Abstract
Background and objectives
Approximately 20% of boys with posterior urethral valves develop ESRD; however, few factors associated with the risk of ESRD have been identified. The objective of this study was to determine if renal parenchymal area, defined as the area of the kidney minus the area of the pelvicaliceal system on first postnatal ultrasound, is associated with the risk of ESRD in infants with posterior urethral valves.
Design, setting, participants, & measurements
A retrospective cohort of boys who were diagnosed with posterior urethral valves at less than 6 months of age between 1988 and 2011 and followed for at least 1 year at a free-standing children’s hospital was assembled. Cox proportional hazard regression and Kaplan–Meier analysis were used to estimate the association between renal parenchymal area and time to ESRD. Cox models were adjusted for age at presentation, minimum creatinine 1 month after bladder decompression, and vesicoureteral reflux.
Results
Sixty patients were followed for 393 person-years. Eight patients developed ESRD. Median renal parenchymal area was 15.9 cm2 (interquartile range=13.0–21.6 cm2). Each 1-cm2 increase in renal parenchymal area was associated with a lower risk of ESRD (hazard ratio, 0.64; 95% confidence interval, 0.42 to 0.98). The rate of time to ESRD was 10 times higher in boys with renal parenchymal area<12.4 cm2 than boys with renal parenchymal area≥12.4 cm2 (P<0.001). Renal parenchymal area could best discriminate children at risk for ESRD when the minimum creatinine in the first 1 month after bladder decompression was between 0.8 and 1.1 mg/dl.
Conclusion
In boys with posterior urethral valves presenting during the first 6 months of life, lower renal parenchymal area is associated with an increased risk of ESRD during childhood. The predictive ability of renal parenchymal area, which is available at time of diagnosis, should be validated in a larger, prospectively-enrolled cohort.
Introduction
Congenital urological disorders account for up to 60% of CKD in children (1–3). Of these disorders, posterior urethral valves (PUVs) are the most common cause of ESRD during childhood (3). The lifetime prevalence of ESRD in boys with PUV is 20%–30% (4–6). Renal failure in patients with PUV is often caused by renal dysplasia that is already established at birth because of fetal obstruction (7). Some children, however, maintain preserved kidney function into adulthood, whereas others develop kidney failure in childhood (8). Previous reports suggested that nadir creatinine predicts the risk of kidney failure in children with PUV. However, the clinical applicability of these prior observations to the infant with PUV is confusing given the wide range of ages at presentation and different definitions of nadir creatinine used (9–14). New prognostic indicators are needed to improve counseling and identify the patients in whom referral to a pediatric nephrologist and early interventions to slow the progression of kidney failure would be most likely to have the greatest efficacy.
Although simple kidney length measurements are associated with magnetic resonance imaging kidney volume in normal children, the correlation decreases in the setting of abnormal anatomy. Since its original description by Cost et al. (15) and Wong et al. (16), renal parenchymal area (RPA) has been used to identify clinically significant reflux nephropathy and ureteropelvic junction obstruction (15,16). RPA determined by ultrasound is highly correlated with kidney volume, even in the presence of hydronephrosis (16). We postulate that RPA correlates with nephron mass and is a surrogate marker of functional renal reserve. Accordingly, we hypothesize that infant boys with less RPA are at higher risk of developing ESRD than boys with more RPA after adjusting for creatinine (17,18). In this study of boys diagnosed with PUV at 6 months of age or less, we determined if RPA measured on the first postnatal ultrasound is associated with ESRD.
Materials and Methods
Patient Cohort Description and Study Design
This study was a retrospective cohort study designed to determine the association between RPA measured on first postnatal ultrasound and time to ESRD. All boys with PUV presenting or referred to our institution between January of 1988 and December of 2011 were assessed for eligibility. Calendar year 1988 was chosen as the start date, because this year represents a modern cohort of patients screened by prenatal ultrasound. We identified this population by searching the inpatient and outpatient billing databases for International Classification of Diseases, Ninth Revision codes for PUV (753.6) and Current Procedural Terminology codes for PUV ablation (52340).
We included boys 6 months old or younger at the time of bladder decompression who were followed for at least 1 year. Bladder decompression was defined as the earliest date on which urethral catheter placement, vesicostomy, or PUV incision was performed. We chose 6 months as the upper age limit to have a cohort that represented patients seen in contemporary clinical practice and limit the heterogeneity of the patient sample with respect to kidney size and estimated GFR based on the 1-month serum creatinine. Patients were excluded if creatinine values at 1 year of follow-up and/or the first postnatal ultrasound were not available. Boys with PUV are monitored for bladder and kidney function per the attending physicians’ judgment. Although there is not currently a standard follow-up algorithm for boys with PUV at our institution, they are never discharged from care.
Patients were followed until ESRD developed, study period end (December 31, 2012), or the last available creatinine if they were lost to follow-up. The Children’s Hospital of Philadelphia Institutional Review Board approved this study. The authors adhered to the Declaration of Helsinki throughout this study.
Outcome Definition and Predictor Variable Measurement
The outcome was ESRD. ESRD was defined as the initiation of dialysis or renal transplantation. The primary predictor was RPA determined by the first postnatal ultrasound. Ultrasounds were reviewed by two investigators (J.E.P. and G.E.T.). The image of the greatest longitudinal dimension of the kidney was imported into ImageJ, which is an open access Java image processing program developed by the National Institutes of Health (19). The method of determining RPA has been previously described in detail (15). Briefly, one investigator (J.E.P.) traced the renal area and pelvicalyceal system separately. The pelvicalyceal area was then subtracted from the renal area, and the resultant product was adjusted to the scale of the ultrasound. Total RPA was defined as the sum of the RPA of both kidneys. Any formal assessment of renal function with renal scintigraphy or magnetic resonance urography that patients underwent was not considered in measuring RPA. Three kidneys in three separate patients had severe cystic dysplasia and were determined by consensus to have no demonstrable parenchyma. The RPA of these kidneys was set to zero. The RPA of a randomly selected subset of eight kidneys was measured by a second investigator (G.E.T.). The intraclass correlation was calculated.
Covariates included age at bladder decompression, vesicoureteral reflux (VUR) defined by voiding cystourethrogram, number of febrile urinary tract infections defined as temperature≥38.0°C and positive urine culture obtained at our hospital or an outside institution, and 1-month creatinine (lowest serum creatinine value measured between 5 days and 1 month after bladder decompression). Voiding cystourethrograms were obtained in all patients. Similar to other studies, vesicoureteral reflux was analyzed as a dichotomous variable, with grade≥4 in both kidneys considered positive (6). The number of documented urinary tract infections was analyzed as a continuous variable because of the biologic plausibility that each infection could affect future renal function. Data were abstracted using manual chart review. Study data were collected and managed using Research Electronic Data Capture hosted at the Children’s Hospital of Philadelphia (20).
Statistical Analyses
We used Cox proportional hazard models to estimate the association between RPA and time to ESRD. Covariates assessed for inclusion in the final model were age at bladder decompression, 1-month creatinine, vesicoureteral reflux, and number of documented urinary tract infections. Regression models were built using manual backward selection of covariates. We included in the final model all covariates associated with ESRD at P<0.10 on univariate analysis, all covariates with a priori–defined face validity (1-month creatinine), and all covariates that exhibited significant confounding of RPA in the final multivariate model. Confounding was defined as a change of ≥15% between the unadjusted and adjusted hazard ratios for RPA. Models were assessed for proportionality using log–log plots. Time to event was measured in person-months.
Sensitivity Analyses
We performed two sensitivity analyses to determine the robustness of association of RPA with ESRD-free survival. First, we determined if observed ESRD-free survival differed between patients with high and low RPA using Kaplan–Meier curves and log rank tests. To determine the optimal cutpoint for RPA, we used receiver operating characteristic curves to determine the value at which the sensitivity and specificity of RPA for ESRD were maximized. We dichotomized RPA at this cutpoint. Second, to determine the range of nadir creatinine values over which RPA maintained its association with ESRD, we fit four Cox regression models with RPA dichotomized above and below the cutpoint and adjusted the models for different baseline renal functions by centering 1-month creatinine values at predefined levels of 0.4, 0.8, 1.1, or 1.5 mg/dl. These analyses generated survival curves that displayed differential renal survival attributable to RPA in hypothetical individuals with a 1-month nadir creatinine of 0.4, 0.8, 1.1, or 1.5 mg/dl. The survivor curves of the RPA strata at each creatinine level were then compared using log rank tests. Tests were two-sided, and the threshold of statistical significance was defined at P<0.05. All analyses were performed with Stata 12 (StataCorp, College Station, TX).
Results
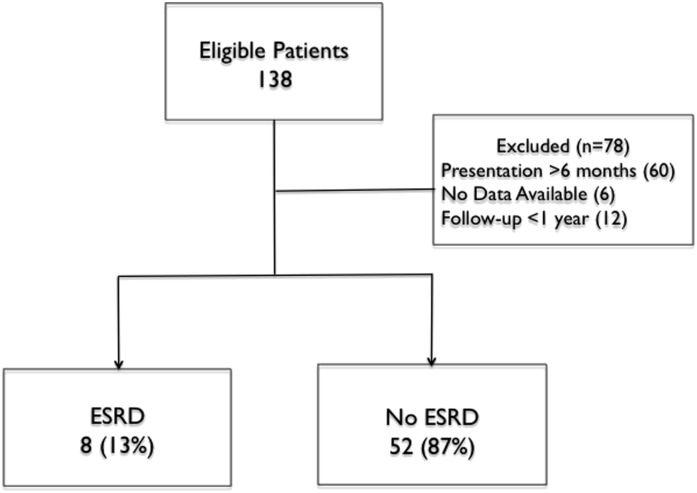
Between 1988 and 2011, 138 patients with PUV presented at, or were referred to, our institution, and they were examined for eligibility; 6 (4%) patients did not have the first postnatal ultrasound available for review, 60 (43%) patients presented after 6 months of life, and 12 (9%) patients were followed for less than 1 year (Figure 1) and, therefore, were ineligible. Sixty patients were in the final cohort and followed for 4711 person-months (393 person-years). Forty-five (73%) patients were followed from birth until study end. Characteristics of the included patients are shown in Table 1.
Figure 1.
Selection of the cohort of boys with posterior urethral valves. Flowchart of patient selection. Sixty patients were included in the analysis.
Table 1.
Characteristics of 60 patients with posterior urethral valves according to kidney function
| Patient Characteristics | Non-ESRD (n=52) | ESRD (n=8) | P Value |
|---|---|---|---|
| Age at presentation, wk (IQR) | 2 (1–11) | 1 (0.5–1.5) | 0.11 |
| Total RPA, cm2 (IQR) | 17.05 (13.69–21.74) | 10.37 (8.21–13.61) | 0.01 |
| Nadir 1-mo creatinine, mg/dl (IQR) | 0.5 (0.4–0.8) | 1.6 (1.4–3.9) | <0.001 |
| VUR≥4 bilaterally (%) | 6/52 (13) | 4/8 (37.5) | 0.02 |
| UTI (IQR) | 0 (0–1) | 1 (0–2) | 0.66 |
Group characteristics: significance tested using Wilcoxon rank sum or Fisher exact test. IQR, interquartile range; RPA, renal parenchymal area; VUR, vesicoureteral reflux; UTI, urinary tract infection.
Eight (13%) patients developed ESRD while being followed at our institution. One patient who developed ESRD died from ESRD-related complications. Median follow-up was 67.1 months (interquartile range [IQR]=2–227 months). Median time to ESRD was 118 months (IQR=2–222 months). Median RPA was 15.96 cm2 (IQR=13.07–21.64 cm2). The intraclass correlation coefficient of RPA measurements was 0.84.
Primary Analyses
RPA, 1-month creatinine, and VUR were associated with ESRD on univariate analysis and included in the final multivariate model. After adjusting for 1-month creatinine and VUR, each 1-cm2 increase in RPA lowered the probability of ESRD by 36% (hazard ratio, 0.64; 95% confidence interval [95% CI], 0.42 to 0.98); 1-month creatinine and VUR were not associated with ESRD on multivariate analysis (Table 2).
Table 2.
Cox proportional hazard ratios for ESRD
| Patient Characteristics | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | P Value | Hazard Ratio | 95% Confidence Interval | P Value | |
| Total RPA, cm2 | 0.76 | 0.63 to 0.92 | 0.01 | 0.64 | 0.42 to 0.98 | 0.04 |
| 1-mo creatinine, mg/dl | 5.00 | 2.09 to 11.95 | <0.001 | 2.33 | 0.90 to 6.04 | 0.08 |
| VUR | 5.43 | 1.2 to 24.5 | 0.03 | 10.7 | 0.59 to 193.9 | 0.11 |
| UTI, number | 0.76 | 0.33 to 1.78 | 0.49 | n/a | n/a | n/a |
n/a, not applicable.
Sensitivity Analyses
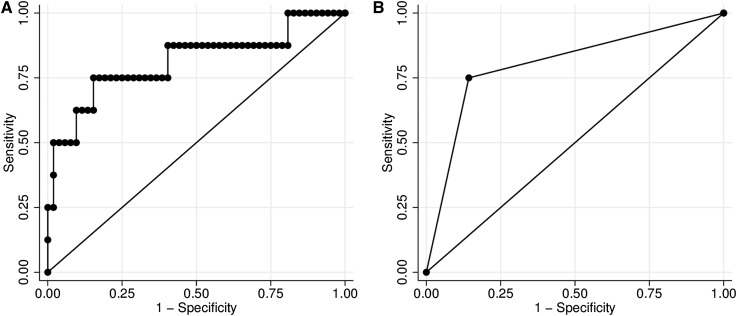
In receiver operating characteristic curve analysis, the sensitivity and specificity of RPA for ESRD were maximized when RPA equaled 12.4 cm2. At 12.4 cm2, the sensitivity and specificity of RPA for future ESRD were 75% and 85.4%, respectively. Little discriminative power was lost after RPA was dichotomized (Figure 2).
Figure 2.
Receiver operating characteristic curves for the association between renal parenchymal area and ESRD in boys with posterior urethral valves. (A) The area under the curve for renal parenchymal area as a continuous measure was 0.81, and (B) it decreased slightly to 0.80 after renal parenchymal area was dichotomized at 12.4 cm2, which was the cutpoint where sensitivity and specificity for ESRD were maximized.
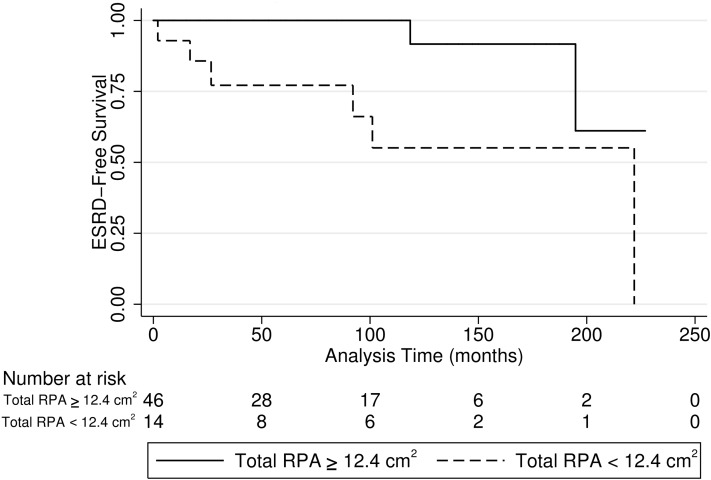
ESRD-free survival was longer in children with RPA≥12.4 cm2 than children with RPA<12.4 cm2 (P=0.003) (Figure 3). In patients with RPA≥12.4 cm2, the rate of ESRD was 0.5 per 1000 patient-months (95% CI, 0.13 to 2.12). In patients with RPA<12.4 cm2, the rate of ESRD was 5.0 per 1000 patient-months (95% CI, 2.3 to 11.2).
Figure 3.
Kaplan–Meier curve for ESRD-free survival for renal parenchymal area (RPA) greater than or equal to 12.4 cm2 and below 12.4 cm2. ESRD-free survival was longer in boys with RPA≥12.4 cm2 (solid line) compared with boys with RPA<12.4 cm2 (dashed line; P<0.001).
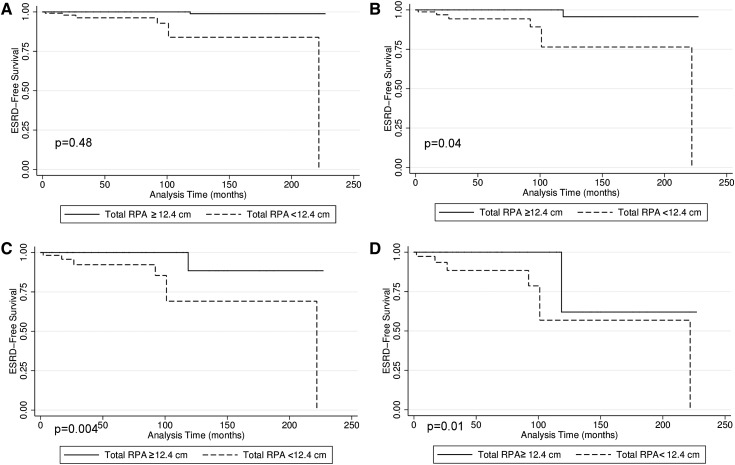
RPA could best discriminate renal survival when the 1-month creatinine was between 0.8 and 1.1 mg/dl. At these creatinine values, estimated ESRD-free survival was greater in patients with RPA≥12.4 cm2 (P=0.001 and P=0.003 for creatinine of 0.8 and 1.1, respectively) (Figure 4). Renal survival was similarly high when 1-month creatinine was centered at 0.4 mg/dl (P=0.40). Renal survival was similarly low when 1-month creatinine was centered at 1.5 mg/dl (P=0.40).
Figure 4.
Estimated ESRD-free survival when 1-month creatinine equals 0.4, 0.8, 1.1, and 1.5 mg/dl. Estimated ESRD-free survival was greater in boys with RPA>12.4 cm2 when 1-month creatinine was (B) 0.8 and (C) 1.1 mg/dl. Estimated ESRD-free survival was (A) similarly high when creatinine was 0.4 mg/dl and (D) similarly low when creatinine was 1.5 mg/dl.
Discussion
RPA measured on first postnatal ultrasound was associated with progression to ESRD in a contemporary population of boys who presented with PUV in the first 6 months of life and were followed up to 18 years. Nearly 75% were followed from birth to the end of the study. Each 1-cm2 increase in RPA was associated with a lower risk of ESRD. Boys with RPA<12.4 cm2 progressed to ESRD at a rate 10-fold higher than boys with RPA≥12.4 cm2. This result supports our hypothesis that the amount of kidney parenchyma may be a surrogate marker for renal functional reserve.
To determine the robustness of the association between RPA and ESRD, we tested how well RPA discriminates risk of ESRD at different baselines of renal function. This analysis assumed a patient population with 1-month creatinine values that ranged from 0.4 to 1.5 mg/dl. We observed that differences in the rates of renal survival between patients with RPA greater or less than 12.4 cm2 were most pronounced when creatinine was between 0.8 and 1.1 mg/dl. We observed no differences in renal survival when creatinine was 0.4 or 1.5 mg/dl. This finding suggests what is clinically obvious: infants with low creatinine values do relatively well, and infants with high creatinine values do poorly; however, there is an intermediate range in which other factors are associated with kidney outcomes. This finding suggests that RPA may have clinical use as a prognostic marker for the risk of ESRD in boys with PUV and intermediate-range creatinine values.
In this study, like in other studies, early nadir creatinine was associated with the risk of future renal failure in univariate analysis (9,13,14). However, the association between 1-month creatinine and ESRD became statistically insignificant in the final model. This result is likely because of a lack of statistical power; additionally, creatinine and RPA are on the same causal pathway to ESRD. Therefore, including both covariates in the model reduces the independent association of each variable. However, we chose to include both RPA and creatinine, because the aim of this study was to assess the association between RPA and ESRD independent of creatinine. We hypothesize that RPA reflects functional renal reserve, whereas 1-month creatinine reflects estimated GFR at the time of assessment. Therefore, 1-month creatinine and RPA represent two correlated, but distinct, measurements of kidney health, and both are on the causal pathway between PUV and ESRD.
Because only eight patients were followed past the age of 13 years, the association between RPA and ESRD that we observed is applicable to the risk of ESRD during childhood. The long-term risk of ESRD cannot be determined from this dataset, and, therefore, it is unknown if this association between RPA and ESRD holds true for patients who develop kidney failure in adulthood. Furthermore, although it is possible that RPA can help predict future kidney outcomes, demonstration of a statistically significant association of biomarker, such as RPA, with ESRD is not sufficient to determine the predictive abilities. Additional prospective studies are needed to validate the association between RPA, ESRD, and other stages of CKD. Additionally, studies designed to determine how RPA improves the correct classification of patients’ risk of kidney failure will increase the clinical use of this novel marker (21,22).
The potential strength of RPA is that it is available at the time of patient presentation. Should our results be validated, we believe that assessing RPA will improve our ability to counsel families about their child’s risk of future kidney failure, help plan follow-up, and identify cohorts of patients in whom early implementation of renal protective therapies may be beneficial (11). For example, recent studies have suggested that angiotensin-converting enzyme inhibitors may slow the progression of kidney function deterioration in children with CKD caused by congenital urological disorders; however, the benefit was seen only in certain cohorts of patients who were selected because they already exhibited late-term sequelae of CKD (8,23).
We used survival analysis methods, which account for different lengths of patient follow-up. These methods avoid the problem that results from logistic regression analyses of a dichotomous outcome (ESRD versus no ESRD) (9,11,12) by not assuming that patients who were lost to follow-up, or who had short follow-up, had similar kidney outcomes as those patients who were followed throughout the study. However, our study has limitations. First, as with all observational studies, selection and misclassification biases may exist. The frequency of creatinine checks varied; thus, we may have observed ESRD sooner in patients who were closely followed than patients whose laboratory draws were spaced farther apart or at an outside laboratory, which could bias our estimates of association to an increased risk if the appearance of the kidneys on the first ultrasound influenced clinician practice patterns. However, because RPA is not currently part of the standard clinical assessment of boys with PUV, we believe that it is unlikely.
Second, laboratory tests were obtained at both our institution and outside laboratories, which may have used different methods for calculating serum creatinine. Thus, the potential exists for misclassification of baseline kidney function; however, this variation should be small. Similarly, the first postnatal ultrasounds were performed at our institution and outside radiology departments. At our institution, kidneys were measured in supine position. Ultrasounds performed at outside institutions may have used a different technique; however, only three ultrasounds were performed at outside institutions, and any differences between prone and supine measurements are likely to be small (24). Additionally, RPA measurements were adjusted for the scale of the images.
Third, we did not evaluate other potentially clinically important factors in our analysis. Notably, we did not systematically assess or include in the regression models the presence of bladder dysfunction, because the definition of bladder dysfunction in PUV is often unclear. Urodynamic testing is the gold standard for defining bladder dysfunction. There was variability in whether urodynamics were performed in our cohort depending on physician judgment and clinical factors, such as recurrent urinary tract infections, kidney function, and ability to empty the bladder. Consequently, including bladder dysfunction in the regression models may have introduced confounding by severity. Similarly, we did not include other interventions that may affect kidney outcomes, such as intermittent catheterization or overnight catheter drainage, because these interventions could, at the same time, potentially improve outcomes and be a marker of more severe underlying disease.
Fourth, some patients included in our cohort had ultrasounds that were obtained over two decades ago, and there have been temporal changes in ultrasound technology since that time. Although we know of no reason why the ability to accurately define a simple anatomic characteristic such as kidney contour would be different now compared with 20 years ago, we cannot exclude this possibility. Fifth, our model may be overfit given the few outcomes relative to the number of independent variables.
In designing future prospective registries of patients with PUV, attention should be paid to measuring height along with creatinine to permit estimation of GFR to allow assessment of CKD stages. Additionally, data on time-varying covariates, such as bladder dysfunction, should be systematically collected. Finally, studies that expand the clinical applicability of our findings are also needed. Currently, the resources required to manually measure RPA limit its use in clinical practice. Automation of RPA measurements would greatly extend its applicability. Finally, the RPA cutpoint that we used for the survival curves was determined by the range of RPA within our particular cohort. Thus, it is unknown if our RPA cutpoints are associated with ESRD in other cohorts of boys with PUV or those patients with other forms of congenital urologic diseases. Development of normalized values for RPA similar to somatic growth curves for children would facilitate generalization of these methods beyond the population studied here (25).
In conclusion, this study shows that lower RPA is associated with an increased risk of ESRD during childhood in boys with PUV presenting during the first 6 months of life. These observations need to be validated externally (preferably in a prospectively enrolled cohort) before they can be used clinically to aid in the identification of patients with PUV at high risk for ESRD.
Disclosures
None.
Acknowledgments
S.L.F. was supported by National Institutes of Health Grant 1K24DK078737. G.E.T. was supported by National Institutes of Health Grant T32HD060550.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Dodson JL, Jerry-Fluker JV, Ng DK, Moxey-Mims M, Schwartz GJ, Dharnidharka VR, Warady BA, Furth SL: Urological disorders in chronic kidney disease in children cohort: Clinical characteristics and estimation of glomerular filtration rate. J Urol 186: 1460–1466, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marra G, Oppezzo C, Ardissino G, Daccò V, Testa S, Avolio L, Taioli E, Sereni F, ItalKid Project : Severe vesicoureteral reflux and chronic renal failure: A condition peculiar to male gender? Data from the ItalKid Project. J Pediatr 144: 677–681, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Seikaly M, Ho PL, Emmett L, Tejani A: The 12th Annual Report of the North American Pediatric Renal Transplant Cooperative Study: Renal transplantation from 1987 through 1998. Pediatr Transplant 5: 215–231, 2001 [PubMed] [Google Scholar]
- 4.Smith GH, Canning DA, Schulman SL, Snyder HM, 3rd, Duckett JW: The long-term outcome of posterior urethral valves treated with primary valve ablation and observation. J Urol 155: 1730–1734, 1996 [PubMed] [Google Scholar]
- 5.Drozdz D, Drozdz M, Gretz N, Möhring K, Mehls O, Schärer K: Progression to end-stage renal disease in children with posterior urethral valves. Pediatr Nephrol 12: 630–636, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Heikkilä J, Holmberg C, Kyllönen L, Rintala R, Taskinen S: Long-term risk of end stage renal disease in patients with posterior urethral valves. J Urol 186: 2392–2396, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Edouga D, Hugueny B, Gasser B, Bussières L, Laborde K: Recovery after relief of fetal urinary obstruction: Morphological, functional and molecular aspects. Am J Physiol Renal Physiol 281: F26–F37, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Neild GH: What do we know about chronic renal failure in young adults? II. Adult outcome of pediatric renal disease. Pediatr Nephrol 24: 1921–1928, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Ansari MS, Gulia A, Srivastava A, Kapoor R: Risk factors for progression to end-stage renal disease in children with posterior urethral valves. J Pediatr Urol 6: 261–264, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Coleman R, King T, Nicoara CD, Bader M, Chandran H, Robb A, Parashar K: Posterior urethral valves: Creatinine velocity, a new early predictor of renal insufficiency. J Pediatr Surg 48: 384–387, 2013 [DOI] [PubMed] [Google Scholar]
- 11.DeFoor W, Clark C, Jackson E, Reddy P, Minevich E, Sheldon C: Risk factors for end stage renal disease in children with posterior urethral valves. J Urol 180[4 Suppl]: 1705–1708, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Kari JA, El-Desoky S, Farag Y, Mosli H, Altyieb AM, Al Sayad A, Radawi O, Ghabra H, Basnawi F, Bahrawi O, Singh A, Farsi H: Renal impairment in children with posterior urethral valves. Pediatr Nephrol 28: 927–931, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Sarhan OM, El-Ghoneimi AA, Helmy TE, Dawaba MS, Ghali AM, Ibrahiem HI: Posterior urethral valves: Multivariate analysis of factors affecting the final renal outcome. J Urol 185[Suppl]: 2491–2495, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Warshaw BL, Hymes LC, Trulock TS, Woodard JR: Prognostic features in infants with obstructive uropathy due to posterior urethral valves. J Urol 133: 240–243, 1985 [DOI] [PubMed] [Google Scholar]
- 15.Cost GA, Merguerian PA, Cheerasarn SP, Shortliffe LM: Sonographic renal parenchymal and pelvicaliceal areas: New quantitative parameters for renal sonographic followup. J Urol 156: 725–729, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Wong IY, Copp HL, Clark CJ, Wu HY, Shortliffe LD: Quantitative ultrasound renal parenchymal area correlates with renal volume and identifies reflux nephropathy. J Urol 182[4 Suppl]: 1683–1687, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Sarma D, Barua SK, Rajeev TP, Baruah SJ: Correlation between differential renal function estimation using CT-based functional renal parenchymal volume and (99m)Tc - DTPA renal scan. Indian J Urol 28: 414–417, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang M, Gao F, Liu H, Pang H, Zuo YP, Yong T: Prospectively estimating the recoverability of renal function after relief of unilateral urinary obstruction by measurement of renal parenchymal volume. Acad Radiol 20: 401–406, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Schneider CA, Rasband WS, Eliceiri KW: NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG: Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42: 377–381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pencina MJ, Larson MG, D’Agostino RB: Choice of time scale and its effect on significance of predictors in longitudinal studies. Stat Med 26: 1343–1359, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Pencina MJ, D'Agostino RB, Sr., D'Agostino RB, Jr., Vasan RS: Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 27: 157–172, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Neild GH, Thomson G, Nitsch D, Woolfson RG, Connolly JO, Woodhouse CR: Renal outcome in adults with renal insufficiency and irregular asymmetric kidneys. BMC Nephrol 5: 12, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carrico CW, Zerin JM: Sonographic measurement of renal length in children: Does the position of the patient matter? Pediatr Radiol 26: 553–555, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Dibley MJ, Goldsby JB, Staehling NW, Trowbridge FL: Development of normalized curves for the international growth reference: Historical and technical considerations. Am J Clin Nutr 46: 736–748, 1987 [DOI] [PubMed] [Google Scholar]