Summary
Membranous nephropathy (MN) is an autoimmune disease usually associated with a nephrotic syndrome and it may progress to ESRD in the long term. Its etiology is often unknown (idiopathic MN), whereas other cases have a recognizable etiology (secondary MN). In idiopathic MN, the glomerular lesions are mainly caused by autoantibodies against a podocyte membrane protein, the M-type of phospholipase A2 receptor 1. The natural course of idiopathic MN is quite varied with spontaneous complete or partial remissions a relatively common occurrence. Patients with asymptomatic non-nephrotic proteinuria seldom progress and need only conservative management. Those with persistent full-blown nephrotic syndrome and those with declining renal function are candidates for specific treatment with any of several regimens. Cyclical therapy with alternating monthly intravenous and oral glucocorticoids combined with a cytotoxic agent can induce remission and preserve renal function in the long term. Cyclosporine or tacrolimus can induce remission, but relapses are frequent after the drug withdrawal. Mycophenolate mofetil monotherapy seems to be ineffective, but may be beneficial when administered together with steroids. The experience with adrenocorticotropic hormone, natural or synthetic, is limited to a few studies with short-term follow-up, but high rates of remission can be seen after prolonged treatment. A high rate of remission and good tolerance have also been reported with rituximab. Patients with moderate renal insufficiency may also benefit from treatment, but at a price of frequent and serious side effects. With these limitations in mind, idiopathic MN may be considered a treatable disease in many patients.
Introduction
Membranous nephropathy (MN) is a pathologic term that defines a specific glomerular lesion characterized by an apparent thickening of glomerular capillary walls by optical microscopy. This thickening is actually mostly due to the subepithelial deposition or in situ formation of immune complexes, as shown by electron and immunofluorescence microscopy. Clinically, MN is associated with proteinuria, often (in approximately 80% of cases) in a nephrotic range (>3.5 g/d). In approximately 75% of adults, the etiology of the lesion is unknown (idiopathic MN), whereas other cases have a well defined etiology (secondary MN). Idiopathic MN has a varied natural course, with some patients (approximately 20%–30%, depending on the severity of proteinuria) entering spontaneous remission, some patients showing a persistent proteinuria fluctuating between a nephrotic and a subnephrotic range, and other patients showing a slow progression to ESRD.
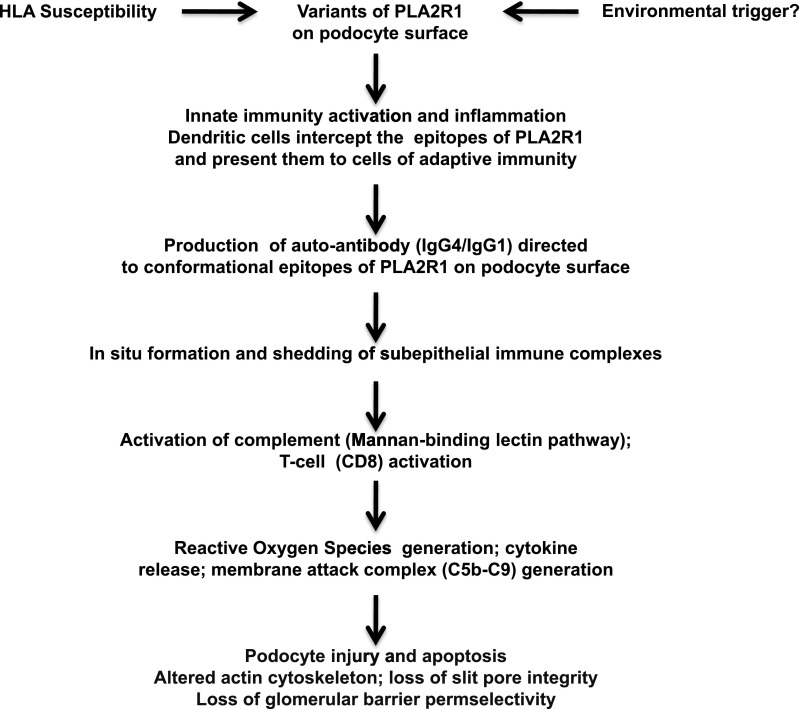
Although the pathogenesis of idiopathic MN remains incompletely defined, an important advance has been obtained by the seminal study of Beck et al., who showed that approximately 70% of patients with idiopathic MN have circulating autoantibodies directed against phospholipase A2 receptor 1 (PLA2R1) located on the surface of normal human podocytes (1). A strong association with the HLA-DQA1 allele has been found in patients with idiopathic MN. This allele may facilitate an autoimmune response against antigenic targets such as variants of PLA2R1 (2). However, these variants were seen only in 4 of 60 patients with PLA2R1-associated MN, suggesting that these rare variants are unlikely to explain the pathogenesis of the disease (3). According to Salant (4), the development of idiopathic MN may be the result of three conditions: (1) the presence of HLA-DQA1 alleles that confer susceptibility to autoimmunity, (2) polymorphism of PLA2R1 that creates a unique conformation that can represent a target for autoantibodies, and (3) production of hypogalactosylated IgG4 anti-PLA2R1 that activates the mannan pathway of the complement. It is also possible that innate immunity is involved. Moreover, endogenous or external (virus?) stimuli may increase the expression of PLA2R1 or shed some “cryptic” epitopes of PLA2R1. In summary, autoantibodies to PLA2R1, usually of the IgG4 subclass, would bind to conformational epitopes on specific domains of PLA2R1 expressed on the podocyte surface, form immune complexes in situ, and induce proteinuria, likely via local activation of the mannan-binding pathway of complement activation (Figure 1). Antibodies against other intracytoplasmic podocyte-related antigens have also been demonstrated in patients with idiopathic MN, although their pathogenetic role is still undefined (5).
Figure 1.
A personal hypothetical mechanistic interpretation of idiopathic MN. In genetically predisposed patients, a polymorphism of PLA2R1 may create a unique conformational variant. Alternatively endogenous or exogenous injuries (viral attack?) may cause structural alterations of PLA2R1 or lead to podocyte injury with exposure of some “hidden” epitopes of PLA2R1. It is possible that these abnormal molecular patterns are recognized as danger signals by the receptors of innate immunity with consequent recruitment of inflammatory cells. In this inflammatory microenvironment, the dendritic cells would become mature, intercept the epitopes of PLA2R1 expressed on the glomerular podocyte surface, and migrate to lymph nodes where they present the antigen to the cells of the adaptive immunity. The B cells would differentiate into plasma cells that secrete specific hypogalactosylated IgG4. These antibodies attack the epitopes of PLA2R1 on podocyte surface, with formation of subepithelial immune complexes and consequent production of ROS, and inflammation. The hypogalactosylated IgG4 activates the complement system through the lectin pathway which initiates the complement cascade, with the final production of the cytolytic C5b-C9 complex, also called the membrane attack complex. As a consequence, there is an increased damage of podocytes, alteration of the actin cytoskeleton, loss of the glomerular barrier permselectivity, and proteinuria. MN, membranous nephropathy; PLA2R1, phospholipase A2 receptor 1; ROS, reactive oxygen species.
The clinical approach to patients with MN entails responding to a series of questions, which are as follows.
Is the MN Lesion Idiopathic or Secondary?
After a lesion of MN is discovered, it is necessary to know whether it is idiopathic or secondary. The most common causes of secondary MN are hepatitis B virus (HBV), drugs (more often nonsteroidal anti-inflammatory agents), SLE, and cancer. However, a large number of other infections and diseases may also be associated with MN (6). An accurate clinical history and a serologic inquiry using specific biomarkers (hepatitis B surface antigen, hepatitis C antibody, anti-DNA antibody, hypocomplementemia) can often uncover an underlying cause for MN; however, in the modern era, the detection of circulating antibodies against PLA2R1, using any one of a number of available assays, can also greatly aid in determining whether MN is idiopathic or secondary (7). These antibodies can be seen in 70%–80% of patients with idiopathic MN, whereas they are seldom detectable in secondary MN (7,8). Glomerular morphologic changes are also important. Extensive mesangial or subendothelial electron dense deposits are not seen in idiopathic MN. With the exception of few cases of MN secondary to IgG4-related disease (9), dominance of deposits of IgG4 on immunofluorescence microscopy generally favors idiopathic MN, whereas deposits of IgG1, IgG2, or IgG3 are more frequent in secondary MN (10). Coexpression of PLA2R1 antigen in glomeruli is also commonly seen in idiopathic but not secondary MN (3,8). Strong capillary wall deposits of C1q, C3, IgG, IgM, and IgA can also be seen in cases of MN associated with lupus (11), whereas leukocyte infiltration may occur in cases of cancer (12). Extensive C1q deposition is rarely seen in idiopathic MN (11).
The association of MN with cancer is frequent, although not always clear cut. A French review showed that 24 of 240 (10%) patients with MN had a malignancy at the time of renal biopsy or within a year thereafter. The frequency of malignancy in MN increased with age up to 20%–25% after age 60 years (12). The solid organ cancers are particularly frequent, mainly in the lung, colon, breast, prostate, or uterus. An evaluation for underlying cancer is recommended for patients with MN, particularly for older individuals (Table 1).
Table 1.
Investigations suggested to detect/exclude an underlying cancer in a patient with apparently idiopathic (primary) MN and repeatedly negative serologic tests for anti-PLA2R1 autoantibody and/or absence of PLA2R1 or IgG4 in glomerular deposits
| Cancer Type | Young Adult | Older Patient |
| Lung | Chest x-ray | Computed tomography |
| Kidney | Ultrasonography, malignant cells in the urine | Ultrasonography, malignant cells in the urine |
| Breast | Physical examination | Mammography |
| Stomach | Fecal occult blood? | Gastroscopy |
| Colon | Fecal occult blood? | Colonoscopy |
| Prostate | Rectal digital examination, percentage PSA | Ultrasonography, prostate biopsy |
| Uterus | Gynecologic examination | Colposcopy |
In young patients, fecal occult blood is usually searched for only in the case of anemia. MN, membranous nephropathy; PLA2R1, phospholipase A2 receptor 1; PSA, prostate specific antigen.
Treatment of secondary MN depends on the etiology. Children with MN secondary to HBV infection do not require specific treatment because they often undergo spontaneous remission of renal disease within 1 year from clinical onset (13). Alternatively, such spontaneous remissions are exceptional in adults (14). Complete remission of proteinuria has been obtained in some cases of HBV-associated MN with nucleotide or nucleoside analogs either given alone or in association with immunosuppressive therapy (15). Most cases of MN secondary to drugs resolve after discontinuation of the offending drug (16). Glucocorticoids and immunosuppressive agents are effective in reducing proteinuria in lupus MN. Eradication of cancer may be followed by complete remission of proteinuria in some patients (17).
When Should a Specific Treatment for Idiopathic MN Be Started?
In view of the potential toxicity of the available treatments, the propensity for spontaneous remissions, and the slow progression of the disorder, some clinicians believe that MN should not routinely be treated with glucocorticoids and immunosuppressive drugs (18). The value of adding angiotensin II blockade to a conservative regimen, with the intent of fostering a “spontaneous” remission, is not firmly proven, and little benefit of such treatment is observed in patients presenting with proteinuria >10 g/d (19), but it may augment spontaneous remissions when lower levels of proteinuria are present (20). Conservative management is justified for patients with persistent subnephrotic proteinuria. However, these patients should be monitored because approximately 60% of them may progress to nephrotic syndrome (20). Patients with proteinuria persistently (>6 months) >4 g/d have an approximately 55% probability of developing ESRD within 10 years, and those with persisting proteinuria >8 g/d and/or abnormal levels of serum creatinine have an approximately 66%–80% probability of developing ESRD within 10 years (21). The decision of whether and when to start a “specific” therapeutic regimen in a patient with idiopathic MN needs to take into account the likelihood of a spontaneous remission as well as the potential adverse consequences of a prolonged exposure to the nephrotic state, including disabling edema, proatherogenic hyperlipidemia and coronary heart disease, the prothrombotic milieu, malnutrition, and enhanced risk of infection. Thus, a wait-and-see attitude might be justified in patients with proteinuria <4 g/d, in those with a steadily diminishing magnitude of proteinuria over time, and in asymptomatic patients with normal renal function. On the other hand, initiation of specific treatment is indicated for patients with declining renal function, disabling symptoms, or full-blown nephrotic syndrome persisting for ≥6 months.
Aggressive immunosuppressive treatment of patients with advanced interstitial fibrosis and tubular atrophy at biopsy and serum creatinine levels >3.5–4.0 mg/dl have a very low prospect of halting or reversing the progression of MN, whereas the risk of severe side effects becomes disproportionately elevated.
What Is the Role for Serologic Markers in Treatment Decisions in Idiopathic MN?
The demonstration of circulating anti-PLA2R1 autoantibodies in idiopathic MN not only helped to elucidate pathogenesis but may also have direct clinical relevance to therapeutic decision making. The sensitivity of anti-PLA2R1 autoantibodies for idiopathic MN is approximately 70%–80% and the specificity is close to 90%–95% (22). Thus, the detection of anti-PLA2R1 autoantibodies can be useful to recognize the presence of MN in patients with nephrotic syndrome and help to diagnosis idiopathic MN in those with a renal biopsy–identified lesion of MN. Furthermore, the levels of circulating anti-PLA2R1 autoantibodies strongly correlate with clinical disease activity (23), and their disappearance often heralds a subsequent decline in proteinuria (24). However, approved tests for PLA2R1 are not commercially available at present in the United States.
What Treatment Regimens Are Effective and Safe for Patients with Idiopathic MN?
Induction of remission, restoration, or preservation of renal function and avoidance of serious side effects are the principal aims of treatment in idiopathic MN. Remission of proteinuria not only may improve the quality of life but is also a good predictor of the long-term outcome. A retrospective analysis of the Toronto Glomerulonephritis Registry showed that patients who entered a complete remission (proteinuria ≤0.3 g/d with normal serum creatinine) only exceptionally developed ESRD in the long term. Partial remission (proteinuria <3.5 g/d and a ≥50% reduction from peak values with improvement of serum albumin) was also associated with a favorable outcome, showing an actuarial renal survival of approximately 70% at 15 years (25).
Specific therapeutic attempts in idiopathic MN have in the past been largely based on glucocorticoids or cytotoxic agents. However, several randomized trials were unable to demonstrate any efficacy of glucocorticoids alone in idiopathic MN, despite early encouraging reports (26–28), whereas cytotoxic agents were often used in extended duration protocols thus exposing patients to an increased risk of disquieting side effects. In 1984, an Italian multicenter open label, randomized controlled trial showed that a treatment based on alternating every month steroids and an alkylating agent obtained significantly more remissions and a better slope of the reciprocal of serum creatinine compared with patients treated with symptomatic therapy alone (29). These findings were later confirmed by other randomized trials (30–32) and by long-term extension of follow-up (33,34).
The 2012 Kidney Disease Improving Global Outcomes guidelines (35) recommended that, in patients qualifying for specific treatment, the initial preferred regimen consists of a 6-month course of alternating monthly cycles of oral and intravenous corticosteroids and an oral alkylating agent (cyclophosphamide is better tolerated than chlorambucil). Such a treatment requires monitoring of leukocyte counts every 7–10 days during alkylating agent administration to help modulate the dosage to avoid serious leucopenia. This regimen may be contraindicated in a number of patients affected by comorbidities such as diabetes or prior malignancy. Concerns over the possible cosmetic (alopecia), gonadotoxic, and oncogenic effects and enhanced infection risk associated with use of alkylating agents can lead to patient refusal of this therapy, despite the documented relatively low risk of adverse events, at least in patients with well preserved renal function.
Further options are available (Table 2). Calcineurin inhibitors (cyclosporine or tacrolimus) are frequently prescribed in idiopathic MN, often as a first or second choice (36). A systematic review showed that cyclosporine could obtain a reduction of proteinuria in 70%–80% of patients with idiopathic MN. The maximum benefit may occur after 3 months or even later. However, most responders (>80%) have a relapse of nephrotic proteinuria after the drug is withdrawn. Therefore, long-term treatment is required. A randomized controlled trial showed that 1-year treatment with low-dose tacrolimus (0.05 mg/kg per day) could obtain remission, usually a partial remission, in 72% of patients. However, almost 50% of responders had a relapse of nephrotic-range proteinuria within 6 months after withdrawal of tacrolimus (37). The major concern with calcineurin inhibitors remains their propensity to induce nephrotoxicity over the long term. However, with careful monitoring of serum creatinine and BP, using the lowest possible dosage for maintenance, and performing repeat renal biopsy in those receiving long-term therapy, this risk can be minimized in patients with initially normal renal function. Whether cyclosporine or tacrolimus is the preferred agent cannot be determined from the available evidence, but tacrolimus can be effective as monotherapy, without concomitant steroids (37).
Table 2.
Possible treatment choices in patients with idiopathic (primary) membranous nephropathy
| Treatment | Results | Notes |
| Steroids alone | No benefit | Although ineffective, frequently used by practitioner |
| Steroids-alkylating agents | Can significantly increase the probability of complete or partial remission. Protect renal function in the long term | The results are confirmed by randomized controlled trials. Risk of side effects (infection, leucopenia). Avoid frequent repetitions (risk of oncogenic or gonadotoxic effects) |
| CNI | Can significantly reduce the amount of proteinuria and increase the probability of complete or partial remission. Little information about their effects on renal function | Relapse of proteinuria is frequent after CNI withdrawal. Risk of hypertension, nephrotoxicity. Little information about long-term safety |
| Mycophenolate salts | Ineffective when given alone. Can reduce proteinuria when given together with steroids | Only small-sized studies with short-term follow-up are available. High relapse rate. No information about the long-term safety and efficacy |
| ACTH | Can reduce proteinuria | Only few small-sized studies with short-term follow-up are available. A randomized controlled trial is in progress |
| Rituximab | Can reduce proteinuria | Large observational studies available. No head-to- head comparison with other treatments |
CNI, calcineurin inhibitors; ACTH, adrenocorticotropic hormone.
Mycophenolate mofetil (MMF) has also been used. Negative results have been reported by a French trial (38), in which 36 patients were randomized to receive MMF as monotherapy (2 g/d for 12 months) or symptomatic therapy. No difference in remission was seen between the two groups. Better results were reported by a retrospective study in which MMF was combined with oral prednisone or methylprednisolone pulses (39). Two small randomized trials, both in Asian patients, reported remissions in approximately 70% of cases treated with MMF and steroids, a rate similar to that observed in patients assigned to a steroid and a cytotoxic drug regimen (40,41). The available data are insufficient to draw any firm conclusions about the use of MMF for induction of remission and the relapse rate is quite high (39).
Synthetic adrenocorticotropic hormone (ACTH; Synacthen), administered by intramuscular or subcutaneous injection at a dosage of 1 mg twice a week for 1 year, can significantly reduce proteinuria in patients with idiopathic MN (42), as confirmed by a randomized clinical trial (43). Similar results have been obtained with natural ACTH (Acthar gel) in a small observational study (44). The mechanism of action of ACTH may be related to the stimulation of melanocortin receptors with inhibition of immune and inflammatory response or a direct action on podocyte stability (45). ACTH can be a reasonable option for patients who have contraindications to or who do not respond to cyclical alkylating agent-steroid therapy or calcineurin inhibitors. It should be noted, however, that so far no randomized trial has been done with the exceedingly expensive ACTH gel.
Rituximab, a mAb to CD20 expressed on B cells, was first used in idiopathic MN by Remuzzi et al. (46). Recently, these investigators reported their observational experience in 100 patients with MN treated with rituximab (47). After a mean follow-up of 29 months, 27 patients were in complete remission and 38 were in partial remission (total response rate of 65%). The median time to response was 7 months. The results were the same if rituximab was used in treatment-naïve patients compared with patients previously treated with other regimens. These are impressive results and no serious side effects were encountered. However, four patients died, three developed cancer, and four progressed to ESRD (47). Good results with rituximab have also been reported in another observational study conducted in North America. Of 20 patients treated with 4 weekly courses of rituximab repeated after 6 months, 2 did not respond, 4 entered complete remission, 12 entered partial remission, 1 had limited response, and 1 relapsed (total response rate of 80%) (48). There is little doubt that rituximab is effective in reducing proteinuria in idiopathic MN, but the mechanisms responsible for this effect remain uncertain. In addition, choices regarding optimal doses (375 mg/m2 ×4 or 1 g ×2), the spacing and duration of re-treatment, the value of monitoring of circulating B cell (B19+) number, and the long-term benefit to harm ratio of rituximab remain incompletely explored. It is also unclear whether patients with renal dysfunction and/or tubulointerstitial lesions will be resistant to such treatment. When using rituximab, a prophylaxis against Pneumocystis jiroveci[i] pneumonia is recommended. One should also consider the very high cost of rituximab and the lack of randomized trials supporting its efficacy.
What Specific Treatments Are Available for Patients with Idiopathic MN and Declining Renal Function?
In 1988, Mathieson et al. administered a 6-month course of alternating monthly cycles of prednisolone and chlorambucil to eight patients with idiopathic MN whose renal function was deteriorating. Proteinuria significantly reduced in all patients and creatinine clearance increased in six patients. Adverse effects of chlorambucil were severe, and the daily dose had to be reduced (49). Since then, other reports suggested the possibility of halting or slowing the progression of MN by glucocorticoids, cyclophosphamide, or cyclosporine. However, most of the available papers were small, uncontrolled, and had short-term follow-up, and only few studies reported the results of renal biopsy.
The results of a randomized controlled trial that compared the effects of three different therapeutic approaches in adult patients with biopsy proven idiopathic MN and declining renal function were recently published (50). Criteria for inclusion included an initial serum creatinine level <300 μmol/L (3.4 mg/dl) and at least a 20% decline in creatinine clearance measured in the 2 years before study entry. The 108 enrolled patients were randomly assigned to receive supportive treatment only, supportive treatment plus 6 months of alternating cycles of prednisolone and chlorambucil, or supportive treatment plus 12 months of cyclosporine (without steroids). At 3 years, the risk of further 20% decline in creatinine clearance was significantly lower in the prednisolone and chlorambucil group than in the supportive care group and this risk did not differ between the cyclosporine and supportive treatment-only groups. Serious adverse events were frequent in all three groups but were higher in the prednisolone and chlorambucil group than in the supportive care-only group (50). It is well known that cyclosporine may be contraindicated in nephrotic patients with serum creatinine >2.00 mg/dl (estimated creatinine clearance around 50 ml/min per 1.73 m2), due to the possibility of nephrotoxicity. Moreover, low-dose steroids may improve the efficacy of cyclosporine (36). However, the benefits of adjunctive steroid administration with cyclosporine in idiopathic MN have not been formally tested in a randomized clinical trial; therefore, any claim that such combined treatment is needed to maximize efficacy and/or safety is entirely anecdotal and not evidence based. Cyclosporine blood concentration should be checked periodically to avoid nephrotoxic levels and minimize adverse events. In chlorambucil-treated patients leukocyte counts should be checked every 7–10 days and the doses halved whenever they are <5000/cmm (29–31). Although patients treated with steroids and chlorambucil had better preservation of renal function, half had a further reduction of creatinine clearance of at least 20%, suggesting that waiting until renal function declines before starting treatment may slow but not halt the progression in patients with MN. If preservation of renal function is a goal, then the alkylating agent-steroid regimen (at reduced dosage intensity) may be preferred in those with impaired renal function.
When Should “Prophylactic” Anticoagulants Be Given to Patients with MN and Persisting Nephrotic Syndrome?
To date, no randomized controlled trials have been designed or executed to address this specific question (51). It is undoubtedly true that patients with MN are at risk for deep venous thrombosis (legs and renal veins) and venous thromboembolic events. The magnitude of this risk appears to be conditioned by the level of serum albumin (as a surrogate for the prothrombotic state). Levels <2.8 g/dl are associated with increased risk of venous thrombosis (52). The coexistence of comorbid factors enhancing thrombotic risk (such as prolonged immobilization, obesity, genetic prothrombotic states, recent abdominal or orthopedic surgery, or a prior venous thrombosis) further increases this risk (52). However, prophylactic anticoagulation (with low-dose heparinoids or oral warfarin) carries its own risks of bleeding and the decision to initiate prophylactic anticoagulation must rest on a careful analysis of the individual patient characteristics and of balancing the estimated benefits (avoidance of vascular thrombosis) versus the known risks (bleeding). Markov decision analysis trees are available to help with this decision making (53,54). If a vascular thrombosis has already occurred, therapeutic anticoagulation is required for the duration of the nephrotic state.
When Should Repeat Biopsies Be Performed in the Course of Treatment of Idiopathic MN?
The main indications to repeat renal biopsy are represented by sudden deterioration of renal function, resistance to treatment, and relapse of the nephrotic syndrome.
Uncommonly superimposed lesions, such as crescentic glomerulonephritis (due to anti-glomerular basement membrane or antineutrophil cytoplasmic autoantibody), late emergence of typical lupus nephritis, or a hypersensitivity interstitial nephritis (due to diuretics, nonsteroidal anti-inflammatory drugs, or allopurinol) require a new approach to treatment. Aside from such superimposed lesions, the extent of glomerular sclerosis may be an indicator of treatment unresponsiveness, but the magnitude of glomerular deposits and underlying basement membrane changes (stages of lesions) may not be very helpful even in patients with persisting nephrotic-range proteinuria. However, extensive thickening of basement membrane accompanied by electron lucent deposits rather than electron dense deposits with scanty IgG, C3, or C4 deposition implies advanced, immunologically inactive disease that will not likely respond to a persistence of treatment or a change in regimen. Such circumstances are often accompanied by negative anti-PLA2R1 autoantibody tests. The best pathologic prognostic marker is represented by severe and diffuse interstitial fibrosis and tubular atrophy. Such changes often signify a poor outcome and only rarely respond (and only partially) to therapy.
Idiopathic MN is an autoimmune disorder that may follow a variety of courses and have different final outcomes. Because spontaneous remissions may occur, waiting several months before starting a specific treatment regimen is reasonable in patients with stable renal function and oligosymptomatic disease, particularly if proteinuria is gradually diminishing. However, specific treatment should be started earlier in patients with massive proteinuria and full-blown symptomatic nephrotic syndrome or in patients with declining renal function. Although not all of the available treatments have been tested by rigorous controlled studies of adequate size and follow-up, some of them have been incorporated into current clinical practice algorithms. Importantly, resistance to one class of regimens does not reliably predict resistance to the effects of another class of regimens. Serology for anti-PLA2R1 antibody and repeat renal biopsy (with staining for PLA2R1 antigen deposition in glomeruli) may be useful tools to guide treatment and estimate its likely efficacy or futility. The possibility of choosing among different therapeutic options can allow the physician to tailor the treatment to the clinical characteristics of the patient.
Disclosures
C.P. was a consultant to Novartis, Italy, until December 2011. R.J.G. is an active consultant to Novartis, Genzyme-Sanofi, Bristol-Myers Squibb, Quest-Cor, Bio-Marin, Ely Lilly, ChemoCentrix, Vifor, Astellas, and Mitsubishi-Tanabe.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Beck LH, Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanescu HC, Arcos-Burgos M, Medlar A, Bockenhauer D, Kottgen A, Dragomirescu L, Voinescu C, Patel N, Pearce K, Hubank M, Stephens HA, Laundy V, Padmanabhan S, Zawadzka A, Hofstra JM, Coenen MJ, den Heijer M, Kiemeney LA, Bacq-Daian D, Stengel B, Powis SH, Brenchley P, Feehally J, Rees AJ, Debiec H, Wetzels JF, Ronco P, Mathieson PW, Kleta R: Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med 364: 616–626, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Coenen MJ, Hofstra JM, Debiec H, Stanescu HC, Medlar AJ, Stengel B, Boland-Augé A, Groothuismink JM, Bockenhauer D, Powis SH, Mathieson PW, Brenchley PE, Kleta R, Wetzels JF, Ronco P: Phospholipase A2 receptor (PLA2R1) sequence variants in idiopathic membranous nephropathy. J Am Soc Nephrol 24: 677–683, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salant DJ: Genetic variants in membranous nephropathy: Perhaps a perfect storm rather than a straightforward conformeropathy? J Am Soc Nephrol 24: 525–528, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Murtas C, Bruschi M, Candiano G, Moroni G, Magistroni R, Magnano A, Bruno F, Radice A, Furci L, Argentiero L, Carnevali ML, Messa P, Scolari F, Sinico RA, Gesualdo L, Fervenza FC, Allegri L, Ravani P, Ghiggeri GM: Coexistence of different circulating anti-podocyte antibodies in membranous nephropathy. Clin J Am Soc Nephrol 7: 1394–1400, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glassock RJ: Secondary membranous glomerulonephritis. Nephrol Dial Transplant 7[Suppl 1]: 64–71, 1992 [PubMed] [Google Scholar]
- 7.Hoxha E, Kneißler U, Stege G, Zahner G, Thiele I, Panzer U, Harendza S, Helmchen UM, Stahl RA: Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int 82: 797–804, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Qin W, Beck LH, Jr, Zeng C, Chen Z, Li S, Zuo K, Salant DJ, Liu Z: Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol 22: 1137–1143, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander MP, Larsen CP, Gibson IW, Nasr SH, Sethi S, Fidler ME, Raissian Y, Takahashi N, Chari S, Smyrk TC, Cornell LD: Membranous glomerulonephritis is a manifestation of IgG4-related disease. Kidney Int 83: 455–462, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Qu Z, Liu G, Li J, Wu LH, Tan Y, Zheng X, Ao J, Zhao MH: Absence of glomerular IgG4 deposition in patients with membranous nephropathy may indicate malignancy. Nephrol Dial Transplant 27: 1931–1937, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Jennette JC, Iskandar SS, Dalldorf FG: Pathologic differentiation between lupus and nonlupus membranous glomerulopathy. Kidney Int 24: 377–385, 1983 [DOI] [PubMed] [Google Scholar]
- 12.Lefaucheur C, Stengel B, Nochy D, Martel P, Hill GS, Jacquot C, Rossert J; GN-PROGRESS Study Group: Membranous nephropathy and cancer: Epidemiologic evidence and determinants of high-risk cancer association. Kidney Int 70: 1510–1517, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Segal PE, Choi MJ: Recent advances and prognosis in idiopathic membranous nephropathy. Adv Chronic Kidney Dis 19: 114–119, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Lai KN, Li PK, Lui SF, Au TC, Tam JS, Tong KL, Lai FM: Membranous nephropathy related to hepatitis B virus in adults. N Engl J Med 324: 1457–1463, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Zheng XY, Wei RB, Tang L, Li P, Zheng XD: Meta-analysis of combined therapy for adult hepatitis B virus-associated glomerulonephritis. World J Gastroenterol 18: 821–832, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radford MG, Jr, Holley KE, Grande JP, Larson TS, Wagoner RD, Donadio JV, McCarthy JT: Reversible membranous nephropathy associated with the use of nonsteroidal anti-inflammatory drugs. JAMA 276: 466–469, 1996 [PubMed] [Google Scholar]
- 17.Forslund T, Kellokumpu I, Elomaa E, Arola J, Nuorva K: Remission of membranous glomerulonephritis after pancreatectomy for pancreatic neuroendocrine neoplasm - a rare coincidence. Clin Nephrol 75[Suppl 1]: 42–46, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Schieppati A, Mosconi L, Perna A, Mecca G, Bertani T, Garattini S, Remuzzi G: Prognosis of untreated patients with idiopathic membranous nephropathy. N Engl J Med 329: 85–89, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Polanco N, Gutiérrez E, Covarsí A, Ariza F, Carreño A, Vigil A, Baltar J, Fernández-Fresnedo G, Martín C, Pons S, Lorenzo D, Bernis C, Arrizabalaga P, Fernández-Juárez G, Barrio V, Sierra M, Castellanos I, Espinosa M, Rivera F, Oliet A, Fernández-Vega F, Praga M; Grupo de Estudio de las Enfermedades Glomerulares de la Sociedad Española de Nefrología: Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol 21: 697–704, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hladunewich MA, Troyanov S, Calafati J, Cattran DC; Metropolitan Toronto Glomerulonephritis Registry: The natural history of the non-nephrotic membranous nephropathy patient. Clin J Am Soc Nephrol 4: 1417–1422, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cattran DC, Pei Y, Greenwood CM, Ponticelli C, Passerini P, Honkanen E: Validation of a predictive model of idiopathic membranous nephropathy: Its clinical and research implications. Kidney Int 51: 901–907, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Beck LH, Jr, Salant DJ: Membranous nephropathy: Recent travels and new roads ahead. Kidney Int 77: 765–770, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Hofstra JM, Beck LH, Jr, Beck DM, Wetzels JF, Salant DJ: Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 6: 1286–1291, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beck LH, Jr, Fervenza FC, Beck DM, Bonegio RG, Malik FA, Erickson SB, Cosio FG, Cattran DC, Salant DJ: Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol 22: 1543–1550, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC; Toronto Glomerulonephritis Registry Group: Idiopathic membranous nephropathy: Definition and relevance of a partial remission. Kidney Int 66: 1199–1205, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Collaborative Study of the Adult Idiopathic Nephrotic Syndrome: A controlled study of short-term prednisone treatment in adults with membranous nephropathy. N Engl J Med 301: 1301–1306, 1979 [DOI] [PubMed] [Google Scholar]
- 27.Cattran DC, Delmore T, Roscoe J, Cole E, Cardella C, Charron R, Ritchie S: A randomized controlled trial of prednisone in patients with idiopathic membranous nephropathy. N Engl J Med 320: 210–215, 1989 [DOI] [PubMed] [Google Scholar]
- 28.Cameron JS, Healy MJ, Adu D; The MRC Glomerulonephritis Working Party: The Medical Research Council trial of short-term high-dose alternate day prednisolone in idiopathic membranous nephropathy with nephrotic syndrome in adults. Q J Med 74: 133–156, 1990 [PubMed] [Google Scholar]
- 29.Ponticelli C, Zucchelli P, Imbasciati E, Cagnoli L, Pozzi C, Passerini P, Grassi C, Limido D, Pasquali S, Volpini T, Sasdelli M, Locatelli F: Controlled trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathy. N Engl J Med 310: 946–950, 1984 [DOI] [PubMed] [Google Scholar]
- 30.Ponticelli C, Zucchelli P, Passerini P, Cagnoli L, Cesana B, Pozzi C, Pasquali S, Imbasciati E, Grassi C, Redaelli B, Sasdelli M, Locatelli F: A randomized trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathy. N Engl J Med 320: 8–13, 1989 [DOI] [PubMed] [Google Scholar]
- 31.Ponticelli C, Zucchelli P, Passerini P, Cesana B; The Italian Idiopathic Membranous Nephropathy Treatment Study Group: Methylprednisolone plus chlorambucil as compared with methylprednisolone alone for the treatment of idiopathic membranous nephropathy. N Engl J Med 327: 599–603, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Ponticelli C, Altieri P, Scolari F, Passerini P, Roccatello D, Cesana B, Melis P, Valzorio B, Sasdelli M, Pasquali S, Pozzi C, Piccoli G, Lupo A, Segagni S, Antonucci F, Dugo M, Minari M, Scalia A, Pedrini L, Pisano G, Grassi C, Farina M, Bellazzi R: A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J Am Soc Nephrol 9: 444–450, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Jha V, Ganguli A, Saha TK, Kohli HS, Sud K, Gupta KL, Joshi K, Sakhuja V: A randomized, controlled trial of steroids and cyclophosphamide in adults with nephrotic syndrome caused by idiopathic membranous nephropathy. J Am Soc Nephrol 18: 1899–1904, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Ponticelli C, Zucchelli P, Passerini P, Cesana B, Locatelli F, Pasquali S, Sasdelli M, Redaelli B, Grassi C, Pozzi C, Bizzarri D, Banfi G: A 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney Int 48: 1600–1604, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Kidney Disease Improving Global Outcomes: KDIGO clinical practice guideline for glomerulonephritis. Kidney Int 2: 139–172, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Cattran DC, Alexopoulos E, Heering P, Hoyer PF, Johnston A, Meyrier A, Ponticelli C, Saito T, Choukroun G, Nachman P, Praga M, Yoshikawa N: Cyclosporin in idiopathic glomerular disease associated with the nephrotic syndrome: Workshop recommendations. Kidney Int 72: 1429–1447, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Praga M, Barrio V, Juárez GF, Luño J; Grupo Español de Estudio de la Nefropatía Membranosa: Tacrolimus monotherapy in membranous nephropathy: A randomized controlled trial. Kidney Int 71: 924–930, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Dussol B, Morange S, Burtey S, Indreies M, Cassuto E, Mourad G, Villar E, Pouteil-Noble C, Karaaslan H, Sichez H, Lasseur C, Delmas Y, Nogier MB, Fathallah M, Loundou A, Mayor V, Berland Y: Mycophenolate mofetil monotherapy in membranous nephropathy: A 1-year randomized controlled trial. Am J Kidney Dis 52: 699–705, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Branten AJ, du Buf-Vereijken PW, Vervloet M, Wetzels JF: Mycophenolate mofetil in idiopathic membranous nephropathy: A clinical trial with comparison to a historic control group treated with cyclophosphamide. Am J Kidney Dis 50: 248–256, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Chan TM, Lin AW, Tang SC, Qian JQ, Lam MF, Ho YW, Tse KC, Chan KW, Lai KN, Tang CS: Prospective controlled study on mycophenolate mofetil and prednisolone in the treatment of membranous nephropathy with nephrotic syndrome. Nephrology (Carlton) 12: 576–581, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Senthil Nayagam L, Ganguli A, Rathi M, Kohli HS, Gupta KL, Joshi K, Sakhuja V, Jha V: Mycophenolate mofetil or standard therapy for membranous nephropathy and focal segmental glomerulosclerosis: A pilot study. Nephrol Dial Transplant 23: 1926–1930, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Berg AL, Nilsson-Ehle P, Arnadottir M: Beneficial effects of ACTH on the serum lipoprotein profile and glomerular function in patients with membranous nephropathy. Kidney Int 56: 1534–1543, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Ponticelli C, Passerini P, Salvadori M, Manno C, Viola BF, Pasquali S, Mandolfo S, Messa P: A randomized pilot trial comparing methylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropic hormone in idiopathic membranous nephropathy. Am J Kidney Dis 47: 233–240, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Bomback AS, Canetta PA, Beck LH, Jr, Ayalon R, Radhakrishnan J, Appel GB: Treatment of resistant glomerular diseases with adrenocorticotropic hormone gel: A prospective trial. Am J Nephrol 36: 58–67, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Lindskog A, Ebefors K, Johansson ME, Stefánsson B, Granqvist A, Arnadottir M, Berg AL, Nyström J, Haraldsson B: Melanocortin 1 receptor agonists reduce proteinuria. J Am Soc Nephrol 21: 1290–1298, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Remuzzi G, Chiurchiu C, Abbate M, Brusegan V, Bontempelli M, Ruggenenti P: Rituximab for idiopathic membranous nephropathy. Lancet 360: 923–924, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Ruggenenti P, Cravedi P, Chianca A, Perna A, Ruggiero B, Gaspari F, Rambaldi A, Marasà M, Remuzzi G: Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol 23: 1416–1425, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fervenza FC, Abraham RS, Erickson SB, Irazabal MV, Eirin A, Specks U, Nachman PH, Bergstralh EJ, Leung N, Cosio FG, Hogan MC, Dillon JJ, Hickson LJ, Li X, Cattran DC; Mayo Nephrology Collaborative Group: Rituximab therapy in idiopathic membranous nephropathy: A 2-year study. Clin J Am Soc Nephrol 5: 2188–2198, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathieson PW, Turner AN, Maidment CG, Evans DJ, Rees AJ: Prednisolone and chlorambucil treatment in idiopathic membranous nephropathy with deteriorating renal function. Lancet 2: 869–872, 1988 [DOI] [PubMed] [Google Scholar]
- 50.Howman A, Chapman TL, Langdon MM, Ferguson C, Adu D, Feehally J, Gaskin GJ, Jayne DR, O’Donoghue D, Boulton-Jones M, Mathieson PW: Immunosuppression for progressive membranous nephropathy: A UK randomised controlled trial. Lancet 381: 744–751, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glassock RJ: Prophylactic anticoagulation in nephrotic syndrome: A clinical conundrum. J Am Soc Nephrol 18: 2221–2225, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Lionaki S, Derebail VK, Hogan SL, Barbour S, Lee T, Hladunewich M, Greenwald A, Hu Y, Jennette CE, Jennette JC, Falk RJ, Cattran DC, Nachman PH, Reich HN: Venous thromboembolism in patients with membranous nephropathy. Clin J Am Soc Nephrol 7: 43–51, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barbour SJ, Greenwald A, Djurdjev O, Levin A, Hladunewich MA, Nachman PH, Hogan SL, Cattran DC, Reich HN: Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int 81: 190–195, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Sarasin FP, Schifferli JA: Prophylactic oral anticoagulation in nephrotic patients with idiopathic membranous nephropathy. Kidney Int 45: 578–585, 1994 [DOI] [PubMed] [Google Scholar]