Abstract
Epithelial-mesenchymal transition (EMT) is a highly conserved cellular program that converts polarized, immotile epithelial cells to migratory mesenchymal cells. In addition, EMT was initially recognized as a key step for morphogenesis during embryonic development. Emerging evidences indicate that this important developmental program promotes metastasis, drug resistance, and tumor recurrence, features that are associated with a poor clinical outcome for patients with breast cancer. Therefore, better understanding of regulation and signaling pathways in EMT is essential to develop novel targeted therapeutics. In this review, we present updated developments underlying EMT in tumor progression and metastasis, and discuss the challenges remaining in breast cancer research.
Keywords: breast cancer, EMT, metastasis, Signaling pathway, Snail
INTRODUCTION
Breast cancer is the most common cancer in women worldwide. A approximately 90% of breast cancer deaths are caused by local invasion and distant metastasis of tumor cells. Metastasis, the spread of cancer cells from the primary tumor to distance organs, is a complex process divided into a number of steps including detachment of tumor cells from the primary tumor, invasion, migration, intravasation, survival in the vasculature, extravasation, and colonization of the secondary site1. During this metastatic cascade, the transformation of normal breast cancer epithelial cells to metastatic ones is a corrupted production of genetic and epigenetic alteration of tumor cells (intrinsic factors) as well as a chaotic microenvironment (extrinsic signals). The major challenge in breast cancer research is to identify extrinsic signals and intrinsic factors that initiate breast cancer metastasis.
Epithelial-mesenchymal transition (EMT) is a phenotypic conversion linked with metastasis2, 3. The concept of EMT was originally defined by developmental biologists as a morphological conversion occurring at specific sites in embryonic epithelia to give rise to individual migratory cells, however, similar observations have been found between developmental EMT and cancer metastasis. In the EMT processes, epithelial cells gain mesenchymal properties and exhibit reduced intercellular adhesion and increased motility; they can also break through the basal membrane and migrate over long distances owing to profound changes in their cytoskeleton architecture2, 4.
Based on the biological context, EMT has been classified into three types2, 5, 6. Type 1 EMT involves embryonic development. Type 2 EMT is associated with wound healing, tissue regeneration, and organ fibrosis. Type 3 EMT occurs in epithelial cancer cells and involves cancer progression and metastasis. Although these three types of EMT represent considerably different biological processes, some genetic elements and mechanisms of regulation are well-conserved. It suggests that tumor cells usurp the developmental EMT program for metastasis. As metastatic breast cancer is largely considered an incurable disease, a better understanding of EMT regulation in the metastatic cascade will lead to the development of novel targeted therapeutic strategies. The role of EMT in breast cancer has been demonstrated via numerous in vitro and in vivo studies7, 8. In this review, we will provide new developments in delineating the extrinsic tumor microenvironmental signals and intrinsic factors leading to EMT induction in breast cancer.
MOLECULAR MECHANISMS INVOLVED IN EMT
Regulators of EMT
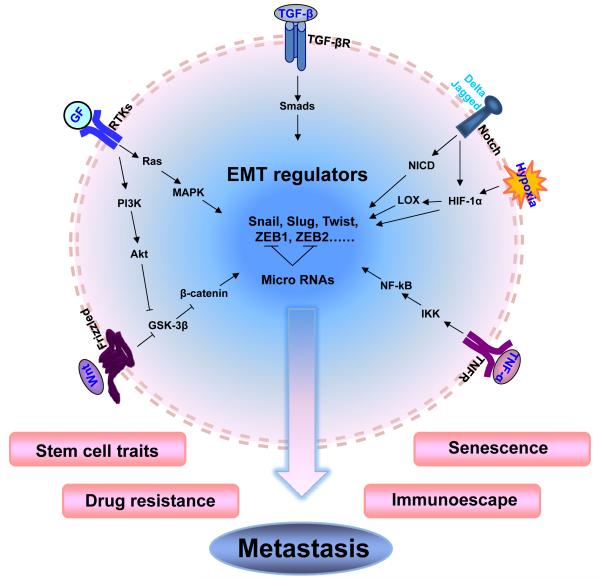
One of the hallmarks of EMT is loss of expression of the key epithelial cell-cell adhesion molecule E-cadherin. As a caretaker of the epithelial phenotype, E-cadherin helps to assemble epithelial cell sheets and maintain the quiescence of the cells within these sheets9. A vast majority of signaling pathways have been implicated in the regulation of EMT. Several transcription factors, for example, the Snail/Slug family, Twist, δEF1/ZEB1, SIP1/ZEB2, and E12/E47, respond to different microenvironmental stimuli and function as master molecular switches of the EMT program10-12 (Figure 1). These transcriptional factors can bind to the so called E-Box at the E-cadherin promoter, recruiting transcriptional co-repressors and histone deacetylases for E-cadherin silencing13. Snail is the most widely studied effector of E-cadherin repression and EMT. It was first described in Drosophila as a repressor of the transcription of shotgun (an E-cadherin homologue) to control embryogenesis, and was later found to play a fundamental role during EMT in mammalian cells10, 14, 15. Snail not only represses the E-cadherin expression, but also down-regulates the expression of other epithelial molecules, including claudins, occludins, and mucin-1, and induces the expression of genes associated with a mesenchymal and invasive phenotype16. High expression levels of Snail were observed in both epithelial and endothelial cells of invasive breast cancer17, 18. It has been linked to tumor grade, metastasis, recurrence and poor prognosis in patients with breast cancer19-21. In addition, Snail family proteins collaborate with other transcription factors, such as Twist and ZEB1, to orchestrate the concerted regulation22.
Figure 1. Embryonic signaling pathways lead to induction of EMT and cancer metastasis.
TGF-β, Wnt, Notch, RTKs and TNF-α signaling pathways can activate EMT regulators, such as Snail, Slug, Twist, ZEB1 and ZEB2, driving immotile epithelial cells to acquire more invasive phenotypes. EMT bestows tumor cells with stem cell-like characters and resistance to escape immune surveillance and senescence as well as offer survivability against chemo- and endocrine therapies during metastasis.
Microenvironmental signaling pathways in EMT induction
EMT is a dynamic process and is triggered by stimuli that emanate from microenvironments, including extracellular matrix (such as collagen and hyaluronic acid) and many secreted soluble factors, such as transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α)/nuclear factor κB (NF-κB), Wnt, epidermal growth factor (EGF), hepatocyte growth factor (HGF), Notch and cytokines23. The identification of several of these developmental signaling pathways in EMT induction and metastasis reinforces the notion that EMT is a dynamic event and that the interaction of microenvironment with cancer cells co-evolves in oncogenesis. A few examples of these signaling events are discussed in detail below.
TGF-β signaling, implicated as the primary inducer of EMT, plays a dual role in cancers. TGF-β suppresses early stages of tumor development by arresting proliferation and inducing cell death, however, it can later contribute to the malignant progression by promoting invasion and metastasis24, 25. The role of TGF-β as a promoter of tumor progression is associated with its ability to induce EMT through activating E-cadherin repressors26. The action of TGF-β is mediated by interaction with type I and type II TGF-β-related serine-threonine kinase receptors (TβRI and TGF-βRII)27. After ligand binding, TβRII transphosphorylates TβRI, which activates the receptor-regulated Smad2 and Smad3. Activated Smad2/3 forms complexes with Smad4, then, the Smad complexes interact with various transcription factors and transcription co-activators to regulate target genes transcription. Overexpression of Smad2 and Smad3 results in increased EMT, and the reduction of the functions of Smad2 and Smad3 decreases metastatic potential of breast cancer cell lines in a xenograft model28. In addition, TGF-β signaling can occur via Smad-independent pathways, including the activation of phosphatidylinositol 3-kinase (PI3K), Akt, mitogen activated protein kinase (MAPK) and small GTPases of the Rho family. Both Smad-dependent and -independent pathways function together to regulate the transcription of EMT master regulators, including Snail and Slug23, 29. In addition, TGF-β collaborates with other signaling pathways to induce complete EMT and maintain the mesenchymal phenotype of invasive/metastatic tumor cells30-32. For instance, the platelet-derived growth factor (PDGF)/PDGF receptor autocrine loop, essential for acquisition of a complete EMT phenotype, can be induced during TGF-β-driven EMT33. Annexin A1 (AnxA1), an inducible endogenous inhibitor of NF-κB, promotes metastasis by enhancing TGF-β/Smad signaling and actin reorganization, which facilitates an EMT-like switch, thereby allowing efficient cell migration and invasion of metastatic breast cancer cells34, 35.
The Wnt/β-catenin pathway is particularly relevant for the tumorigenesis of breast cancer, since activation of this pathway by mouse mammary tumor virus (MMTV) in the Wnt1 locus induces breast cancer in mice36. β-catenin, one of the downstream signaling molecules activated by Wnt signaling, may exist in different functional forms. On one hand, it acts as a bridge to enhance cell-cell adhesion when bound to cadherin complexes in adherens junctions. On the other hand, it functions as a transcription co-factor with DNA-binding proteins of the T cell factor (TCF)/lymphoid enhancer factor (LEF) family. Thus, β-catenin is considered an ideal target for studying the molecular basis of both EMT and malignant cancer formation. In the absence of Wnt, cytoplasmic β-catenin is phosphorylated by a destruction complex consisting of Axin, adenomatous polyposis coli (APC), glycogen synthase kinase-3β (GSK-3β) and casein kinase. The phosphorylated β-catenin is recognized by the E3 ubiquitin ligase β-Trcp, which targets β-catenin for proteosomal degradation. In the presence of Wnt ligands, Wnt binds to 7-transmembrane domain receptor Frizzled and the lipoprotein receptor-related protein (LRP5 or LRP6) complexes to inactivate GSK-3β in the destruction complex, thus stabilizing β-catenin. Stabilized β-catenin accumulates and travels into the nucleus, where it interacts with TCF/LEF complex, leading to the transcription of Wnt target genes, such as c-myc, cyclin D, and survivin37, 38. Within the mammary gland, the Wnt signaling pathway has emerged as a critical regulator of mammary stem cells and is required for both mammary gland development and morphogenesis39, 40. Aberrant activation of this pathway has been associated with over 50% of breast carcinomas41, 42, suggesting that the pathway is subverted in cancer cells to allow malignant proliferation and survival. Interestingly, only nuclear β-catenin has been correlated with an aggressive tumor phenotype and poor survival, whereas E-cadherin bound membrane β-catenin is associated with a favorable outcome. In addition, nuclear β-catenin is mainly found in the dedifferentiated mesenchymal-like tumor cells at the invasive front (tumor-host boundary) of malignant tumors, whereas in the central areas of tumors, β-catenin is localized to the membrane and cytoplasm42, 43. These observations indicate that the nuclear translocation of β-catenin is crucial for tumor progression and is controlled by intrinsic factors as well as extrinsic signals from the tumor microenvironment.
In this pathway, GSK-3β is a nodal protein, which not only negatively regulates stability and activity of β-catenin, but also mediates phosphorylation of Snail44. We have reported that GSK-3β binds to and phosphorylates Snail at two consensus motifs to dually regulate its function. The first phosphorylation motif regulates its ubiquitination mediated by β-Trcp, whereas the second phosphorylation motif controls its subcellular localization45. Thus, inhibition of GSK-3β can lead to Snail accumulation and E-cadherin downregulation, which releases β-catenin from the membrane complex and induces more nuclear translocation of β-catenin. This synergistic effect of Snail and β-catenin provides the cancer cells with the ability to survive during dissemination and invasion. Furthermore, in human breast cancer cells, canonical Wnt signaling activates the EMT program by inducing the expression of intracellular protein Axin2 to stabilize Snail. Axin2 acts as a nucleocytoplasmic chaperone for GSK-3β and forming a Wnt-Axin2-GSK-3β cascade46. Therefore, by blocking the activity of GSK-3β, Wnt can stabilize the level of Snail and β-catenin to induce EMT and cancer metastasis. In addition, Wnt signaling can activate the extracellular signal-regulated kinase1/2 (ERK1/2) pathway in mouse mammary epithelial cells via epidermal growth factor receptor (EGFR) transactivation. Wnt and EGFR signaling pathways crosstalk and transactivate one another in cancer. Furthermore, EGFR has been shown to form a complex with β-catenin and increase the invasion and metastasis of cancer cells47.
The Notch signaling pathway is an evolutionarily conserved signaling pathway. It not only maintains a balance between cell proliferation, differentiation and apoptosis, but plays a critical role in maintaining the progenitor cell population as well. Four Notch receptors (Notch1-4) interact with a diverse group of ligands from the Delta (Deltalike1, 3 and 4) and Serrate/Jagged family (Jagged1 and 2). Notch signaling is normally initiated by ligand-receptor binding between adjacent cells, followed by receptor cleavage by γ-secretase and eventual release of Notch intracellular domain (NICD). Once released from the membrane receptor, NICD translocates to the nucleus where it interacts with transcription factor CSL to activate canonical Notch target genes including those belonging to HES and HRT (HEY) families48. These target genes are critical for maintenance of stem cells. Alteration of Notch signaling has been associated with various types of cancer in which Notch can act as an oncogene or a tumor suppressor depending on the cellular context. Whichever the case, Notch signaling requires coordinated interaction with other oncogenic pathways and molecules to induce EMT. For example, Notch signaling leads to the initiation of EMT by activating NF-κB or altering TGF-β signaling49, 50. TGF-β increases Notch activity through Smad3, which upregulates both Jagged1 and HEY1. Elevated Jagged1 and Notch promote Slug expression, thereby suppressing E-cadherin and leading to an EMT phenotype. Slug-induced EMT is also accompanied by activation of β-catenin and resistance to anoikis, which contributes to breast cancer metastasis51. In addition, Notch serves as a critical intermediate in conveying the hypoxic response into EMT. Hypoxia has received considerable attention as an inducer of tumor metastasis. In this process, Notch signaling controls Snail expression by two distinct but synergistic mechanisms, including direct transcriptional activation of Snail and an indirect mechanism operating via lysyl oxidase (LOX). Notch increases LOX expression by recruiting hypoxia-inducible factor 1α (HIF-1α) to LOX promoter, which stabilizes the Snail protein, resulting in up-regulation of EMT and migration and invasion of cancer cells48, 52. Recent research shows that hypoxia-induced Jagged2 promotes breast cancer metastasis and self-renewal of cancer stem-like cells53.
TNF-α is a pivotal cytokine involved in inflammation, immunity, cellular homeostasis and tumor progression. TNF-α mediates its effect through two distinct cell surface receptors, TNFR1 and TNFR2. TNFR1-associated death domain protein (TRADD) recruits TNF receptor-associated factor (TRAF2) and receptor-interacting protein (RIP). TRAF2, in turn, recruits IκB kinase (IKK) complex, which is then activated in an RIP-dependent manner. The IKK complex phosphorylates IκB, which normally binds to NF-κB and inhibits its translocation. After phosphorylation, IκB proteins undergo rapid ubiquitination and proteasome-mediated degradation, releasing NF-κB. The free NF-κB translocates to the nucleus, binds to the κB site and activates gene transcription54. Unequivocal evidence shows that NF-κB activation is associated with the induction of many EMT regulators, such as Snail, Slug, Twist, and ZEB1/ZEB255. Our study found that Snail stabilization and EMT induction mediated by TNF-α are critical for metastasis. Snail can be stabilized by TNF-α through the activation of NF-κB, which prevents Snail phosphorylation by GSK-3β and subsequent degradation56. NF-κB also binds to Snail promoter, resulting in increased Snail transcription57. Knockdown of Snail expression not only inhibits TNF-α-induced cancer cell migration and invasion in vitro but also suppresses lipopolysaccharide-mediated metastasis in vivo. Recent studies show that Raf kinase inhibitor protein (RKIP), a metastatic suppressor, inhibits NF-κB activity, and conversely, Snail can repress the expression of RKIP. Therefore, there is a circuitry between RKIP, NF-κB and Snail, in which overexpression of Snail inhibits RKIP and induces EMT58-60. In addition, NF-κB mediates important aspects of TGF-β signaling essential for inducing and maintaining EMT in RAS-transformed mammary epithelial cells, facilitating an invasive/metastatic tumor phenotype61.
Furthermore, numerous other RTKs have also been found to contribute to EMT and cancer metastasis62. Growth factors, such as HGF, EGF and fibroblast growth factors (FGF), transduce signals via activation of RTKs and their central downstream effector Ras63, 64. HGF that signals via the RTK c-Met and ERK/MAPK cascade, was among the first observed to play a role in reshaping epithelial differentiation towards a scattering phenotype characterized by robust down-regulation of E-cadherin and with critical links to tumor metastasis65. Most frequently, the constitutive activation of RTKs and their downstream signaling effectors such as MAPK or PI3K endow epithelial cells with an increased rate of proliferation and are crucial events in establishing hyperplastic/premalignant lesions66. Ras-activated MAPK was found to promote EMT and metastasis of breast tumor cells via increasing phosphorylation and stabilization of Twist167. Although RTKs signaling plays a critical role in EMT, oncogenic pathways whose signaling involves RTKs may not be sufficient to elicit EMT. These oncogenic pathways fail to induce a mesenchymal and migratory phenotype even if they have the ability to destroy polarity and tight junction assembly68. This is compatible with the overwhelming evidence that EMT involves an intricate interplay of multiple signaling pathways, including TGF-β, Wnt, Notch, PDGF, and downstream β-catenin, NF-κB, and ERK/MAPK pathways69.
The role of microRNAs in EMT
MicroRNAs (miRNAs) are master regulators of gene expression in mammary gland development and breast cancer. They are single-stranded, small, 20-22 nucleotide-long, non-coding RNAs that modulate gene expression at the post-transcriptional level by inhibiting translation or initiating mRNA destruction. MicroRNAs have been implicated in regulating diverse cellular pathways, such as cell differentiation, proliferation and programmed cell death70, 71. Current findings show that some miRNAs have appeared as powerful regulators of EMT. When EMT is induced with different stimuli, the most consistently striking change is the reduction of miR-200 family (miR-200a, miR-200b, miR-200c, miR-141 and miR-429) and miR-205 levels72. The miR-200 family up-regulates E-cadherin expression in cancer cell lines by directly targeting the ZEB1 and ZEB2 mRNA, thereby enforcing epithelial phenotype72. Loss of miR-200 is commonly found in invasive breast cancer cell lines with mesenchymal phenotype. Through the regulation of EMT, miRNAs are also involved in modulating invasion and metastasis. For instance, miR-10b is highly expressed in metastatic breast cancer cells and positively regulates cell migration and invasion. Expression of miR-10b is induced by Twist, and proceeds to inhibit translation of HOXD10, resulting in increased expression of RhoC, and thus facilitates metastasis of breast cancer cells73. In addition, miR-155 is found to be mechanistically involved in TGF-β-induced EMT and cell migration and invasion by targeting RhoA, which plays an important role in formation and stabilization of cell junctions. The level of miR-155 is increased during TGF-β-induced EMT in mammary epithelial cell model systems74. Similar studies identified that miR-29a and miR-21 levels are also up-regulated upon TGF-β-induced EMT and are higher in many mesenchymal cell lines compared to epithelial cell lines75. However, the precise relationship between miR-29a and EMT is context dependent since the over-expression of miR-29a in non-tumorigenic murine mammary epithelial cells does not induce EMT. A contrasting role has been associated with miR-335, which was identified as a suppressor of invasion and metastasis. It suppresses metastasis and migration through targeting the progenitor cell transcription factor SOX4 and extracellular matrix component tenascin C76. The emerging overall scenario indicates that up-regulation or down-regulation of different miRNAs may be critical for the regulation of EMT and cancer metastasis. Manipulation of these EMT related miRNAs may present a novel therapeutic strategy for the treatment of advanced breast cancer although many obstacles remain.
Genetic and epigenetic control of EMT
Aberrant gene function and altered patterns of gene expression are key features of cancer. Growing evidence shows that several genes involved in the EMT process are altered in tumors through genetic and epigenetic events. For instance, E-cadherin can be inactivated by gene mutation and epigenetic silencing that occurs in invasive lobular carcinoma and invasive ductal carcinoma, respectively. Although E-cadherin promoter DNA hypermethylation was recognized as the major mechanism responsible for the epigenetic silencing of this molecule in EMT and invasive breast ductal carcinoma for a decade77, the sequential event and the molecular mechanism leading to DNA methylation at the E-cadherin promoter in breast cancer remain not well characterized. Our previous study shows that the N-terminal SNAG domain of Snail resembles a histone H3-like structure for recruiting histone lysine-specific demethylase 1 (LSD1) to the E-cadherin promoter for the de-methylation on histone H3 lysine 4 (denoted as H3K4), a transcriptional mark associated with gene activation78. However, H3K4 demethylation is known to be an initial step in gene repression79, suggesting that an intermediate step is required to bridge H3K4 demethylation to the DNA methylation on the E-cadherin promoter. We recently identified that Snail also interacted with H3K9 methyltransferase G9a and Suv39H1, two major methyltransferases responsible for H3K9 methylation that intimately link to DNA methylation80. These studies delineate a detail mechanism underlying E-cadherin silencing in breast cancer. Another example of multiple connections between epigenetics, stemness, and EMT is the polycomb protein BMI1. BMI1 repressed tumor suppressor PTEN by binding to the PTEN promoter to induce the EMT and promote metastasis81; overexpression of miR-200c decreased BMI1 expression to reverse EMT and stem-cell like properties81, 82. In addition, overactive TGF-β-Smad signaling was proposed to play a major role in the “epigenetic memory” and maintenance of EMT through a unique DNA methylation pattern on epithelial specific gene promoters83.
ROLE OF EMT IN CANCER METASTASIS
EMT and cancer stem cells
Stem cells have a large replicative and long life potential, making them good candidates to be the cells of origin in cancer. This potential has resulted in increased focus on cancer stem cells (CSCs) by cancer researchers as well as developmental biologists84-86. CSCs are defined by their ability to seed new tumors, self-renew, and produce non-stem differentiated cells87. Although the concept of CSCs has been long proposed, recent advances in the identification of CSCs from several human tumors, including human acute myeloid leukemia malignancies, breast tumors, colon tumors and others, have provided more convincing evidence for this concept88-90. For example, cells isolated from breast tumors can also form tumorspheres. These cells have an enriched CD44+/CD24− cell surface profile, which marks the same population of cells that have increased tumor-initiation capability in vivo88,91. Recently, a connection between EMT and CSCs has been demonstrated. The EMT process facilitates the generation of cancer cells with the mesenchymal traits required for dissemination as well as self-renewal properties needed for initiating secondary tumors92. The induction of EMT in immortalized human mammary epithelial cells (HMLE) by ectopic expression of Snail or Twist, or exposure to TGF-β, results in an increased ability to form tumorspheres and yield cells with a CD44high/CD24low stem cell signature. Such cells adopt a mesenchymal phenotype are greatly enriched in tumor initiating cells, and are akin to breast CSCs93. This evidence suggests that there may be a direct link between EMT and the gain of CSC-like properties, which may be prerequisites for cancer cell metastasis. The TGF-β signaling pathway is a major driving force for these processes94. Analysis of breast cancer tissue has identified a significant correlation between the claudin-low subtype of breast cancers with gene expression signatures for both EMT and CSCs, strengthening the EMT-CSCs connection. Interestingly, the miR-200 family members, which are down-regulated in these tumors, have been shown to target BMI1 polycomb ring finger oncogene BMI1 and SUZ12, a subunit of a PRC2. BMI1 is a known regulator of stem cell self-renewal and more highly expressed in breast cancer metastases when compared to match primary tumors, again bridging EMT and CSCs phenotypes with metastasis82, 95-97. In addition, the ZEB/miR-200 feedback loop is speculated to account for such an EMT-CSCs link at the molecular level. This feedback loop is a driving force for cancer progression towards metastasis by controlling the state of CSCs98. Our recent study shows that activation of β-catenin and Akt pathways by Twist is critical for the maintenance of EMT-associated, CSC-like features99.
EMT and therapeutic resistance
Advancement in early detection technologies and cancer therapies has greatly improved the survival of cancer patients, however, more than 45% to 50% of patients diagnosed with breast cancer will ultimately develop refractory or resistant disease92. The existence of a subpopulation of tumor cells with stem cell-like characteristics, such as very slow replication and resistance to conventional cancer therapy (chemotherapy, hormone therapy and radiotherapy), poses a new concept to explain the phenomena of drug resistance and tumor recurrence99, 100. Compared to the major population of more differentiated breast cancer cells, the subpopulation of CSCs with a CD44high/CD24low cell surface marker profile was more resistant to cancer therapies101. An in vitro study using radiation therapy reported increased resistance to treatment in cells grown as mammospheres, which contain a relatively high CSC population, versus cells grown in monolayer cultures102. In human breast tumors, neoadjuvant chemotherapeutic treatment of patients with locally advanced breast cancer results in the enrichment of CD44+/CD24−/low cells and increased efficiency of mammosphere formation103. Considering the relationship between CSCs and EMT, indirect resistance of CSCs to therapies suggests that the EMT program contributes to drug resistance. Indeed, evidence has been found for the connection between the EMT phenotype and therapeutic resistance. For example, EGFR-induced EMT has been linked to tamoxinfen resistance and increased invasiveness in the breast cancer cell line MCF7104. Another study shows that doxorubicin treatment increased the fraction of EMT-like cells, and the cells that underwent an oncogenic EMT were resistant to vincristine and pacilitaxel105. In addition, basal subtype breast cancer, which has a mesenchymal morphology in phenotype, is highly resistant to chemotherapies and is associated with higher mortality rates92. The mechanism by which the EMT program links to drug resistance is not well understood. It requires thorough investigations to determine whether chemotherapies select resistant cells with high EMT features or whether chemotherapies facilitate the EMT process to acquire drug-resistance in tumor cells. Recent studies show that Twist may play a role in this process. Increased expression of Twist was found in highly invasive breast cancer cell lines, and the elevated Twist was reported to up-regulate the transcription of AKT2 to promote cell survival and resistance to paclitaxel106. Furthermore, aberrant expression of Snail and/or Slug in breast cancer cells has also been related with invasive growth potential. Snail or Slug expression promotes cellular resistance to programmed cell death in MCF7 cells elicited by the DNA-damaging chemotherapeutic agent doxorubicin and promotes acquisition of invasive growth properties107. Identification of the molecular mechanism underlying the contribution of EMT and CSCs to drug resistance is crucial for the development of novel therapeutic strategies to treat metastatic disease.
EMT and senescence
Cellular senescence is characterized by the inability of cells to proliferate and by the maintenance of cell viability and metabolic activity108. It constitutes a failsafe program against cancer progression by allowing cells to enter a state of permanent cell cycle arrest. Senescence can be triggered by short or malfunctioning telomeres, but also by activation of oncogenes and loss of tumor suppressor genes. Currently, it has been proposed that activation of EMT is linked to suppression of cellular senescence in the context of EMT regulators. For example, Twist may launch cancer cells into their trajectories long before these cells exhibit invasive and metastatic powers by suppressing the expression of cyclin-dependent kinase inhibitors p16INK4A and p21cip1—two inducers of the senescence program109. Another EMT-regulator, ZEB1, was found to protect mouse embryonic fibroblasts from senescence110. Conversely, senescence players have also been found to affect EMT. For example, p21cip1 attenuates Ras and c-Myc-induced features of EMT and CSC-like gene expression in vivo111. It is interesting that EMT and senescence seem to cross paths. Better understanding these two mechanisms driving cancer progression and metastasis will eventually help to improve strategies for therapeutic intervention.
EMT and immunoescape
Cancer immune surveillance is considered to be an important host protection process to inhibit tumorigenesis and to maintain cellular homeostasis. Recently, the ability of cancers to escape immune surveillance via immunosuppression is attracting increasing attention. Cancer cells that have undergone EMT acquire immunosuppressive properties3, suggesting that cancer cells may utilize EMT program to escape immune-mediated destruction. Researchers have found that cancer immunosuppression evolves via an immunosuppressive network, which is mediated by several tumor-derived soluble factors (TDSFs), such as TGF-β, interleukin-10 (IL-10) and vascular endothelial growth factor (VEGF), and extends from the primary tumor site to secondary lymphoid organs and peripheral vessels. TDSFs act as strong chemoattractants to recruit immature myeloid cells, including immature dendritic cells (DCs) and macrophages, from bone marrow to the tumor site in accordance with tumor progression. This recruitment results in the inhibition of DC maturation and T-cell activation in a tumor-specific immune response112. It has been demonstrated that Snail-induced EMT accelerate metastasis through induction of immunosuppressive cytokines and regulatory T cells (Treg), as well as impairment of DCs and cytotoxic T lymphocytes113. Knockdown of Snail significantly inhibits tumor growth and metastasis by increasing tumor-infiltrating lymphocytes and systemic immune responses113. These results suggest that Snail is an effective target for preventing metastasis. In addition, several signaling pathways involved in EMT induction mentioned above also activate immunosuppressive T cells. For example, Wnt/β-catenin is reported to be involved in the generation of regulatory DCs and the enhancement of Treg survivals114. Therefore, therapies directed to inhibit EMT represent both anti-metastasis and anti-immunosuppression approach in treating cancer patients3.
THERAPEUTIC STRATEGIES FOR CANCER METASTASIS
Some traditional anticancer therapies were initially designed to prevent tumor growth and dissemination. These therapies have limitations since they are not designed to kill CSCs or mesenchymal cells derived from EMT process. CSCs and mesenchymal cells are more resistant to traditional therapeutic regimens than most epithelial non-CSCs. As a result, residual cancer cells may display both EMT and tumor-initiating characteristics after conventional therapies115. For example, some angiogenic inhibitors that favor hypoxia have been developed for the treatment of cancer, both in monotherapy and in combination with chemo- and radiotherapy, however researchers have now found that these inhibitors reduce primary tumor growth but promote tumor invasiveness and metastasis116. Tumor cells are proficient in escaping the noxious hypoxic microenvironment by switching on EMT and CSC programs in order to escape anti-angiogenic treatment117. Therefore, considerable effort is being devoted to develop specific therapies that target both EMT and CSCs118. Inhibition of RTK pathways, such as EGFR, ErbB and PDGFR, using receptor antagonists or small molecule inhibitors remains an effective approach for suppressing EMT119. To target CSCs, inhibition of Notch and Wnt/β-catenin signaling pathways may represent an excellent approach. High-throughput screening strategies have discovered some compounds, such as salinomycin, which seems to show selectivity for CSCs120. It appears that the characterization of pathways preferentially activated in CSCs will provide a more effective target to eliminate CSCs.
CONCLUSION AND REMARKS
Metastasis is the most poorly understood aspect in breast cancer, a problem that is responsible for approximately 90% of cancer-associated patient mortality. Despite numerous efforts made in the past several decades, progress in metastasis research has stagnated due to lack of effective tools to understand the complex network of signaling pathways that drives this multistep process. Recent studies found that EMT is a complex stepwise phenomenon that is not only involved in embryonic development but also in other physiological and pathological conditions, with a role in sustaining stem cell-like features as well as in enhancing invasive and metastatic behavior of tumor cells. It seems that cancer cells hijack the normal developmental networks for tumor metastasis. In addition, the link between EMT and CSCs has sparked considerable interest. The finding that EMT confers tumor cells with traits of CSCs provides a plausible molecular mechanism for tumor metastasis and recurrence. Therefore inhibition of EMT is an attractive therapeutic approach that could have significant effect on disease outcome. EMT is triggered by the signals from tumor microenvironment, including: extracellular matrix, blood vasculature, inflammatory cells and fibroblasts. This combinatorial signaling event not only synergizes the induction of EMT in metastasis but also contributes to the homing of tumor cells to specific tissues and organs. Furthermore, microenvironmental signaling events along with the heterogeneity of breast cancer cells elicit an invincible challenge to dissect the role and function of individual particular molecule or pathway in EMT, as well as their roles in other parallel biological processes, such as those in survival and proliferation. In order to push EMT and cancer metastasis research forward, it is obvious that several fields have to be further developed. New methodologies, innovative technologies, and conceptual ingenuities are needed to unravel the molecular mechanisms of cancer progression, in particular the formation of metastasis. For instance, EMT and MET are transient and dynamic events, particularly at the tumor invasive front, and a powerful imaging system that can capture these events may reveal the mystery of EMT. Large-scale analysis of transcripts by sequencing or microarray can be applied to uncover new candidates for EMT. Utilizing next generation technology for whole-genome sequencing of primary tumors and matched metastases can advance the ability to identify metastasis-specific driver mutations. Additionally, proteomics-based approaches can advance at a rapid pace as mass spectrometry technologies continue to evolve. Moreover, the improved molecular toolkit will permit more extensive evaluation of the heterogeneity that exists within tumor cell populations, as well as interrogation of its functional significance. In summary, targeting EMT represents an effective therapeutic strategy for preventing metastasis.
HALLMARKS IMPACT.
Metastasis is responsible for a majority of breast cancer patient deaths. EMT is involved in the metastatic cascade of many solid tumors and represents as a hallmark of this event. It not only enhances cell motility and invasiveness but also confers tumor cells with stem cell-like characters and immunosuppressive properties, and provides them with therapeutic resistance. Therefore, targeting EMT is an attractive therapeutic approach for inhibiting breast cancer metastasis and will have significant impact on disease outcome.
ACKNOWLEDGMENTS
We thank Ms. Hope Johnson for critical reading and editing of this manuscript. This work was supported by grants from NIH (RO1CA125454), Susan G Komen Foundation (KG081310), and Mary Kay Ash Foundation (to B.P. Zhou).
REFERENCE
- 1.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 2.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 2009;119:1417–1419. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blick T, et al. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis. 2008;25:629–642. doi: 10.1007/s10585-008-9170-6. [DOI] [PubMed] [Google Scholar]
- 8.Trimboli AJ, et al. Direct evidence for epithelial-mesenchymal transitions in breast cancer. Cancer Res. 2008;68:937–945. doi: 10.1158/0008-5472.CAN-07-2148. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 11.Hartwell KA, et al. The Spemann organizer gene, Goosecoid, promotes tumor metastasis. Proc Natl Acad Sci U S A. 2006;103:18969–18974. doi: 10.1073/pnas.0608636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Pardali K, Moustakas A. Actions of TGF-beta as tumor suppressor and pro-metastatic factor in human cancer. Biochim Biophys Acta. 2007;1775:21–62. doi: 10.1016/j.bbcan.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, Zhou BP. Snail: More than EMT. Cell Adh Migr. 2010;4:199–203. doi: 10.4161/cam.4.2.10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y, Zhou BP. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br J Cancer. 2010;102:639–644. doi: 10.1038/sj.bjc.6605530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin TA, Goyal A, Watkins G, Jiang WG. Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann Surg Oncol. 2005;12:488–496. doi: 10.1245/ASO.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Parker BS, et al. Alterations in vascular gene expression in invasive breast carcinoma. Cancer Res. 2004;64:7857–7866. doi: 10.1158/0008-5472.CAN-04-1976. [DOI] [PubMed] [Google Scholar]
- 19.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 20.Moody SE, et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Chen WJ, et al. Multidrug resistance in breast cancer cells during epithelial-mesenchymal transition is modulated by breast cancer resistant protein. Chin J Cancer. 2010;29:151–157. doi: 10.5732/cjc.009.10447. [DOI] [PubMed] [Google Scholar]
- 22.Casas E, et al. Snail2 is an essential mediator of Twist1-induced epithelial mesenchymal transition and metastasis. Cancer Res. 2011;71:245–254. doi: 10.1158/0008-5472.CAN-10-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gavert N, Ben-Ze’ev A. Epithelial-mesenchymal transition and the invasive potential of tumors. Trends Mol Med. 2008;14:199–209. doi: 10.1016/j.molmed.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Massague J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heldin CH, Landstrom M, Moustakas A. Mechanism of TGF-beta signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr Opin Cell Biol. 2009;21:166–176. doi: 10.1016/j.ceb.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1:303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 28.Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. Embo J. 2004;23:1155–1165. doi: 10.1038/sj.emboj.7600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vincent T, et al. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta mediated epithelial-mesenchymal transition. Nat Cell Biol. 2009 doi: 10.1038/ncb1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nawshad A, Lagamba D, Polad A, Hay ED. Transforming growth factor-beta signaling during epithelial-mesenchymal transformation: implications for embryogenesis and tumor metastasis. Cells Tissues Organs. 2005;179:11–23. doi: 10.1159/000084505. [DOI] [PubMed] [Google Scholar]
- 31.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 32.Neth P, et al. The Wnt signal transduction pathway in stem cells and cancer cells: influence on cellular invasion. Stem Cell Rev. 2007;3:18–29. doi: 10.1007/s12015-007-0001-y. [DOI] [PubMed] [Google Scholar]
- 33.Jechlinger M, et al. Expression profiling of epithelial plasticity in tumor progression. Oncogene. 2003;22:7155–7169. doi: 10.1038/sj.onc.1206887. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, Huang L, Zhao W, Rigas B. Annexin 1 induced by anti-inflammatory drugs binds to NF-kappaB and inhibits its activation: anticancer effects in vitro and in vivo. Cancer Res. 2010;70:2379–2388. doi: 10.1158/0008-5472.CAN-09-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Graauw M, et al. Annexin A1 regulates TGF-beta signaling and promotes metastasis formation of basal-like breast cancer cells. Proc Natl Acad Sci U S A. 2010;107:6340–6345. doi: 10.1073/pnas.0913360107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Hively WP, Varmus HE. Use of MMTV-Wnt-1 transgenic mice for studying the genetic basis of breast cancer. Oncogene. 2000;19:1002–1009. doi: 10.1038/sj.onc.1203273. [DOI] [PubMed] [Google Scholar]
- 37.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Wu Y, Zhou BP. New insights of epithelial-mesenchymal transition in cancer metastasis. Acta Biochim Biophys Sin (Shanghai) 2008;40:643–650. doi: 10.1111/j.1745-7270.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turashvili G, Bouchal J, Burkadze G, Kolar Z. Wnt signaling pathway in mammary gland development and carcinogenesis. Pathobiology. 2006;73:213–223. doi: 10.1159/000098207. [DOI] [PubMed] [Google Scholar]
- 40.Woodward WA, Chen MS, Behbod F, Rosen JM. On mammary stem cells. J Cell Sci. 2005;118:3585–3594. doi: 10.1242/jcs.02532. [DOI] [PubMed] [Google Scholar]
- 41.Brennan KR, Brown AM. Wnt proteins in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2004;9:119–131. doi: 10.1023/B:JOMG.0000037157.94207.33. [DOI] [PubMed] [Google Scholar]
- 42.Howe LR, Brown AM. Wnt signaling and breast cancer. Cancer Biol Ther. 2004;3:36–41. doi: 10.4161/cbt.3.1.561. [DOI] [PubMed] [Google Scholar]
- 43.Brabletz T, et al. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A. 2001;98:10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fuxe J, Vincent T, de Herreros AG. Transcriptional crosstalk between TGFbeta and stem cell pathways in tumor cell invasion: Role of EMT promoting Smad complexes. Cell Cycle. 2010;9 doi: 10.4161/cc.9.12.12050. [DOI] [PubMed] [Google Scholar]
- 45.Zhou BP, et al. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 46.Yook JI, et al. A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nat Cell Biol. 2006;8:1398–1406. doi: 10.1038/ncb1508. [DOI] [PubMed] [Google Scholar]
- 47.Hu T, Li C. Convergence between Wnt-beta-catenin and EGFR signaling in cancer. Mol Cancer. 2010;9:236. doi: 10.1186/1476-4598-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grego-Bessa J, Diez J, Timmerman L, de la Pompa JL. Notch and epithelial-mesenchyme transition in development and tumor progression: another turn of the screw. Cell Cycle. 2004;3:718–721. [PubMed] [Google Scholar]
- 49.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 50.Wang Z, et al. Down-regulation of notch-1 inhibits invasion by inactivation of nuclear factor-kappaB, vascular endothelial growth factor, and matrix metalloproteinase-9 in pancreatic cancer cells. Cancer Res. 2006;66:2778–2784. doi: 10.1158/0008-5472.CAN-05-4281. [DOI] [PubMed] [Google Scholar]
- 51.Leong KG, et al. Jagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through Slug-induced repression of E-cadherin. J Exp Med. 2007;204:2935–2948. doi: 10.1084/jem.20071082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci U S A. 2008;105:6392–6397. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xing F, et al. Hypoxia-induced Jagged2 promotes breast cancer metastasis and self-renewal of cancer stem-like cells. Oncogene. 2011 doi: 10.1038/onc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Basseres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25:6817–6830. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- 55.Min C, Eddy SF, Sherr DH, Sonenshein GE. NF-kappaB and epithelial to mesenchymal transition of cancer. J Cell Biochem. 2008;104:733–744. doi: 10.1002/jcb.21695. [DOI] [PubMed] [Google Scholar]
- 56.Wu Y, et al. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–428. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barbera MJ, et al. Regulation of Snail transcription during epithelial to mesenchymal transition of tumor cells. Oncogene. 2004;23:7345–7354. doi: 10.1038/sj.onc.1207990. [DOI] [PubMed] [Google Scholar]
- 58.Wu K, Bonavida B. The activated NF-kappaB-Snail-RKIP circuitry in cancer regulates both the metastatic cascade and resistance to apoptosis by cytotoxic drugs. Crit Rev Immunol. 2009;29:241–254. doi: 10.1615/critrevimmunol.v29.i3.40. [DOI] [PubMed] [Google Scholar]
- 59.Katsman A, Umezawa K, Bonavida B. Chemosensitization and immunosensitization of resistant cancer cells to apoptosis and inhibition of metastasis by the specific NF-kappaB inhibitor DHMEQ. Curr Pharm Des. 2009;15:792–808. doi: 10.2174/138161209787582156. [DOI] [PubMed] [Google Scholar]
- 60.Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446:475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 61.Huber MA, et al. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114:569–581. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 63.Rommel C, Hafen E. Ras--a versatile cellular switch. Curr Opin Genet Dev. 1998;8:412–418. doi: 10.1016/s0959-437x(98)80111-1. [DOI] [PubMed] [Google Scholar]
- 64.Shields JM, Pruitt K, McFall A, Shaub A, Der CJ. Understanding Ras: ‘it ain’t over ‘til it’s over’. Trends Cell Biol. 2000;10:147–154. doi: 10.1016/s0962-8924(00)01740-2. [DOI] [PubMed] [Google Scholar]
- 65.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 66.Gotzmann J, et al. Molecular aspects of epithelial cell plasticity: implications for local tumor invasion and metastasis. Mutat Res. 2004;566:9–20. doi: 10.1016/s1383-5742(03)00033-4. [DOI] [PubMed] [Google Scholar]
- 67.Hong J, et al. Phosphorylation of Serine 68 of Twist1 by MAPKs Stabilizes Twist1 Protein and Promotes Breast Cancer Cell Invasiveness. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-10-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aranda V, et al. Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat Cell Biol. 2006;8:1235–1245. doi: 10.1038/ncb1485. [DOI] [PubMed] [Google Scholar]
- 69.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 70.Stadler BM, Ruohola-Baker H. Small RNAs: keeping stem cells in line. Cell. 2008;132:563–566. doi: 10.1016/j.cell.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008;9:219–230. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- 72.Gregory PA, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 73.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 74.Kong W, et al. MicroRNA-155 is regulated by the transforming growth factor beta/Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Mol Cell Biol. 2008;28:6773–6784. doi: 10.1128/MCB.00941-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gebeshuber CA, Zatloukal K, Martinez J. miR-29a suppresses tristetraprolin, which is a regulator of epithelial polarity and metastasis. EMBO Rep. 2009;10:400–405. doi: 10.1038/embor.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tavazoie SF, et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lombaerts M, et al. E-cadherin transcriptional downregulation by promoter methylation but not mutation is related to epithelial-to-mesenchymal transition in breast cancer cell lines. Br J Cancer. 2006;94:661–671. doi: 10.1038/sj.bjc.6602996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin Y, et al. The SNAG domain of Snail1 functions as a molecular hook for recruiting lysine-specific demethylase 1. EMBO J. 2010;29:1803–1816. doi: 10.1038/emboj.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rudolph T, et al. Heterochromatin formation in Drosophila is initiated through active removal of H3K4 methylation by the LSD1 homolog SU(VAR)3-3. Mol Cell. 2007;26:103–115. doi: 10.1016/j.molcel.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 80.Dong C, et al. G9a interacts with Snail and is critical for Snail-mediated E-cadherin repression in human breast cancer. J Clin Invest. 2012;122:1469–1486. doi: 10.1172/JCI57349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Song LB, et al. The polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells. J Clin Invest. 2009;119:3626–3636. doi: 10.1172/JCI39374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shimono Y, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Papageorgis P, et al. Smad signaling is required to maintain epigenetic silencing during breast cancer progression. Cancer Res. 2010;70:968–978. doi: 10.1158/0008-5472.CAN-09-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smalley M, Ashworth A. Stem cells and breast cancer: A field in transit. Nat Rev Cancer. 2003;3:832–844. doi: 10.1038/nrc1212. [DOI] [PubMed] [Google Scholar]
- 85.Reiman JM, Knutson KL, Radisky DC. Immune promotion of epithelial-mesenchymal transition and generation of breast cancer stem cells. Cancer Res. 2010;70:3005–3008. doi: 10.1158/0008-5472.CAN-09-4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang QZ, Lu YH, Jiang N, Diao Y, Xu RA. The asymmetric division and tumorigenesis of stem cells. Chin J Cancer. 2010;29:248–253. doi: 10.5732/cjc.009.10668. [DOI] [PubMed] [Google Scholar]
- 87.Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat Med. 2009;15:1010–1012. doi: 10.1038/nm0909-1010. [DOI] [PubMed] [Google Scholar]
- 88.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ricci-Vitiani L, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 90.Singh SK, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 91.Ponti D, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 92.Hollier BG, Evans K, Mani SA. The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. J Mammary Gland Biol Neoplasia. 2009;14:29–43. doi: 10.1007/s10911-009-9110-3. [DOI] [PubMed] [Google Scholar]
- 93.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Herschkowitz JI, et al. Comparative oncogenomics identifies breast tumors enriched in functional tumor-initiating cells. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1018862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iliopoulos D, et al. Loss of miR-200 inhibition of Suz12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells. Mol Cell. 2010;39:761–772. doi: 10.1016/j.molcel.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu S, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brabletz S, Brabletz T. The ZEB/miR-200 feedback loop--a motor of cellular plasticity in development and cancer? EMBO Rep. 2010;11:670–677. doi: 10.1038/embor.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li J, Zhou BP. Activation of beta-catenin and Akt pathways by Twist are critical for the maintenance of EMT associated cancer stem cell-like characters. BMC Cancer. 2011;11:49. doi: 10.1186/1471-2407-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yu H, Zhang CM, Wu YS. Research progress in cancer stem cells and their drug resistance. Chin J Cancer. 2010;29:261–264. doi: 10.5732/cjc.009.10487. [DOI] [PubMed] [Google Scholar]
- 101.Nicolini A, et al. Stem cells: their role in breast cancer development and resistance to treatment. Curr Pharm Biotechnol. 2011;12:196–205. doi: 10.2174/138920111794295657. [DOI] [PubMed] [Google Scholar]
- 102.Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 103.Li X, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 104.Hiscox S, et al. Tamoxifen resistance in MCF7 cells promotes EMT-like behaviour and involves modulation of beta-catenin phosphorylation. Int J Cancer. 2006;118:290–301. doi: 10.1002/ijc.21355. [DOI] [PubMed] [Google Scholar]
- 105.Li QQ, et al. Twist1-mediated adriamycin-induced epithelial-mesenchymal transition relates to multidrug resistance and invasive potential in breast cancer cells. Clin Cancer Res. 2009;15:2657–2665. doi: 10.1158/1078-0432.CCR-08-2372. [DOI] [PubMed] [Google Scholar]
- 106.Cheng GZ, et al. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007;67:1979–1987. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- 107.Kajita M, McClinic KN, Wade PA. Aberrant expression of the transcription factors snail and slug alters the response to genotoxic stress. Mol Cell Biol. 2004;24:7559–7566. doi: 10.1128/MCB.24.17.7559-7566.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 109.Ansieau S, et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14:79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 110.Liu Y, El-Naggar S, Darling DS, Higashi Y, Dean DC. Zeb1 links epithelial-mesenchymal transition and cellular senescence. Development. 2008;135:579–588. doi: 10.1242/dev.007047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu M, et al. p21CIP1 attenuates Ras- and c-Myc-dependent breast tumor epithelial mesenchymal transition and cancer stem cell-like gene expression in vivo. Proc Natl Acad Sci U S A. 2009;106:19035–19039. doi: 10.1073/pnas.0910009106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim R, Emi M, Tanabe K. Cancer immunosuppression and autoimmune disease: beyond immunosuppressive networks for tumour immunity. Immunology. 2006;119:254–264. doi: 10.1111/j.1365-2567.2006.02430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 114.Manicassamy S, et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Creighton CJ, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106:13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell. 2009;15:167–170. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 117.Brahimi-Horn MC, Chiche J, Pouyssegur J. Hypoxia and cancer. J Mol Med (Berl) 2007;85:1301–1307. doi: 10.1007/s00109-007-0281-3. [DOI] [PubMed] [Google Scholar]
- 118.Alison MR, Lim SM, Nicholson LJ. Cancer stem cells: problems for therapy? J Pathol. 2011;223:147–161. doi: 10.1002/path.2793. [DOI] [PubMed] [Google Scholar]
- 119.Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol. 2011;27:347–376. doi: 10.1146/annurev-cellbio-092910-154036. [DOI] [PubMed] [Google Scholar]
- 120.Gupta PB, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]