Abstract
Background and objectives
The epidemiology of AKI and CKD has been described. However, the epidemiology of progressively worsening kidney function (subacute kidney injury [s-AKI]) developing over a longer time frame than defined for AKI (7 days), but shorter than defined for CKD (90 days), is completely unknown.
Design, setting, participants, & measurements
This retrospective study used a hospital laboratory and admission database. Adult patients admitted to a teaching hospital in Tokyo, Japan, between April 1, 2008, and October 31, 2011, were included. s-AKI was classified into three grades of severity (mild, moderate, severe) in accordance with the Risk, Injury, and Failure categories of the Risk, Injury, Failure, Risk, Loss, and ESRD classification, but did not use its time frame. Kidney injury (AKI and s-AKI) occurring during each hospital stay was identified, and logistic regression analysis was performed to assess their effect on hospital mortality.
Results
Of 56,567 patients admitted to the hospital during the study period, 49,518 were included. Of these, 87.8% had no evidence of kidney dysfunction, 11.0% had AKI, and 1.1% had s-AKI. Patients with s-AKI had mild renal dysfunction in 82.7% of cases, moderate in 12.1%, and severe in 5.0%. Worsening s-AKI category was linearly correlated with hospital mortality, as previously described for AKI (no injury: 1.2%, mild: 6.5%, moderate: 12.9%, severe: 20.7%). Although mortality (8.0% versus 17.5%) and need for renal replacement therapy (0.2% versus 2.2%) were lower in patients with s-AKI than in those with AKI, multivariable regression analysis confirmed that s-AKI was an independent risk factor for hospital mortality (odds ratio (OR), 5.44; 95% confidence interval [95% CI], 3.89 to 7.44); the OR with AKI was 14.8 (95% CI, 13.2 to 16.7).
Conclusions
Close to 1% of hospitalized patients develop s-AKI. This condition is independently associated with increased hospital mortality, and the risk for death increases with s-AKI severity. Patients with s-AKI had a better outcome and were less likely to require renal replacement therapy than patients with AKI.
Introduction
AKI is a major clinical problem among hospital patients (1). Definitions of AKI based on changes in serum creatinine and urine output within each time frame have now been developed and are widely accepted and used (2–4). Since these criteria were released, the epidemiology and characteristics of patients with AKI have been well described, and even mild AKI is independently associated with increased mortality rates (5–7). Similarly, consensus classifications of CKD exist and are also widely applied to define the epidemiology of this condition (8). However, a group of hospital patients develop renal dysfunction but appear not to fulfill the time-frame criteria for AKI (7 days) or CKD (>90 days). These patients could be said to have subacute kidney injury (s-AKI). However, it is not clear whether these patients are truly different from patients with AKI and what the associated epidemiology might be.
Accordingly, we conducted a retrospective study to describe the epidemiology of s-AKI. Our aim was to identify hospital patients with s-AKI and to understand the epidemiology and independent association with outcome.
Materials and Methods
This retrospective observational study included all patients admitted to a 1074-bed academic hospital in Tokyo, Japan, between April 1, 2008, and October 31, 2011. The computerized hospital admissions and discharges database was screened and variables, such as age, sex, all dates and results of serum creatinine measured during the study period, admission units, intensive care unit admission, and hospital mortality, were retrieved. Patients were excluded if they were younger than 15 years of age, had CKD stage 5 at admission or baseline and received renal replacement therapy (RRT) during the admission, or stayed in the hospital for less than 2 days. The institutional ethics committee waived the need for informed consent because this study did not require any intervention and patient data were anonymized.
AKI was defined by serum creatinine criteria according to the RIFLE (Risk, Injury, Failure, Risk, Loss, and ESRD) classification, and s-AKI was defined to describe a more slowly progressive subacute kidney functional impairment, as shown in Table 1. Baseline serum creatinine was defined by the most recent value obtained at an outpatient clinic 1–12 months before admission, or, if unavailable, calculated by the simplified Modification of Diet in Renal Disease (MDRD) formula for Japanese, assuming a GFR of 75 ml/min per 1.73 m2, as previously reported (9).
Table 1.
Definition and staging of AKI by RIFLE (Risk, Injury, Failure, Risk, Loss, and ESRD) classification and subacute kidney injury
| Classification per Type of Kidney Injury | Criteria |
|---|---|
| AKI | |
| Risk | 1.5–1.9 times baseline within 7 d |
| Injury | 2.0–2.9 times baseline within 7 d |
| Failure | 3.0 times baseline within 7 d or increase in serum creatinine to ≥4.0 mg/dl with an acute rise of ≥0.5 mg/dl within 7 d |
| Subacute kidney injury | |
| Mild | 1.5–1.9 times baseline in >7 d |
| Moderate | 2.0–2.9 times baseline in >7 d |
| Severe | 3.0 times baseline in >7 d or increase in serum creatinine to ≥4.0 mg/dl with a rise of ≥0.5 mg/dl in >7 d |
Because our database did not include urine output, we used only creatinine criteria. For analysis, RIFLE class was calculated using serum creatinine levels with reference to the preadmission baseline creatinine (or calculated from the MDRD equation), or the lowest creatinine within the first 7 days after admission. After day 8, the reference value was the lowest creatinine within the last 7 days.
The maximum RIFLE category during hospitalization was reported. We classified s-AKI into three grades of severity based on gradual changes of serum creatinine with reference to the preadmission baseline creatinine (or calculated from the MDRD equation) taking >7 days, as described in Table 1, and the maximum category was reported.
Categorical data are reported as proportions, and numeric data are reported as medians with interquartile ranges. Multiple comparisons between the group without kidney injury, s-AKI group, and AKI group were performed using the Fisher exact test with Bonferroni adjustment to a P value<0.02, which was considered statistically significant for categorical data, and by the Dunn method using a P value<0.05 for numerical data. Multivariable logistic regression analysis was used to assess the association of each category of AKI and s-AKI with hospital mortality. A model was adjusted for age, sex, baseline serum creatinine levels, intensive care unit admission, admission units, and hospital length of stay. Model fit was assessed by the goodness-of-fit test. Sensitivity analyses were conducted for patients who had baseline creatinine levels at the outpatient clinic and after exclusion of patients who stayed in the hospital for <7 days (because they could not develop s-AKI). Results are presented as odds ratios (ORs) with 95% confidence intervals (95% CIs). A P value<0.05 was considered to represent statistically significant differences for the regression analysis. All analyses were performed using JMP Pro 9.0.3 (SAS Institute, Inc., Cary, NC).
Results
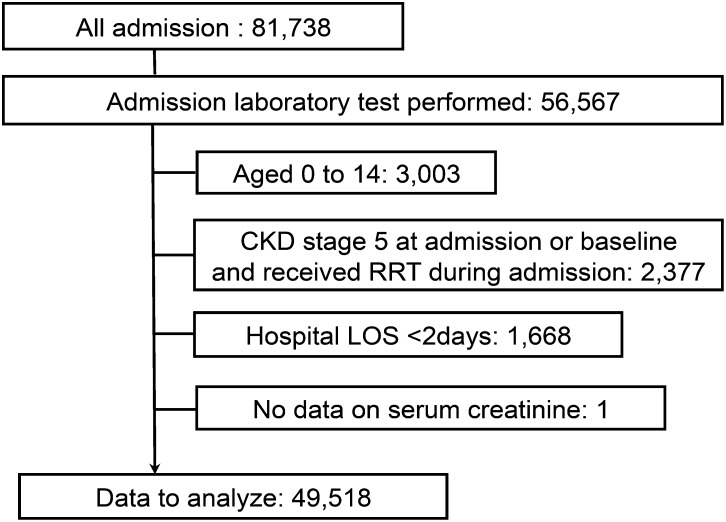
During the study period, we screened all 81,738 admissions; 56,567 of them had laboratory data on renal function during the hospital admission. Of these, we included 49,518 of those who fulfilled the criteria for analysis (Figure 1). Among these patients, 43,493 did not develop kidney injury (87.8%), while AKI (stratified by the RIFLE criteria with a 7-day time frame) occurred in 5451 admissions (11.0%) and s-AKI occurred in 574 admissions (1.1%).
Figure 1.
Study flow chart. LOS, length of stay; RRT, renal replacement therapy.
Patient characteristics are described in Table 2. Significantly fewer patients in the s-AKI group and AKI group had available data for baseline creatinine values (43.2% and 47.8%, respectively) than the group without kidney injury (62.9%). We found that s-AKI was more common in older male patients and in patients admitted to the intensive care unit compared with the group without kidney injury. Patients with s-AKI and those with AKI were similar in age and percentage of men.
Table 2.
Characteristics of patients with no kidney injury, subacute kidney injury, and AKI
| Characteristic | No Kidney Injury | s-AKI | AKI | No Kidney Injury versus s-AKI | s-AKI versus AKI |
|---|---|---|---|---|---|
| Patients, n (%) | 43,493 (87.8) | 574 (1.1) | 5,451 (11.0) | ||
| Age (IQR) (yr) | 60 (43–70) | 68 (59–75) | 70 (60–78) | <0.001 | 0.11 |
| Men (%) | 53.5 | 59.9 | 62.9 | 0.002 | 0.15 |
| Baseline creatinine (IQR) (mg/dl) | 0.78 (0.65–0.91) | 0.76 (0.60–0.86) | 0.77 (0.66–1.02) | 0.07 | 0.02 |
| Patients with baseline data available, n (%) | 27,335 (62.8) | 248 (43.2) | 2,605 (47.7) | <0.001 | 0.04 |
| Medical units (%) | |||||
| Cardiology | 6.13 | 4.7 | 6.02 | 0.18 | 0.22 |
| Endocrinology | 3.73 | 4.01 | 2.84 | 0.73 | 0.11 |
| Emergency medicine | 5.23 | 5.68 | 11.89 | 0.71 | <0.001 |
| Gastroenterology | 9.87 | 9.76 | 8.93 | 1.00 | 0.49 |
| Nephrology | 2.38 | 5.05 | 8.11 | <0.001 | 0.01 |
| Neurology | 1.63 | 1.57 | 1.27 | 1.00 | 0.55 |
| Oncology | 3.85 | 6.1 | 7.41 | 0.01 | 0.27 |
| Psychiatry | 1.49 | 1.74 | 0.44 | 0.59 | <0.001 |
| Respiratory medicine | 2.86 | 6.79 | 3.69 | <0.001 | <0.001 |
| Other | 2.61 | 4.88 | 2.49 | 0.002 | 0.002 |
| Surgical units | |||||
| Cardiac surgery | 0.84 | 3.31 | 3.38 | <0.001 | 1.00 |
| General surgery | 13.12 | 12.37 | 10.33 | 0.66 | 0.13 |
| Neurosurgery | 4.12 | 0.7 | 2.26 | <0.001 | 0.01 |
| Obstetrics and gynecology | 15.61 | 6.27 | 5.39 | <0.001 | 0.38 |
| Orthopedics | 7.67 | 5.75 | 2.38 | 0.09 | <0.001 |
| Otorhinolaryngology | 3.4 | 9.06 | 2.94 | <0.001 | <0.001 |
| Thoracic surgery | 2.25 | 1.74 | 1.03 | 0.56 | 0.13 |
| Urology | 5.31 | 5.57 | 10.03 | 0.77 | <0.001 |
| Vascular surgery | 3.93 | 2.96 | 7.54 | 0.27 | <0.001 |
| Other | 3.52 | 2.44 | 1.63 | 0.20 | 0.17 |
| ICU admission (%) | 8.0 | 11.6 | 18.2 | <0.001 | <0.001 |
s-AKI, subacute kidney injury; IQR, interquartile range; ICU, intensive care unit.
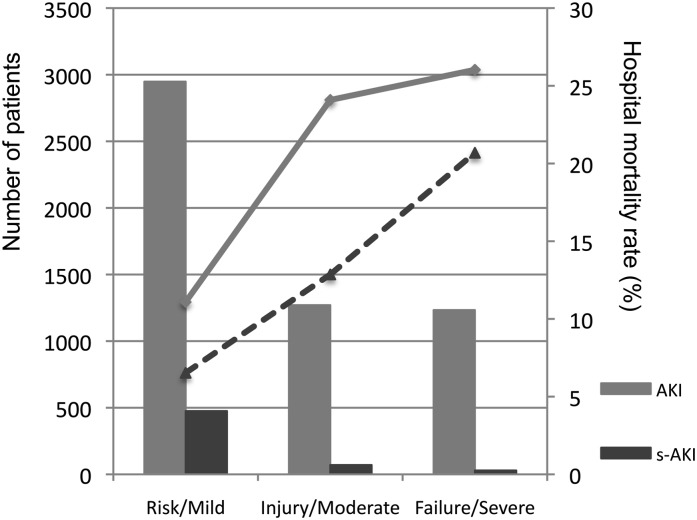
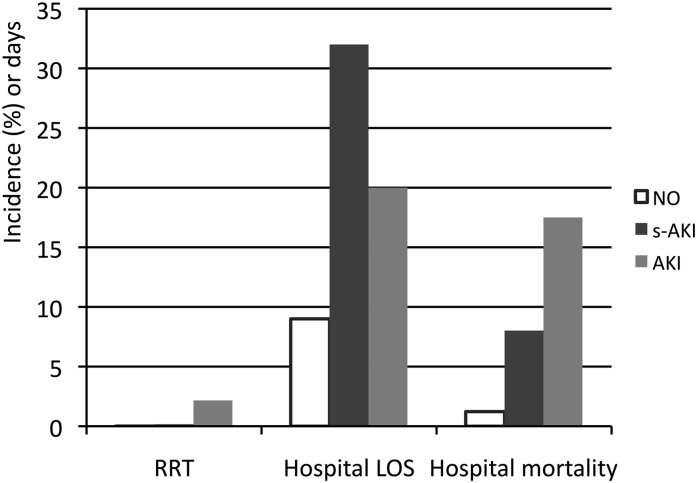
Per the RIFLE classification system, patients with AKI were classified into the Risk category in 2948 (54.0%) patients, Injury category in 1270 (24.2%), and Failure category in 1233 (22.6%); for those with s-AKI, 475 (82.7%) were classified as mild, 70 (12.1%) as moderate, and 29 (5.0%) as severe. The mortality proportionally increased as severity of impairment progressed (Figure 2). Analysis of hospital mortality (Figure 3) revealed that patients with s-AKI had significantly higher mortality than those without kidney injury (8.0% versus 1.2% for s-AKI and no kidney injury, respectively; P<0.001) but lower mortality than the patients with AKI (8.0% versus 17.5%; P<0.001). Compared with the AKI group, fewer patients with s-AKI needed RRT (0.17% for s-AKI versus 0.01% for no kidney injury [P<0.001] and versus 2.16% for AKI [P<0.002]), although their hospital length of stay was longer (32 days for s-AKI [interquartile range, 19–52 days] versus 9 days for no kidney injury [interquartile range, 5–17 years; P<0.001] and versus 20 days for AKI [interquartile range, 10–43; P<0.001]), as shown in Figure 3.
Figure 2.
Incidence and hospital mortality for each category of AKI and subacute kidney injury (s-AKI). Column graph shows numbers of patients in each group, and the line chart shows the mortality rate.
Figure 3.
Outcomes of each group.
In multivariable regression analysis, s-AKI was associated with a significant increase in hospital mortality, although not to the extent seen with AKI (Table 3). Sensitivity analyses for patients who had baseline creatinine levels at the outpatient clinic and for those who stayed in hospital for >7 days confirmed that s-AKI and AKI had strong independent associations with hospital mortality (Table 3).
Table 3.
Multivariable logistic regression analysis for hospital mortality
| Variable | All Patients (n=49,518) | Baseline Data Available (n=30,188) | Hospital Length of Stay>7 d (n=30,273) | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Renal function | ||||||
| No kidney injury | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Sub-AKI | 5.44 (3.89 to 7.44) | <0.001 | 5.58 (3.63 to 8.31) | <0.001 | 3.62 (2.60 to 5.03) | <0.001 |
| AKI | 14.8 (13.2 to 16.7) | <0.001 | 15.4 (13.35 to 17.87) | <0.001 | 10.56 (9.26 to 12.03) | <0.001 |
| Age (1 yr) | 1.02 (1.02 to 1.02) | <0.001 | 1.02 (1.02 to 1.03) | <0.001 | 1.02 (1.01 to 1.02) | <0.001 |
| Male | 1.14 (1.00 to 1.29) | 0.036 | 1.25 (1.07 to 1.46) | 0.004 | 1.22 (1.06 to 1.40) | 0.003 |
| Baseline creatinine | 1.16 (1.06 to 1.26) | 0.001 | 0.98 (0.87 to 1.09) | 0.80 | 1.08 (0.96 to 1.20) | <0.16 |
| ICU admission | 1.43 (1.19 to 1.71) | <0.001 | 0.76 (0.59 to 0.97) | 0.03 | 1.01 (0.83 to 1.22) | 0.91 |
| Admission units | ||||||
| Cardiology | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Emergency medicine | 2.93 (2.08 to 4.19) | <0.001 | 3.79 (2.50 to 5.92) | <0.001 | 2.46 (1.65 to 3.67) | <0.001 |
| Gastroenterology | 4.09 (2.94 to 5.83) | <0.001 | 3.56 (2.37 to 5.52) | <0.001 | 3.62 (2.44 to 5.36) | <0.001 |
| Endocrinology | 0.86 (0.47 to 1.50) | 0.60 | 1.00 (0.50 to 1.93) | 0.98 | 0.75 (0.40 to 1.42) | 0.39 |
| Nephrology | 0.36 (0.19 to 0.63) | <0.001 | 0.25 (0.10 to 0.53) | <0.001 | 0.34 (0.18 to 0.65) | <0.001 |
| Neurology | 2.80 (1.63 to 4.72) | <0.001 | 2.4 (1.16 to 4.75) | 0.02 | 1.25 (0.65 to 2.42) | 0.49 |
| Oncology | 8.90 (6.36 to 12.7) | <0.001 | 5.89 (3.89 to 9.19) | <0.001 | 6.83 (4.58 to 10.17) | <0.001 |
| Psychiatry | 0.50 (0.08 to 1.68) | 0.30 | 0.30 (0.01 to 1.49) | 0.16 | 0.27 (0.06 to 1.17) | 0.08 |
| Respiratory medicine | 10.5 (7.43 to 15.2) | <0.001 | 6.42 (4.18 to 10.12) | <0.001 | 7.61 (5.07 to 11.42) | <0001 |
| Other | 2.05 (1.21 to 3.38) | 0.007 | 1.64 (0.85 to 3.08) | 0.13 | 1.69 (0.97 to 2.92) | 0.06 |
| Cardiac surgery | 0.57 (0.28 to 1.07) | 0.08 | 0.71 (0.28 to 1.61) | 0.42 | 0.43 (0.20 to 0.94) | 0.03 |
| General surgery | 3.41 (2.45 to 4.84) | <0.001 | 3.14 (2.10 to 4.84) | <0.001 | 3.26 (2.20 to 4.81) | <0.001 |
| Neurosurgery | 0.97 (0.54 to 1.69) | 0.97 | 1.41 (0.65 to 2.87) | 0.36 | 0.65 (0.33 to 1.28) | 0.65 |
| Obstetrics and gynecology | 2.88 (1.95 to 4.29) | <0.001 | 3.28 (2.06 to 5.34) | <0.001 | 3.66 (2.34 to 5.73) | <0.001 |
| Orthopedics | 0.32 (0.14 to 0.64) | <0.001 | 0.24 (0.08 to 0.58) | <0.001 | 0.26 (0.12 to 0.57) | <0.001 |
| Otorhinolaryngology | 2.86 (1.88 to 4.38) | <0.001 | 2.87 (1.72 to 4.84) | <0.001 | 1.81 (1.11 to 2.94) | 0.02 |
| Thoracic surgery | 4.11 (2.55 to 6.58) | <0.001 | 4.92 (2.83 to 8.57) | <0.001 | 3.64 (2.13 to 6.23) | <0.001 |
| Urology | 1.77 (1.22 to 2.60) | 0.002 | 1.66 (1.06 to 2.67) | 0.02 | 1.69 (1.10 to 2.60) | 0.02 |
| Vascular surgery | 0.66 (0.42 to 1.05) | 0.08 | 0.86 (0.48 to 1.53) | 0.62 | 0.70 (0.41 to 1.19) | 0.19 |
| Other surgery | 1.49 (0.83 to 2.56) | 0.17 | 1.89 (0.94 to 3.61) | 0.06 | 1.27 (0.69 to 2.35) | 0.43 |
| Hospital length of stay (1 d) | 1.00 (1.00 to 1.00) | 0.99 | 1.01 (1.00 to 1.02) | <0.001 | 1.00 (1.003 to 1.005) | <0.001 |
The R2 values of the whole model are 0.26, 0.28, and 0.24, respectively, and the goodness-of-fit P value for all is 1.00. OR, odds ratio; 95% CI, 95% confidence interval.
Discussion
Key Findings
We conducted a retrospective database analysis of close to 50,000 hospital admissions to evaluate the epidemiology of s-AKI.
We defined s-AKI by the same criterion of serum creatinine increase as in the RIFLE classification (2) for AKI but over a longer time frame, and we assessed its characteristics and independent association with hospital mortality. We found that s-AKI occurred in 1.1% of all admissions while AKI occurred in 11.0%. Age and sex did not significantly differ between the AKI group and the s-AKI group, although baseline creatinine values in the s-AKI group were slightly lower than those in the AKI group.
We also found that most patients with s-AKI were classified as having mild injury, and worsening category of s-AKI was linearly correlated with hospital mortality, as was previously described for AKI with RIFLE classification (5,10). Although mortality and need for RRT were markedly lower than in patients with AKI, multivariable regression analysis confirmed that s-AKI itself could be an independent risk factor for hospital mortality.
Relationship with Previous Studies
To our knowledge, we are the first investigators to describe the epidemiology, outcomes, and associations of s-AKI. However, some of our findings are clinically plausible. For example, half of the admission wards for patients with s-AKI (nephrology, respiratory medicine, and oncology) are wards where low-grade or subacute sepsis frequently occurs and nephrotoxic drugs, such as antineoplastic agents, are used (11). These states may be more likely to cause slowly progressive renal dysfunction (12). In comparison with patients with AKI, those with s-AKI who were admitted to orthopedics and otorhinolaryngology services were dominant compared with those admitted to neurosurgery, urology, and vascular surgery services, where use of radiocontrast agents for intravascular radiologic intervention, urethral obstructive disorder, or acute hemodynamic change during the perioperative period are more likely to be involved in the development of AKI (11). Patients undergoing cardiac surgery were included in both the s-AKI and AKI groups. Cardiac surgery is well known to be associated with AKI, especially because of perioperative hemodynamic instability, existing comorbid conditions (e.g., advanced age and atherosclerosis), and administration of drugs that affect kidney autoregulation (e.g., nonsteroidal anti-inflammatory drugs, renin-angiotensin system inhibitors, and radiocontrast agents) (13). In our study, these patients were at risk of s-AKI as well as AKI.
Implications for Clinicians
We have shown that most patients with s-AKI had mild kidney dysfunction; however, s-AKI itself was significantly independently associated with hospital mortality. Recognition of such worsening kidney function may lead to a search for its causes or possible intervention, which may, in turn, improve outcome.
Strengths and Limitations
Our study had several strengths. To our knowledge, this study is the first to explore the concept of s-AKI. We found that such patients represent a subpopulation of hospital patients with clear differences from those with AKI; their characteristics, clinical course, and outcome also differ from patients without AKI. These patients represent 1% of admissions and consume a substantial amount of resources, as shown by the duration of hospital stay.
Our study involved a large cohort of all patients admitted to a tertiary hospital. Our inclusion process minimized selection bias and aimed to present a complete picture of kidney dysfunction that occurred in hospitalized patients. Sensitivity analysis confirmed our findings.
However, this study was conducted in a single center, which may affect its external validity. Because it was a retrospective study, data on causes of kidney injury, interventions, and urine output were not available. This limited further assessment of the characteristics and cause of s-AKI. The study is also limited by lack of information for the causes of admissions, comorbid conditions, and organ failure scores from our hospital databases: the model of fit in the regression analyses for hospital mortality was poor. We used recorded creatinine values whenever possible and used the MDRD equation to estimate baseline function when recorded data were not available. Because fewer patients with s-AKI and AKI had data on baseline creatinine at the outpatient clinic than in the patients without kidney injury, we performed a sensitivity analysis of hospital mortality in patients with recorded baseline creatinine values to eliminate the effect of MDRD equation to the outcomes, which confirmed our overall findings. Patients who developed both s-AKI and AKI during one admission were classified as having AKI. Thus, we could not evaluate the additional effect of AKI on s-AKI. Because patients with acute-on-chronic renal failure may have worse renal recovery than those with AKI alone (14), the prognosis of patients with s-AKI who subsequently developed AKI should be investigated separately.
In conclusion, this study showed that s-AKI occurred in 1% of hospitalized patients and that s-AKI was independently associated with increased hospital mortality. Although mortality increased with increased severity, patients with s-AKI were different from patients with AKI, had a better outcome, were less likely to require RRT, had milder disease, and had a longer hospital stay than those with AKI. Further studies to elucidate the cause and triggers of s-AKI appear desirable.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Singbartl K, Kellum JA: AKI in the ICU: Definition, epidemiology, risk stratification, and outcomes. Kidney Int 81: 819–825, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup : Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury Network : Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group : KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 5.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C: An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med 34: 1913–1917, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Shema L, Ore L, Geron R, Kristal B: Hospital-acquired acute kidney injury in Israel. Isr Med Assoc J 11: 269–274, 2009 [PubMed] [Google Scholar]
- 7.Fang Y, Ding X, Zhong Y, Zou J, Teng J, Tang Y, Lin J, Lin P: Acute kidney injury in a Chinese hospitalized population. Blood Purif 30: 120–126, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 3: 1–150, 2013 [Google Scholar]
- 9.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR : Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Ricci Z, Cruz D, Ronco C: The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int 73: 538–546, 2008 [DOI] [PubMed] [Google Scholar]
- 11.de Jonge MJ, Verweij J: Renal toxicities of chemotherapy. Semin Oncol 33: 68–73, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Kagoya Y, Kataoka K, Nannya Y, Kurokawa M: Pretransplant predictors and posttransplant sequels of acute kidney injury after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 17: 394–400, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, Dupuis JY, Fremes SE, Kent B, Laflamme C, Lamy A, Legare JF, Mazer CD, McCluskey SA, Rubens FD, Sawchuk C, Beattie WS: Acute kidney injury after cardiac surgery: Focus on modifiable risk factors. Circulation 119: 495–502, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, Macleod A: Incidence and outcomes in acute kidney injury: A comprehensive population-based study. J Am Soc Nephrol 18: 1292–1298, 2007 [DOI] [PubMed] [Google Scholar]