Abstract
Background and objectives
The Avosentan on Time to Doubling of Serum Creatinine, End Stage Renal Disease or Death (ASCEND) trial tested the renoprotective effect of the endothelin receptor antagonist avosentan in patients with diabetes and nephropathy, but the study was terminated due to an excess of congestive heart failure (CHF) events in the avosentan arms, likely due to fluid retention. The aim of this study was to identify risk markers of CHF after treatment with avosentan.
Design, setting, participants, & measurements
In a post hoc analysis of the ASCEND trial (N=1392 participants), we assessed which baseline characteristics predicted CHF risk during avosentan treatment. Furthermore, postrandomization changes between baseline and the first available measurement of body weight and hemoglobin were examined as potential clinical indicators of fluid retention for their relationship with CHF development.
Results
Relative to placebo, avosentan increased CHF risk (hazard ratio, 2.76; 95% confidence interval, 1.68 to 4.54). The avosentan-related CHF risk was higher with lower baseline cholesterol levels (P interaction=0.003) and concomitant statin use (P interaction=0.06), whereas it was lower with a lower estimated GFR (P interaction=0.04). Patients allocated to avosentan had a median body weight increase of 0.6 kg (interquartile range, 0.0 to 2.0 kg) and a median hemoglobin decrease of 1.4 g/dl (interquartile range, −2.1 to −0.7 g/dl) at the first postrandomization measurement. The body weight increase induced by avosentan was associated with CHF development (P interaction=0.04), whereas hemoglobin decrease was not (P interaction=0.64). The increase in body weight was particularly pronounced in patients with a cardiovascular disease history and in patients using statins.
Conclusions
In avosentan-treated patients, body weight increase, but not hemoglobin decrease, was associated with CHF development, indicating that close body weight monitoring could provide an early signal of CHF development in future trials with endothelin receptor antagonists.
Introduction
In recent years, several clinical trials involving individuals with type 2 diabetes reported harmful effects of investigational drugs on cardiovascular outcomes, despite a promising efficacy profile on surrogate markers (1–4). An often-cited example is the peroxisome proliferator–activated receptor agonist (PPAR) rosiglitazone, which has a favorable effect on glucose metabolism but harbors an increased risk for cardiovascular outcomes (1,5). Part of this risk is due to the fact that PPARs induce an increase in sodium uptake in the kidney, which may result in fluid retention, peripheral edema, and, ultimately, congestive heart failure (CHF) (6). The risk is exacerbated in patients with diabetes because these individuals are more prone to retaining sodium (7).
A large hard outcome trial, Avosentan on Time to Doubling of Serum Creatinine, End Stage Renal Disease or Death (ASCEND), was recently terminated due to an excess of CHF events in the active treatment arm. ASCEND was a randomized double-blind, placebo-controlled parallel group study to assess the effect of the endothelin receptor antagonist (ERA) avosentan on delaying the time to doubling of serum creatinine, ESRD, or death in patients with type 2 diabetes mellitus and nephropathy (8). Earlier clinical studies in patients with heart failure had indicated that ERAs (e.g., bosentan, ambrisentan) caused fluid retention, as signaled by body weight increase, peripheral edema, and a decrease in hemoglobin levels (9,10). Fluid retention was also reported in a pilot study with avosentan, especially at the highest dosage of 50 mg/d (11). Despite these findings, no specific risk management plan was specified in the ASCEND trial to prevent or manage fluid retention.
The unfavorable risk profile of avosentan prompted us to conduct a post hoc analysis of the ASCEND trial. The aim of the analysis was to identify baseline characteristics associated with CHF development. Moreover, we assessed whether postrandomization changes in clinical indicators of fluid retention could be used to identify patients at increased CHF risk. We paid specific attention to changes in body weight and hemoglobin, because significant changes in these markers were previously observed during treatment with ERAs as well as PPARs (6,11,12). The analysis serves as an impetus for clinical trial design of other agents with similar fluid retention risk profiles, especially for ERAs and PPARs that are currently in clinical development for patients with diabetes and nephropathy (13,14).
Materials and Methods
Patients
ASCEND was an international randomized double-blind, placebo-controlled clinical trial (Clinicaltrials.gov NCT0012038) that compared the effect of two doses of avosentan (25 mg/d and 50 mg/d) versus placebo in patients with type 2 diabetes and overt nephropathy. The complete study design, inclusion/exclusion criteria, and treatment protocol were reported previously (8). In short, all patients were taking conventional therapy for diabetic nephropathy that had to include an angiotensin converting enzyme inhibitor, an angiotensin receptor blocker, or their combination for at least 6 months before screening. Major inclusion criteria were a urinary albumin-to-creatinine ratio≥300 mg/g, a serum creatinine level between 1.3 and 3.0 mg/dl in men and between 1.2 and 3.0 mg/dl in women, a diabetes duration>3 years, and treatment with oral antidiabetic agents or insulin. Exclusion criteria included type 1 diabetes mellitus, proteinuria of known nondiabetic origin, renal transplant, estimated GFR (eGFR)≤15 ml/min per 1.73 m2, sitting BP≥160/100 mmHg with or without antihypertensive medication, recent (60 days) history of acute myocardial infarction, unstable angina, stroke or transient ischemic attack, and coronary interventions or a New York Heart Association class III or IV score. Participants were randomized 2 weeks after screening and were followed-up monthly thereafter. BP, body weight, and adverse events were recorded at each visit. Most plasma and urine parameters, including hemoglobin, were measured at baseline and every 3 months thereafter. Due to the early termination of the trial, the median follow-up was 4 months in the avosentan arms and 5 months in the placebo arm. At that time, 1392 participants had been randomized. All participants gave written informed consent. Approval from all local and central ethics committees and by regulatory authorities was obtained consistent with the principles of the Declaration of Helsinki.
In this post hoc analysis, we analyzed the association between patient characteristics at baseline and CHF events. In addition, we tested whether postrandomization changes in body weight or hemoglobin were associated with CHF development in the subpopulation of participants who had documented body weight measurement after 1 month of treatment (n=761) and documented hemoglobin measurement after 3 months of treatment (n=727). Previous studies showed that fluid-retaining effects of avosentan can be detected within several days (15), and, therefore, we took the earliest time point of risk marker measurement in the trial.
The primary outcome of this analysis was defined as all CHF events recorded in the study. The Event Adjudication Charter stipulated that for CHF the patient had to have typical signs and/or symptoms of heart failure, receive new therapy for CHF, and be admitted to the hospital for at least 24 hours. We also considered the nonadjudicated CHF events to increase statistical power of the analysis. The nonadjudicated events were taken from the case report forms, which were based on signs and symptoms of CHF as judged by the investigators. Because the reported results were essentially similar for the adjudicated and nonadjudicated CHF events, we combined these events in our primary analysis. We report separate results for adjudicated and nonadjudicated CHF events in the Supplemental Material.
Statistical Analyses
Univariable comparisons of baseline characteristics between patients who developed CHF and those who did not were performed in both the placebo arm and the combined avosentan arm with Wilcoxon rank-sum tests for continuous variables and Fisher exact tests for categorical variables. The cumulative incidence function of CHF was presented by treatment arm, and the treatment effect of avosentan was estimated using Cox proportional hazard regression.
Postrandomization changes in body weight and hemoglobin were computed as the absolute difference between baseline and the first available measurement after treatment start. Changes in risk markers were visualized in box plots and kernel density plots using optimal widths. Statistical differences in body weight change and hemoglobin change between treatment arms were assessed using Wilcoxon rank-sum tests. Cumulative incidence functions of CHF were presented by above or below median increases in body weight and hemoglobin for each treatment arm separately.
Baseline characteristics and postrandomization changes in body weight and hemoglobin were evaluated in a univariable Cox proportional hazard regression. The regression model included interaction terms between treatment and baseline characteristics or postrandomization changes to test whether the treatment effect on CHF was modified by baseline characteristics or mediated by postrandomization changes. In a multivariable Cox proportional hazard regression, we tested whether the risk of CHF was mediated by avosentan-induced body weight change while controlling for the following baseline predictors: age, weight, systolic BP, diastolic BP, LDL cholesterol, hemoglobin A1C, eGFR, albumin-to-creatinine ratio, history of heart failure, history of cardiovascular disease, and concomitant use of calcium channel blockers, β-blockers, statins, diuretics, or insulin.
To assess whether diuretic treatment reversed avosentan-induced body weight increase, we matched all patients taking avosentan who did not receive diuretic treatment at baseline and started diuretics during one of the study visits with a control group that had similar body weight gain, but did not receive diuretics at baseline or during follow-up. We considered patients that started diuretics during any of the study visits. The change in body weight between the visit at which diuretic treatment was initiated and the next scheduled visit was assessed and compared between patients starting diuretics and those not starting diuretics using Wilcoxon rank-sum tests. A P value<0.05 was considered statistically significant. Analyses were conducted with STATA software (version 11.2; StataCorp, College Station, TX).
Results
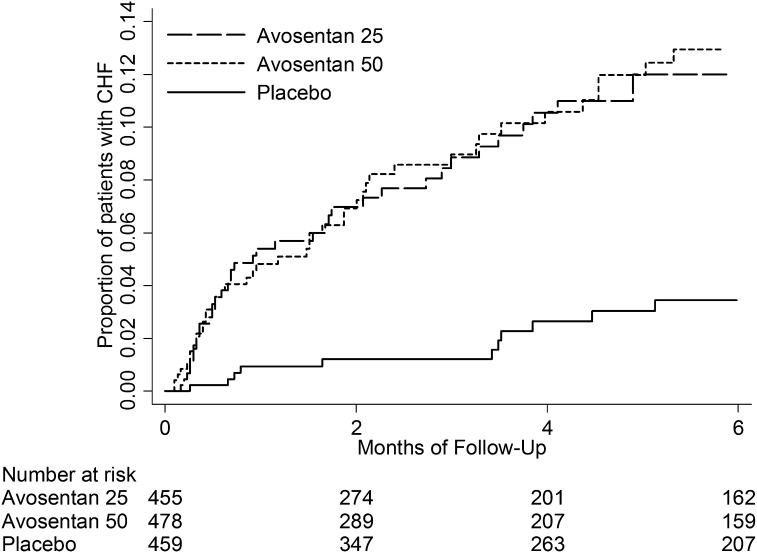
Figure 1 shows the CHF incidence in the avosentan and placebo treatment arms. A total of 19 CHF events occurred in the placebo arm, 43 CHF events occurred in the 25-mg avosentan arm, and 46 CHF events occurred in the 50-mg avosentan arm. The relative risk of developing CHF was significantly higher in both the 25-mg avosentan arm (95% confidence interval [95% CI], 1.64 to 4.95) and the 50-mg avosentan arm (95% CI, 1.64 to 4.78) compared with placebo, but did not differ between avosentan treatment arms (95% CI, 0.76 to 1.55). We therefore combined the avosentan arms for further analysis and found a hazard ratio (HR) for CHF events of 2.76 (95% CI, 1.68 to 4.54) compared with placebo.
Figure 1.
Kaplan–Meier curve showing the cumulative incidence of CHF by avosentan and placebo treatment arm. CHF, congestive heart failure.
Baseline Predictors of Avosentan-Induced CHF
Table 1 depicts the baseline characteristics of participants by treatment allocation and CHF development. Baseline characteristics were typical for patients with stage 3–4 CKD and overt diabetic nephropathy and did not differ between randomized groups (8). In both the placebo and avosentan arms, patients were more likely to develop CHF when they had a history of heart failure and a lower eGFR. We tested whether any of the baseline characteristics modified the risk of CHF related to avosentan treatment. The avosentan-related CHF risk was higher in patients with a lower LDL cholesterol (P interaction=0.003, far right column of Table 1) and in patients with higher eGFR (P interaction=0.04), and tended to be higher in patients treated with statins (P interaction=0.06).
Table 1.
Baseline characteristics by risk of CHF
| Characteristic | Placebo | Avosentan | P for Interactiona | ||||
|---|---|---|---|---|---|---|---|
| No CHF (n=440) | CHF (n=19) | P Value | No CHF (n=844) | CHF (n=89) | P Value | ||
| Age, yr | 60.7 (9.0) | 63.2 (7.3) | 0.29 | 60.9 (9.0) | 63.3 (8.2) | 0.01 | 0.93 |
| Women | 149 (33.9) | 6 (31.6) | 1.00 | 265 (31.4) | 32 (36.0) | 0.40 | 0.68 |
| Physical examination | |||||||
| Systolic BP, mmHg | 135.2 (15.1) | 138.4 (16.2) | 0.29 | 136.8 (13.9) | 139.3 (15.9) | 0.07 | 0.80 |
| Diastolic BP, mmHg | 77.0 (9.6) | 79.8 (8.4) | 0.23 | 77.8 (8.8) | 77.0 (9.6) | 0.78 | 0.09 |
| Weight, kg | 83.7 (20.2) | 90.1 (12.0) | 0.06 | 84.1 (21.0) | 91.1 (19.7) | <0.001 | 0.95 |
| Laboratory results | |||||||
| Hemoglobin A1C, % | 8.0 (1.5) | 7.8 (1.6) | 0.56 | 8.1 (1.5) | 7.7 (1.4) | 0.02 | 0.69 |
| Hemoglobin, g/dl | 12.1 (1.7) | 12.0 (1.9) | 0.85 | 12.2 (1.8) | 12.0 (1.8) | 0.37 | 0.98 |
| LDL cholesterol, mg/dl | 107.1 (47.1) | 128.1 (48.9) | 0.07 | 107.3 (46.6) | 95.4 (41.4) | 0.03 | 0.003 |
| eGFR, ml/min per 1.73 m2 | 33.3 (10.5) | 25.6 (8.9) | 0.001 | 33.7 (11.1) | 31.1 (10.8) | 0.05 | 0.04 |
| ACR, mg/mmolb | 1512 (793–2704) | 2830 (1084–4364) | 0.06 | 1414 (731–2444) | 1718 (906–2664) | 0.11 | 0.26 |
| Disease history | |||||||
| Heart failure | 44 (10.0) | 5 (26.3) | 0.04 | 73 (8.6) | 23 (25.8) | <0.001 | 0.61 |
| Cardiovascular diseasec | 130 (29.5) | 8 (42.1) | 0.31 | 221 (26.2) | 43 (48.3) | <0.001 | 0.31 |
| Edema | 58 (13.2) | 4 (21.1) | 0.31 | 115 (13.6) | 14 (15.7) | 0.63 | 0.42 |
| Medication at baseline | |||||||
| ACEI | 264 (60.0) | 10 (52.6) | 0.63 | 539 (63.9) | 55 (61.8) | 0.73 | 0.83 |
| ARB | 202 (45.9) | 7 (36.8) | 0.49 | 389 (46.1) | 44 (49.4) | 0.58 | 0.26 |
| ACEI+ARB | 65 (14.8) | 2 (10.5) | 1.00 | 150 (17.8) | 17 (19.1) | 0.77 | 0.52 |
| CCB | 242 (55.0) | 12 (63.2) | 0.64 | 440 (52.1) | 56 (62.9) | 0.06 | 0.77 |
| β-Blockers | 199 (45.2) | 13 (68.4) | 0.06 | 372 (44.1) | 48 (53.9) | 0.09 | 0.33 |
| Diuretics | 282 (64.1) | 16 (84.2) | 0.09 | 547 (64.8) | 71 (79.8) | 0.004 | 0.64 |
| Loop diuretics | 197 (44.8) | 12 (63.2) | 0.16 | 380 (45.0) | 57 (64.0) | 0.001 | 0.98 |
| Other diuretics | 85 (19.3) | 4 (21.1) | 0.77 | 167 (19.8) | 14 (15.7) | 0.40 | 0.63 |
| Statin | 255 (58.0) | 9 (47.4) | 0.48 | 442 (52.4) | 58 (65.2) | 0.03 | 0.06 |
| Sulfonylurea | 153 (34.8) | 5 (26.3) | 0.62 | 284 (33.6) | 23 (25.8) | 0.16 | 0.98 |
| Glitazone | 50 (11.4) | 2 (10.5) | 1.00 | 88 (10.4) | 10 (11.2) | 0.86 | 0.87 |
| Insulin | 281 (63.9) | 14 (73.7) | 0.47 | 559 (66.2) | 71 (79.8) | 0.01 | 0.60 |
Data are presented as n (%) or median (interquartile range). Comparison testing was performed with Wilcoxon rank-sum tests for continuous variables and Fisher exact tests for dichotomous variables. CHF, congestive heart failure; eGFR, estimated GFR; ACR, albumin-to-creatinine ratio; ACEI, angiotensin converting enzyme inhibitor; ARB angiotensin receptor blocker; CCB, calcium channel blocker.
P value for interaction term on the base of an unadjusted Cox regression that also includes main terms.
ACR values are log-normalized for testing purposes.
History of cardiovascular disease includes coronary artery disease, myocardial infarction, cerebrovascular accidents.
Postrandomization Mediators of Avosentan-Induced CHF
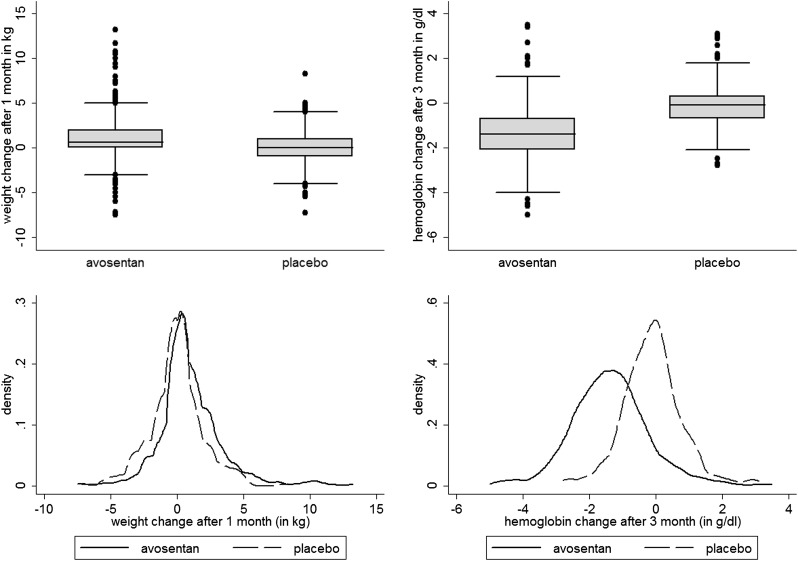
Figure 2 shows changes in body weight (left panel) and hemoglobin (right panel) by treatment arms. Median changes for body weight after 1 month of treatment were 0.0 kg (interquartile range [IQR], −1.0 to 1.0 kg) in the placebo group and 0.6 kg (IQR, 0.0 to 2.0 kg) in the combined avosentan arms. Median changes for hemoglobin after 3 months of treatment were −0.1 g/dl (IQR, −0.7 to 0.3 g/dl) in the placebo group and −1.4 g/dl (IQR, −2.1 to −0.7g/dl) in the combined avosentan arms. For both body weight and hemoglobin change, these differences were significant between placebo and avosentan arms but not between avosentan arms.
Figure 2.
Postrandomization changes in body weight and hemoglobin. Box plots (upper panel) and kernel density plots (lower panel) of body weight change after 1 month of treatment (left panel) and hemoglobin change after 3 months of treatment (right panel).
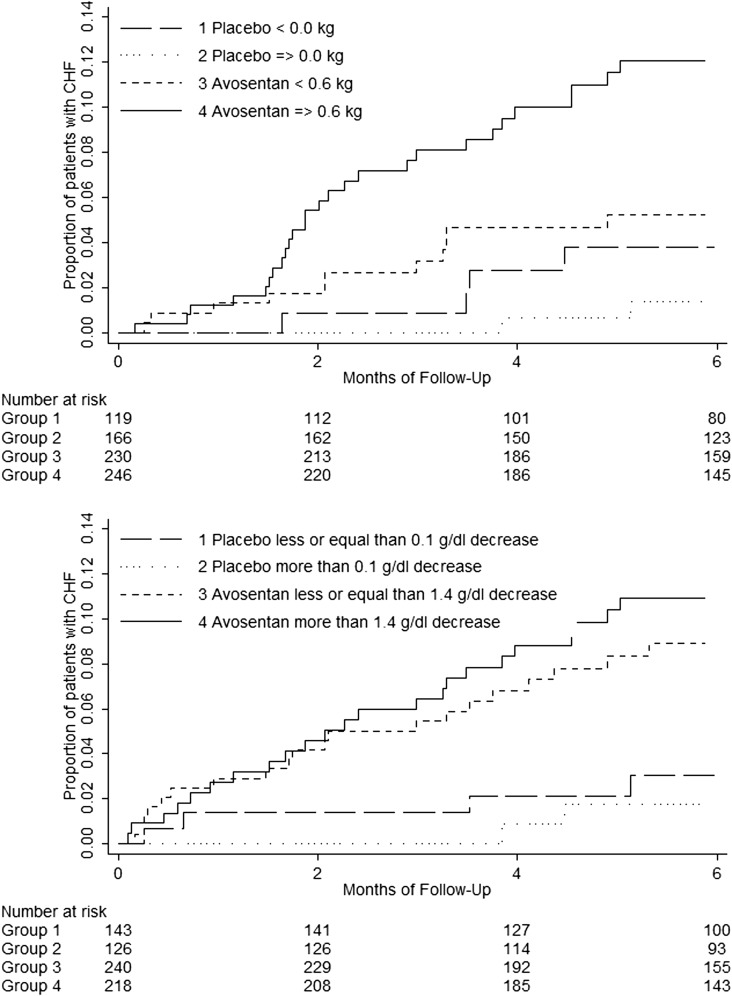
Figure 3 shows the cumulative incidence of CHF stratified by above and below median body weight change after 1 month of treatment (upper panel) and by above and below hemoglobin change after 3 months of treatment (lower panel). Body weight change (per kilogram) was positively associated with CHF risk in the avosentan arm (HR, 1.10; 95% CI, 1.00 to 1.23), but not in the placebo arm (HR, 0.85; 95% CI, 0.65 to 1.11), whereas hemoglobin change (per gram per deciliter) was not associated with CHF risk in the avosentan arm (HR, 0.97; 95% CI, 0.76 to 2.24) or in the placebo arm (HR, 1.12; 95% CI, 0.61 to 2.07). The effect of avosentan-induced body weight change (per kilogram) on CHF development was significant in univariate analysis and persisted when controlling for other significant predictors of CHF (Table 2). After controlling for avosentan-induced body weight change, the HR of avosentan attenuated (2.28; 95% CI, 1.15 to 4.51) (Table 2).
Figure 3.
Higher CHF risk in patients with greater than median body weight change. Kaplan–Meier curves showing the cumulative incidence of CHF by avosentan and placebo treatment arms and greater or smaller than median body weight change (upper panel) or greater or smaller than median hemoglobin change (lower panel). In both panels, CHF risk is higher in the avosentan treatment arm. Among avosentan-allocated patients, however, CHF risk compared with placebo is only higher in those with above median body weight change and not in those with below median body weight change (P for interaction=0.04). In contrast, avosentan-induced CHF risk is higher in patients with either above or below median hemoglobin change (P for interaction=0.64).
Table 2.
Cox proportional hazard regression on risk of CHF in all patients with body weight measurement after 1 month (n=761)
| Risk Marker | Hazard Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| Avosentan | 2.28 (1.15 to 4.51) | 0.02 |
| Age, yr | 1.03 (1.00 to 1.07) | 0.05 |
| Hemoglobin A1C, % | 0.83 (0.68 to 1.00) | 0.06 |
| eGFR, ml/min per 1.73 m2 | 0.98 (0.95 to 1.00) | 0.09 |
| ACR, mg/mmola | 1.39 (0.98 to 1.96) | 0.06 |
| History of cardiovascular disease | 1.97 (1.15 to 3.37) | 0.01 |
| Insulin use | 2.32 (1.17 to 4.59) | 0.02 |
| Weight change after month 1, kg | 0.80 (0.60 to 1.07) | 0.13 |
| Avosentan×weight change after month 1, kg | 1.38 (1.02 to 1.88) | 0.04 |
Backward stepwise regression containing all predictors that showed a relation with CHF in univariable analysis (P<0.10). ACR, albumin-to-creatinine ratio.
ACR values are log-normalized.
We conducted a number of sensitivity analyses to check the robustness of the findings. We included other significant interaction terms in the multivariable analyses. In this model, the interaction term between avosentan and body weight change remained significant (P=0.03), and treatment interactions with eGFR (P=0.04) and LDL cholesterol (P=0.03) were also significant. We also repeated the multivariable analysis, excluding patients that were diagnosed with CHF before or on the first postrandomization study visit. The association between body weight gain and CHF persisted in this model (P=0.04). Subsequently, we also excluded patients that had a previous CHF event before study onset and found a P value for interaction of 0.06.
Baseline Predictors of Avosentan-Induced Body Weight Change
Because avosentan-induced body weight change was associated with CHF risk, we established which baseline characteristics were associated with body weight change during avosentan treatment. We found that the risk of avosentan-induced body weight change was higher with lower LDL cholesterol levels (P interaction=0.03), a history of heart failure (P interaction=0.001), a history of cardiovascular disease (P interaction=0.04), concomitant diuretic use (P interaction = 0.04), and concomitant statin use (P interaction=0.06). Median body weight change was higher in avosentan-treated patients who used statins at baseline (Table 3).
Table 3.
Body weight change between baseline and month 1 by statin use
| Statin | Patients (n) | Median (Interquartile Range) | P Value |
|---|---|---|---|
| Placebo | 285 | 0.0 (−1.0 to 1.0) | <0.001 |
| Avosentan | 476 | 0.6 (0.0 to 2.0) | |
| Avosentan+no statin | 225 | 0.0 (−0.1 to 1.0) | <0.001 |
| Avosentan+statin | 251 | 1.0 (0.0 to 2.5) | |
| Avosentan+simvastatin (major)a | 98 | 1.1 (0.0 to 2.4) | |
| Avosentan+lovastatin (major)a | 29 | 1.1 (0.0 to 2.5) | |
| Avosentan+atorvastatin (moderate)a | 105 | 0.8 (0.0 to 2.6) | 0.44 |
| Avosentan+pravastatin (minor)a | 4 | 2.6 (1.1 to 5.1) | |
| Avosentan+rosuvastatin (no)a | 6 | 0.8 (−1.0 to 1.7) | |
| Avosentan+fluvastatin (no)a | 10 | 0.6 (0.0 to 2.0) |
One patient was treated with both simvastatin and lovastatin. Comparison testing with Wilcoxon rank-sum tests for two groups and with one-way ANOVA for more than two groups.
Degree of CYP3a4 metabolism as discussed by Williams and Feely (25).
Table 4 analyzes whether body weight decreased in patients that started diuretics during avosentan treatment. The 32 patients that started diuretics had a median body weight increase of 1.2 kg between baseline and diuretic start. The rise in body weight stabilized (median 0.0 kg; IQR, −1.8 to 0.5 kg) in the month after diuretic initiation, whereas the body weight of patients that did not start diuretics continued to increase (median 1.0 kg; IQR, 0.0 to 2.2 kg).
Table 4.
Median body weight change in kilograms (interquartile range) in patients that started diuretics during the study (cases) and patients that did not start diuretics but had similar body weight at baseline and body weight change before diuretic start (controls)
| Body Weight | Patients Starting Diuretics (n=32) | Patients Not Starting Diuretics (n=32) | P Value |
|---|---|---|---|
| Baseline | 80.6 (67.6 to 95.6) | 73.5 (66.5 to 89.1) | 0.46 |
| Change before diuretic start | 1.25 (−1 to 2.3) | 1.2 (−1 to 2) | 0.83 |
| Change after diuretic start | 0 (−1.8 to 0.5) | 1 (−0.05 to 2.25) | <0.001 |
Data are presented as the median (interquartile range). Comparison testing with Wilcoxon rank-sum tests for continuous variables.
Discussion
The ASCEND trial showed that avosentan decreased albuminuria by >40%, but at the same time exposed patients to an unacceptable risk of CHF, leading to the termination of the renal development program of this drug. This study explored strategies that could be used to identify patients at high risk for CHF in future trials with ERAs. We found that body weight increase in avosentan-treated patients was an early clinical indicator of fluid retention and CHF development. We also showed that the risk of CHF and body weight increase was higher when avosentan-treated patients had a lower LDL cholesterol level, when they had a higher eGFR, and when they were taking concomitant medication with statins.
Although ASCEND was the first study that showed an increased CHF risk after ERA treatment, earlier studies with both nonselective ERAs (9,10) as well as with endothelin-A selective antagonists (11,16–18) showed clinical signs suggestive of fluid retention. The exact mechanisms responsible for these fluid-retaining effects are not yet fully understood. In humans, activation of endothelin receptor subtype A (ET-A) promotes vasoconstriction, whereas stimulation of endothelin receptor subtype B (ET-B) induces vasodilation. Activation of ET-B receptors also has a natriuretic and diuretic effect through direct inhibition of sodium and water reabsorption (19,20). The increased risk of fluid retention and CHF in the ASCEND trial may therefore be a result of avosentan’s relatively low ET-A selectivity and its ability to block the ET-B receptor when administered at higher doses (19). This idea is supported by a mechanistic study showing that the effect of avosentan on weight gain and hemodilution is particularly present when administered at high doses of 50 mg/d compared with low doses (≤5 mg/d) (15).
Given these findings and the ASCEND study results, in retrospect it seems that the design of ASCEND could have benefited from a better understanding of the drug’s dose response and its associated risk–benefit profile before trial initiation (21,22). The dose-selection issue was further amplified by the enrollment of a vulnerable patient population with overt diabetic nephropathy who are more prone to retain fluid (7). These observations, however, also suggest that the development of ERAs should not yet be completely abandoned, because lower doses of ERAs and more selective ET-A antagonists may exert renoprotective effects without inducing significant fluid retention and CHF risk.
Recommendations
A couple of lessons can be learned from our observations. First, future clinical trials with ERAs should specify protocol guidelines on how to manage and prevent weight gain. Regular and precise measurements of body weight should be part of these protocols because weight gain may serve as an early clinical indicator of fluid retention and CHF development. In the ASCEND trial, body weight was only measured after 1 month of study. We recommend that body weight should be monitored on a weekly basis in future studies because fluid retention may occur directly after treatment start (15). Moreover, patients should be instructed to self-monitor their body weight during clinical trials. These recommendations are similar to the clinical guidelines for use of thiazolidinediones, which also exert sodium-retaining effects and harbor an increased CHF risk (6).
However, given that patients without observed body weight gain also developed CHF in the ASCEND trial, sole monitoring of body weight is not sufficient in itself as a surveillance strategy. We therefore suggest that all patients should also be treated with concomitant diuretic therapy when starting ERA therapy. We showed that patients starting diuretics had a decrease in body weight compared with matched patients that did not start diuretics. This suggests the possibility that concomitant diuretic treatment may be a useful strategy as an adjunct to body weight monitoring. However, prospective studies are required to prove the efficacy of this strategy, given the small number of patients that started diuretics and the post hoc design of this study.
Future studies with drugs that have sodium-retaining effects should also carefully select their patient population, possibly during an active drug run-in period. Specific attention should be paid to vulnerable patient populations that already have an increased risk of CHF irrespective of ERA treatment, including patients with a history of heart failure and impaired renal function. We suggest that patients with New York Heart Association class II–IV scores should be excluded from these studies, and other patients should be carefully selected and monitored on a regular basis using markers such as N-terminal prohormone of brain natriuretic peptide, which is released in the setting of volume overload (23).
Finally, careful monitoring of the interaction between treatment with ERAs and statins is necessary in future trials. This study shows that the use of avosentan in combination with statins resulted in an almost 2-fold increase in median body weight compared with treatment with avosentan alone. An earlier study showed that exposure of avosentan may be increased up to 6-fold in the presence of potent CYP3a4 inhibitors (24). The potential interaction of avosentan with statin use in the ASCEND trial was confirmed by a significant decrease in lipid levels in patients receiving avosentan, suggesting that plasma levels of statins and avosentan were disproportionally high in ASCEND.
Limitations
Our study has limitations. Body weight measurement was only performed after 1 month of study and on just half of the patient population. The subpopulation in whom body weight was measured differed from the population in whom measurements were missing. Fewer patients in the avosentan arm had a body weight measurement at month 1 and those patients had a lower eGFR and were less likely to have a history of CHF. This suggests that body weight was insufficiently monitored in patients at high risk for body weight increase and CHF. Moreover, the protocol did not include specific guidelines on how to measure body weight. Consequently, we observed a large within-patient variability in body weight change. It should, however, be noted that missing data for high-risk patients and poor measurement precision likely led to an underestimation of the reported association between weight gain and CHF risk.
Second, we cannot explain the significant interaction between eGFR and avosentan treatment and are not aware of previous evidence that can substantiate our finding. We speculate that the interaction may be due to altered avosentan pharmacokinetics or pharmacodynamics in patients with low eGFR, but cannot exclude the possibility of a chance finding.
Finally, laboratory measurements, including hemoglobin measurements, were merely performed after 3 months of study. As a result, we assessed the association of hemoglobin change and CHF after 3 months of treatment. However, given the observed weak association, it is unlikely that we would have observed a significant mediating effect of hemoglobin change on CHF development in case earlier measurements were available.
Our data suggest that treatment with avosentan was associated with CHF development. Body weight gain during the first weeks of treatment with avosentan provided an early signal of subsequent CHF development. Monitoring of body weight change during the first weeks of treatment is therefore recommended. These measures are not only applicable to ERAs, but should also be considered for other agents with similar risk profiles.
Disclosures
H.J.L.H. reports having received consultancy fees from Abbvie, Astellas, Johnson & Johnson, Reata Pharmaceuticals, and Vitae; all honoraria are paid to his institution. G.V. reports having received consultancy fees from GlaxoSmithKline, Daichii Sankyo. Mitsubishi Pharma, Abbott, and Roche. J.F.E.M. reports having received consulting and lecture fees from Abbott, NovoNordisk, Novartis, Amgen, Roche, and Boehringer Ingelheim, and other funds through his institution from Roche, Celgene, and the European Union (Framework-7 Grant SysKid 241544). D.d.Z. reports having received consultancy fees from Abbvie, Astellas, Bristol-Meyers Squibb, Hemocue, Johnson & Johnson, Merck Sharpe & Dohme, Novartis, Reata Pharmaceuticals, and Vitae; all honoraria are paid to his institution. G.V. and J.F.E.M. were members of the steering committee of the ASCEND trial.
Acknowledgments
The ASCEND trial was supported by Speedel Ltd. This analysis was conducted independently from the original study sponsor and was supported by a grant from the Dutch Top Institute Pharma (T6-503).
Data in this article were presented at the annual meetings of the American Society of Nephrology, October 30–November 4, 2012, in San Diego, CA, and at the European Association for the Study of Diabetes, September 23–27, 2013, in Barcelona, Spain.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07040713/-/DCSupplemental.
References
- 1.Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR, DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators : Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomised controlled trial. Lancet 368: 1096–1105, 2006 [DOI] [PubMed] [Google Scholar]
- 2.James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, Torp-Pedersen C, Sharma AM, Shepherd GM, Rode RA, Renz CL, SCOUT Investigators : Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med 363: 905–917, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B, ILLUMINATE Investigators : Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 357: 2109–2122, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Persson F, Desai AS, Nicolaides M, Richard A, Xiang Z, Brunel P, Pfeffer MA, ALTITUDE Investigators : Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 367: 2204–2213, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Nissen SE, Wolski K: Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356: 2457–2471, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S, Young LH, Kahn R, American Heart Association. American Diabetes Association : Thiazolidinedione use, fluid retention, and congestive heart failure: A consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation 108: 2941–2948, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Mogensen CE, Christensen CK, Vittinghus E: The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes 32[Suppl 2]: 64–78, 1983 [DOI] [PubMed] [Google Scholar]
- 8.Mann JF, Green D, Jamerson K, Ruilope LM, Kuranoff SJ, Littke T, Viberti G, ASCEND Study Group : Avosentan for overt diabetic nephropathy. J Am Soc Nephrol 21: 527–535, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalra PR, Moon JC, Coats AJ: Do results of the ENABLE (Endothelin Antagonist Bosentan for Lowering Cardiac Events in Heart Failure) study spell the end for non-selective endothelin antagonism in heart failure? Int J Cardiol 85: 195–197, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Battistini B, Berthiaume N, Kelland NF, Webb DJ, Kohan DE: Profile of past and current clinical trials involving endothelin receptor antagonists: The novel “-sentan” class of drug. Exp Biol Med (Maywood) 231: 653–695, 2006 [PubMed] [Google Scholar]
- 11.Wenzel RR, Littke T, Kuranoff S, Jürgens C, Bruck H, Ritz E, Philipp T, Mitchell A, SPP301 (Avosentan) Endothelin Antagonist Evaluation in Diabetic Nephropathy Study Investigators : Avosentan reduces albumin excretion in diabetics with macroalbuminuria. J Am Soc Nephrol 20: 655–664, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karalliedde J, Buckingham R, Starkie M, Lorand D, Stewart M, Viberti G, Rosiglitazone Fluid Retention Study Group : Effect of various diuretic treatments on rosiglitazone-induced fluid retention. J Am Soc Nephrol 17: 3482–3490, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Kohan DE, Pritchett Y, Molitch M, Wen S, Garimella T, Audhya P, Andress DL: Addition of atrasentan to renin-angiotensin system blockade reduces albuminuria in diabetic nephropathy. J Am Soc Nephrol 22: 763–772, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herz M, Gaspari F, Perico N, Viberti G, Urbanowska T, Rabbia M, Wieczorek Kirk D: Effects of high dose aleglitazar on renal function in patients with type 2 diabetes. Int J Cardiol 151: 136–142, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Smolander J, Vogt B, Maillard M, Zweiacker C, Littke T, Hengelage T, Burnier M: Dose-dependent acute and sustained renal effects of the endothelin receptor antagonist avosentan in healthy subjects. Clin Pharmacol Ther 85: 628–634, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Anand I, McMurray J, Cohn JN, Konstam MA, Notter T, Quitzau K, Ruschitzka F, Lüscher TF, EARTH investigators : Long-term effects of darusentan on left-ventricular remodelling and clinical outcomes in the EndothelinA Receptor Antagonist Trial in Heart Failure (EARTH): Randomised, double-blind, placebo-controlled trial. Lancet 364: 347–354, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Nelson JB, Fizazi K, Miller K, Higano C, Moul JW, Akaza H, Morris T, McIntosh S, Pemberton K, Gleave M: Phase 3, randomized, placebo-controlled study of zibotentan (ZD4054) in patients with castration-resistant prostate cancer metastatic to bone. Cancer 118: 5709–5718, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Galié N, Badesch D, Oudiz R, Simonneau G, McGoon MD, Keogh AM, Frost AE, Zwicke D, Naeije R, Shapiro S, Olschewski H, Rubin LJ: Ambrisentan therapy for pulmonary arterial hypertension. J Am Coll Cardiol 46: 529–535, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Kohan DE, Pollock DM: Endothelin antagonists for diabetic and non-diabetic chronic kidney disease. Br J Clin Pharmacol 76: 573–579, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhaun N, Webb DJ, Kluth DC: Endothelin-1 and the kidney—beyond BP. Br J Pharmacol 167: 720–731, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baltatu OC, Iliescu R, Zaugg CE, Reckelhoff JF, Louie P, Schumacher C, Campos LA: Antidiuretic effects of the endothelin receptor antagonist avosentan. Front Physiol 3: 103, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heerspink HL, de Zeeuw D: Pharmacology: defining the optimal dose of a new drug: A crucial decision. Nat Rev Nephrol 5: 498–500, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Slagman MC, Waanders F, Vogt L, Damman K, Hemmelder M, Navis G, Laverman GD: Elevated N-terminal pro-brain natriuretic peptide levels predict an enhanced anti-hypertensive and anti-proteinuric benefit of dietary sodium restriction and diuretics, but not angiotensin receptor blockade, in proteinuric renal patients. Nephrol Dial Transplant 27: 983–990, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Dieterle W, Mann J: Pharmacokinetic interaction between ketoconazole and SPP301 in healthy volunteers. Int J Clin Pharmacol Ther 44: 326–330, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Williams D, Feely J: Pharmacokinetic-pharmacodynamic drug interactions with HMG-CoA reductase inhibitors. Clin Pharmacokinet 41: 343–370, 2002 [DOI] [PubMed] [Google Scholar]