Abstract
Background and objectives
Kidney stones are common in general clinical practice, and their prevalence is increasing. Kidney stone formers often have risk factors associated with atherosclerosis, but it is uncertain whether having a kidney stone is associated with higher risk of cardiovascular events. This study sought to assess the association between one or more kidney stones and the subsequent risk of cardiovascular events.
Design, setting, participants, & measurements
Cohort study of 3,195,452 people aged≥18 years registered in the universal health care system in Alberta, Canada, between 1997 and 2009 (median follow-up of 11 years). People undergoing dialysis or with a kidney transplant at baseline were excluded. The primary outcome was the first acute myocardial infarction (AMI) during follow-up. We also considered other cardiovascular events, including death due to coronary heart disease, percutaneous transluminal coronary angioplasty (PTCA), coronary artery bypass grafting (CABG), and stroke.
Results
In total, 25,532 (0.8%) participants had at least one kidney stone, and 91,465 (3%) individuals had at least one cardiovascular event during follow-up. Compared with people without kidney stones and after adjustment for cardiovascular risk factors and other potential confounders, people who had at least one kidney stone had a higher risk of subsequent AMI (adjusted hazard ratio [HR], 1.40; 95% confidence interval [95% CI], 1.30 to 1.51), PTCA/CABG (HR, 1.63; 95% CI, 1.51 to 1.76), and stroke (HR, 1.26; 95% CI, 1.12 to 1.42). The magnitude of the excess risk associated with a kidney stone appeared more pronounced for younger people than for older people (P<0.001) and for women than men (P=0.01).
Conclusions
The occurrence of a kidney stone is associated with a higher risk of cardiovascular events, including AMI, PTCA/CABG, and stroke.
Introduction
Kidney stones are associated with hypertension, diabetes, and dyslipidemia (1–4), all known risk factors for cardiovascular disease (CVD). Moreover, kidney stones are associated with a higher risk of CKD (5) and ESRD (1), two additional risk factors for CVD. Kidney stone formers have increased cardiovascular disease and mortality risk scores (6) as well as evidence of subclinical atherosclerosis (7). However, evidence linking kidney stones to CVD events is limited. Previous studies dating back more than three decades found an association between kidney stone development and myocardial infarction (8–11), but other studies performed at that time failed to observe an association (12,13). More recently, data from the Health Professionals Follow-up Study and the Nurses Health Study demonstrate a higher risk of acute myocardial infarction (AMI) and revascularization procedures in women, but not men, with kidney stones (14). A second study done using a cohort of 4564 kidney stone formers in Olmsted County, Minnesota, found that both men and women who developed a kidney stone were at higher risk of AMI (2).
Because kidney stones are common and potentially preventable, accurately determining their long-term sequelae is important for informing health interventions. Therefore, we used a large population-based cohort of people receiving care in a universal health care system in the Canadian province of Alberta to investigate this association. We hypothesized that even a single kidney stone episode would be associated with an excess risk of cardiovascular events, including AMI, death from coronary heart disease (CHD), coronary revascularization (percutaneous transluminal coronary angioplasty [PTCA] or coronary artery bypass grafting [CABG]), and stroke.
Materials and Methods
Population and Data Sources
This study used the Alberta Kidney Disease Network database, which incorporates data from Alberta Health (AH; the provincial health ministry), the Northern and Southern Alberta Renal Programs, and the clinical laboratories in Alberta (15). The database was used to assemble a cohort of adults aged ≥18 years who resided in Alberta, Canada, between April 1997 and March 2009. All people registered with AH were eligible for inclusion. All Alberta residents are eligible for insurance coverage by AH, and >99% participate in coverage. We excluded participants with ESRD (defined as documented long-term dialysis or prior kidney transplant) at baseline because of their known higher risk of CVD. We followed participants with at least 3 years of prebaseline data from April 1997, their 18th birthday, or registration with AH (whichever was later), until March 2009. The prebaseline data were used to assess the presence or absence of comorbidity; baseline was defined as the end of the 3-year period following cohort entry. Participants without 3 years of prebaseline data were excluded.
Presentation with Kidney Stone Episodes
We used physician claims, hospitalization and ambulatory care utilization data, and International Classification of Diseases (ICD), Ninth Revision, Clinical Modification (ICD-9-CM) codes (592, 594, 274.11) and ICD, Tenth Revision, Canada codes (N20.0, N20.1, N20.2, N20.9, N21.0, N21.1, N21.8, N21.9, N22.0, N22.8) to identify kidney stone presentations. The accuracy of these codes for defining a kidney stone episode has been previously validated (5). We also used physician claims, hospitalization, and ambulatory care utilization data to assess for a history of kidney stone at baseline (i.e., in at least 3 years before follow-up) using the ICD codes listed above.
Other Covariates
Demographic variables included age (categorized as 18–49, 50–69, and ≥70 years), sex, Aboriginal status (registered First Nations or recognized Inuit), social assistance, and distance to nearest nephrologist. We used validated algorithms to define Charlson comorbidity index and hypertension (16) at baseline from physician claims, hospitalization, and ambulatory care utilization data. The Charlson score was based on the Deyo classification (17) of the following comorbid conditions: cerebrovascular disease, peripheral vascular disease, congestive heart failure, cancer, chronic obstructive pulmonary disease, dementia, diabetes with and without complications, AIDS/HIV, metastatic solid tumor, myocardial infarction, mild liver disease, moderate/severe liver disease, paralysis, peptic ulcer disease, and rheumatic disease.
Clinical and Laboratory Outcomes
We used validated algorithms based on claims and hospitalization data to assess the outcomes (18–20). The primary outcome was first hospitalization for AMI, defined as ICD-9-CM 410 and ICD-10-CA I21 and I22 hospitalization claims during follow-up. Secondary outcomes included death from CHD, PTCA/CABG, and stroke (see Supplemental Table 1 for claims codes). We also evaluated a composite endpoint (i.e., the occurrence of any of the outcomes). In a subset of participants with available data on serum creatinine (sCr) (“laboratory cohort”), participants were followed from their first available outpatient sCr measurement (May 2002 or after) until March 2009. From this subset, we excluded participants with documented estimated GFR (eGFR)<15 ml/min per 1.73 m2 at baseline, in addition to those treated with dialysis or a kidney transplant at baseline. The eGFR was estimated using the CKD-Epidemiology Collaboration equation (21). Baseline eGFR was categorized as ≥60, 45–59.9, 30–44.9, and 15–29.9 ml/min per 1.73 m2, and baseline albuminuria (first after index eGFR) was categorized as none (albumin-to-creatinine ratio [ACR]<3.39 mg/mmol or urine dipstick negative), mild (ACR, 3.39–59.9 or urine dipstick trace or 1+), and heavy or severe (ACR>59.9 or urine dipstick 2+ or 3+). Kidney stones occurring on or before the index date were considered to be exposed from baseline, but only cardiovascular events occurring after the index date were included as outcomes in our analyses.
Statistical Analyses
We did analyses with Stata/MP 11.2 (www.stata.com) and reported baseline descriptive statistics as counts and percentages or medians and interquartile ranges, as appropriate. Absolute rates were calculated by totaling the number of events and dividing by the total follow-up time. They are presented as events per 1000 person-years in strata defined by the presence/absence of at least one kidney stone during or before follow-up. Using each participant as the unit of analysis, we estimated the associations between kidney stone episode and outcomes using multivariable Cox proportional hazards models adjusted for all variables listed in Table 1.
Table 1.
Demographic and clinical characteristics of participants
| Characteristic | Primary Cohort | Laboratory Cohort | ||||
|---|---|---|---|---|---|---|
| Stone | No Stone | P Value | Stone | No Stone | P Value | |
| Patients (n) | 25,532 | 3,169,920 | — | 13,262 | 1,989,792 | — |
| Age (yr) | 46 (34.8,60.2) | 35.1 (23,48.4) | <0.001 | 52.7 (41.7,65.3) | 46.3 (34.2,58.5) | <0.001 |
| Men | 16,361 (64.1) | 1,598,312 (50.4) | <0.001 | 8422 (63.5) | 886,774 (44.6) | <0.001 |
| Aboriginal | 568 (2.2) | 87,941 (2.8) | <0.001 | 293 (2.2) | 48,737 (2.4) | 0.08 |
| Social assistance | 968 (3.8) | 83,528 (2.6) | <0.001 | 535 (4) | 59,377 (3) | <0.001 |
| Residencea | <0.001 | <0.001 | ||||
| ≤50 km | 17,201 (67.4) | 2,303,140 (72.7) | 9306 (70.2) | 1,491,766 (75) | ||
| 50.1–200 km | 5612 (22) | 538,693 (17) | 2618 (19.7) | 313,192 (15.7) | ||
| >200 km | 2719 (10.6) | 328,087 (10.4) | 1338 (10.1) | 184,834 (9.3) | ||
| Comorbid conditions | ||||||
| AIDS/HIV | 21 (0.1) | 1138 (0) | <0.001 | 15 (0.1) | 1533 (0.1) | 0.14 |
| Cancer | 1108 (4.3) | 65,469 (2.1) | <0.001 | 925 (7) | 76,171 (3.8) | <0.001 |
| Cerebral vascular disease | 586 (2.3) | 37,922 (1.2) | <0.001 | 453 (3.4) | 42,236 (2.1) | <0.001 |
| COPD | 4441 (17.4) | 391,613 (12.4) | <0.001 | 2426 (18.3) | 265,908 (13.4) | <0.001 |
| CHDb | 325 (1.3) | 14,078 (0.4) | <0.001 | 668 (5) | 49,360 (2.5) | <0.001 |
| Dementia | 116 (0.5) | 17,097 (0.5) | 0.07 | 140 (1.1) | 22,350 (1.1) | 0.46 |
| Diabetes | 1589 (6.2) | 82,682 (2.6) | <0.001 | 1835 (13.8) | 133,808 (6.7) | <0.001 |
| Heart failure | 586 (2.3) | 41,204 (1.3) | <0.001 | 543 (4.1) | 44,471 (2.2) | <0.001 |
| Hypertension | 4140 (16.2) | 255,621 (8.1) | <0.001 | 4425 (33.4) | 429,520 (21.6) | <0.001 |
| Mild liver disease | 226 (0.9) | 15,052 (0.5) | <0.001 | 191 (1.4) | 16,638 (0.8) | <0.001 |
| Moderate/severe liver disease | 27 (0.1) | 1494 (0) | <0.001 | 20 (0.2) | 1792 (0.1) | 0.02 |
| Paraplegia | 240 (0.9) | 10,419 (0.3) | <0.001 | 146 (1.1) | 7669 (0.4) | <0.001 |
| PVD | 409 (1.6) | 23,619 (0.7) | <0.001 | 331 (2.5) | 25,146 (1.3) | <0.001 |
| Peptic ulcer | 1205 (4.7) | 72,530 (2.3) | <0.001 | 432 (3.3) | 39,480 (2) | <0.001 |
| Rheumatologic disease | 344 (1.3) | 23,219 (0.7) | <0.001 | 264 (2) | 22,853 (1.1) | <0.001 |
| Charlson comorbidity scorec | 0 (0,1) | 0 (0,0) | <0.001 | 0 (0,1) | 0 (0,1) | <0.001 |
| eGFR | — | — | — | <0.001 | ||
| ≥60 ml/min per 1.73 m2 | 11,645 (87.8) | 1,854,612 (93.2) | ||||
| 45–59.9 ml/min per 1.73 m2 | 1071 (8.1) | 92,869 (4.7) | ||||
| 30–44.9 ml/min per 1.73 m2 | 415 (3.1) | 32,528 (1.6) | ||||
| 15–29.9 ml/min per 1.73 m2 | 131 (1) | 9783 (0.5) | ||||
| Albuminuria | — | — | — | <0.001 | ||
| None | 10,574 (79.7) | 1,829,246 (91.9) | ||||
| Mild | 2221 (16.7) | 139,820 (7) | ||||
| Heavy | 467 (3.5) | 20,726 (1) | ||||
Unless otherwise noted, values are expressed as number (percentage) of patients or median (interquartile range) where appropriate. COPD, chronic obstructive pulmonary disease; CHD coronary heart disease; PVD, peripheral vascular disease; eGFR, estimated glomerular filtration rate using the CKD-Epidemiology Collaboration formula.
Residence is distance in kilometers to the nearest nephrologist, used as a proxy for access to specialty medical care.
Coronary heart disease includes acute myocardial infarction, percutaneous transluminal coronary angioplasty, and coronary artery bypass graft.
Charlson score includes AIDS/HIV, metastatic cancer, nonmetastatic cancer, cerebral vascular disease, chronic obstructive pulmonary disease, dementia, diabetes, heart failure, mild liver disease, moderate/severe liver disease, myocardial infarction, paraplegia, peptic ulcer, peripheral vascular disease, and rheumatologic disease.
In a secondary analysis we adjusted for the potentially confounding factors of baseline eGFR and albuminuria. Stone episodes were used to update exposure status during follow-up (i.e., as a time varying covariate), meaning that an individual experiencing a single stone episode during the course of follow-up would contribute person-time to the no-stone hazard before the stone episode and person-time to the stone hazard following the stone episode (1). In the Supplemental Material, we also present unadjusted and age- and sex-adjusted hazard ratios.
In separate sensitivity analyses, we (1) excluded participants with a baseline history of kidney stones, (2) excluded participants with a baseline history of CVD (this term includes individuals with AMI, PTCA, CABG, and stroke), and (3) examined for the possibility of a dose-response association, classifying the number of health care encounters for a kidney stone (dose) for each hazard. We censored follow-up when a participant died, moved out of the province, or reached the end of the study (March 31, 2009). We determined that the proportional hazard assumption was satisfied by examining plots of the log-negative-log of within-group survivorship probabilities versus log-time. The threshold P value for statistical significance was set at 0.05. Age, sex, and comorbid conditions (diabetes mellitus, hypertension, coronary heart disease, cancer, Charlson comorbidity score in both cohorts, and baseline eGFR<60 ml/min per 1.73 m2 and ACR in the laboratory cohort only) were explored as potential modifiers of the association between kidney stones and clinical outcomes.
The institutional review boards at the University of Alberta and the University of Calgary approved the study.
Results
Characteristics of Study Participants
There were 3,195,452 eligible individuals with at least 3 years of prebaseline data to allow the assessment of comorbid conditions at baseline (Figure 1). Of those, 2,003,054 had sCr measurements and were included in the laboratory cohort. The characteristics of the study population with and without kidney stone episodes are shown in Table 1. Individuals with kidney stones were older; were more often male; and were more likely to have diabetes, hypertension, baseline eGFR≥60 ml/min per 1.73 m2, albuminuria, and a history of kidney stones.
Figure 1.
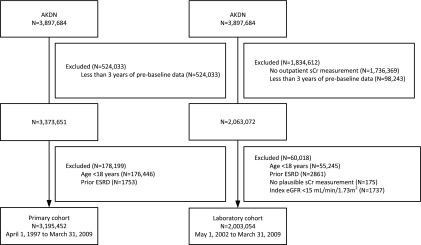
Participant flow. AKDN, Alberta Kidney Disease Network; eGFR, estimated GFR using the CKD-Epidemiology Collaboration formula; sCr, serum creatinine.
During a median follow-up of 11 years (range, 1 day to 12 years), 91,465 (3%) individuals experienced a cardiovascular event, 202,261 (6%) died, and 263,948 (8%) moved outside Alberta. In the subset of participants with sCr data, the median follow-up period was 4 years (range, 1 day to 7 years). During this period, 58,800 (3%) participants in the laboratory cohort experienced a cardiovascular event, 97,124 (5%) died, and 75,582 (4%) moved outside Alberta.
Relation between Kidney Stone Episodes and Cardiovascular Events
In the primary cohort, the adjusted risk for AMI was significantly higher among individuals with one or more kidney stone episodes (hazard ratio [HR], 1.40; 95% confidence interval [95% CI], 1.30 to 1.51) (Figure 2). Significant associations were also observed between the development of at least one kidney stone and PTCA/CABG (HR, 1.63; 95% CI, 1.51 to 1.76), stroke (HR, 1.26; 95% CI, 1.12 to 1.42), and the composite outcome of the individual events (HR, 1.30; 95% CI, 1.24 to 1.37) but not for CHD death (HR, 0.97; 95% CI, 0.90 to 1.05) (Figure 2). The magnitude of the excess risk after full adjustment is comparatively smaller than the magnitude of the excess risk as assessed by unadjusted and age-sex adjusted models (Supplemental Table 1).
Figure 2.

Forest plots of cardiovascular events in the primary and laboratory cohorts. The plots show the relative adjusted hazards (with corresponding 95% confidence intervals) of cardiovascular events associated with first kidney stone presentation during the study follow-up. Cardiovascular events include acute myocardial infarction (AMI), coronary heart disease (CHD) death (includes angina), percutaneous transluminal coronary angioplasty (PTCA)/coronary artery bypass grafting (CABG), and stroke. The left panel shows participants in the primary cohort (n=3,195,452); the right panel shows participants in the laboratory cohort (n=2,003,054). Hazard ratios from both cohorts were adjusted for age, sex, Aboriginal status, social assistance, residence location, and comorbid conditions (diabetes, hypertension, coronary heart disease, cancer, AIDS/HIV, cerebral vascular disease, chronic obstructive pulmonary disease, dementia, heart failure, mild liver disease, moderate/severe liver disease, paraplegia, peptic ulcer, peripheral vascular disease, and rheumatologic disease). Hazard ratios from the laboratory cohort have also been adjusted for baseline eGFR and albuminuria. 95% CI, 95% confidence interval; CV, cardiovascular; HR, hazard ratio.
When analyses were repeated in the laboratory cohort, the risk for AMI remained significantly increased among people who developed a kidney stone, although it was attenuated compared with the corresponding result in the primary cohort (HR, 1.16; 95% CI, 1.06 to 1.27) (Figure 2). The risk of PTCA/CABG (HR, 1.24; 95% CI, 1.14 to 1.34) and the composite outcome of the individual events (HR, 1.08; 95% CI, 1.02 to 1.14) (Figure 2) were similarly increased—but not the risk of stroke alone (HR, 1.06; 95% CI, 0.94 to 1.20) or CHD death alone (HR, 0.88; 95% CI, 0.79 to 0.97).
Results were similar in a sensitivity analysis using the primary cohort that excluded participants with a history of kidney stones at baseline. Specifically, in these analyses, participants who experienced one or more kidney stones had a significantly higher risk of the primary outcome (HR for AMI, 1.46; 95% CI, 1.31 to 1.63). The risk of AMI was similarly increased in further sensitivity analyses that excluded individuals with prior CHD (HR, 1.44; 95% CI, 1.33 to 1.55). We did not observe evidence of a dose-response association (HR for AMI associated with one stone, 1.39 [95% CI, 1.26 to 1.55]; two stones, 1.43 [95% CI, 1.20 to 1.71]; three stones, 1.33 [95% CI, 1.04 to 1.71]; four stones, 1.15 [95% CI, 0.80 to 1.66]; five or more stones, 1.51 [95% CI, 1.26 to 1.81]).
Effect Modification by Age, Sex, and Comorbid Conditions
Analyses in the primary cohort that evaluated separately for effect modification by sex and age found that the risk of AMI associated with stones was greater for women than for men (P=0.01) and for younger participants (aged<50 years) than for older participants (P<0.001); the excess risk associated with kidney stones was attenuated or absent among participants aged≥70 years (Figure 3). The excess risk of AMI associated with kidney stones was also increased among people without a baseline history of diabetes (P=0.001), CHD (P<0.001), or hypertension (P=0.002) and with lower baseline Charlson comorbidity score (P<0.001) (Figure 3). Baseline cancer did not alter the risk of AMI associated with kidney stones (P=0.33). There was evidence that the risk of AMI associated with kidney stones was increased at higher baseline eGFR (P<0.001) and among people without albuminuria (P=0.05) (Figure 3).
Figure 3.
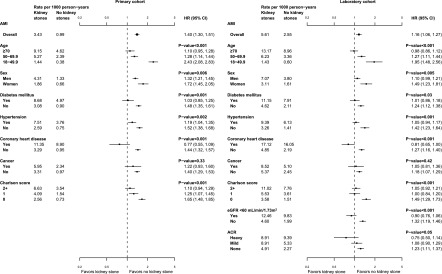
Forest plots of AMI events in the primary and laboratory cohorts, by strata. The plots show the relative adjusted hazards (with corresponding 95% confidence intervals) of AMI associated with the first kidney stone presentation during the study follow-up, overall (top square point symbol) and in subgroups. The left panel shows participants in the primary cohort (n=3,195,452); the right panel shows participants in the laboratory cohort (n=2,003,054). The P values are a measure of the interaction between each characteristic and the risk of AMI associated with the first kidney stone presentation. Hazard ratios from both cohorts were adjusted for age, sex, Aboriginal status, social assistance, residence location, and comorbid conditions (diabetes, hypertension, coronary heart disease, cancer, AIDS/HIV, cerebral vascular disease, chronic obstructive pulmonary disease, dementia, heart failure, mild liver disease, moderate/severe liver disease, paraplegia, peptic ulcer, peripheral vascular disease, and rheumatologic disease). Hazard ratios from the laboratory cohort have also been adjusted for baseline estimated GFR and albuminuria. ACR, albumin-to-creatinine ratio.
Discussion
Kidney stones are a common, potentially preventable condition associated with significant morbidity. We used a cohort of more than 3 million people receiving care from a universal health care system to study the relation between kidney stone formation and the risk of several clinically relevant cardiovascular events: AMI, CHD death, PTCA/CABG, and stroke. Despite adjustment for potential confounders we observed a significant association between kidney stones and the risk of cardiovascular events.
Tests for interaction showed that the relative risk of cardiovascular events associated with a kidney stone was greater in women; younger participants; and those without a history of diabetes, hypertension, CHD, reduced eGFR, or albuminuria at baseline. This effect modification was independent of multiple potential confounders and may be due to the absence of competing risk factors for cardiovascular events in these generally healthy subgroups, although this explanation is speculative. The reduced risk for all outcomes in the laboratory cohort is probably due to adjustment for the confounding effect of CKD, although differences in the characteristics of participants with and without laboratory data cannot be excluded.
Although we found a higher risk of AMI and cardiac revascularization procedures in people who had a kidney stone, the risk of death due to CHD was not significantly increased. Inaccuracies in classifying cause of death (reducing effective statistical power) may have accounted for this finding. Alternatively, patients with a history of stones may receive therapies such as thiazide diuretics for hypercalciuria, reducing the severity of new cardiovascular events and, accordingly, the likelihood of death from cardiovascular causes. However, these possibilities are speculative and cannot be addressed by our dataset.
Comparison with Other Studies
Consistent with our data, the Coronary Artery Risk Development in Young Adults Study study showed an association between a history of a kidney stone and subclinical atherosclerosis (14,22). However, this study did not provide evidence of an association between kidney stones and clinical outcomes such as AMI. Other older studies have also found an association between kidney stone formation and myocardial infarction (8–11). These studies used smaller populations, were mostly retrospective, and often did not select coronary heart disease as the primary outcome. In contrast, two reports where coronary heart disease was the primary outcome did not demonstrate such an association (12,13), perhaps because of limited statistical power. Since then, Rule et al. (5) reported a significantly higher risk of AMI in 4564 people with a history of kidney stones (2), even after adjustment for dyslipidemia, tobacco use, and obesity. However, they did not evaluate other cardiovascular outcomes, such as CHD death, coronary revascularization, or stroke. A second prospective study of >11,000 individuals found that those who sustained a stroke were more likely to have had a kidney stone (23). More recently, Ferraro et al. (14) reported a higher risk of AMI and cardiac revascularization procedures in women (but not men) with kidney stones. The explanation for the lack of an association in men in the Ferraro study is unclear but could be due to a lack of statistical power (only 45,748 of the 242,105 participants were male, and only 3517 had a history of a stone).
Potential Mechanisms for the Findings
These results suggest that kidney stones may be an important contributor to the risk of future cardiovascular events. Treatment of symptomatically-identified kidney stones is unlikely to explain the observed association. Hyperuricemia is certainly a risk factor for uric acid stones and vascular disease, potentially explaining our findings. However, most kidney stones are composed of calcium, and the greatest risk factor for the development of kidney stones is hypercalciuria. It is tempting to therefore speculate that the underlying pathophysiology leading to the formation of calcium precipitations in the renal tubule might also contribute to calcium precipitation in other parts of the body, including the intracranial region and coronary vessels. These precipitations would in turn cause the clinical sequelae of AMI and stroke observed herein. Consistent with this, a recent study found that the formation of Randall plaque (the inciting stone nidus) is similar in composition to vascular plaque (24). Moreover, inhibitors of calcification are found both in blood and urine, such as pyrophosphates (25). It is conceivable that a deficiency of such inhibitors in both blood and urine could explain the apparent link between kidney stone formation and cardiovascular disease. However, we cannot exclude the possibility of residual confounding by characteristics that increase the risk of CVD as well as kidney stones.
Limitations
First, individuals with kidney stones were identified by presentation to health services, and consequently our results do not apply to those who did not seek medical care for their stone or were unaware that they had a stone. Second, we used validated algorithms applied to claims and hospitalization data to assess the incidence of kidney stones and cardiovascular outcomes. However, because these validation studies were done outside Alberta, it is possible that between-jurisdiction differences in ICD-9 coding may have influenced the diagnostic properties of the algorithms we used. Similarly, it is notoriously difficult to accurately classify the cause of death in clinical settings, and thus analyses considering the outcome of CHD death may be less reliable than the others. Third, although we adjusted for numerous potential confounders, we could not adjust for obesity, alcohol intake, smoking, or dyslipidemia, all of which are associated with both kidney stones and cardiovascular events. Similarly, although we adjusted for the presence of hypertension, our dataset did not include information on BP or diet—both of which are plausible potential confounders. Finally, we used data from death certificates rather than review of medical records to assess CHD death, which may have led to imprecision in classifying the cause of death.
We found an independent association between kidney stones and the risk of several clinically relevant cardiovascular events. Future research should be directed at delineating the mechanisms underlying this association and to treat the risk factors for CVD in people with kidney stones.
Disclosures
None.
Acknowledgments
This work was supported by a team grant to the Interdisciplinary Chronic Disease Collaboration from the Alberta Heritage Foundation for Medical Research (AHFMR) and the Canadian Institutes for Health Research. R.T.A. is supported by a Clinician-Scientist award from the Canadian Institute of Health Research and an Alberta Innovates Health Solutions Clinical Investigator Award. S.S. is supported by a Kidney Research Scientist Care Education and National Training Program New Investigator award and a Canadian Child Health Clinician Scientist Program Career Development award. M.T. is supported by an AHFMR Population Health Scholar award and a Government of Canada Research Chair in the optimal care of people with CKD. This study is based in part by data provided by Alberta Health and Alberta Health Services. The interpretation and conclusions are those of the researchers and do not represent the views of the Government of Alberta. Our funders have had no role in the design or analysis of this study, nor in drafting or approval of this manuscript. M.T. has access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04960513/-/DCSupplemental.
References
- 1.Alexander RT, Hemmelgarn BR, Wiebe N, Bello A, Morgan C, Samuel S, Klarenbach SW, Curhan GC, Tonelli M, Alberta Kidney Disease Network : Kidney stones and kidney function loss: A cohort study. BMJ 345: e5287, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rule AD, Roger VL, Melton LJ, 3rd, Bergstralh EJ, Li X, Peyser PA, Krambeck AE, Lieske JC: Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol 21: 1641–1644, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obligado SH, Goldfarb DS: The association of nephrolithiasis with hypertension and obesity: A review. Am J Hypertens 21: 257–264, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Taylor EN, Stampfer MJ, Curhan GC: Diabetes mellitus and the risk of nephrolithiasis. Kidney Int 68: 1230–1235, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Rule AD, Bergstralh EJ, Melton LJ, 3rd, Li X, Weaver AL, Lieske JC: Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol 4: 804–811, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aydin H, Yencilek F, Erihan IB, Okan B, Sarica K: Increased 10-year cardiovascular disease and mortality risk scores in asymptomatic patients with calcium oxalate urolithiasis. Urol Res 39: 451–458, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Reiner AP, Kahn A, Eisner BH, Pletcher MJ, Sadetsky N, Williams OD, Polak JF, Jacobs DR, Jr, Stoller ML: Kidney stones and subclinical atherosclerosis in young adults: The CARDIA study. J Urol 185: 920–925, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elmfeldt D, Vedin A, Wilhelmsson C, Tibblin G, Wilhelmsen L: Morbidity in representative male survivors of myocardial infarction compared to representative population samples. J Chronic Dis 29: 221–231, 1976 [DOI] [PubMed] [Google Scholar]
- 9.Zimmerer T, Weiss C, Hammes HP, Braun C, Hesse A, Alken P, Knoll T: Evaluation of urolithiasis: A link between stone formation and diabetes mellitus? Urol Int 82: 350–355, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Hamano S, Nakatsu H, Suzuki N, Tomioka S, Tanaka M, Murakami S: Kidney stone disease and risk factors for coronary heart disease. Int J Urol 12: 859–863, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Lindén V: Vitamin D and myocardial infarction. BMJ 3: 647–650, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ljunghall S, Hedstrand H: Renal stones and coronary heart disease. Acta Med Scand 199: 481–485, 1976 [DOI] [PubMed] [Google Scholar]
- 13.Westlund K: Urolithiasis and coronary heart disease: A note on association. Am J Epidemiol 97: 167–172, 1973 [DOI] [PubMed] [Google Scholar]
- 14.Ferraro PM, Taylor EN, Eisner BH, Gambaro G, Rimm EB, Mukamal KJ, Curhan GC: History of kidney stones and the risk of coronary heart disease. JAMA 310: 408–415, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemmelgarn BR, Clement F, Manns BJ, Klarenbach S, James MT, Ravani P, Pannu N, Ahmed SB, MacRae J, Scott-Douglas N, Jindal K, Quinn R, Culleton BF, Wiebe N, Krause R, Thorlacius L, Tonelli M: Overview of the Alberta Kidney Disease Network. BMC Nephrol 10: 30, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quan H, Khan N, Hemmelgarn BR, Tu K, Chen G, Campbell N, Hill MD, Ghali WA, McAlister FA, Hypertension Outcome and Surveillance Team of the Canadian Hypertension Education Programs : Validation of a case definition to define hypertension using administrative data. Hypertension 54: 1423–1428, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA: Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43: 1130–1139, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Austin PC, Daly PA, Tu JV: A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J 144: 290–296, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee : Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation 125: e2–e220, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kokotailo RA, Hill MD: Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke 36: 1776–1781, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Levin A, Hemmelgarn B, Culleton B, Tobe S, McFarlane P, Ruzicka M, Burns K, Manns B, White C, Madore F, Moist L, Klarenbach S, Barrett B, Foley R, Jindal K, Senior P, Pannu N, Shurraw S, Akbari A, Cohn A, Reslerova M, Deved V, Mendelssohn D, Nesrallah G, Kappel J, Tonelli M, Canadian Society of Nephrology : Guidelines for the management of chronic kidney disease. CMAJ 179: 1154–1162, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoller ML, Meng MV, Abrahams HM, Kane JP: The primary stone event: A new hypothesis involving a vascular etiology. J Urol 171: 1920–1924, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Li C, Engström G, Hedblad B, Berglund G, Janzon L: Risk factors for stroke in subjects with normal blood pressure: a prospective cohort study. Stroke 36: 234–238, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Khan SR, Rodriguez DE, Gower LB, Monga M: Association of Randall plaque with collagen fibers and membrane vesicles. J Urol 187: 1094–1100, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlieper G, Westenfeld R, Brandenburg V, Ketteler M: Inhibitors of calcification in blood and urine. Semin Dial 20: 113–121, 2007 [DOI] [PubMed] [Google Scholar]


