Abstract
Background and objectives
Ferric citrate hydrate is a novel iron-based phosphate binder being developed for hyperphosphatemia in patients with CKD.
Design, setting, participants, & measurements
A phase 3, multicenter, randomized, double blind, placebo-controlled study investigated the efficacy and safety of ferric citrate hydrate in nondialysis-dependent patients with CKD. Starting in April of 2011, 90 CKD patients (eGFR=9.21±5.72 ml/min per 1.73 m2) with a serum phosphate≥5.0 mg/dl were randomized 2:1 to ferric citrate hydrate or placebo for 12 weeks. The primary end point was change in serum phosphate from baseline to the end of treatment. Secondary end points included the percentage of patients achieving target serum phosphate levels (2.5–4.5 mg/dl) and change in fibroblast growth factor-23 at the end of treatment.
Results
The mean change in serum phosphate was −1.29 mg/dl (95% confidence interval, −1.63 to −0.96 mg/dl) in the ferric citrate hydrate group and 0.06 mg/dl (95% confidence interval, −0.20 to 0.31 mg/dl) in the placebo group (P<0.001 for difference between groups). The percentage of patients achieving target serum phosphate levels was 64.9% in the ferric citrate hydrate group and 6.9% in the placebo group (P<0.001). Fibroblast growth factor-23 concentrations were significantly lower in patients treated with ferric citrate hydrate versus placebo (change from baseline [median], −142.0 versus 67.0 pg/ml; P<0.001). Ferric citrate hydrate significantly increased serum iron, ferritin, and transferrin saturation compared with placebo (P=0.001 or P<0.001). Five patients discontinued active treatment because of treatment-emergent adverse events with ferric citrate hydrate treatment versus one patient with placebo. Overall, adverse drug reactions were similar in patients receiving ferric citrate hydrate or placebo, with gastrointestinal disorders occurring in 30.0% of ferric citrate hydrate patients and 26.7% of patients receiving placebo.
Conclusion
In patients with nondialysis-dependent CKD, 12-week treatment with ferric citrate hydrate resulted in significant reductions in serum phosphate and fibroblast growth factor-23 while simultaneously increasing serum iron parameters.
Introduction
In early CKD, serum phosphate is maintained within the normal range by a compensatory increase in parathyroid hormone (PTH) and fibroblast growth factor-23 (FGF-23), which inhibit reabsorption of phosphate by the proximal renal tubules (1–3). However, as CKD progresses, the risk of hyperphosphatemia increases, because the limit of the ability of the kidney to excrete phosphate is reached (4–6). In patients with CKD, elevated serum phosphate is associated with vascular calcification (7,8), progression of CKD, and increased mortality (4,9–12). Consequently, the Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD) (13) and the Japanese Society for Dialysis Therapy (14) recommend maintaining serum phosphate of CKD patients within the normal range.
Several intestinal phosphate binders (calcium carbonate, calcium acetate, sevelamer carbonate, and lanthanum carbonate) are presently used to treat hyperphosphatemia in CKD. There is a concern that calcium preparations may induce hypercalcemia and accelerate ectopic calcification, even in nondialysis-dependent CKD (15,16). The efficacy of noncalcium-containing phosphate binders, such as sevelamer and lanthanum, has been confirmed for this indication (17,18); however, sevelamer must be taken in large quantities to achieve adequate efficacy and is commonly associated with gastrointestinal side effects, such as constipation and bloating (19). Lanthanum accumulation in bone and other tissues has also been observed, although no safety issues related to tissue accumulation have been identified so far (20). There remains an unmet need for a highly efficacious and well tolerated phosphate binder that can be used in patients with nondialysis-dependent CKD.
Ferric citrate hydrate (JTT-751) is a novel phosphate binder containing ferric citrate as an active ingredient, and it is being developed for hyperphosphatemia in patients with CKD, including those patients who are dialysis-dependent (21). JTT-751 has a larger surface area and faster dissolution rate than ferric citrate, a food additive. In patients receiving hemodialysis, JTT-751 has been shown to be efficacious and well tolerated (22).
This study was a phase 3 trial to evaluate the efficacy and safety of JTT-751 in Japanese nondialysis CKD patients with hyperphosphatemia.
Materials and Methods
Study Design
This study was a randomized, double blind, placebo-controlled trial conducted in 36 centers in Japan. The study consisted of a 2- to 4-week screening period followed by a 12-week treatment period. The methods were conducted in accordance with the Declaration of Helsinki and the Guidelines for Good Clinical Practice of the Japanese Ministerial Ordinance. This trial was registered with the Japan Pharmaceutical Information Center as CTI-111435.
Study Population
Patients (≥20 years) with stable nondialysis CKD (stages 3–5) were recruited. All patients received standard conservative therapy in Japan to prevent CKD progression (e.g., protein restriction of 0.6–0.8 g/kg per day) for ≥3 months before the initial screening date.
Inclusion criteria included a serum phosphate≥5.0 and <8.0 mg/dl during the screening period, and if patients were receiving treatment with a phosphate-lowering drug or vitamin D preparation, it was required that the dosage remained constant for ≥4 weeks before the initial screening date.
Exclusion criteria included patients scheduled for dialysis or renal transplantation≤4 months after the initial screening date; AKI≤3 months before the initial screening date; active gastrointestinal disease (e.g., peptic ulcer, chronic ulcerative colitis, or regional enteritis); previous gastrectomy or duodenectomy; hemochromatosis, ferritin>500 ng/ml, or transferrin saturation>50%; corrected serum calcium<8.0 or >11.0 mg/dl; and any significant comorbidity that the investigators deemed would interfere with completion of study procedures.
Treatment Protocol
Drugs for hyperphosphatemia were discontinued at the initial screening visit. Patients were randomized (2:1 ratio) to receive either JTT-751 or placebo. The investigational products, JTT-751 tablets containing 250 mg JTT-751 as an anhydride and placebo tablets, were indistinguishable in appearance and provided by Japan Tobacco, Inc.
The starting dose of the investigational product was 1.5 g/d (6 tablets per day of JTT-751 or placebo tablets) administered orally three times daily immediately after a meal. The dose was increased to 3.0 g/d at week 2. At week 4, the dose was adjusted between 1.5 and 6.0 g/d according to the target range of serum phosphate (2.5–4.5 mg/dl). When serum phosphate exceeded 4.5 mg/dl, the dose was increased by two tablets per dose, and when serum phosphate fell below 2.5 mg/dl, the dose was reduced by two tablets per dose. Decisions to change the dosage were made on weeks 4, 6, and 8. Thereafter, the dose was maintained, except in certain cases, such as when adverse events (AEs) occurred.
During the study, concomitant use of drugs with phosphate-binding properties (including oral iron, magnesium, calcium, and aluminum preparations) and drugs that affect serum phosphate (such as niceritrol and colestimide) were prohibited. The doses of vitamin D preparations were kept constant. Intravenous iron preparations as iron replacement therapy for renal anemia were permitted. No change in prescribed diet was allowed during the trial.
Discontinuation criteria included investigator decision to introduce RRT; ferritin≥800 ng/ml; two consecutive serum phosphates<2.5 or ≥8.0 mg/dl; and corrected serum calcium<7.5 mg/dl.
End Points
The primary end point in this trial was the change in serum phosphate from baseline to the end of treatment (EOT). Secondary end points were percentage of patients achieving target serum phosphate levels (defined as 2.5–4.5 mg/dl), corrected serum calcium, calcium–phosphate product, urinary phosphate excretion, intact PTH, and FGF-23. Serum iron and ferritin concentrations, total iron-binding capacity, and transferring saturation were also measured throughout the study duration.
Safety end points were clinically significant AEs determined by symptoms, physical findings, and abnormal changes in physiologic tests (i.e., vital signs and electrocardiogram) and laboratory results (i.e., blood hematology and biochemistry). All AEs were coded using Medical Dictionary for Regulatory Activities version 13.1.
All blood and urine samples were tested centrally at SRL, Inc. (Tokyo, Japan), including measurements for evaluating efficacy end points. FGF-23 was determined by measuring biologically active full-length intact FGF-23 (Kainos Laboratories, Inc., Tokyo, Japan). eGFR was calculated before and after treatment (23), and estimated protein intake (24) was calculated using urine urea nitrogen to ascertain dietary therapy (protein intake).
Statistical Analyses
Patients who had an efficacy assessment at week 2 were included in the full analysis set (FAS), whereas all patients who received any investigational drug and were assessed for safety end points were included in the safety analysis set.
Changes at the EOT in serum phosphate, corrected serum calcium, and calcium–phosphate product between groups were assessed by analysis of covariance using the treatment group as the factor and the baseline value as the covariate. With respect to the percentage of patients achieving target serum phosphate levels at the EOT, comparison between groups was assessed using Fisher’s exact test. Differences in changes at the EOT in urinary phosphate excretion, intact PTH, and FGF-23 were assessed using the nonparametric Wilcoxon rank-sum test.
For the sample size determination, it was assumed that the change in serum phosphate would be −0.75±0.75 mg/dl in the JTT-751 group and −0.18±0.75 mg/dl in the placebo group. The sample size (assignment ratio; 2:1) to provide a 90% probability of showing that the upper limit of the 95% confidence interval for the least squares mean of the difference between groups in change in serum phosphate is lower than 0 mg/dl was calculated using SAS software (SAS System for Windows Release 9.2).
Results
Patient Demographics and Disposition
Written informed consent was obtained from 157 patients. Of these patients, 90 patients were randomly assigned 2:1 to the JTT-751 (n=60) or placebo group (n=30) in a double blind fashion. In total, 46 patients in the JTT-751 group and 23 patients in the placebo group completed the 12-week treatment (Figure 1); 4 patients did not return for the week 2 visit, and 86 patients were included in the FAS population (JTT-751, n=57; placebo, n=29).
Figure 1.
90 patients were randomized in the ratio of 2:1 (JTT-751:placebo), and 69 patients completed the 12-week treatment. Four patients were withdrawn from the study without assessment at week 2 and excluded from full analysis set population. IC, informed consent; JTT-751, ferric citrate hydrate; P, serum phosphate.
There were no significant differences between groups in the FAS population, and 93.0% of patients were classified as having CKD stage 5 (Table 1). The percentage of patients who had been receiving vitamin D preparations before study entry was similar across groups (38.6%, JTT-751; 37.9%, placebo).
Table 1.
Baseline demographic clinical characteristics (full analysis set analysis population)
| Characteristic | JTT-751 (n=57) | Placebo (n=29) | P Value |
|---|---|---|---|
| Sex, n (%) | 0.95a | ||
| Men | 33 (57.9) | 17 (58.6) | |
| Women | 24 (42.1) | 12 (41.4) | |
| Age, yr (mean±SD) | 65.3±10.2 | 64.6±13.5 | 0.78b |
| Primary disease of CKD, n (%) | 0.63a | ||
| Chronic GN | 15 (26.3) | 10 (34.5) | |
| Diabetic nephropathy | 16 (28.1) | 10 (34.5) | |
| Nephrosclerosis | 7 (12.3) | 3 (10.3) | |
| Polycystic kidney disease | 3 (5.3) | 3 (10.3) | |
| Chronic GN and diabetic nephropathy | 1 (1.8) | 0 (0.0) | |
| Unknown | 11 (19.3) | 2 (6.9) | |
| Otherc | 4 (7.0) | 1 (3.4) | |
| KDOQI CKD classification, n (%) | 0.30a | ||
| Stage 3 | 0 (0.0) | 1 (3.4) | |
| Stage 4 | 4 (7.0) | 1 (3.4) | |
| Stage 5 | 53 (93.0) | 27 (93.1) | |
| eGFR in CKD stage 5 (ml/min per 1.73 m2), n (%) | 0.68a | ||
| 10 to <15 | 12 (22.6) | 6 (22.2) | |
| 5 to <10 | 38 (71.7) | 18 (66.7) | |
| <5 | 3 (5.7) | 3 (11.1) | |
| Prior phosphate binder therapy, n (%) | 0.66a | ||
| Calcium carbonate | 10 (17.5) | 4 (13.8) | |
| Prior vitamin D therapy, n (%) | 0.95a | ||
| Oral preparations | 22 (38.6) | 11 (37.9) | |
| Efficacy parameters (mean±SD) | |||
| Serum phosphate (mg/dl) | 5.66±0.75 | 5.57±0.63 | 0.56b |
| Corrected serum calcium (mg/dl) | 8.61±0.52 | 8.57±0.44 | 0.72b |
| Calcium–phosphate product (mg2/dl2) | 48.75±6.90 | 47.68±5.28 | 0.47b |
| Urinary phosphate (mg/d)d | 380.6 (289.8, 520) | 341.9 (273.6, 492.7) | 0.82e |
| Intact PTH (pg/ml)d | 246 (129, 364) | 236 (156, 331) | 0.95e |
| FGF-23 (pg/ml)d | 453 (215, 800) | 358 (237, 656) | 0.47e |
JTT-751, ferric citrate hydrate; KDOQI, Kidney Disease Outcomes Quality Initiative; PTH, parathyroid hormone; FGF-23, fibroblast growth factor-23.
Chi-squared test.
t test.
Other includes gouty kidney, obesity-related nephropathy, abdominal aortic aneurysm, FSGS in the JTT-751 group, and Alport’s syndrome in the placebo group.
Data are expressed as median (25th, 75th percentile interval).
Wilcoxon rank-sum test.
The mean dose of JTT-751 prescribed over the trial period was 3.50 g/d. The mean number of tablets for the JTT-751 and placebo groups were 14.0 and 15.0 tablets/d, and treatment compliance rates were 95.7% and 94.4%, respectively.
Efficacy
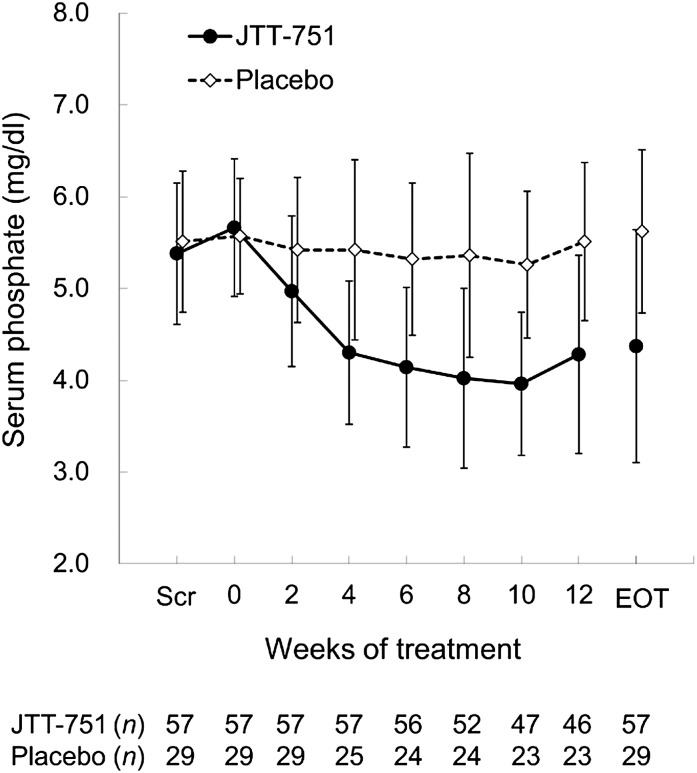
The efficacy parameters values at baseline and EOT are presented in Table 2. At baseline, serum phosphate concentrations in the JTT-751 and placebo groups were similar. Serum phosphate at the EOT was significantly reduced from baseline by −1.29 mg/dl in the JTT-751 group (P<0.001) and unchanged in the placebo group (0.06 mg/dl; P=0.66) (Figure 2). The least squares mean of the difference between groups was −1.31 mg/dl (95% confidence, −1.80 to −0.82 mg/dl, P<0.001). Estimates of protein intake were approximately equal between groups at baseline (Table 3). Change in estimated protein intake in the JTT-751 group was similar to change in estimated protein intake in the placebo group (P=0.75). The percentage of patients achieving target serum phosphate levels at the EOT was 64.9% in the JTT-751 group and 6.9% in the placebo group (P<0.001).
Table 2.
Efficacy parameter values (full analysis set analysis population)
| Parameter | JTT-751 (n=57) | Placebo (n=29) | Intergroup Differenceb; P Value | ||||
|---|---|---|---|---|---|---|---|
| BL | EOT | Change from BLa; P Value | BL | EOT | Change from BLa; P Value | ||
| P (mg/dl) | 5.66±0.75 | 4.37±1.27 | −1.29 (−1.63 to −0.96); <0.001c | 5.57±0.63 | 5.62±0.89 | 0.06 (−0.20 to 0.31); 0.66c | −1.31 (−1.80 to −0.82); <0.001d |
| Ca (mg/dl) | 8.61±0.52 | 8.82±0.57 | 0.21 (0.07 to 0.34); 0.004c | 8.57±0.44 | 8.57±0.43 | −0.01 (−0.14 to 0.13); 0.92c | 0.23 (0.03 to 0.42); 0.02d |
| Ca×P (mg2/dl2) | 48.75±6.90 | 38.27±10.30 | −10.47 (−13.26 to −7.68); <0.001c | 47.68±5.28 | 48.02±6.83 | 0.34 (−1.59 to 2.28); 0.72c | −10.33 (−14.27 to −6.40); <0.001d |
| uPe (mg/d) | 380.6 (289.8, 519.6) | 211.2 (129.6, 307.1) | −192.8 (−284, −136.8); <0.001f | 384 (272.8, 516) | 355.1 (263.5, 450) | −71 (−114.4, 19.1); 0.03f | <0.001g |
| iPTH (pg/ml) | 246 (129, 364) | 193 (117, 302) | −25 (−111, 21); 0.001f | 236 (156, 331) | 214 (128, 339) | 7 (−30, 48); 0.67f | 0.03g |
| FGF-23 (pg/ml) | 453 (215, 800) | 209 (124, 510) | −142 (−365, −40); <0.001f | 358 (237, 656) | 478 (239, 1100) | 67 (−17, 541); 0.002f | <0.001g |
Data are expressed as mean±SD or median (25th, 75th percentile interval). BL, baseline (the day of the start of treatment except for uP [week −2 to the day of the start of treatment]); EOT, end of treatment (week 12 or the observation day at the time of discontinuation); P, phosphate; Ca, calcium; uP, urinary phosphate; iPTH, intact PTH.
Values of change from BL are expressed as mean (95% confidence interval), except for uP, iPTH, and FGF-23, which are expressed as median (25th, 75th percentile).
Values of intergroup difference in P, Ca, and Ca×P are expressed as least squares mean difference (95% confidence interval).
Paired t test.
Analysis of covariance model.
Full analysis set population is 53 patients in the JTT-751 group and 26 patients in the placebo group. Urine samples were obtained from 1-day urine collection.
Wilcoxon signed rank test.
Wilcoxon rank-sum test.
Figure 2.
Serum phosphate levels decreased during 12-week treatment with JTT-751, but were unchanged in the placebo group. Data are expressed as mean±SD. EOT, end of treatment (week 12 or the observation day at the time of discontinuation); Scr, the initial screening date.
Table 3.
Special clinical laboratory evaluations (safety analysis population)
| Parameter | JTT-751 | Placebo | Intergroup Difference P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | BL | EOT | P Value | n | BL | EOT | P Value | ||
| Serum iron (μg/dl) | 60 | 71.5±27.2 | 105.2±46.7 | <0.001a | 30 | 64.2±32.4 | 70.0±32.6 | 0.42a | 0.001b |
| Ferritin (ng/ml) | 60 | 69.00±50.92 | 204.01±106.54 | <0.001a | 30 | 105.98±95.58 | 93.66±82.70 | 0.45a | <0.001b |
| TIBC (μg/dl) | 60 | 269.2±42.8 | 242.0±34.9 | <0.001a | 30 | 263.4±48.0 | 264.0±37.5 | 0.61a | <0.001b |
| TSAT (%) | 60 | 27.22±11.30 | 44.19±20.88 | <0.001a | 30 | 24.99±12.75 | 27.03±12.60 | 0.70a | <0.001b |
| Hemoglobin (g/dl) | 60 | 10.25±1.46 | 10.71±1.88 | 0.04c | 30 | 10.52±1.27 | 10.58±1.26 | 0.74c | 0.23d |
| 1,25-(OH)2 VDe (pg/ml) | 57 | 17.66±8.96 | 18.12±6.99 | 0.65c | 29 | 17.45±7.83 | 19.18±10.01 | 0.19c | 0.46d |
| 25-OH VDe (ng/ml) | 57 | 21.3±7.6 | 21.9±7.7 | 0.38c | 29 | 20.9±8.0 | 20.6±8.5 | 0.78c | 0.46d |
| Cystatin C (mg/L) | 60 | 4.526±0.778 | 4.692±1.007 | 0.03c | 30 | 4.369±0.668 | 4.516±0.748 | 0.09c | 0.88d |
| eGFR (ml/min per 1.73 m2) | 60 | 8.61±3.93 | 7.86±4.32 | 0.001c | 30 | 9.79±8.23 | 8.98±7.34 | 0.03c | 0.88d |
| uCa (mg/d) | 55 | 27.33±17.73 | 26.64±16.57 | 0.72c | 27 | 19.89±12.09 | 24.04±16.40 | 0.22c | 0.17d |
| ePIe (g/d) | 49 | 41.80±15.37 | 38.17±11.14 | 0.01c | 26 | 40.38±12.46 | 37.54±12.59 | 0.23c | 0.75d |
Data are expressed as mean±SD. BL, baseline; TIBC, total iron-binding capacity; TSAT, transferring saturation; 1,25-(OH)2 VD, 1,25-dihydroxy vitamin D; 25-OH VD, 25-hydroxy vitamin D; uCa, urinary calcium; ePI, estimated protein intake.
Wilcoxon signed rank test.
Wilcoxon rank-sum test.
Paired t test
t test.
Data are expressed as full analysis set population.
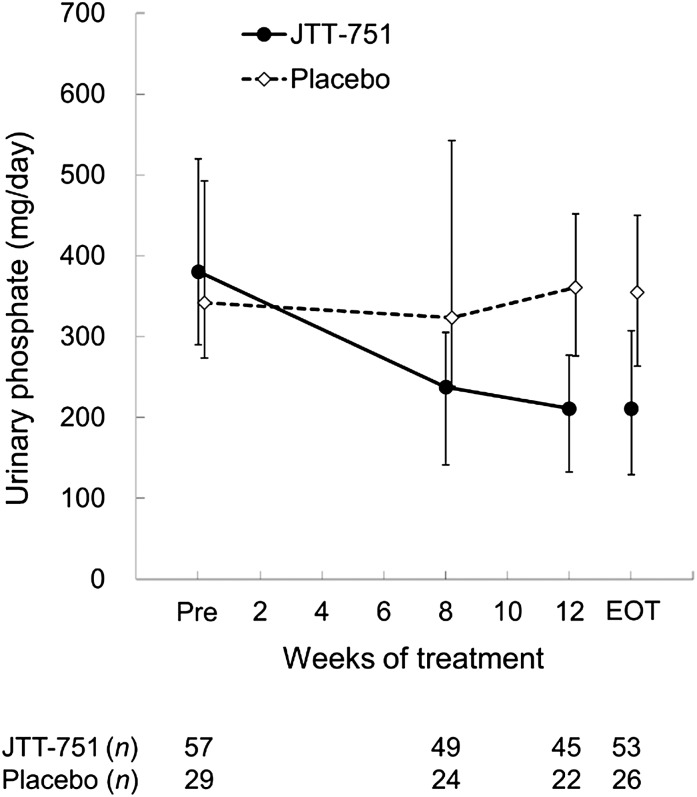
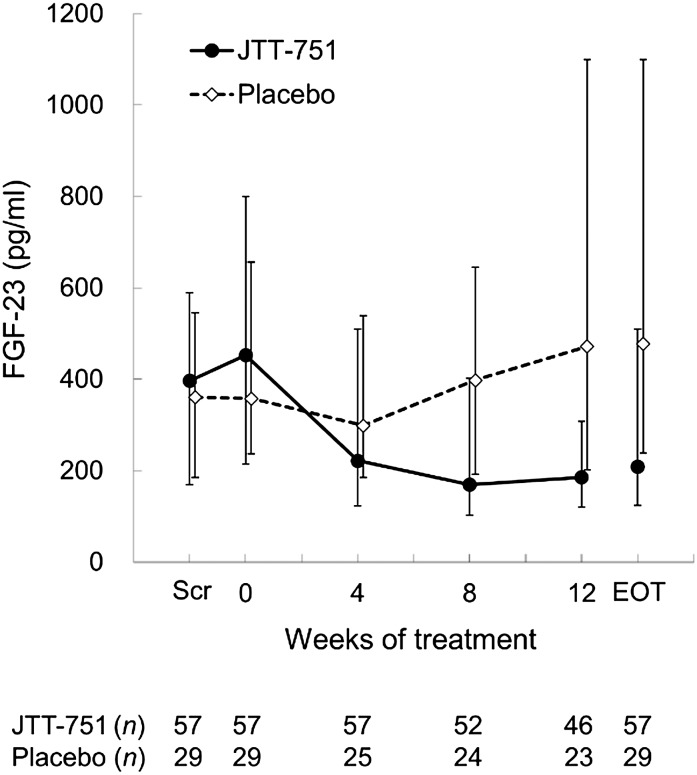
The change (median) in urinary phosphate excretion from baseline to the EOT was −192.76 mg/d in the JTT-751 group (P<0.001), with significant differences between groups (P<0.001) (Figure 3). FGF-23 at the EOT was significantly reduced from baseline by −142.0 pg/ml (median) in the JTT-751 group (P<0.001), whereas it significantly increased by 67.0 pg/ml in the placebo group (P=0.002), with significant intergroup differences (P<0.001) (Figure 4). In the JTT-751 group, intact PTH and corrected serum calcium were significantly reduced and increased, respectively, compared with the levels in the placebo group (P=0.03 and P=0.02, respectively). The change in calcium–phosphate product was consistent with the change in serum phosphate.
Figure 3.
Urinary phosphate excretion reduced by 50% during 12-week treatment with JTT-751. Data are expressed as median (25th or 75th percentile interval). Pre, before treatment (week −2 to the day of the start of treatment).
Figure 4.
The levels of fibroblast growth factor-23 (FGF-23) decreased during 12-week treatment with JTT-751, but increased in the placebo group. Data are expressed as median (25th or 75th percentile interval).
Change in Iron Parameters and Hemoglobin
Treatment with JTT-751 resulted in significant increases in serum iron, ferritin, and transferrin saturation and a decrease in total iron-binding capacity (Table 3) (P=0.001 or P<0.001 for difference between groups). In addition, although there was no significant difference between groups, hemoglobin concentration increased from 10.3 to 10.7 g/dl (P=0.04) in the JTT-751 group.
Safety
In total, 90 patients (JTT-751, n=60; placebo, n=30) were included in the safety analysis set. Nine severe AEs occurred in eight patients (13.3%) in the JTT-751 group, and four severe AEs occurred in three patients (10.0%) in the placebo group (Table 4). There was one death caused by hyponatremia (JTT-751), which was deemed unrelated to the study medication. Overall, 70% of patients treated with JTT-751 and 60% of patients treated with placebo experienced an AE. Five patients discontinued participation because of AEs in the JTT-751 group versus one patient in the placebo group.
Table 4.
Summary of adverse events in safety analysis population
| AEsa | JTT-751 (n=60) | Placebo (n=30) | ||
|---|---|---|---|---|
| Patients | Percent | Patients | Percent | |
| Any AE | 41 | 68.3 | 18 | 10.0 |
| Any serious AEb | 8 | 13.3 | 3 | 10.0 |
| Any adverse drug reaction | 19 | 31.7 | 8 | 26.7 |
| Adverse drug reactionsc | ||||
| Diarrhea | 8 | 13.3 | 2 | 6.7 |
| Constipation | 7 | 11.7 | 2 | 6.7 |
| Abdominal discomfort | 3 | 5.0 | 3 | 10.0 |
| Abdominal distension | 3 | 5.0 | 0 | |
| Duodenal ulcer | 2 | 3.3 | 0 | |
| Nausea | 1 | 1.7 | 2 | 6.7 |
AE, adverse event.
Medical Dictionary for Regulatory Activities version 13.1 preferred terms.
Except death (one patient in the JTT-751 group), nine serious AEs (duodenal ulcer, azotemia, congestive cardiac failure, shunt stenosis, gouty arthritis, nephrogenic anemia, cellulitis, chronic renal failure, and bronchopneumonia) occurred in the JTT-751 group and four serious AEs (bacterial gastroenteritis, contusion, chronic GN, and mycoplasma infection) occurred in the placebo group.
Adverse drug reactions occurring in two or more patients in either treatment group are listed.
In total, 35 adverse drug reactions (ADRs) occurred in 19 patients (31.7%) in the JTT-751 group, whereas 12 ADRs occurred in 8 patients (26.7%) in the placebo group. In both groups, the most common ADR was gastrointestinal disorder. In the JTT-751 group, the frequencies of diarrhea (13.3%), constipation (11.7%), and abdominal distension (5.0%) were higher than in the placebo group (6.7%, 6.7%, and 0%, respectively).
Discussion
The results of this study show, in patients with nondialysis-dependent CKD and hyperphosphatemia, that treatment with JTT-751 resulted in a clinically and statistically significant decrease in serum phosphate. Furthermore, nearly two thirds of patients achieved a serum phosphate below the upper limit of normal (4.5 mg/dl), a result consistent with the KDIGO Clinical Practice Guidelines for CKD-MBD, which recommend maintaining serum phosphate of nondialysis patients with CKD (stages 3–5) within the normal range (13).
In contrast to patients on dialysis, nondialysis patients with CKD still maintain some degree of residual kidney function and therefore, retain the ability to excrete excess phosphate into the urine. Although patients with CKD absorb less phosphate (and thus, excrete less phosphate) compared with normal healthy subjects, when in steady state, the amount of phosphate that is excreted into the urine reflects the amount of phosphate absorbed from the intestine (6). Treatment with JTT-751 resulted in almost 50% reduction of urinary phosphate excretion, showing potency in limiting intestinal phosphate absorption.
Increases in serum phosphate≥4.0 mg/dl have been associated with a variety of important AEs, including progression of underlying CKD, development of ESRD, attenuation of the protective effects of renin angiotensin aldosterone inhibitors, cardiovascular events, and all-cause mortality (12,25,26). However, no randomized trial of phosphate lowering has yet shown any improvement in these hard clinical end points. A recent 9-month double blind, randomized, controlled trial of all three commercially available phosphate binders in patients with nondialysis-dependent CKD showed an approximate reduction of urine phosphate by 20%–25%, which is only a modest ability of these drugs to lower serum phosphate (a mean change of 0.3 mg/dl over 9 months) and suggested the possibility of increased harm related to excessive calcium intake (16). Thus, the ability of this novel noncalcium phosphate binder to effectively reduce urinary phosphate excretion by 50% and reduce serum phosphate into the normal range is promising for the management of CKD-MBD in patients with nondialysis-dependent CKD.
FGF-23 is an endocrine hormone that is secreted from osteocytes and osteoblasts. Its main physiologic actions are to increase urinary phosphate excretion by downregulating the luminal expression of sodium–phosphate cotransporters in the proximal renal tubules and decrease synthesis of 1,25(OH)2 vitamin D by inhibiting 1-α-hydroxylase in the kidney (27). In patients with CKD, PTH and FGF-23 are increased as a compensatory response to maintain the phosphate balance from the early stages of CKD (2–5). It has recently been suggested that hyperphosphatemia and FGF-23 are risks for vascular calcification, CKD progression, and death (28–32).
In this 12-week study, treatment with JTT-751 gave a statistically significant reduction of PTH with a simultaneous and noticeable (nearly 50%) reduction in FGF-23.
There are clinical studies that have investigated the effect of administration of phosphate binders on FGF-23 in CKD patients on hemodialysis who had hyperphosphatemia or nondialysis patients with CKD who had normal or increased serum phosphate (33–36). Block et al. (16), in a pilot clinical trial conducted in nondialysis patients with CKD, found that sevelamer carbonate significantly decreased FGF-23, calcium acetate significantly increased FGF-23, and lanthanum carbonate caused no significant change compared with placebo. These findings suggest that, although various phosphate binders may have similar effects on binding phosphate in the gastrointestinal tract and urinary excretion, the effect on FGF-23 differs by the type of phosphate binder. The potency of JTT-751, shown by the reduction of both serum phosphate and urinary phosphate excretion, may underlie the more substantial effect of JTT-751 on FGF-23 compared with previous trials. However, it is also now recognized that iron deficiency anemia stimulates FGF-23 synthesis and that administration of intravenous iron preparations has an immediate effect on circulating levels of FGF-23 (37–39). One intravenous iron preparation was found to increase FGF-23—resulting in increased urinary phosphate excretion and a decrease in serum phosphate (39). Given the apparent efficacy with which JTT-751 reduces urinary and serum phosphate as well as FGF-23, it will be compelling to see if future long-term trials of JTT-751 in patients with CKD show attenuated progression of kidney disease, vascular calcification, or incidence of cardiovascular events. Furthermore, additional studies in different ethnic populations might be required because of the difference in the restriction therapy of dietary protein for CKD patients.
The most common ADRs with JTT-751 were gastrointestinal disorders, such as diarrhea and constipation. Most symptoms were mild, and there was no substantial difference in the occurrence of ADRs between the JTT-751 and placebo groups. Sevelamer frequently causes constipation and abdominal distension, and lanthanum frequently causes nausea and vomiting, whereas the occurrence of these ADRs with JTT-751 treatment was infrequent.
The effect of JTT-751 on iron parameters is potentially a clinically important ancillary effect. The KDIGO Clinical Practice Guideline for Anemia in CKD recommends that ferritin be ≤500 ng/ml (and transferrin saturation be ≤30%) when intravenous iron preparations are used (40). In the JTT-751 group, the ferritin concentration at the EOT was 204.01±106.54 ng/ml, significantly higher than baseline (P<0.001). However, the ferritin concentration did not exceed 500 ng/ml during the 12-week treatment period with JTT-751, and there were no AEs or changes in liver function test results that were thought to be caused by iron overload. It has been presumed that iron overload is less likely to occur in the setting of oral versus intravenous iron administration because of the intact ability to regulate intestinal iron absorption through the action of hepcidin and other factors (41,42). The absorption of some of the ingested iron was clearly used for hematopoiesis—shown by the significant increase in hemoglobin of 0.5 g/dl in the JTT-751–treated group. The long-term administration of JTT-751 might reduce the need for intravenous iron or erythropoietin-stimulating agents to support erythropoiesis. However, we recognize that, although there are theoretical advantages to oral iron delivery (versus intravenous; such as reduced inflammatory and oxidative stress and intact intestinal regulation of iron absorption), it will be critical to further assess the long-term safety of iron-based phosphate binders with regard to indices of iron overload (43). Therefore, iron status in patients treated with JTT-751 should be regularly monitored. Additionally, citrate, which is a component of JTT-751, has been shown to markedly increase intestinal absorption of aluminum (44,45). Use of aluminum is, in general, contraindicated in the setting of CKD.
In conclusion, we have shown in this 12-week placebo controlled trial that JTT-751 effectively reduces serum phosphate in nondialysis-dependent patients with CKD with hyperphosphatemia, with a safety profile and tolerability similar to placebo. Most patients were able to achieve normal serum phosphate concentrations with simultaneous reductions in FGF-23, increases in serum iron, and improvements in CKD-related anemia. Although additional studies are required to assess the longer-term safety, all of these biochemical effects suggest that JTT-751 may be an important addition to the current therapeutic options to treat CKD-MBD at earlier stages of kidney dysfunction, and indeed, it may offer an opportunity to test the role of CKD-MBD on the exaggerated cardiovascular risk seen in this patient population.
Disclosures
K.Y. has received research funding and consulting fees from Japan Tobacco, Inc. H.H., T.A., M.F., M.N., K.S., and Y.K. have received consulting fees from Japan Tobacco, Inc.
Acknowledgments
We thank the physicians, nurses, and patients of the participating centers for their support. We acknowledge the editorial support of Emedits Global (www.emedits.com).
Financial support for this study was provided by Japan Tobacco, Inc.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Ritz E, Gross ML: Hyperphosphatemia in renal failure. Blood Purif 23: 6–9, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Jüppner H, Wolf M: Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16: 2205–2215, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL: Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16: 520–528, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL: Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int 71: 31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Craver L, Marco MP, Martínez I, Rue M, Borràs M, Martín ML, Sarró F, Valdivielso JM, Fernández E: Mineral metabolism parameters throughout chronic kidney disease stages 1-5—achievement of K/DOQI target ranges. Nephrol Dial Transplant 22: 1171–1176, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Russo D, Corrao S, Miranda I, Ruocco C, Manzi S, Elefante R, Brancaccio D, Cozzolino M, Biondi ML, Andreucci VE: Progression of coronary artery calcification in predialysis patients. Am J Nephrol 27: 152–158, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Adeney KL, Siscovick DS, Ix JH, Seliger SL, Shlipak MG, Jenny NS, Kestenbaum BR: Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol 20: 381–387, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G, Cholesterol And Recurrent Events Trial Investigators : Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 112: 2627–2633, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Eddington H, Hoefield R, Sinha S, Chrysochou C, Lane B, Foley RN, Hegarty J, New J, O’Donoghue DJ, Middleton RJ, Kalra PA:Serum phosphate and mortality in patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 2251–2257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovesdy CP, Anderson JE, Kalantar-Zadeh K: Outcomes associated with serum phosphorus level in males with non-dialysis dependent chronic kidney disease. Clin Nephrol 73: 268–275, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Zoccali C, Ruggenenti P, Perna A, Leonardis D, Tripepi R, Tripepi G, Mallamaci F, Remuzzi G, REIN Study Group : Phosphate may promote CKD progression and attenuate renoprotective effect of ACE inhibition. J Am Soc Nephrol 22: 1923–1930, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 113: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Fukagawa M, Yokoyama K, Koiwa F, Taniguchi M, Shoji T, Kazama JJ, Komaba H, Ando R, Kakuta T, Fujii H, Nakayama M, Shibagaki Y, Fukumoto S, Fujii N, Hattori M, Ashida A, Iseki K, Shigematsu T, Tsukamoto Y, Tsubakihara Y, Tomo T, Hirakata H, Akizawa T, CKD-MBD Guideline Working Group. Japanese Society for Dialysis Therapy : Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial 17: 247–288, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Russo D, Miranda I, Ruocco C, Battaglia Y, Buonanno E, Manzi S, Russo L, Scafarto A, Andreucci VE: The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int 72: 1255–1261, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, Allison MA, Asplin J, Smits G, Hoofnagle AN, Kooienga L, Thadhani R, Mannstadt M, Wolf M, Chertow GM: Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 23: 1407–1415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ketteler M, Rix M, Fan S, Pritchard N, Oestergaard O, Chasan-Taber S, Heaton J, Duggal A, Kalra PA: Efficacy and tolerability of sevelamer carbonate in hyperphosphatemic patients who have chronic kidney disease and are not on dialysis. Clin J Am Soc Nephrol 3: 1125–1130, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sprague SM, Abboud H, Qiu P, Dauphin M, Zhang P, Finn W: Lanthanum carbonate reduces phosphorus burden in patients with CKD stages 3 and 4: A randomized trial. Clin J Am Soc Nephrol 4: 178–185, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tonelli M, Pannu N, Manns B: Oral phosphate binders in patients with kidney failure. N Engl J Med 362: 1312–1324, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Hutchison AJ, Barnett ME, Krause R, Kwan JT, Siami GA, SPD405-309 Lanthanum Study Group : Long-term efficacy and safety profile of lanthanum carbonate: Results for up to 6 years of treatment. Nephron Clin Pract 110: c15–c23, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinsakul M, Sika M, Koury M, Shapiro W, Greene T, Dwyer J, Smith M, Korbet S, Lewis J, Collaborative Study Group : The safety and tolerability of ferric citrate as a phosphate binder in dialysis patients. Nephron Clin Pract 121: c25–c29, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama K, Hirakata H, Akiba T, Sawada K, Kumagai Y: Effect of oral JTT-751 (ferric citrate) on hyperphosphatemia in hemodialysis patients: Results of a randomized, double-blind, placebo-controlled trial. Am J Nephrol 36: 478–487, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR : Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Maroni BJ, Steinman TI, Mitch WE: A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int 27: 58–65, 1985 [DOI] [PubMed] [Google Scholar]
- 25.Sim JJ, Bhandari SK, Smith N, Chung J, Liu IL, Jacobsen SJ, Kalantar-Zadeh K: Phosphorus and risk of renal failure in subjects with normal renal function. Am J Med 126: 311–318, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D’Agostino RB, Sr., Gaziano JM, Vasan RS: Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 167: 879–885, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Wolf M: Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int 82: 737–747, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desjardins L, Liabeuf S, Renard C, Lenglet A, Lemke HD, Choukroun G, Drueke TB, Massy ZA, European Uremic Toxin (EUTox) Work Group : FGF23 is independently associated with vascular calcification but not bone mineral density in patients at various CKD stage. Osteoporos Int 23: 2017–2025, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, König P, Kraatz G, Mann JF, Müller GA, Köhler H, Riegler P, MMKD Study Group : Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: The Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol 18: 2600–2608, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, Chonchol M, HOST Investigators : FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 22: 1913–1922, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koiwa F, Kazama JJ, Tokumoto A, Onoda N, Kato H, Okada T, Nii-Kono T, Fukagawa M, Shigematsu T, ROD21 Clinical Research Group : Sevelamer hydrochloride and calcium bicarbonate reduce serum fibroblast growth factor 23 levels in dialysis patients. Ther Apher Dial 9: 336–339, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Oliveira RB, Cancela AL, Graciolli FG, Dos Reis LM, Draibe SA, Cuppari L, Carvalho AB, Jorgetti V, Canziani ME, Moysés RM: Early control of PTH and FGF23 in normophosphatemic CKD patients: A new target in CKD-MBD therapy? Clin J Am Soc Nephrol 5: 286–291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez-Parra E, Gonzalez-Casaus ML, Galán A, Martinez-Calero A, Navas V, Rodriguez M, Ortiz A: Lanthanum carbonate reduces FGF23 in chronic kidney disease Stage 3 patients. Nephrol Dial Transplant 26: 2567–2571, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Yilmaz MI, Sonmez A, Saglam M, Yaman H, Kilic S, Eyileten T, Caglar K, Oguz Y, Vural A, Yenicesu M, Mallamaci F, Zoccali C: Comparison of calcium acetate and sevelamer on vascular function and fibroblast growth factor 23 in CKD patients: A randomized clinical trial. Am J Kidney Dis 59: 177–185, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Hryszko T, Rydzewska-Rosolowska A, Brzosko S, Koc-Zorawska E, Mysliwiec M: Low molecular weight iron dextran increases fibroblast growth factor-23 concentration, together with parathyroid hormone decrease in hemodialyzed patients. Ther Apher Dial 16: 146–151, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Takeda Y, Komaba H, Goto S, Fujii H, Umezu M, Hasegawa H, Fujimori A, Nishioka M, Nishi S, Fukagawa M: Effect of intravenous saccharated ferric oxide on serum FGF23 and mineral metabolism in hemodialysis patients. Am J Nephrol 33: 421–426, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Wolf M, Koch TA, Bregman DB: Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res 28: 1793–1803, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Drüeke TB, Parfrey PS: Summary of the KDIGO guideline on anemia and comment: Reading between the (guide)line(s). Kidney Int 82: 952–960, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Nemeth E: Iron regulation and erythropoiesis. Curr Opin Hematol 15: 169–175, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Kemna EH, Tjalsma H, Willems HL, Swinkels DW: Hepcidin: From discovery to differential diagnosis. Haematologica 93: 90–97, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Vaziri ND: Understanding iron: Promoting its safe use in patients with chronic kidney failure treated by hemodialysis. Am J Kidney Dis 61: 992–1000, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Coburn JW, Mischel MG, Goodman WG, Salusky IB: Calcium citrate markedly enhances aluminum absorption from aluminum hydroxide. Am J Kidney Dis 17: 708–711, 1991 [DOI] [PubMed] [Google Scholar]
- 45.Drüeke TB: Intestinal absorption of aluminium in renal failure. Nephrol Dial Transplant 17[Suppl 2]: 13–16, 2002 [DOI] [PubMed] [Google Scholar]