-γ1b, although ultimately negative, proved an important watershed for the field of interstitial lung disease. For the first time, it was demonstrated that it is possible to recruit and follow up for 12 months sufficient numbers of patients with IPF to make appropriately powered RCTs a feasible prospect. It should be noted that an important foundation stone to this study was the development and publication of the first international guidelines and consensus statement on the diagnosis of IPF [6]. Without internationally accepted diagnostic criteria (and separation of IPF from other forms of idiopathic interstitial pneumonitis), it would not have been possible for multi-center international studies to develop. Since the publication of these consensus guidelines and the interferon-γ study, there has been an exponential growth in clinical trial activity in IPF [7]. Whilst many of these trials have ultimately proved negative (as detailed in Table 1), each individual study has provided important insights in to optimal trial design and choice of end-points. Furthermore, as will be described, these studies have culminated in the licensing, in Europe, Japan, India, and Canada, of the first specific treatment for IPF, pirfenidone [8].
Table 1. A summary overview of negative randomized placebo-controlled trials undertaken in idiopathic pulmonary fibrosis.
| Compound | Study | Study design | Patients | Primary end-point | Results |
|---|---|---|---|---|---|
|
Warfarin (anticoagulant) |
ACE-IPF [45] 2012 | Phase III Warfarin vs. placebo for 48 weeks |
n = 256 terminated at 145 | Composite of all-cause mortality, hospitalization and ≥10% absolute FVC decline | Increased mortality in warfarin arm (14 vs. 3 deaths in placebo arm P = 0.005). Study terminated at unscheduled interim analysis by the DSMB. |
|
Everolimus (mTOR inhibitor) |
Malouf et al. [46] 2011 | Phase II 4 mg bd Everolimus vs. placebo for 3 years |
n = 89 | Time to disease progression defined by the time to the second of any two of a 10% change in FVC or TLC; 15% change in DLCO; a 4% point change in resting room air SaO2 | Increased disease progression in everolimus arm (180 days vs. 450 days in placebo arm P <0.01). Higher frequency of AEs in patients in everolimus arm. |
|
Bosentan (dual endothelin receptor antagonist) |
BUILD1 [47] 2008 | Phase III Bosentan 125 mg bd vs. placebo for 1 year |
n = 158 | BUILD1: change in 6MWT at 12 months | No significant change in 6MWT between treatment groups but post hoc analysis showed a trend towards reduced mortality in patients in the bosentan arm who had undergone a surgical lung biopsy. |
| BUILD3 [48] 2011 | Phase III Bosentan 125 mg bd vs. placebo for 3 years |
n = 616 | BUILD3: time to disease progression (≥10% absolute FVC decline or ≥15% decline in DLCO or acute exacerbation) | No significant difference in time to disease progression in patients diagnosed with a surgical lung biopsy. | |
|
Ambrisentan (type A endothelin receptor antagonist) |
ARTEMIS [49] IPF 2013 | Phase III Ambrisentan 10 mg vs. placebo for 18 months. |
n = 660 terminated at 492 |
Time to disease progression defined by death or respiratory hospitalization, ≥10% absolute FVC decline or ≥15% decline in DLCO or acute exacerbation | Terminated at interim analysis due to lack of efficacy. Increased disease progression in ambrisentan arm (n = 90 (27.4%) vs. n = 28 (17.2%) in placebo arm P = 0.01). Increased respiratory hospitalizations in ambrisentan arm (n = 44 (13.4%) vs. n = 9 (5.5%) in placebo arm P = 0.007). |
|
Macitentan (dual endothelin receptor antagonist) |
MUSIC trial [50] 2013 | Phase II Macitentan 10 mg vs. placebo for 1 year |
n = 178 | Change in FVC at 12 months | No significant difference in change in absolute FVC between treatment groups. |
|
Sildenafil (phosphodiesterase-5 inhibitor) |
STEP-IPF [51] 2010 | Phase III Sildenafil 20 mg tds vs. placebo for 12 weeks, followed by 12-week open-label extension with all patients receiving sildenafil |
n = 180 | ≥20% increase in the 6MWT at 12 weeks | No significant difference in the number of patients with ≥20% increase in 6MWT distance, but significant improvement in secondary outcomes DLCO and QOL scores. |
|
Interferon γ -1b (Th1 cytokine) |
Raghu et al. [5] 2004 | Phase III 200 µg subcutaneous IFNγ-1b vs. placebo 3 times weekly for 48 weeks |
n = 330 | Progression-free survival | No significant difference in progression-free survival. |
|
INSPIRE [52] 2009 |
Phase III 200 µg subcutaneous IFNγ-1b vs. placebo 3 times weekly for 96 weeks |
n = 826 | Overall survival time from randomization | No significant survival benefit with IFNγ-1b at second interim analysis hence study terminated at this point. | |
|
Etanercept (recombinant soluble human TNF-α receptor) |
Raghu et al. [53] 2008 | Phase II Etanercept 25 mg vs. placebo twice weekly for 48 weeks |
n = 88 | Changes from baseline in FVC% predicted, DLCO% predicted and P(a–a)O2 at rest. |
No significant difference in primary end-points between etanercept and placebo groups. |
|
Imatinib (tyrosine kinase inhibitor with activity against PDGF receptors, c-kit and c-abl) |
Daniels et al. [54] 2010 | Phase II Imatinib 600 mg vs. placebo for 96 weeks |
n = 119 | Time to disease progression (10% decline in percent predicted FVC from baseline) or time to death. | No significant difference in survival or lung function between treatment groups. |
|
Co-trimoxazole (antibiotic) |
Shulgina et al. [55] 2013 | Phase II Co-trimoxazole 960 mg twice daily vs. placebo for 52 weeks |
n = 181 | 12 month change in FVC | No significant difference between groups in FVC but improved survival in treatment adherent subjects |
6MWT, 6-minute walk distance; AE, adverse effect; ARTEMIS; Randomized, Placebo-Controlled Study to Evaluate Safety and Effectiveness of Ambrisentan in IPF; bd, bis in die (twice daily); BUILD, Bosentan Use in Interstitial Lung Disease; DLCO, carbon monoxide diffusing capacity; DSMB, Data and Safety Monitoring Board; FVC, forced vital capacity; IFN, interferon; INSPIRE, interferon gamma-1b on survival in patients with idiopathic pulmonary fibrosis; IPF, idiopathic pulmonary fibrosis; mTOR, mammalian target of rapamycin; MUSIC, Macitentan for the treatment of idiopathic pulmonary fibrosis; PDGF, platelet-derived growth factor; QOL, quality of life; SaO2, oxygen saturation; STEP-IPF, Sildenafil Trial of Exercise Performance in Idiopathic Pulmonary Fibrosis; TLC, total lung capacity; TNF, tumor necrosis factor.
N-acetyl-cysteine
The IFIGENIA (Idiopathic Pulmonary Fibrosis International Group Exploring N-Acetylcysteine I Annual) study, a multi-center, RCT of high-dose oral N-acetyl-cysteine (NAC) compared with placebo, published in 2005 [9], was the first RCT in IPF to achieve its primary end-point. In this trial, NAC was given in addition to what, at the time, was the recommended standard therapy for IPF: a tapering dose of oral prednisolone combined with the immunosuppressant azathioprine.
NAC is a precursor of glutathione, a free radical scavenger and an important endogenous antioxidant in the lung [10]. Oxidative stress-mediated injury of alveolar epithelial cells is important in the pathogenesis of IPF [11]. However, glutathione levels are markedly reduced in the lungs of individuals with IPF [12]. Small pilot studies in IPF demonstrated that orally administered NAC results in increased lung glutathione levels [13,14]. This observation paved the way for the IFIGENIA trial.
In the IFIGENIA study, when compared with placebo, treatment with 1800 mg/day NAC led to a significant reduction in absolute forced vital capacity (FVC) decline (9% P < 0.02) and diffusion capacity (DLCO) decline (24% P < 0.003) [9]. There was no difference in the number of deaths between arms at one year; however, the study was not powered for survival. NAC was safe and well tolerated. Although positive, the study has attracted some criticism; firstly, the numbers of subjects completing treatment at 12 months were relatively small. Secondly, all patients were on immunosuppression and so the true effect of NAC alone is unknown; and, thirdly, the findings have yet to be replicated. Despite this and because of its good safety profile, NAC is widely used as a treatment for IPF.
To address the issues raised by the IFIGENIA study, the National Institutes of Health-funded United States IPF network designed the PANTHER-IPF study (Prednisolone, Azathioprine and N-acetylcysteine: A Study That Evaluates Response in Idiopathic Pulmonary Fibrosis) [15]. PANTHER-IPF is a 60-week, double-blind, RCT with three treatment arms: (i) combination (triple) therapy of azathioprine, prednisolone, and NAC; (ii) NAC monotherapy; and (iii) placebo. At interim safety analysis, triple therapy was associated with an increase in all-cause mortality (8 patients in the combination arm vs. 1 in placebo arm), an increase in the rate of hospitalization (29% vs. 8%), and an excess of serious adverse events (31% vs. 9%) [15]. Furthermore, triple therapy failed to show any treatment benefit (measured using FVC) when compared with placebo. In light of these results, the independent Data and Safety Monitoring Board terminated treatment in the triple-therapy arm. The NAC monotherapy and placebo arms of the study were continued, and the last visit of the last patient has now occurred. Results are anticipated in early 2014. In the meantime, the results from the triple-therapy arm of the PANTHER-IPF study have had significant repercussions and led to a rapid shift away from the use of immunosuppressants as a treatment for IPF.
Pirfenidone
Pirfenidone, a pleotropic, orally available, small molecule with anti-fibrotic, anti-oxidant, and anti-inflammatory effects [8], has become the first drug to be licensed specifically for the treatment of IPF. Pirfenidone reduces growth factor-driven fibroblast proliferation and extracellular matrix production in vitro and attenuates experimentally induced pulmonary fibrosis in vivo in animal models [16,17].
Following several small open-label studies [18-20], the first RCT of pirfenidone for IPF was completed in Japan in 2005. The study by Azuma et al. was terminated early because of an excess of acute exacerbations in the placebo arm (a finding not repeated in any subsequent study) [21]. The study did not achieve its primary end-point of change in lowest arterial oxygen saturation during a 6-minute walk. There was, however, a significant reduction in FVC decline at 9 months in the treatment arm (P = 0.0366). Subsequent to this study, there have been three completed phase III RCTs of pirfenidone compared with placebo in IPF. Two of these studies have met their primary end-point of reduction in FVC decline.
The first phase III trial was a Japanese multi-center double-blind placebo-controlled trial conducted over 52 weeks [22]. Patients were randomized to high-dose (1800 mg/day) pirfenidone, low-dose (1200 mg/day) pirfenidone, or placebo. A significant improvement in FVC decline (−0.09 L vs. −0.16 L P <0.0416) and progression-free survival was seen in the high-dose treatment arm compared with placebo. The CAPACITY (Clinical Studies Assessing Pirfenidone in IPF: Research of Efficacy and Safety Outcomes) 1 and 2 phase III multinational randomized double-blind placebo trials ran concurrently [23]. CAPACITY 1 (n = 435) assessed the efficacy of high-dose (2403 mg/day) pirfenidone, low-dose pirfenidone (1197 mg/day), and placebo. CAPACITY 2 (n = 344) had only two treatment arms; high-dose pirfenidone (2403 mg/day) and placebo. The end-point for both trials was change in FVC at week 72. In CAPACITY 1, the high-dose treatment arm met the primary end-point with a significant reduction in decline in FVC at week 72 compared with placebo (−8% vs. −12.4% P = 0.001). Unfortunately, CAPACITY 2 failed to meet its primary end-point, although improvements were seen in some secondary end-points. Notably, no effect on quality-of-life scores was observed in any of these trials. Whilst generally safe, pirfenidone does cause a number of side effects, including gastrointestinal upset and photosensitivity rash, which impact its tolerability.
A recent Cochrane meta-analysis of the four published pirfenidone trials demonstrated that treatment with pirfenidone reduced the risk of disease progression by 30% (hazard ratio 0.70, 95% confidence interval 0.56 to 0.88) [24]. On the basis of these studies, the European Medicines Agency approved the use of pirfendone in 2011. Pirfenidone is also licensed in Japan, India, and Canada. In contrast, the US Food and Drug Administration, having discounted the results of the two Japanese studies, refused approval of pirfenidone based on the failure of the CAPACITY 2 trial to meet its primary end-point. Consequently, a further US-based phase III RCT, the ASCEND (Assessment of Pirfenidone to Confirm Efficacy and Safety in IPF) trial (NCT01366209), commenced in 2011. The primary end-point for the study is change in percent predicted FVC over a 52-week period. Results are expected in early 2014.
Nintedanib
There has been an exponential increase in the number of compounds entering clinical trials for IPF in the last decade [25]. Of the drugs currently in development, the closest to clinic is the novel tyrosine kinase inhibitor nintedanib (Boehringer Ingelheim, Ingelheim am Rhein, Germany) (formerly known by its development name BIBF 1120) [26]. Originally developed as an angiogenesis inhibitor for use in oncology indications, nintedanib proved to have anti-fibrotic activity in vitro in human lung fibroblasts and in vivo in the rodent model of bleomycin-induced pulmonary fibrosis [27]. In the phase II, double-blinded RCT TOMORROW (To imprOve pulMOnaRy fibROsis With BIBF1120) study, 432 patients with IPF were treated with placebo or one of four escalating doses of nintedanib for 52 weeks [28]. The primary end-point of change in FVC was narrowly missed when the highest-dose (150 mg twice daily) treatment arm was compared with placebo (P = 0.06). Treatment with nintetanib was associated with fewer acute exacerbations and improved quality-of-life scores. The results of the TOMORROW study were sufficiently compelling for two parallel phase III studies (NCT01335464 and NCT01335477) of nintedanib in IPF to be undertaken. These two trials have recently been completed, and results are anticipated in early 2014.
Potential future idiopathic pulmonary fibrosis treatments
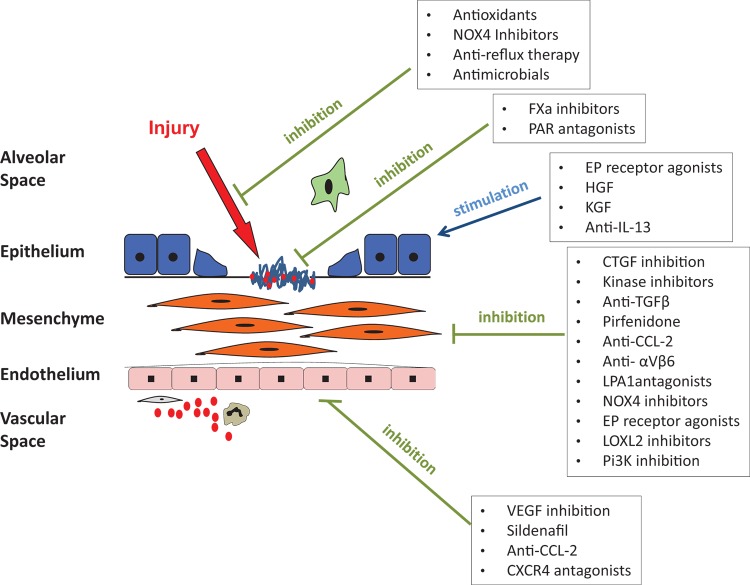
As understanding of the pathogenesis of IPF has improved, the number of potential therapeutic targets has increased dramatically. Strategies that have been considered include (a) prevention of alveolar epithelial injury, (b) targeting the coagulation cascade, (c) stimulating epithelial proliferation, inhibiting epithelial apoptosis, and increasing epithelial resistance to injury, (d) inhibition of fibroblast proliferation and collagen synthesis, (e) direct targeting of the extracellular matrix, (f) inhibition of transforming growth factor-beta (TGF-β) and other pro-fibrotic cytokines, and (g) targeting the capillary endothelium, principally to prevent the development of pulmonary hypertension. These potential approaches and compounds in development are outlined in Figure 2 [29].
Figure 2. A schematic outlining potential new idiopathic pulmonary fibrosis (IPF) therapies as they relate to the proposed pathogenetic events involved in the development of idiopathic pulmonary fibrosis.
Modified from [29] with permission.
A multitude of novel compounds are currently in various stages of clinical trial development. It is beyond the scope of this article to consider all but the most developed of these potential therapies. For a more detailed review of compounds in development, please refer to [29]. The three drugs in most advanced development are covered briefly below.
a) Simtuzumab (Gilead Sciences, Foster City, CA, USA – formerly known as AB0024) is a humanized monoclonal antibody against lysyl oxidase-like 2 (LOXL2) [30-32]. The lysyl oxidases are a group of enzymes that catalyze the cross-linking of type 1 collagen molecules. LOXL2 expression is increased in IPF and promotes collagen accumulation and deposition [31]. In the murine bleomycin model, simtuzumab effectively attenuates the development of fibrosis [30]. Simtuzumab is now being tested in a large multi-center phase IIb RCT in IPF (NCT0176196).
b) Lebrikizumab (Roche, Basel, Switzerland) is a humanized monoclonal antibody against interleukin (IL)-13. In the lungs of patients with IPF, IL-13 is found at increased concentrations [33]. In vitro IL-13 stimulates fibroblast proliferation, whilst in vivo, IL-13 overexpression results in increased fibrosis in mice in response to bleomycin [34]. Lebrikizumab has been shown to have efficacy in severe asthma [35] and is now being tested in IPF in a large phase IIb study (NCT01872689).
c) STX-100 (Biogen Idec, Weston, MA, USA) is a humanized monoclonal antibody targeted against the integrin αvβ6. TGF-β is a critical pro-fibrotic growth factor that is believed to play a critical role in the development of fibrosis [36]. The αvβ6 integrin functions as an activator of TGF-β. In mice, inhibition of αvβ6 attenuates the development of bleomycin-induced fibrosis [37]. Consequently, STX-100 is being tested in a phase 2 study in IPF (NCT01371035).
Challenges and controversies
After decades of stagnation, it is very exciting to see the great strides that are being taken in the search for treatments for IPF. Whilst current developments are a cause for great optimism, there are a number of important areas within the field of IPF clinical trials that require addressing.
a) In the last year or two, there has been considerable controversy around the choice of optimal end-point for IPF studies. It has been suggested that survival should be used as the primary end-point in registration studies as it is both clinically meaningful and robust [38]. While this might be the case, important practical considerations such as the number of subjects needed to power a survival study and the ethical difficulty of asking study subjects to continue in a study through to death when they may be receiving placebo treatment alone means that annual change in FVC currently remains the end-point of choice [39-42]. Better quantification of the relationship between FVC change and both mortality and quality of life is necessary so that clinical trial outcomes can be better understood [43].
b) In recent times, all phase 2b and phase 3 studies have excluded the use of other “anti-fibrotic” therapies. Whilst this was feasible in an era where there were no licensed treatments for IPF, it is an approach that is becoming harder to justify. The situation at the moment is complicated by the fact that pirfenidone is not yet licensed in all territories; however, this is likely to change if the ASCEND study is positive. Whilst on the face of it permitting the use of other therapies in clinical trials should not create major issues, it will narrow the therapeutic window in which efficacy of a new compound can be measured and it also raises the prospect of interactions and altered tolerability.
c) To date, the majority of new compounds trialed for IPF have gone straight in to late-phase studies with very few having been rigorously tested in early-phase studies. The principal reason for this has been the lack of robust short-term end-points demonstrating inhibition of fibrotic pathways. The lack of an agreed-upon proof-of-concept study design is a barrier to entry in to the IPF arena for many smaller pharmaceutical companies. It is important that the IPF field focus on developing robust, validated surrogate end-points, which will enable the easier translational development of compounds from the laboratory through to the clinic [44].
Conclusions
IPF is a devastating disease with a poorer prognosis than many cancers. In recent times, a multitude of different cellular pathways have been identified as potential therapeutic targets. Accompanying this, there has been a huge increase in the number of compounds entering clinical trials. Although the majority of trials have yielded negative results, a great deal has been learnt about the natural history of IPF and its pathogenesis. The lack of efficacy and detrimental effects of immunosuppression has led to a paradigm shift with IPF no longer being thought of as an inflammatory disease. Consequently, pharmacotherapy has evolved to focus on anti-fibrotic strategies. To date, pirfenidone is the only treatment to show a significant effect in slowing the inevitable declining lung function that characterizes IPF. It is to be hoped, however, that compounds currently in clinical trials will lead to further transformation of the clinical landscape in IPF. In turn, these ongoing developments should be a source of hope to individuals with IPF and to their treating physicians.
Abbreviations
- ASCEND
(Assessment of Pirfenidone to Confirm Efficacy and Safety in IPF
- CAPACITY
Clinical Studies Assessing Pirfenidone in Idiopathic pulmonary fibrosis: Research of Efficacy and Safety Outcomes
- FVC
forced vital capacity
- IFIGENIA
Idiopathic Pulmonary Fibrosis International Group Exploring N-Acetylcysteine I Annual)
- IL
interleukin
- IPF
idiopathic pulmonary fibrosis
- LOXL2
lysyl oxidase-like 2
- NAC
N-acetyl-cysteine
- PANTHER-IPF
Prednisolone, Azathioprine and N-acetylcysteine: A Study That Evaluates Response in Idiopathic Pulmonary Fibrosis
- RCT
randomized placebo-controlled trial
- TGF-β
transforming growth factor-beta
- TOMORROW
To imprOve pulMOnaRy fibROsis With BIBF1120
Disclosures
Toby Maher is in receipt of unrestricted academic industry grants from GlaxoSmithKline and Novartis. In the last three years, Toby or his institution has received advisory board or consultancy fees from Actelion, Boehringer Ingelheim, GlaxoSmithKline, InterMune, Novartis, Lanthio, Takeda, Sanofi, and UCB. Toby has received speaker’s fees from UCB, Boehringer Ingelheim, InterMune, and AstraZeneca and has participated as an investigator in industry-sponsored clinical trials run by Boehringer Ingelheim, GlaxoSmithKline, InterMune, Novartis, Roche, and Celgene. Hannah Woodcock has no disclosures.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/m/6/16
References
- 1.Maher TM, Wells AU, Laurent GJ. Idiopathic pulmonary fibrosis: multiple causes and multiple mechanisms? Eur Respir J. 2007;30:835–9. doi: 10.1183/09031936.00069307. [DOI] [PubMed] [Google Scholar]
- 2.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–6. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718265909
- 3.Navaratnam V, Fleming KM, West J, Smith CJP, Jenkins RG, Fogarty A, Hubbard RB. The rising incidence of idiopathic pulmonary fibrosis in the U.K. Thorax. 2011;66:462–7. doi: 10.1136/thx.2010.148031. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718265913
- 4.King TE, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–61. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 5.Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DA, King TE. A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2004;350:125–33. doi: 10.1056/NEJMoa030511. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718265915
- 6.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718265918
- 7.Luppi F, Spagnolo P, Cerri S, Richeldi L. The big clinical trials in idiopathic pulmonary fibrosis. Curr Opin Pulm Med. 2012;18:428–32. doi: 10.1097/MCP.0b013e3283567ff9. [DOI] [PubMed] [Google Scholar]
- 8.Maher TM. Pirfenidone in idiopathic pulmonary fibrosis. Drugs Today. 2010;46:473–82. doi: 10.1358/dot.2010.46.7.1488336. [DOI] [PubMed] [Google Scholar]
- 9.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, MacNee W, Thomeer M, Wallaert B, Laurent F, Nicholson AG, Verbeken EK, Verschakelen J, Flower CDR, Capron F, Petruzzelli S, Vuyst P de, van den Bosch JMM, Rodriguez-Becerra E, Corvasce G, Lankhorst I, Sardina M, Montanari M. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–42. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718265920
- 10.Borok Z, Buhl R, Grimes GJ, Bokser AD, Hubbard RC, Holroyd KJ, Roum JH, Czerski DB, Cantin AM, Crystal RG. Effect of glutathione aerosol on oxidant-antioxidant imbalance in idiopathic pulmonary fibrosis. Lancet. 1991;338:215–6. doi: 10.1016/0140-6736(91)90350-X. [DOI] [PubMed] [Google Scholar]
- 11.Kinnula VL, Fattman CL, Tan RJ, Oury TD. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med. 2005;172:417–22. doi: 10.1164/rccm.200501-017PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantin AM, Hubbard RC, Crystal RG. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1989;139:370–2. doi: 10.1164/ajrccm/139.2.370. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718265923
- 13.Behr J, Degenkolb B, Krombach F, Vogelmeier C. Intracellular glutathione and bronchoalveolar cells in fibrosing alveolitis: effects of N-acetylcysteine. Eur Respir J. 2002;19:906–11. doi: 10.1183/09031936.02.00204902. [DOI] [PubMed] [Google Scholar]
- 14.Behr J, Maier K, Degenkolb B, Krombach F, Vogelmeier C. Antioxidative and clinical effects of high-dose N-acetylcysteine in fibrosing alveolitis. Adjunctive therapy to maintenance immunosuppression. Am J Respir Crit Care Med. 1997;156:1897–901. doi: 10.1164/ajrccm.156.6.9706065. [DOI] [PubMed] [Google Scholar]
- 15.Raghu G, Anstrom KJ, King TE, Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366:1968–77. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/716097808
- 16.Nakayama S, Mukae H, Sakamoto N, Kakugawa T, Yoshioka S, Soda H, Oku H, Urata Y, Kondo T, Kubota H, Nagata K, Kohno S. Pirfenidone inhibits the expression of HSP47 in TGF-beta1-stimulated human lung fibroblasts. Life Sci. 2008;82:210–7. doi: 10.1016/j.lfs.2007.11.003. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718265927
- 17.Oku H, Shimizu T, Kawabata T, Nagira M, Hikita I, Ueyama A, Matsushima S, Torii M, Arimura A. Antifibrotic action of pirfenidone and prednisolone: different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur J Pharmacol. 2008;590:400–8. doi: 10.1016/j.ejphar.2008.06.046. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718265928
- 18.Raghu G, Johnson WC, Lockhart D, Mageto Y. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label Phase II study. Am J Respir Crit Care Med. 1999;159:1061–9. doi: 10.1164/ajrccm.159.4.9805017. [DOI] [PubMed] [Google Scholar]
- 19.Nagai S, Hamada K, Shigematsu M, Taniyama M, Yamauchi S, Izumi T. Open-label compassionate use one year-treatment with pirfenidone to patients with chronic pulmonary fibrosis. Intern Med. 2002;41:1118–23. doi: 10.2169/internalmedicine.41.1118. [DOI] [PubMed] [Google Scholar]
- 20.Gahl WA, Brantly M, Troendle J, Avila NA, Padua A, Montalvo C, Cardona H, Calis KA, Gochuico B. Effect of pirfenidone on the pulmonary fibrosis of Hermansky-Pudlak syndrome. Mol Genet Metab. 2002;76:234–42. doi: 10.1016/S1096-7192(02)00044-6. [DOI] [PubMed] [Google Scholar]
- 21.Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, Taguchi Y, Nagai S, Itoh H, Ohi M, Sato A, Kudoh S. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040–7. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718270310
- 22.Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, Taguchi Y, Takahashi H, Nakata K, Sato A, Takeuchi M, Raghu G, Kudoh S, Nukiwa T. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:821–9. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/3599956
- 23.Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, King TE, Lancaster L, Sahn SA, Szwarcberg J, Valeyre D, Du Bois RM. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760–9. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718270311
- 24.Spagnolo P, Del Giovane C, Luppi F, Cerri S, Balduzzi S, Walters EH, D’Amico R, Richeldi L. Non-steroid agents for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD003134.pub2. CD003134. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718270312
- 25.Adamali HI, Maher TM. Current and novel drug therapies for idiopathic pulmonary fibrosis. Drug Des Devel Ther. 2012;6:261–72. doi: 10.2147/DDDT.S29928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woodcock HV, Molyneaux PL, Maher TM. Reducing lung function decline in patients with idiopathic pulmonary fibrosis: potential of nintedanib. Drug Des Devel Ther. 2013;7:503–10. doi: 10.2147/DDDT.S38833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaudhary NI, Roth GJ, Hilberg F, Müller-Quernheim J, Prasse A, Zissel G, Schnapp A, Park JE. Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur Respir J. 2007;29:976–85. doi: 10.1183/09031936.00152106. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718270313
- 28.Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, Brown KK, Flaherty KR, Noble PW, Raghu G, Brun M, Gupta A, Juhel N, Klüglich M, Du Bois RM. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365:1079–87. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/13340022
- 29.Maher TM. Idiopathic pulmonary fibrosis: pathobiology of novel approaches to treatment. Clin Chest Med. 2012;33:69–83. doi: 10.1016/j.ccm.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C, Garcia CA, Velayo AC, Jorgensen B, Biermann D, Tsai D, Green J, Zaffryar-Eilot S, Holzer A, Ogg S, Thai D, Neufeld G, van Vlasselaer P, Smith V. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16:1009–17. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/5492957
- 31.Chien JW, Richards TJ, Gibson KF, Zhang Y, Lindell KO, Shao L, Lyman SK, Adamkewicz JI, Smith V, Kaminski N, O’Riordan T. Serum lysyl oxidase like-2 levels and idiopathic pulmonary fibrosis disease progression. Eur Respir J. 2013 doi: 10.1183/09031936.00141013. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718163308
- 32.Akiri G, Sabo E, Dafni H, Vadasz Z, Kartvelishvily Y, Gan N, Kessler O, Cohen T, Resnick M, Neeman M, Neufeld G. Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo. Cancer Res. 2003;63:1657–66. [PubMed] [Google Scholar]
- 33.Hancock A, Armstrong L, Gama R, Millar A. Production of interleukin 13 by alveolar macrophages from normal and fibrotic lung. Am J Respir Cell Mol Biol. 1998;18:60–5. doi: 10.1165/ajrcmb.18.1.2627. [DOI] [PubMed] [Google Scholar]
- 34.Belperio JA, Dy M, Burdick MD, Xue YY, Li K, Elias JA, Keane MP. Interaction of IL-13 and C10 in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2002;27:419–27. doi: 10.1165/rcmb.2002-0009OC. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718275832
- 35.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, Mosesova S, Eisner MD, Bohen SP, Matthews JG. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–98. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/12609956
- 36.Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, Simon KJ, Hahm K, Allaire NE, Rinaldi NJ, Goyal J, Feghali-Bostwick CA, Matteson EL, O’Hara C, Lafyatis R, Davis GS, Huang X, Sheppard D, Violette SM. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med. 2008;177:56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718275833
- 38.Raghu G, Collard HR, Anstrom KJ, Flaherty KR, Fleming TR, King TE, Martinez FJ, Brown KK. Idiopathic pulmonary fibrosis: clinically meaningful primary endpoints in phase 3 clinical trials. Am J Respir Crit Care Med. 2012;185:1044–8. doi: 10.1164/rccm.201201-0006PP. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/715348056
- 39.Vancheri C, Du Bois RM. A progression-free end-point for idiopathic pulmonary fibrosis trials: lessons from cancer. Eur Respir J. 2013;41:262–9. doi: 10.1183/09031936.00115112. [DOI] [PubMed] [Google Scholar]
- 40.Du Bois RM, Nathan SD, Richeldi L, Schwarz MI, Noble PW. Idiopathic pulmonary fibrosis: lung function is a clinically meaningful endpoint for phase III trials. Am J Respir Crit Care Med. 2012;186:712–5. doi: 10.1164/rccm.201206-1010PP. [DOI] [PubMed] [Google Scholar]
- 41.Spagnolo P, Luppi F, Maher TM, Wuyts WA, Grutters JC. Primary endpoints in phase 3 clinical trials in idiopathic pulmonary fibrosis: one step at a time. Am J Respir Crit Care Med. 2013;187:1271–2. doi: 10.1164/rccm.201209-1755LE. [DOI] [PubMed] [Google Scholar]
- 42.Wells AU, Behr J, Costabel U, Cottin V, Poletti V, Richeldi L. Hot of the breath: mortality as a primary end-point in IPF treatment trials: the best is the enemy of the good. Thorax. 2012;67:938–40. doi: 10.1136/thoraxjnl-2012-202580. [DOI] [PubMed] [Google Scholar]
- 43.Du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, Lancaster L, Noble PW, Raghu G, Sahn SA, Szwarcberg J, Thomeer M, Valeyre D, King TE. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:459–66. doi: 10.1164/rccm.201011-1790OC. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/13356022
- 44.Maher TM. PROFILEing idiopathic pulmonary fibrosis: rethinking biomarker discovery. Eur Respir Rev. 2013;22:148–52. doi: 10.1183/09059180.00000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noth I, Anstrom KJ, Calvert SB, Andrade J de, Flaherty KR, Glazer C, Kaner RJ, Olman MA. A placebo-controlled randomized trial of warfarin in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186:88–95. doi: 10.1164/rccm.201202-0314OC. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717951800
- 46.Malouf MA, Hopkins P, Snell G, Glanville AR. An investigator-driven study of everolimus in surgical lung biopsy confirmed idiopathic pulmonary fibrosis. Respirology. 2011;16:776–83. doi: 10.1111/j.1440-1843.2011.01955.x. [DOI] [PubMed] [Google Scholar]
- 47.King TE, Behr J, Brown KK, Du Bois RM, Lancaster L, Andrade JA de, Stähler G, Leconte I, Roux S, Raghu G. BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:75–81. doi: 10.1164/rccm.200705-732OC. [DOI] [PubMed] [Google Scholar]
- 48.King TE, Brown KK, Raghu G, Du Bois RM, Lynch DA, Martinez F, Valeyre D, Leconte I, Morganti A, Roux S, Behr J. BUILD-3: a randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:92–9. doi: 10.1164/rccm.201011-1874OC. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/12474956
- 49.Raghu G, Behr J, Brown KK, Egan JJ, Kawut SM, Flaherty KR, Martinez FJ, Nathan SD, Wells AU, Collard HR, Costabel U, Richeldi L, Andrade J de, Khalil N, Morrison LD, Lederer DJ, Shao L, Li X, Pedersen PS, Montgomery AB, Chien JW, O’Riordan TG. Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med. 2013;158:641–9. doi: 10.7326/0003-4819-158-9-201305070-00003. [DOI] [PubMed] [Google Scholar]
- 50.Raghu G, Million-Rousseau R, Morganti A, Perchenet L, Behr J. Macitentan for the treatment of idiopathic pulmonary fibrosis: the randomised controlled MUSIC trial. Eur Respir J. 2013;42:1622–32. doi: 10.1183/09031936.00104612. [DOI] [PubMed] [Google Scholar]
- 51.Zisman DA, Schwarz M, Anstrom KJ, Collard HR, Flaherty KR, Hunninghake GW. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010;363:620–8. doi: 10.1056/NEJMoa1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/4808956
- 52.King TE, Albera C, Bradford WZ, Costabel U, Hormel P, Lancaster L, Noble PW, Sahn SA, Szwarcberg J, Thomeer M, Valeyre D, Du Bois RM. Effect of interferon gamma-1b on survival in patients with idiopathic pulmonary fibrosis (INSPIRE): a multicentre, randomised, placebo-controlled trial. Lancet. 2009;374:222–8. doi: 10.1016/S0140-6736(09)60551-1. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1162867
- 53.Raghu G, Brown KK, Costabel U, Cottin V, Du Bois RM, Lasky JA, Thomeer M, Utz JP, Khandker RK, McDermott L, Fatenejad S. Treatment of idiopathic pulmonary fibrosis with etanercept: an exploratory, placebo-controlled trial. Am J Respir Crit Care Med. 2008;178:948–55. doi: 10.1164/rccm.200709-1446OC. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1120772
- 54.Daniels CE, Lasky JA, Limper AH, Mieras K, Gabor E, Schroeder DR. Imatinib treatment for idiopathic pulmonary fibrosis: Randomized placebo-controlled trial results. Am J Respir Crit Care Med. 2010;181:604–10. doi: 10.1164/rccm.200906-0964OC. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/3558960
- 55.Shulgina L, Cahn AP, Chilvers ER, Parfrey H, Clark AB, Wilson ECF, Twentyman OP, Davison AG, Curtin JJ, Crawford MB, Wilson AM. Treating idiopathic pulmonary fibrosis with the addition of co-trimoxazole: a randomised controlled trial. Thorax. 2013;68:155–62. doi: 10.1136/thoraxjnl-2012-202403. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717964789