Abstract
Background and objectives
Chinese patients with ESRD have different comorbidity patterns than white patients with ESRD and require a validated comorbidity index. The objective of this study was to develop a new index for mortality prediction in 2006–2009 Taiwanese incident hemodialysis patients.
Design, setting, participants, & measurements
Data were retrieved from 2005 to 2010 Taiwan National Health Insurance claim records, and follow-up was available until December 31, 2010. The same comorbid conditions as the US Renal Data System (USRDS) index that occurred during a 12-month period from 9 months before to 3 months after dialysis initiation were used to construct the index. Integer weight of the comorbid conditions was derived from coefficient estimates of Cox regression for all-cause mortality, and the index was internally validated. The performance of the index was assessed by discrimination, calibration, and reclassification.
Results
A total of 30,303 hemodialysis patients were included in this study. The weight for individual comorbid conditions of this index differed from that of the USRDS index. The performance of this index was similar to that of USRDS and Charlson indices in terms of model fit statistics, overall predictive ability, discrimination, and calibration. Hosmer–Lemeshow test showed that all three indices demonstrated significant differences between predicted and observed mortality rates. When patients were categorized by the predicted 2.5-year survival probabilities, the index achieved a net reclassification improvement of 4.71% (P<0.001), referenced to USRDS index.
Conclusions
Compared with USRDS index, this new index demonstrated better reclassification ability, but future studies should address the clinical significance.
Introduction
Comparisons of clinical outcomes for patients with ESRD may be confounded by multiple comorbid conditions (1–3). A comorbidity score summarizing overall comorbid conditions may be useful for confounder or case-mix adjustment in observational studies of ESRD (4).
A new index was recently developed using data from the US Renal Data System (USRDS) (5). Eleven comorbid conditions were identified from medical evidence forms and claims during a 6-month period, and the index was shown to have good predictive abilities for all-cause mortality, hospitalization, and medical costs. However, <3% of the USRDS cohort was of Asian ethnicity (5), and studies have shown that East Asian patients with ESRD have different comorbidity patterns than white patients with ESRD (6–9). Thus, an index fully validated in Chinese patients with ESRD is required for epidemiologic research.
CKD (10) and ESRD (11) are highly prevalent in Taiwan, with an ESRD prevalence of 2451 per million population in 2009 (3). Most patients with ESRD received hemodialysis as their initial dialysis modality in Taiwan, and the prevalence of hemodialysis use was 89.7% in 2009 (3). Because dialysis-related expenditures in Taiwan were reimbursed solely by the National Health Insurance (NHI) system, comprehensive information regarding dialysis therapy can be extracted from the NHI claim records. Therefore, we developed a comorbidity index in 2006–2009 Taiwanese incident hemodialysis patients. Baseline comorbid conditions were derived from a 12-month claim period, and the predictive ability of our index for mortality risk was compared with that of the USRDS and Charlson indices (12,13). We focused our investigation on the differential effects of these comorbid conditions on mortality risk in different ESRD populations.
Materials and Methods
Study Population
The 2005–2010 NHI claims data, which contain records for 99% of the 23 million residents of Taiwan (14), were obtained from the Collaboration Center of Health Information Application, Ministry of Health and Welfare, Executive Yuan, Taiwan. All identification numbers and hospital names were encrypted. The medical history of each beneficiary was established by linking birth date and identification number between several administrative data sets and the death registry. Our study was approved by the Research Ethics Committee of Taipei Veterans General Hospital.
Records for incident patients aged 20 years and older, who started dialysis therapy between January 1, 2006, and December 31, 2009, and who had received dialysis treatment for >90 days were reviewed for our study. We excluded patients who had received long-term dialysis or a kidney transplant before January 1, 2006. The initial dialysis modality was determined by the modality used on the 90th day after initiating dialysis, and, in this study, we included only patients who received hemodialysis as initial modality.
Covariates
The primary causes of ESRD were not included in the NHI database and were therefore not used as covariates in our study. The baseline comorbid conditions were defined as conditions diagnosed using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for which at least one inpatient stay or two outpatient examinations on separate dates occurred during the 12-month period beginning 9 months before and ending 3 months after the date of the first dialysis treatment (15). To construct our new index, we used the same 11 comorbid conditions and the corresponding ICD-9-CM codes used in the USRDS index (5), including diabetes, atherosclerotic heart disease (ASHD), congestive heart failure (CHF), cerebrovascular accident or transient ischemic attack (CVA/TIA), peripheral vascular disease (PVD), chronic obstructive pulmonary disease (COPD), gastrointestinal bleeding, liver disease, dysrhythmia, cancer, and other cardiac diseases (heart transplant, heart valve replacement, cardiac devices, pericarditis, endocarditis, myocarditis, and other complications of heart disease).
Model Development
The study outcome was all-cause mortality. Patients were followed from the first day of dialysis treatment until the date of death, transplantation, loss to follow-up, recovery of renal function, or December 31, 2010. We performed 1000 simulations by randomly dividing the cohort into derivation and validation sets in a 2:1 ratio. Age, sex, and 11 comorbid conditions were included in the Cox regression. Using a stepwise regression method, Cox regression was performed in each derivation set, and variables with a P value<0.2 were selected (16–18). To enhance the stability of our model, only variables selected >750 times from the 1000 simulations were included in the final prediction model. The selected comorbid conditions were assigned a weight by multiplying the mean of the point estimation by 10 and rounding the product to the nearest integer (16–18). An individual patient’s comorbidity score was the sum of the weighted comorbid conditions. The internal validation of the index was performed using the 1000 derivation and validation sets.
Model Performance and Statistical Analyses
The performance of our index was then compared with that of the USRDS and Charlson indices, using the full data set. The overall model fit was compared using the Akaike information criterion (18). The Kent and O'Quigley R2 was used to assess the overall predictive ability (19,20). The Harrell global c-statistic was calculated to assess the probability that a patient from the event group had a higher predicted probability of having an event than a patient from the nonevent group (21). The calibration was assessed by comparing the predicted and the observed death rates (22). Patients were grouped into deciles using estimated survival probability based on Martingale residual, and the Hosmer–Lemeshow test was applied (23). In general, P>0.05 suggests an accurate model, with no differences between the predicted and observed results. To better compare model performance in clinical risk prediction, reclassification tables were drawn by classifying patients according to predicted survival probability estimated by comorbidity score only (without age and sex), using our index and USRDS index (24). Net reclassification improvement (NRI) of our index was calculated to account for the net fraction of reclassifying patients’ risk categories appropriately (25), using USRDS index as the reference model. All statistical analyses were conducted using SAS statistical software, version 9.2 (SAS Institute, Cary, NC). A two-tailed P value<0.05 was considered to indicate a statistically significant difference.
Results
Characteristics of Study Participants
A total of 30,303 hemodialysis patients were followed up for a median of 27.1 months (Table 1). Diabetes (61.2%), ASHD (29.7%), CHF (33%), and gastrointestinal bleeding (28.7%) were the most prevalent comorbid conditions in our patients. Until the end of follow-up, 8537 (28.2%) patients died, 385 (1.3%) patients received a kidney transplant, 151 patients recovered their renal function, and 153 patients were lost to follow-up.
Table 1.
Demographic data for the 2006–2009 Taiwanese incident hemodialysis patients
| Variable | Value |
|---|---|
| Patients (n) | 30,303 |
| Mean age ± SD (yr) | 64.3 ± 13.3 |
| Age groups, n (%) | |
| 40 yr | 1251 (4.1) |
| 40–64 yr | 12,925 (42.7) |
| ≥65 yr | 16,127 (53.2) |
| Men, n (%) | 15,636 (51.6) |
| Comorbid conditions, n (%) | |
| Diabetes | 18,539 (61.2) |
| ASHD | 9011 (29.7) |
| CHF | 9996 (33.0) |
| CVA/TIA | 5112 (16.9) |
| PVD | 2374 (7.8) |
| Other cardiac disease | 2929 (9.7) |
| COPD | 3235 (10.7) |
| GI bleeding | 8699 (28.7) |
| Liver disease | 2514 (8.3) |
| Dysrhythmia | 2558 (8.4) |
| Cancer | 2695 (8.9) |
Other cardiac diseases included heart transplant, heart valve replacement, cardiac devices, pericarditis, endocarditis, myocarditis, and other complications of heart disease. ASHD, atherosclerotic heart disease; CHF, congestive heart failure; CVA/TIA, cerebrovascular accident or transient ischemic attack; PVD, peripheral vascular disease; COPD, chronic obstructive pulmonary disease; GI, gastrointestinal.
Model Development and Validation
The results of stepwise Cox regression are shown in Table 2. Older age was independently associated with all-cause mortality. The risk of mortality among men was higher in our patients. Other cardiac disease was excluded from the final model because of selection <750 times in the 1000 simulations. Weight assigned to predictors for all-cause mortality in our index differed considerably from those of USRDS index (5). Cancer had a weight of 6. CVA/TIA and liver disease had a weight of 4. Diabetes, CHF, COPD, and dysrhythmia had a weight of 3. PVD and gastrointestinal bleeding had a weight of 2. ASHD had a weight of only 1. The sum of the weighted comorbid conditions represented a patient’s risk score for mortality and ranged from 0 to 31 in our patients. The median comorbidity score for our patients was 7. Unlike the USRDS index, a weight could not be assigned to the primary cause of ESRD in our index because information regarding the primary cause of ESRD is not included in the NHI claim records (5). Internal validation revealed that the means of the c-statistic of the 1000 derivation and validation sets were 0.76 and 0.76 in our hemodialysis patients.
Table 2.
Cox regression for all-cause mortality of 1000 simulations in 2006–2009 Taiwanese incident hemodialysis patients
| Variable | Frequency Selecteda | Coefficient Estimate: Mean±SDb | Hazard Ratio (95% CI) | Weight |
|---|---|---|---|---|
| Age groups | ||||
| <40 yr | 1000 | −1.47 ± 0.09 | 0.23 (0.19 to 0.27) | |
| 40–64 yr | 1000 | −0.79 ± 0.02 | 0.46 (0.44 to 0.47) | |
| ≥65 yr | 0 | 1 | ||
| Men versus women | 1000 | 0.11 ± 0.02 | 1.11 (1.07 to 1.15) | |
| Comorbid conditions | ||||
| Diabetes | 1000 | 0.29 ± 0.02 | 1.34 (1.29 to 1.39) | 3 |
| ASHD | 996 | 0.10 ± 0.02 | 1.10 (1.06 to 1.14) | 1 |
| CHF | 1000 | 0.26 ± 0.02 | 1.30 (1.25 to 1.35) | 3 |
| CVA/TIA | 1000 | 0.44 ± 0.02 | 1.55 (1.48 to 1.61) | 4 |
| PVD | 1000 | 0.21 ± 0.03 | 1.23 (1.15 to 1.30) | 2 |
| Other cardiac disease | 680 | 0.09 ± 0.02 | 1.09 (1.06 to 1.14) | |
| COPD | 1000 | 0.34 ± 0.03 | 1.40 (1.33 to 1.47) | 3 |
| GI bleedingc | 1000 | 0.25 ± 0.02 | 1.28 (1.23 to 1.33) | 2 |
| Liver disease | 1000 | 0.38 ± 0.03 | 1.46 (1.37 to 1.55) | 4 |
| Dysrhythmia | 1000 | 0.31 ± 0.03 | 1.36 (1.28 to 1.43) | 3 |
| Cancer | 1000 | 0.56 ± 0.03 | 1.75 (1.66 to 1.85) | 6 |
Other cardiac diseases included heart transplant, heart valve replacement, cardiac devices, pericarditis, endocarditis, myocarditis, and other complications of heart disease. 95% CI, confidence interval; ASHD, atherosclerotic heart disease; CHF, congestive heart failure; CVA/TIA, cerebrovascular accident or transient ischemic attack; PVD, peripheral vascular disease; COPD, chronic obstructive pulmonary disease; GI, gastrointestinal.
By inclusion criteria of P<0.2, comorbid conditions selected >750 times were assigned a weight by multiplying mean of the coefficient estimate by 10 and rounding it to the nearest integer.
Data are mean±SD of 1000 simulations.
GI bleeding had a coefficient estimate of 0.25 and therefore was assigned a weight of 2.
Comparison of Model Performance
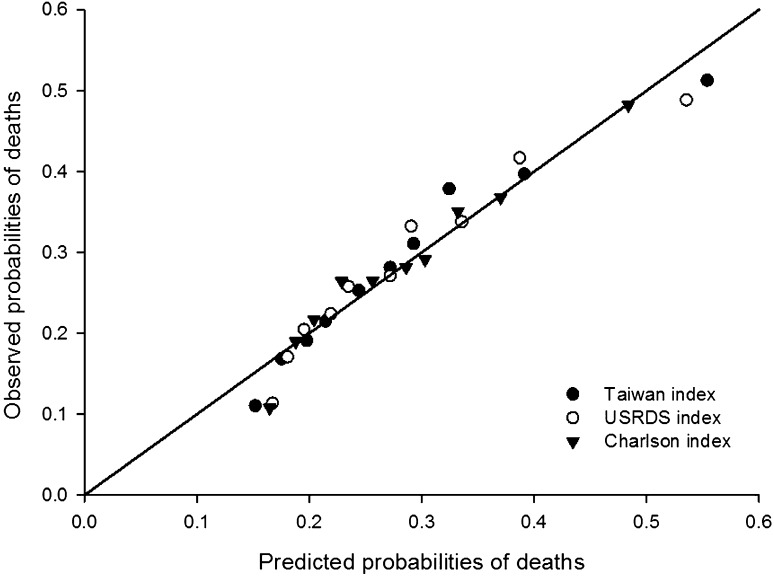
The performance of our index was compared with that of USRDS and Charlson indices, using the full data set, including the derivation and validation sets (Table 3). These 3 indices demonstrated similar model fit statistics (Akaike information criterion, 164437.5–164693.2), overall predictive ability (R2=0.29–0.3), and discrimination (c-statistic=0.76–0.77). All three indices demonstrated significant differences between the predicted and observed probability of mortality, but our index had the smallest test statistic in the Hosmer–Lemeshow test (chi-square=78.39; P<0.001; degrees of freedom=8) (Figure 1).
Table 3.
Comparisons of model performance between the Taiwan, U.S. Renal Data System, and Charlson indices using all-cause mortality prediction in 2006–2009 Taiwanese incident hemodialysis patients
| Index | Model Fit Statistics (AIC) | Overall Performance (R2) | Discrimination (c-Statistic) | Calibration (H-L Testa) |
|---|---|---|---|---|
| Taiwan index | 164,437.5 | 0.30 | 0.76 | Chi-square=78.39, P<0.001 |
| USRDS index | 164,693.2 | 0.29 | 0.77 | Chi-square =100.16, P<0.001 |
| Charlson index | 164,683.4 | 0.30 | 0.77 | Chi-square=84.98, P<0.001 |
AIC, Akaike information criterion; USRDS, US Renal Data System; H-L, Hosmer–Lemeshow.
Degrees of freedom=8.
Figure 1.
Calibration curve for the predicted and observed probability of mortality in the 2006–2009 Taiwanese incident hemodialysis patients. Patients were grouped by deciles of estimated probability of deaths. USRDS, US Renal Data System.
The numbers of patients whose risk categories were estimated by our index or USRDS index are shown in Table 4. The median follow-up of our patients was 27.1 months and, on the basis of the prediction by our index (without age and sex), >95% of patients had survival probability between 50% and 90% at 2.5 years after dialysis. We therefore categorized our patients into five risk groups according to the predicted 2.5-year survival probability of ≥80%, 70% to <80%, 60% to <70%, 50% to <60%, and <50%. We assigned patients’ final status as death (with event) or living (without event, including those who received a kidney transplant or were censored because of other reasons) with vital status at the end of the study. Compared with the USRDS index, our index significantly reclassified more patients to the correct risk categories and achieved an NRI of 4.71% (P<0.001).
Table 4.
Reclassification table and net reclassification improvement estimated by Taiwan index in 2006–2009 Taiwanese incident hemodialysis patients
| USRDS Indexa | Taiwan Index Survival Probability (Comorbidity Score)b | ||||
|---|---|---|---|---|---|
| P≥0.8 (0–4) | 0.7≤P<0.8 (5–8) | 0.6≤P<0.7 (9–11) | 0.5≤P<0.6 (12–13) | P<0.5 (≥ 14) | |
| Death (n=8537) | |||||
| Survival probability (comorbidity score)b | |||||
| P≥0.8 (0–2) | 1766 | 134 | 0 | 0 | 0 |
| 0.7≤P<0.8 (3–5) | 346 | 2129 | 606 | 75 | 0 |
| 0.6≤P<0.7 (6–7) | 15 | 528 | 940 | 217 | 95 |
| 0.5≤P<0.6 (8–9) | 0 | 98 | 336 | 422 | 220 |
| P<0.5 (≥ 10) | 0 | 0 | 54 | 144 | 412 |
| Living (n=21,766) | |||||
| Survival probability (comorbidity score) | |||||
| P≥0.8 (0–2) | 8821 | 336 | 0 | 0 | 0 |
| 0.7≤P<0.8 (3–5) | 1361 | 5553 | 884 | 68 | 0 |
| 0.6≤P<0.7 (6–7) | 44 | 1068 | 1520 | 241 | 73 |
| 0.5≤P<0.6 (8–9) | 0 | 119 | 480 | 470 | 191 |
| P<0.5 (≥10) | 0 | 0 | 57 | 132 | 348 |
| NRI | 4.71% | ||||
| P value | <0.001 | ||||
NRI, net reclassification improvement.
USRDS index was used as reference prediction model.
Range of survival probability and the corresponding range of comorbidity scores.
Discussion
A new index containing different weights for comorbid conditions was developed and validated to predict mortality in Taiwanese hemodialysis patients.
The prevalence of comorbidity observed in our patients substantially differed from that of 1999–2001 USRDS cohorts, from which the USRDS index was derived (5). Differences in diet, lifestyle, and ethnicity may have contributed to the differences observed. Diabetes was a comorbid condition in 61.2% of 2006–2009 incident hemodialysis patients in Taiwan compared with 63.6% in the 1999 USRDS cohort. The prevalence of ASHD, CHF, PVD, and dysrhythmia were remarkably lower in our cohort, with rates of 29.7%, 33%, 7.8%, and 8.4%, respectively, compared with rates of 51.5%, 54.3%, 44.4%, and 30.6%, respectively, in the 1999 USRDS cohort (5). Gastrointestinal bleeding and liver disease were substantially more frequent among Taiwanese hemodialysis patients (26), with prevalence of 28.7% and 8.3% in our cohort, compared with rates of only 10.8% and 5.3%, respectively, in the USRDS cohort (5).
Our index was simpler and was composed of fewer predictors (10 comorbid conditions), than the USRDS index (11 comorbid conditions and 3 primary causes of ESRD) and the Charlson index (17 comorbid conditions). During the derivation of our index, only predictors selected for >750 times in the 1000 simulations were included in the final model, greatly enhancing the stability of our model. The weights for individual comorbid conditions were considerably different from those observed in the USRDS cohort (5), reflecting the unequal association of comorbid conditions and mortality in different ESRD populations. In the USRDS index, diabetes had a weight of 4 if it was also the primary cause of ESRD. CHF and ASHD had a weight of 3 and 1, respectively, and other comorbid conditions were all assigned a weight of 2. In our index, cancer, CVA/TIA, and liver disease received a weight of 6, 4, and 4, respectively. Diabetes, CHF, COPD, and dysrhythmia all had a weight of 3. A previous study showed that compared with nondialysis patients, more cardiac arrest and in-hospital death were reported in dialysis patients hospitalized for acute myocardial infarction (27). However, ASHD received a weight of only 1 in both the USRDS index and our index. Further study is required to understand the effect of baseline ASHD on prognosis of patients with ESRD.
The comparisons of index performance in our study consisted of an internal validation of our index and an external (fully independent) validation of the USRDS index. The c-statistic indicates whether a risk score appropriately rank-orders risks for patients with and without events (21). Calibration provides information about how well the estimated risks match the actual outcomes (24). Overall, despite the differences in prevalence of comorbid conditions and mortality risks between the Taiwanese and USRDS cohorts in which the indices were developed, our index and the USRDS index had similar performance for mortality prediction in the Taiwanese hemodialysis population. Recently, reclassification has received increasing attention for the evaluation of incremental improvement of a new marker or a new prediction model (25). The NRI indicates how much more frequently appropriate reclassification occurs than inappropriate reclassification with use of the new model. Referenced to the USRDS index, results showed that the proportion of patients reclassified appropriately by our index was 4.71% greater than that reclassified inappropriately. Although the NRI of 4.71% was statistically significant, its clinical significance cannot be assured in this study. However, the difference in weights for comorbid conditions and the positive NRI suggest that Taiwan index might be more appropriate for other clinical outcomes, which will be clarified in the future. The difference in weights for comorbid conditions also provides directions for implementing specific treatment strategy to improve patient outcomes in different ESRD populations.
Despite the longer life expectancy of women in the general population (28,29), several European studies have shown that sex is not a prognostic factor in patients with ESRD (30–32). In contrast, our data showed that sex was an independent risk factor for mortality in Chinese hemodialysis patients. Thus, when information regarding sex is available, our index may more accurately estimate risks in future studies on mortality in Chinese patients with ESRD.
The strengths of our study included the large sample size and the comprehensive data from all levels of dialysis facilities in Taiwan. In addition, the USRDS index was based on medical evidence form and Medicare claims data during months 4–9 after the initiation of dialysis treatment. By contrast, our index was based on a 12-month period that began 9 months before and ended 3 months after the date of the first dialysis treatment. We believe that our index more accurately reflected the disease burden at baseline and can be used to predict clinical outcomes within the first year of dialysis, during which the mortality risk is greatest (33).
However, our methods have limitations. Our index was based on data from patients with ESRD in Taiwan who were of Chinese ancestry. Because of the potential contributions of environmental factors to mortality risks in patients with ESRD, our index might not completely apply to Chinese patients in mainland China, Hong Kong, or other parts of the world. Further external (fully independent) validation or temporal validation using a more recent dialysis cohort is required to extend the general applicability of our index to other Asian populations. Furthermore, we cannot exclude the possibility that comorbid conditions included in our index were overestimated because the NHI remunerated the care of all these comorbid conditions. Data on certain risk factors for ESRD, such as nutrition status (34), inflammation, and frailty (35), were absent from the NHI database. Although a previous study showed that the severity of comorbid conditions did not influence the discriminating power of indices (13), residual confounding may have occurred because the NHI claims records did not describe the severity of comorbid conditions (36). A validation study is required to determine whether the severity of comorbid conditions can affect the performance of our index. Of note, the ability of our index to predict hospitalization duration and medical expenditures in Taiwan or elsewhere remains untested.
In conclusion, a new index containing different weights for comorbid conditions was developed and validated to predict mortality in Taiwanese hemodialysis patients. Compared with the USRDS index, our index demonstrated better reclassification ability, but the clinical significance needs to be addressed in future studies.
Disclosures
None.
Acknowledgments
Our study was supported by research grants from the Yen Tjing Ling Medical Foundation (CI-100-29), Taipei Veteran General Hospital (V101C-202), and Cheng Hsin General Hospital (101-15), Taipei, Taiwan.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Khan IH, Catto GR, Edward N, Fleming LW, Henderson IS, MacLeod AM: Influence of coexisting disease on survival on renal-replacement therapy. Lancet 341: 415–418, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Davies SJ, Russell L, Bryan J, Phillips L, Russell GI: Comorbidity, urea kinetics, and appetite in continuous ambulatory peritoneal dialysis patients: Their interrelationship and prediction of survival. Am J Kidney Dis 26: 353–361, 1995 [DOI] [PubMed] [Google Scholar]
- 3.U.S. Renal Data System : USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2011 [Google Scholar]
- 4.Beddhu S, Bruns FJ, Saul M, Seddon P, Zeidel ML: A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am J Med 108: 609–613, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Huang Z, Gilbertson DT, Foley RN, Collins AJ: An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int 77: 141–151, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Hwang SJ, Yang WC, Lin MY, Mau LW, Chen HC, Taiwan Society of Nephrology : Impact of the clinical conditions at dialysis initiation on mortality in incident haemodialysis patients: A national cohort study in Taiwan. Nephrol Dial Transplant 25: 2616–2624, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Chae JW, Song CS, Kim H, Lee KB, Seo BS, Kim DI: Prediction of mortality in patients undergoing maintenance hemodialysis by Charlson Comorbidity Index using ICD-10 database. Nephron Clin Pract 117: c379–c384, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Hou F, Jiang J, Chen J, Yu X, Zhou Q, Chen P, Mei C, Xiong F, Shi W, Zhou W, Liu X, Sun S, Xie D, Liu J, Zhang P, Yang X, Zhang Y, Zhang Y, Liang X, Zhang Z, Lin Q, Yu Y, Wu S, Xu X: China collaborative study on dialysis: A multi-centers cohort study on cardiovascular diseases in patients on maintenance dialysis. BMC Nephrol 13: 94, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashino Y, Fukuhara S, Akiba T, Akizawa T, Asano Y, Saito A, Bragg-Gresham JL, Ramirez SP, Port FK, Kurokawa K: Diabetes, glycaemic control and mortality risk in patients on haemodialysis: The Japan Dialysis Outcomes and Practice Pattern Study. Diabetologia 50: 1170–1177, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, Chiang PH, Hsu CC, Sung PK, Hsu YH, Wen SF: All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet 371: 2173–2182, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Yang WC, Hwang SJ, Taiwan Society of Nephrology : Incidence, prevalence and mortality trends of dialysis end-stage renal disease in Taiwan from 1990 to 2001: The impact of national health insurance. Nephrol Dial Transplant 23: 3977–3982, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: 373–383, 1987 [DOI] [PubMed] [Google Scholar]
- 13.van Manen JG, Korevaar JC, Dekker FW, Boeschoten EW, Bossuyt PM, Krediet RT, NECOSAD Study Group. Netherlands Co-operative Study on the Adequacy of Dialysis-2 : How to adjust for comorbidity in survival studies in ESRD patients: A comparison of different indices. Am J Kidney Dis 40: 82–89, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Chang CH, Lin JW, Wu LC, Lai MS, Chuang LM, Chan KA: Association of thiazolidinediones with liver cancer and colorectal cancer in type 2 diabetes mellitus. Hepatology 55: 1462–1472, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Chang TI, Shilane D, Kazi DS, Montez-Rath ME, Hlatky MA, Winkelmayer WC: Multivessel coronary artery bypass grafting versus percutaneous coronary intervention in ESRD. J Am Soc Nephrol 23: 2042–2049, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrell FE, Jr, Lee KL, Mark DB: Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15: 361–387, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Harrell F: Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis, New York, NY, Springer, 2001 [Google Scholar]
- 18.Steyerberg E: Clinical Prediction Models: A Practical Approach to Development, Validation and Updating, New York, NY, Springer, 2009 [Google Scholar]
- 19.O’Quigley J, Xu R, Stare J: Explained randomness in proportional hazards models. Stat Med 24: 479–489, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Choodari-Oskooei B, Royston P, Parmar MK: A simulation study of predictive ability measures in a survival model I: Explained variation measures. Stat Med 31: 2627–2643, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Pencina MJ, D’Agostino RB: Overall C as a measure of discrimination in survival analysis: Model specific population value and confidence interval estimation. Stat Med 23: 2109–2123, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, Pencina MJ, Kattan MW: Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology 21: 128–138, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cook NR: Statistical evaluation of prognostic versus diagnostic models: Beyond the ROC curve. Clin Chem 54: 17–23, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Cook NR: Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation 115: 928–935, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Pencina MJ, D'Agostino RB, Sr., D'Agostino RB, Jr., Vasan RS: Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 27: 157-172; discussion 207-112, 2008 [DOI] [PubMed]
- 26.Luo JC, Leu HB, Huang KW, Huang CC, Hou MC, Lin HC, Lee FY, Lee SD: Incidence of bleeding from gastroduodenal ulcers in patients with end-stage renal disease receiving hemodialysis. CMAJ 183: E1345–E1351, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herzog CA, Littrell K, Arko C, Frederick PD, Blaney M: Clinical characteristics of dialysis patients with acute myocardial infarction in the United States: A collaborative project of the United States Renal Data System and the National Registry of Myocardial Infarction. Circulation 116: 1465–1472, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Murray CJ, Lopez AD: Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 349: 1269–1276, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Wingard DL, Suarez L, Barrett-Connor E: The sex differential in mortality from all causes and ischemic heart disease. Am J Epidemiol 117: 165–172, 1983 [DOI] [PubMed] [Google Scholar]
- 30.Villar E, Remontet L, Labeeuw M, Ecochard R: Effect of age, gender, and diabetes on excess death in end-stage renal failure. J Am Soc Nephrol 18: 2125–2134, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Carrero JJ, de Mutsert R, Axelsson J, Dekkers OM, Jager KJ, Boeschoten EW, Krediet RT, Dekker FW, NECOSAD Study Group : Sex differences in the impact of diabetes on mortality in chronic dialysis patients. Nephrol Dial Transplant 26: 270–276, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Carrero JJ, de Jager DJ, Verduijn M, Ravani P, De Meester J, Heaf JG, Finne P, Hoitsma AJ, Pascual J, Jarraya F, Reisaeter AV, Collart F, Dekker FW, Jager KJ: Cardiovascular and noncardiovascular mortality among men and women starting dialysis. Clin J Am Soc Nephrol 6: 1722–1730, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Bradbury BD, Fissell RB, Albert JM, Anthony MS, Critchlow CW, Pisoni RL, Port FK, Gillespie BW: Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol 2: 89–99, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Iseki K, Kawazoe N, Fukiyama K: Serum albumin is a strong predictor of death in chronic dialysis patients. Kidney Int 44: 115–119, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimbürger O, Massy Z: Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol 3: 505–521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindholm B, Davies S: End-stage renal disease: A new comorbidity index for estimating mortality risk in ESRD. Nat Rev Nephrol 6: 391–393, 2010 [DOI] [PubMed] [Google Scholar]