Abstract
Background and objectives
The influence of deceased-donor AKI on post-transplant outcomes is poorly understood. The few published studies about deceased-donor preimplant biopsy have reported conflicting results regarding associations between AKI and recipient outcomes.
Design, setting, participants, & measurements
This multicenter study aimed to evaluate associations between deceased-donor biopsy reports of acute tubular necrosis (ATN) and delayed graft function (DGF), and secondarily for death-censored graft failure, first adjusting for the kidney donor risk index and then stratifying by donation after cardiac death (DCD) status.
Results
Between March 2010 and April 2012, 651 kidneys (369 donors, 4 organ procurement organizations) were biopsied and subsequently transplanted, with ATN reported in 110 (17%). There were 262 recipients (40%) who experienced DGF and 38 (6%) who experienced graft failure. DGF occurred in 45% of kidneys with reported ATN compared with 39% without ATN (P=0.31) resulting in a relative risk (RR) of 1.13 (95% confidence interval [95% CI], 0.9 to 1.43) and a kidney donor risk index–adjusted RR of 1.11 (95% CI, 0.88 to 1.41). There was no significant difference in graft failure for kidneys with versus without ATN (8% versus 5%). In stratified analyses, the adjusted RR for DGF with ATN was 0.97 (95% CI, 0.7 to 1.34) for non-DCD kidneys and 1.59 (95% CI, 1.23 to 2.06) for DCD kidneys (P=0.02 for the interaction between ATN and DCD on the development of DGF).
Conclusions
Despite a modest association with DGF for DCD kidneys, this study reveals no significant associations overall between preimplant biopsy-reported ATN and the outcomes of DGF or graft failure. The potential benefit of more rigorous ATN reporting is unclear, but these findings provide little evidence to suggest that current ATN reports are useful for predicting graft outcomes or deciding to accept or reject allograft offers.
Introduction
The scarcity of kidney allografts coupled with the challenges of performing multicenter deceased-donor research are fundamental obstacles in transplantation (1). The allograft shortage prolongs waiting list times, resulting in deteriorations in health and quality of life for patients maintained on chronic dialysis. To expand organ supplies, we increasingly utilize kidneys from deceased donors with risk factors for allograft dysfunction (e.g., older age, high terminal serum creatinine) (2–4). However, the procurement of kidneys with possible injury along with the lack of precise tools to characterize that injury have led to high organ discard rates and increasing numbers of recipients with long-term allograft dysfunction (5,6). To address these problems, the transplant community needs effective tools to assess early injury and prognosis. Such tools could facilitate the allocation of viable kidneys, lead to novel therapies for early allograft injury, and improve recipient outcomes.
After donation, all transplanted organs undergo ischemia followed by reperfusion in the recipient. Even before procurement, however, a donor may experience organ ischemia during the primary insult leading to death (e.g., trauma), the process of dying itself (e.g., circulatory or brain death), or tests/interventions used during donor management (e.g., contrast-induced nephropathy or vasopressor administration). These insults can lead to renal injury that may be clinically and/or histologically quantified. A growing body of literature in nontransplant settings suggests that AKI initiates and propagates CKD (7,8), and we hypothesize that analogous mechanisms are important in transplantation. Whereas over half of all allograft failures are due to recipient death with functional allografts (9), the remaining result from different alloantigen-dependent and independent causes that typically follow progressive declines in transplant function. The severity of ischemic kidney injury at procurement may be contributing to this allograft dysfunction.
Several factors directly or indirectly related to AKI have been evaluated in transplantation, including preservation technique, cold-ischemia time, and donor terminal creatinine (10), although many authors argue against discarding kidneys based on high creatinine levels alone (2,11–13). Little attention, however, has been given to assessing the degree of structural AKI in the donor apart from conventional “proxy” measures such as cold-ischemia time or creatinine. Histopathologic examination can be considered the gold standard for identifying structural AKI. A better understanding of how acute structural kidney injury is currently quantified by donor biopsy and reported to prospective transplant centers may provide new insights about decisions regarding organ allocation, discard/refusal, and preservation options. Thus, the goal of this study was to determine the association of preimplant kidney biopsy-reported acute tubular necrosis (ATN) with the development of delayed graft function (DGF) and secondarily with death-censored graft failure.
Materials and Methods
We performed a multicenter cohort study of deceased-donor kidney transplants from participating organ procurement organizations (OPOs) between March 2010 and April 2012. The OPOs performed wedge biopsies immediately after procurement, and different pathology services associated with these OPOs evaluated frozen sections to generate reports for review by potential transplant centers. We used standardized adjudication to retrospectively categorize reports for the presence of ATN. As the exposure of interest, we considered ATN as present if any evidence of acute tubular injury was mentioned in the report (without regard to severity). Reports that specified the absence of ATN or had no mention of tubular injury were categorized as no ATN. All analyses were performed using ATN as present or absent (i.e., dichotomous); however, for descriptive purposes, we categorized ATN as mild, moderate, or severe if that terminology was used in the report or if tubular involvement was noted to be <25%, 25%–50% or >50%, respectively. If no description of severity or tubular involvement was provided, we categorized the ATN as moderate. All available consecutive donor biopsy reports from transplanted kidneys were included and linked to recipients via the United Network for Organ Sharing (UNOS) database.
We used the UNOS definition of DGF as the outcome (i.e., at least one dialysis session in the first week of transplant). We also evaluated death-censored graft failure (return to dialysis or retransplantation) as a secondary outcome. OPOs also provided the following donor/allograft information and characteristics (not available from UNOS) to test as potential confounders: admission creatinine, terminal urine output, use of any vasoactive medications (dopamine, epinephrine, norepinephrine, phenylephrine, arginine vasopressin, dobutamine, or sodium nitroprusside) within 24 hours of procurement, and machine perfusion characteristics (perfusate flow and renal resistance) at 4 hours for kidneys that were preserved with machine perfusion.
We adhered to the ethical principles of the Declaration of Helsinki (14), and the institutional review boards and/or scientific review committees for all organizations involved approved this study. Data for deceased donors were collected if donor families agreed to research, whereas waiver of consent was approved for recipient outcomes given our use of deidentified UNOS data. The clinical and research activities reported are also consistent with the principles outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Statistical Analyses
Analyses were assessed at a two-tailed significance value of 0.05. We compared recipient, donor, and clinical characteristics by the presence of reported ATN on preimplant kidney biopsy and DGF using t tests for continuous variables and chi-squared tests or Fisher’s exact tests for categorical variables. We calculated the kidney donor risk index (KDRI) based on the following donor characteristics: age, height, weight, race, hypertension, diabetes, cause of death, terminal creatinine, hepatitis C, and donation after cardiac death (DCD) status (15,16). Given that DCD is known to be highly associated with DGF, we performed a prespecified stratified analysis by DCD status. We used log-binomial regression to determine the relative risk (RR) for the primary outcome based on the pathology report of any ATN alone and after adjusting for KDRI. We also used Cox proportional hazards models to evaluate the individual effects of ATN, DCD, and DGF on the secondary outcome of death-censored graft failure.
We performed a sensitivity analysis for the primary outcome after excluding all kidneys from the OPO with the lowest rate of reported ATN and obtained rereads of available preimplant biopsy slides from a subset of these kidneys. A single renal pathologist was asked to examine the slides and comment about the presence/absence, as well as the severity/extent, of ATN. SAS 9.2 statistical software for Windows (SAS Institute, Cary, NC) was used for all analyses.
Results
Prospective Cohort Description
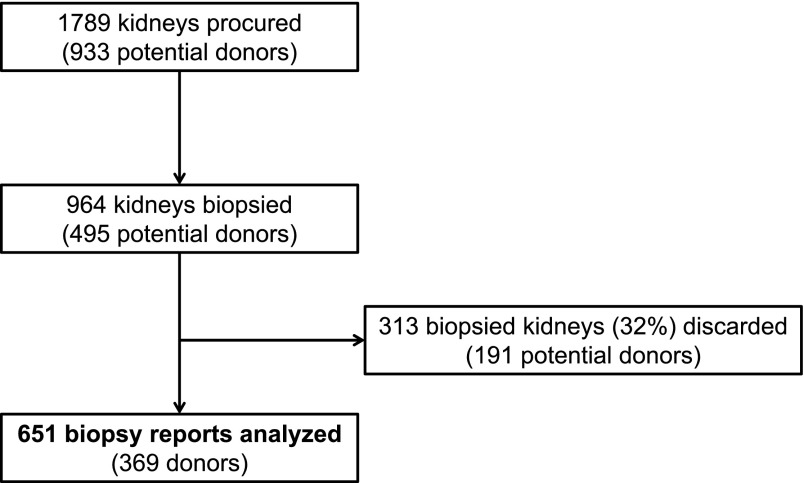
Four participating OPOs procured 1789 kidneys, 964 of which (54%) were chosen for biopsy. We analyzed 651 transplanted kidneys with available preimplant biopsy reports from 369 donors (Figure 1). Of note, 156 of all procured kidneys were DCD, 83 (53%) of which were chosen for biopsy. In addition, 75 of the 825 kidneys not chosen for biopsy (9%) were discarded, whereas 313 biopsied kidneys (32%) were discarded. Only 12 (4%) of the biopsied and discarded kidneys were DCD.
Figure 1.
Total deceased-donor kidneys procured by participating organ procurement organizations and development of the study cohort.
Clinical characteristics for recipients and allografts are shown in Table 1. ATN was noted in 110 biopsies (17%). We adjudicated ATN as mild, moderate, and severe in 82 (13%), 25 (4%), and 3 (<1%) biopsy reports, respectively. There was excellent concordance for ATN reports between kidney pairs, with only four listing ATN in one kidney but not the other for the same donor. Compared with no ATN, the ATN group had more HLA mismatches (although likely not clinically significant). There were no other significant differences in recipient/allograft characteristics by preimplant biopsy-reported ATN, including DCD status, KDRI, or donor admission/terminal creatinine or terminal urine output.
Table 1.
Recipient and allograft characteristics by preimplant biopsy-reported ATN
| Characteristic | All (N=651) | ATN (n=110) | No ATN (n=541) | P Valuea |
|---|---|---|---|---|
| Recipient | ||||
| Age, yr | 59 (51–67) | 60 (50–67) | 59 (51–67) | 0.9 |
| Men | 407 (63) | 71 (65) | 336 (62) | 0.63 |
| Body mass index, kg/m2 | 28 (25–33) | 29 (25–33) | 28 (25–32) | 0.32 |
| Black race | 268 (41) | 42 (38) | 226 (42) | 0.49 |
| Dialysis duration, mo | 52 (31–71) | 49 (35–69) | 52 (31–72) | 0.97 |
| Cause of ESRD | 0.09 | |||
| Hypertension | 211 (32) | 25 (23) | 186 (34) | |
| Diabetes | 194 (30) | 38 (35) | 156 (29) | |
| GN | 94 (14) | 21 (19) | 73 (13) | |
| Graft failure (prior transplant) | 40 (6) | 9 (8) | 31 (6) | |
| Other | 112 (17) | 17 (15) | 95 (18) | |
| HLA mismatches | 5 (4–5) | 5 (4–6) | 5 (4–5) | 0.04 |
| HLA-DR mismatches | 0.04 | |||
| 0 | 86 (13) | 16 (15) | 70 (13) | |
| 1 | 278 (43) | 35 (32) | 243 (45) | |
| 2 | 286 (44) | 59 (54) | 227 (42) | |
| Allograft (donor) | ||||
| Age, yr | 51 (44–58) | 53 (47–60) | 51 (43–58) | 0.44 |
| Men | 407 (63) | 62 (56) | 345 (64) | 0.14 |
| Body mass index, kg/m2 | 28 (24–33) | 28 (24–34) | 28 (24–33) | 0.73 |
| Black race | 117 (18) | 13 (12) | 104 (19) | 0.23 |
| Hypertension | 338 (52) | 48 (44) | 290 (54) | 0.06 |
| Diabetes | 109 (17) | 16 (15) | 93 (17) | 0.5 |
| Cause of death | 0.16 | |||
| Head trauma | 126 (19) | 22 (20) | 104 (19) | |
| Stroke | 290 (45) | 46 (42) | 244 (45) | |
| Anoxia | 216 (33) | 42 (38) | 174 (32) | |
| Other | 126 (19) | 22 (20) | 104 (19) | |
| Hepatitis C | 13 (2) | 2 (2) | 11 (2) | 0.88 |
| Expanded-criteria donor | 243 (37) | 43 (39) | 200 (37) | 0.67 |
| Donation after cardiac death | 154 (24) | 22 (20) | 132 (24) | 0.32 |
| Kidney donor risk index | 1.44 (1.23–1.67) | 1.43 (1.23–1.59) | 1.45 (1.21–1.69) | 0.28 |
| Kidney donor profile index, % | 84 (70–92) | 83 (71–89) | 84 (69–92) | 0.27 |
| Admission serum creatinine, mg/dl | 1.0 (0.8–1.3) | 1.0 (0.8–1.3) | 1.0 (0.7–1.2) | 0.37 |
| Terminal serum creatinine, mg/dl | 1.0 (0.7–1.4) | 1.0 (0.7–1.4) | 1.0 (0.7–1.4) | 0.91 |
| Terminal urine output, ml/h | 129 (88–218) | 130 (87–219) | 126 (97–196) | 0.60 |
| Any vasoactive agent usedb | 550 (84) | 460 (85) | 90 (82) | 0.40 |
| Cold-ischemia time, h | 17 (13–22) | 16 (12–23) | 17 (13–22) | 0.53 |
| Kidney machine perfused | 433 (67) | 81 (74) | 352 (65) | 0.09 |
| Perfusate flow at 4 h, ml/min | 115 (96–130.5) | 116 (96–131) | 109 (96–129) | 0.28 |
| Renal resistance at 4 h, mmHg/ml per min | 0.23 (0.19–0.29) | 0.23 (0.18–0.29) | 0.25 (0.2–0.3) | 0.16 |
Values are the median (interquartile range) or n (%). ATN, acute tubular necrosis.
P values comparing kidneys with to those without ATN by t tests for continuous variables or chi-squared tests for categorical variables.
Considered “yes” if at least one of the following medications were administered as part of deceased-donor management within 24 hours of organ procurement: dopamine, epinephrine, norepinephrine, phenylephrine, arginine vasopressin, dobutamine, or sodium nitroprusside.
Primary Outcome—DGF
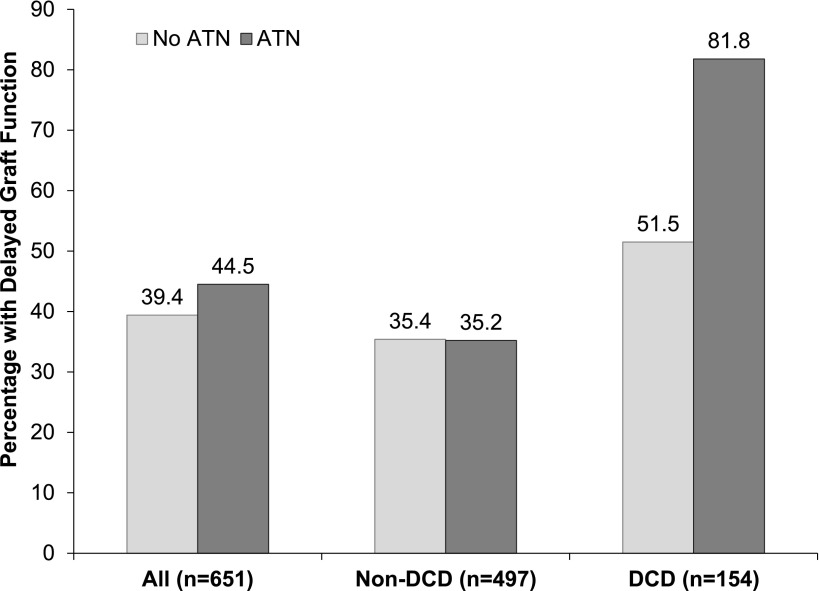
DGF occurred in 262 recipients (38%), with a nonsignificantly higher rate for kidneys with reported ATN (45% versus 39%; P=0.31). The only characteristics that were significantly different between allografts with and without DGF were recipient race (49% versus 36% black; P<0.001), donor body mass index (29 versus 27 kg/m2; P=0.02), donor hepatitis C seropositivity (4% versus 1%; P<0.001), and DCD status (33% versus 17%; P<0.001). Figure 2 shows the occurrence of DGF by ATN for the entire cohort and stratified by DCD status. Although there was no difference in DGF rates by ATN status in the non-DCD group, DCD kidneys with reported ATN had a significantly higher rate of DGF than those without ATN (82% versus 52%; P=0.01). Of note, the overall median values for terminal creatinine in non-DCD and DCD kidneys were 1.1 mg/dl (interquartile range [IQR], 0.8–1.6) and 0.9 mg/dl (IQR, 0.6–1.2), respectively (P<0.001).
Figure 2.
Percentage of all recipients with DGF by preimplant biopsy-reported ATN and stratified by donation after DCD status. The P value for the interaction term (ATN*DCD) on the development of DGF was significant at 0.02. P values for the chi-squared tests comparing the proportion with DGF in kidneys with versus without ATN for the entire cohort, non-DCD kidneys and DCD kidneys were 0.31, 0.97, and 0.01, respectively. ATN, acute tubular necrosis; DCD, donation after cardiac death; DGF, delayed graft function.
In regression analysis, the RR of DGF associated with a preimplant report of ATN was 1.13 (95% confidence interval [95% CI], 0.9 to 1.43), and the KDRI-adjusted RR was 1.11 (95% CI, 0.88 to 1.41). There was significant interaction between DCD status and ATN on the development of DGF (P=0.02). In non-DCD kidneys, the adjusted RR of DGF with ATN was 0.97 (95% CI, 0.7 to 1.34) compared with 1.59 (95% CI, 1.23 to 2.06) in DCD kidneys.
Secondary Outcome—Graft Failure
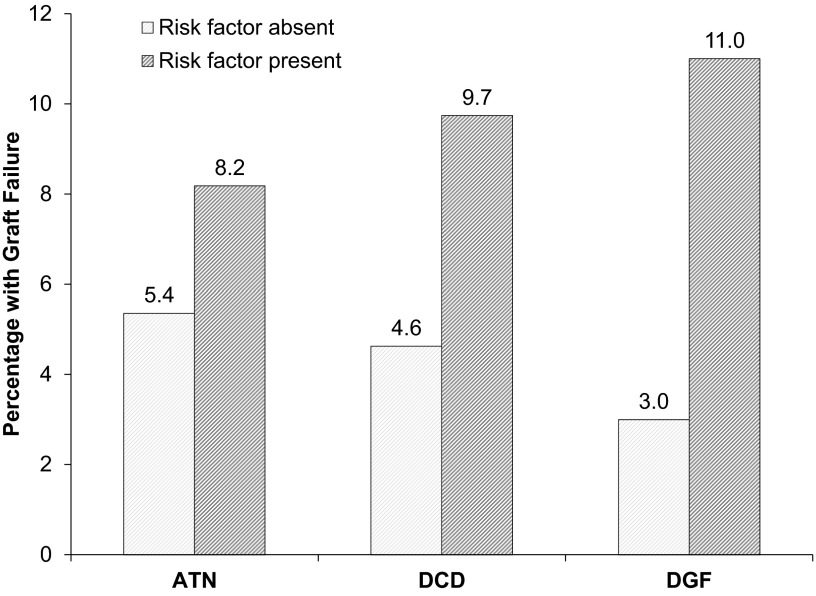
Median follow-up was 356 days (IQR, 196–400 days). Death-censored graft failure occurred in 38 recipients (6%). ATN was not significantly associated with this outcome with a hazard ratio of 1.5 (95% CI, 0.7 to 3.1; P=0.31). Stratifying by either DCD or DGF status did not affect the insignificant relationship between ATN and graft failure. However, on their own, DCD and DGF were both significant risk factors with hazard ratios of 2.3 (95% CI, 1.2 to 4.4; P=0.01) and 4.6 (95% CI, 2.2 to 9.4; P<0.001), respectively. Figure 3 shows the proportion of the cohort that developed graft failure by the presence or absence of each risk factor (Kaplan–Meier curves are provided in Supplemental Figures 1–3).
Figure 3.
Percentage of recipients (N=651) with death-censored graft failure (median follow-up 356 days; interquartile range, 196–400 days) by the presence or absence of risk factors. The risk factors were as follows: preimplant biopsy-reported ATN (present in 110, absent in 541), DCD (present in 154, absent in 497), and DGF (present in 262, absent in 389). The Cox proportional hazard ratios were 1.5 (95% CI, 0.7 to 3.1; P=0.31), 2.3 (95% CI, 1.2 to 4.4; P=0.01), and 4.6 (95% CI, 2.2 to 9.4; P<0.001), respectively. 95% CI, 95% confidence interval.
Sensitivity Analyses
We noted substantial variation in ATN reporting. OPOs provided evidence of ATN in 0%, 14%, 19%, and 25% of the preimplant biopsies, respectively. To determine the influence of the absence of ATN reporting, analyses were repeated after excluding all 54 kidneys (30 donors) from the OPO that provided biopsy reports without mention of ATN. The adjusted RR for the remaining subset was not substantially different at 1.08 (95% CI, 0.85 to 1.37). Results were similarly unchanged in non-DCD and DCD kidneys with adjusted RRs of 0.95 (95% CI, 0.69 to 1.32) and 1.46 (95% CI, 1.12 to 1.89), respectively. In addition, renal pathology rereads of available biopsy slides for the excluded donors revealed evidence of tubular injury in six of nine kidneys, all of which were reportedly mild in terms of severity. Multiple combinations for ATN and DCD/expanded-criteria status were noted in this small subset of donor biopsies, with no clear pattern of association with DGF in the recipients (Supplemental Table 1).
Discussion
This multicenter study is the largest cohort to date to examine associations between reported AKI by preimplant histology and allograft outcomes. We demonstrated that preimplant biopsy-reported ATN, as provided with actual organ offers, is modestly associated with the development of DGF only in DCD kidneys. More generally, however, our findings suggest that these ATN reports provide little utility for determining the overall risk of DGF or premature death-censored graft failure in deceased-donor kidney transplantation. In addition, we noted substantial variability in pathology reports with evidence to suggest that acute structural injury is frequently underreported in preimplant kidney biopsies.
In aggregate, prior studies of histologic AKI by preimplantation biopsy have reported inconsistent associations with allograft outcomes (Table 2) (17–23). None of the studies that we identified provided magnitudes of association or confidence intervals for histologic AKI, and only one study analyzed multicenter data (21). Three studies noted a significant association between histologic AKI and DGF (17,21,22), but two others did not (18,19). In their analysis of 92 kidneys, Sulikowski et al. also described worse longer-term allograft function (reported as “higher concentration of serum urea at 12 and 36 months”) for preimplant biopsies with more severe ATN (22), but the two largest prior cohorts (200 and 371 kidneys, respectively) failed to demonstrate a significant association between this exposure and graft survival (20,23). In fact, the latter study by Munivenkatappa et al. which proposed the Maryland Aggregate Pathologic Index to predict graft failure based on preimplant biopsy findings, did not retain ATN for scoring given its lack of association at a mean follow-up of 38±30 months. Apart from these studies of direct associations, however, other types of evidence have suggested the importance of early allograft injury.
Table 2.
Studies that assessed preimplant kidney biopsies for acute injury in relation to allograft or recipient outcomes
| First Author | Year | Country | Study Type | N | Biopsy Details | ATN Definition | Short-Term Recipient Outcomes | Long-Term Recipient Outcomes |
|---|---|---|---|---|---|---|---|---|
| Goumenos (17) | 2010 | Greece | Prospective, single center | 74 | Core biopsies done at recipient hospital; frozen and permanent sections compared; number of pathologists not specified | ATN reported as mild (affecting <25% of cortical area), moderate (25%–50%), or severe (>50%) | Recipients with 3-month serum creatinine>2 mg/dl had (in retrospect) more severe ATN | Not reported |
| Matignon (18) | 2008 | France | Retrospective, single center | 172 | Wedge biopsies done at recipient hospital; permanent sections stained for H&E, PAS, Masson trichrome and silver; read by one pathologist | Not clear, reported “presence of” ATN or osmotic nephrosis | Neither ATN nor osmotic nephrosis was associated with DGF | Not reported |
| Munivenkatappa (23) | 2008 | United States | Retrospective, single center | 371 | Wedge biopsies done at procurement; frozen and permanent sections compared, stained for H&E and Masson trichrome; read by one pathologist, also used automatic morphometric analysis | Not clear, ATN reported as “any” | Not reported | ATN was assessed as a potential variable for a score to predict graft failure, but it was not statistically significant and therefore not retained in the score |
| Oberbauer (19) | 1999 | Austria | Retrospective, single center | 92 | Wedge biopsies done at recipient hospital; permanent sections stained for H&E, PAS, silver, TUNEL, anti-PCNA and anti-bcl-2; number of pathologists not specified | Histologic “acute tubular damage” scored from 0 to 3 (0, none; 1, mild; 2, moderate; 3, severe) | Histologic acute tubular damage was not associated with DGF or rejection in the first 7 d; however, there was variably more TUNEL and bcl-2 positivity in kidneys with DGF or acute rejection compared with kidneys with immediate function | Not reported |
| Pokorná (20) | 2000 | Czech Republic | Prospective, single center | 200 | Wedge biopsies done at procurement; permanent sections stained for H&E, PAS, silver, aniline fuchsin, and orange G; read by one pathologist | Tubular vacuolization and desquamation scored separately (0, absent; 1, mild; 2, moderate; 3, severe) | Tubular vacuolization was associated with graft function at 1 wk, 3 wk, and 6 mo. Tubular desquamation was associated with graft function at 1 wk, 3 wk, and 3 mo | Neither injury type was significantly associated with graft function at 24 mo or with graft survival |
| Rohr (21) | 1983 | United States | Prospective, multicenter | 57 | Wedge biopsies done at procurement; permanent sections compared with repeat biopsy after cold storage at the recipient hospital; read by one pathologist | General histologic descriptions of tubule cell injury (e.g., luminal cytoplasmic debris, sloughing, vacuolization) | Abnormalities consistent with acute injury were observed in 19 of 22 preimplant biopsies with DGF and 22 of 22 post-cold storage biopsies with DGF | Not reported |
| Sulikowski (22) | 2010 | Poland | Retrospective, single center | 92 | Core biopsies done “before kidney perfusion at mono- or multiorgan harvest”; permanent sections stained for H&E, PAS, silver, and Mallory; number of pathologists not specified | Not clear, ATN reported as − (absent), +, or ++ | Absence of ATN was associated with immediate graft function. ATN was associated with DGF, PNF, and higher serum creatinine at 6 mo | ATN was associated with higher serum urea at 1 and 3 yr, but was not associated with graft failure |
Listed here are the results of a systematic search of the Cochrane and Medline databases for human studies of preimplant kidney biopsies for assessing early allograft injury in relation to allograft or recipient outcomes without date or language limitations. The following search terms were used: kidney, transplant, biopsy, donor, and preimplantation. A total of 30 publications were initially identified; 26 were excluded due to lack of histologic AKI reporting, animal studies, editorials, and 3 additional publications were identified by manual review of references. DGF, delayed graft function; H&E, hematoxylin and eosin; PAS, periodic acid–Schiff; PCNA, proliferating cell nuclear antigen; PNF, primary nonfunction; TUNEL, terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling.
Several investigators have studied newer techniques (e.g., quantifiable protein or other biomarkers) that may ultimately improve upon current approaches to preimplant biopsies and modify the way we assess acute structural kidney injury. Schröppel et al. demonstrated that kidney injury molecule-1 (KIM-1) expression in pretransplant biopsies corresponded with donor renal function, but concomitant injury histology was not described (24). Zhang et al. assessed KIM-1 staining in biopsies obtained on average 16–79 weeks after transplant (25). KIM-1 staining was present in all 25 cases that showed histologic evidence of tubular injury, and it was even detected in 7 of 25 cases without histologic injury. Mishra et al. found staining intensity for neutrophil gelatinase–associated lipocalin (NGAL) in 1-hour reperfusion biopsies from 25 children to be associated with donor type (deceased versus living), cold-ischemia time, and peak postoperative creatinine (26). Our investigator group previously reported the first biomarker evidence of a detrimental effect of AKI (detected perioperatively) on 1-year outcomes in a prospective, multicenter deceased-donor kidney transplant cohort (27). We showed that higher first postoperative day urine NGAL and IL-18 concentrations were associated with >5-fold higher adjusted odds for the return to dialysis or an eGFR<30 ml/min per 1.73 m2 at 1 year. Furthermore, results from a recent trial showed that therapies successful in ameliorating early allograft injury, as assessed by a reduction in the rate of DGF, can improve 1-year allograft survival (28). Thus, there is mounting evidence to suggest that early AKI is affecting outcomes in kidney transplantation, but that the value of measuring this injury depends on the method of detection. We believe more studies are needed to validate KIM-1, NGAL, IL-18, and other promising biomarkers, with appropriate comparison to histology (via fixed and stained core-needle biopsies read by blinded renal pathologists), to measure AKI at procurement in hopes of improving our ability to predict important allograft outcomes.
Considering the essentially negative results of this study, it is reasonable to question whether ATN, or AKI in general for that matter, truly causes important allograft outcomes. Preimplant ATN can only reflect one aspect of the injury involved (i.e., ischemic injury) and cannot capture the contribution from recipient reperfusion. As such, preimplant biopsies may simply be too early in the process to adequately address the importance of AKI in transplant. Alternatively, currently utilized biopsies may have significant limitations in this context because of several practical concerns related to rushed interpretations by nonrenal pathologists based on frozen sections from wedge biopsy specimens. One might also consider whether kidneys selected for transplant, and thereby included in this cohort, had somewhat minor ATN in the setting of otherwise favorable characteristics such that the overall spectrum of acute injury in these kidneys was relatively inconsequential. Our finding of a positive association in DCD kidneys may actually support this notion regarding other influential characteristics.
Because of the time inherent in allocating and transporting a deceased-donor kidney, some degree of ischemic structural injury may be unavoidable, which may be even more severe in DCD settings given longer periods of warm ischemia before the kidney can be flushed and procured. We were interested to see that ATN was not more frequently reported in DCD kidneys, and that biopsy-reported ATN was associated with DGF only in this subgroup. Others have highlighted the importance of DCD status when evaluating the effect of treating ischemia via machine perfusion on the development of DGF (29). Although reported ATN per se was not associated with premature death-censored graft failure in our cohort, DCD status itself was, a finding that conflicts with prior studies (30,31). This inconsistency may be related to DCD acceptance patterns, in that DCD kidneys accepted for transplant and included in most cohort studies otherwise tend to be of relatively high quality. Evidence for this differential donor quality can be seen by the lower terminal creatinines in our DCD kidneys compared with non-DCD kidneys. Nonetheless, our cohort consisted of kidneys that were specifically chosen to undergo preimplant biopsy, which likely excluded some high-quality DCD kidneys that were not chosen for biopsy.
Although other methods exist for assessing kidney injury at procurement, the current findings are important to transplantation because histopathology can be considered the gold standard measure for this risk factor. As previously noted, however, the quality of currently utilized preimplant biopsies for detecting acute injury may be limited for several reasons. Preimplant biopsies require rapid turnaround for results within the time constraints of organ allocation. As such, these specimens are typically frozen sections from wedge biopsy samples read by on-call pathologists rather than immediately fixed and subsequently stained core-needle biopsies read by dedicated renal pathologists. In addition, the focus of the preimplant pathology evaluation is often on chronic disease rather than AKI. Thus, histologic ATN can be overlooked in many cases, and our data regarding variability in ATN reporting between OPOs as well as misclassification based on a manual review of a small number of biopsies from one OPO provide strong evidence for this. To address this potential bias, we performed a sensitivity analysis in which we excluded all kidneys from the one OPO with no reports of ATN, which did not affect our study conclusions. Most importantly, however, our conclusions speak to the utility of currently utilized pathology reports for identifying ATN during organ offers and not the theoretical value of truly quantifying all renal tubular injury.
As for other limitations, our cohort was defined by available preimplant biopsy reports for kidneys that were ultimately transplanted in accordance with current allocation practices. Thus, we could not evaluate the effect of biopsy-reported ATN on allograft outcomes in kidneys that were discarded for any reason (typically older, higher-risk donors) or in kidneys not chosen to undergo preimplant biopsy (typically younger, healthier donors). These important selection biases affect any observational study of preimplant kidney biopsy, and a properly designed randomized controlled trial will be required to adequately address this uncertainty for the transplant community. In addition, we utilized the UNOS database to determine outcomes given the inclusion of kidneys from different OPOs; however, we view our multicenter study design as a strength with regard to generalizability, and we analyzed additional key donor and allograft characteristics not available from UNOS as potential confounders.
In summary, we found a modest association between preimplant biopsy-reported ATN and the development of DGF for DCD kidneys, but no significant associations overall between reported ATN and the outcomes of DGF or premature death-censored graft failure. The potential effects of more rigorous ATN reporting remain unclear; however, our findings indicate that ATN, as described in typical deceased-donor pathology reports at the time of organ offer, is unlikely to be a good classifier of important recipient outcomes on its own. Innovative clinical trial data are needed to inform the transplant community about the true utility of preimplant kidney biopsy and how biopsy should affect organ acceptance/rejection patterns. Ancillary studies to such a trial could also compare newer methods of defining/detecting AKI against traditional methods such as histology as well as evaluate the overall clinical significance of structural ischemic kidney injury, especially in relation to other increasingly common and important factors such as DCD status.
Disclosures
None.
Acknowledgments
We thank Gift of Life Philadelphia, the Michigan Organ and Tissue Donation Program, the New Jersey Sharing Network, and the New York Organ Donor Network for their active support and participation in this study.
This work was supported in part by awards from the American Heart Association (Grant 12FTF12080082 to I.E.H.), the Roche Organ Transplantation Research Foundation (to C.R.P.), the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (Grant R01-DK093770 to C.R.P.), and the Health Resources and Services Administration (Contract 234-2005-37011C).
The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the US Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. The funding organizations were not involved in study design, analysis, interpretation, or manuscript creation. I.E.H., K.H., and C.R.P. had full access to all data and take responsibility for its integrity and the accuracy of all analyses.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08270813/-/DCSupplemental.
See related editorial, “Procurement Biopsies in Kidneys Retrieved for Transplantation,” on pages 443–444.
References
- 1.Abt PL, Marsh CL, Dunn TB, Hewitt WR, Rodrigue JR, Ham JM, Feng S: Challenges to research and innovation to optimize deceased donor organ quality and quantity. Am J Transplant 13: 1400–1404, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Anil Kumar MS, Khan SM, Jaglan S, Heifets M, Moritz MJ, Saeed MI, Fyfe B, Sustento-Reodica N, Kumar A: Successful transplantation of kidneys from deceased donors with acute renal failure: Three-year results. Transplantation 82: 1640–1645, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Nathan HM, Hasz RD, Radolovic C, West SM, Abrams JD, Moritz MJ: 159 acute renal failure deceased donor kidneys transplanted: One organ procurement organization’s 15-year experience. Presented at the 2009 Organ Donation Congress, Berlin, Germany, October 4–7, 2009 [Google Scholar]
- 4.Schold JD, Segev DL: Increasing the pool of deceased donor organs for kidney transplantation. Nat Rev Nephrol 8: 325–331, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE, Metzger RA, Ojo AO, Port FK: Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA 294: 2726–2733, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Sung RS, Christensen LL, Leichtman AB, Greenstein SM, Distant DA, Wynn JJ, Stegall MD, Delmonico FL, Port FK: Determinants of discard of expanded criteria donor kidneys: Impact of biopsy and machine perfusion. Am J Transplant 8: 783–792, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG, University of Toronto Acute Kidney Injury Research Group : Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 302: 1179–1185, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ: Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Renal Data System : Transplantation. In: USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Vol. 2, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2011, pp 247–256 [Google Scholar]
- 10.Port FK, Bragg-Gresham JL, Metzger RA, Dykstra DM, Gillespie BW, Young EW, Delmonico FL, Wynn JJ, Merion RM, Wolfe RA, Held PJ: Donor characteristics associated with reduced graft survival: An approach to expanding the pool of kidney donors. Transplantation 74: 1281–1286, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Kayler LK, Garzon P, Magliocca J, Fujita S, Kim RD, Hemming AW, Howard R, Schold JD: Outcomes and utilization of kidneys from deceased donors with acute kidney injury. Am J Transplant 9: 367–373, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Morgan C, Martin A, Shapiro R, Randhawa PS, Kayler LK: Outcomes after transplantation of deceased-donor kidneys with rising serum creatinine. Am J Transplant 7: 1288–1292, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Klein R, Galante NZ, de Sandes-Freitas TV, de Franco MF, Tedesco-Silva H, Medina-Pestana JO: Transplantation with kidneys retrieved from deceased donors with acute renal failure. Transplantation 95: 611–616, 2013 [DOI] [PubMed] [Google Scholar]
- 14.World Medical Association: WMA Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects. Available at: http://www.wma.net/en/30publications/10policies/b3/ Accessed September 10, 2013 [DOI] [PubMed]
- 15.Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, Port FK, Sung RS: A comprehensive risk quantification score for deceased donor kidneys: The kidney donor risk index. Transplantation 88: 231–236, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Organ Procurement and Transplantation Network: A guide to calculating and interpreting KDPI. Available at: http://optn.transplant.hrsa.gov/ContentDocuments/Guide_to_Calculating_Interpreting_KDPI.pdf Accessed July 15, 2013
- 17.Goumenos DS, Kalliakmani P, Tsamandas AC, Maroulis I, Savidaki E, Fokaefs E, Papachristou E, Karavias D, Vlachojannis JG: The prognostic value of frozen section preimplantation graft biopsy in the outcome of renal transplantation. Ren Fail 32: 434–439, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Matignon M, Desvaux D, Noël LH, Roudot-Thoraval F, Thervet E, Audard V, Dahan K, Lang P, Grimbert P: Arteriolar hyalinization predicts delayed graft function in deceased donor renal transplantation. Transplantation 86: 1002–1005, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Oberbauer R, Rohrmoser M, Regele H, Mühlbacher F, Mayer G: Apoptosis of tubular epithelial cells in donor kidney biopsies predicts early renal allograft function. J Am Soc Nephrol 10: 2006–2013, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Pokorná E, Vítko S, Chadimová M, Schück O, Ekberg H: Proportion of glomerulosclerosis in procurement wedge renal biopsy cannot alone discriminate for acceptance of marginal donors. Transplantation 69: 36–43, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Rohr MS: Renal allograft acute tubular necrosis. II. A light and electron microscopic study of biopsies taken at procurement and after revascularization. Ann Surg 197: 663–671, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sulikowski T, Tejchman K, Ziętek Z, Urasińska E, Domański L, Sieńko J, Romanowski M, Safranow K, Zukowski M, Ciechanowicz A, Ciechanowski K, Ostrowski M: Histopathologic evaluation of pretransplantation biopsy as a factor influencing graft function after kidney transplantation in 3-year observation. Transplant Proc 42: 3375–3381, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Munivenkatappa RB, Schweitzer EJ, Papadimitriou JC, Drachenberg CB, Thom KA, Perencevich EN, Haririan A, Rasetto F, Cooper M, Campos L, Barth RN, Bartlett ST, Philosophe B: The Maryland aggregate pathology index: A deceased donor kidney biopsy scoring system for predicting graft failure. Am J Transplant 8: 2316–2324, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Schröppel B, Krüger B, Walsh L, Yeung M, Harris S, Garrison K, Himmelfarb J, Lerner SM, Bromberg JS, Zhang PL, Bonventre JV, Wang Z, Farris AB, Colvin RB, Murphy BT, Vella JP: Tubular expression of KIM-1 does not predict delayed function after transplantation. J Am Soc Nephrol 21: 536–542, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang PL, Rothblum LI, Han WK, Blasick TM, Potdar S, Bonventre JV: Kidney injury molecule-1 expression in transplant biopsies is a sensitive measure of cell injury. Kidney Int 73: 608–614, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishra J, Ma Q, Kelly C, Mitsnefes M, Mori K, Barasch J, Devarajan P: Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol 21: 856–863, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Hall IE, Doshi MD, Reese PP, Marcus RJ, Thiessen-Philbrook H, Parikh CR: Association between peritransplant kidney injury biomarkers and 1-year allograft outcomes. Clin J Am Soc Nephrol 7: 1224–1233, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moers C, Smits JM, Maathuis MH, Treckmann J, van Gelder F, Napieralski BP, van Kasterop-Kutz M, van der Heide JJ, Squifflet JP, van Heurn E, Kirste GR, Rahmel A, Leuvenink HG, Paul A, Pirenne J, Ploeg RJ: Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med 360: 7–19, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Lodhi SA, Lamb KE, Uddin I, Meier-Kriesche HU: Pulsatile pump decreases risk of delayed graft function in kidneys donated after cardiac death. Am J Transplant 12: 2774–2780, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Singh RP, Farney AC, Rogers J, Zuckerman J, Reeves-Daniel A, Hartmann E, Iskandar S, Adams P, Stratta RJ: Kidney transplantation from donation after cardiac death donors: Lack of impact of delayed graft function on post-transplant outcomes. Clin Transplant 25: 255–264, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Jochmans I, Moers C, Smits JM, Leuvenink HG, Treckmann J, Paul A, Rahmel A, Squifflet JP, van Heurn E, Monbaliu D, Ploeg RJ, Pirenne J: Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: A multicenter, randomized, controlled trial. Ann Surg 252: 756–764, 2010 [DOI] [PubMed] [Google Scholar]