Abstract
It is commonly believed that the ability to integrate information from different senses develops according to associative learning principles as neurons acquire experience with co-active cross-modal inputs. However, previous studies have not distinguished between requirements for co-activation versus co-variation. To determine whether cross-modal co-activation is sufficient for this purpose in visual–auditory superior colliculus (SC) neurons, animals were reared in constant omnidirectional noise. By masking most spatiotemporally discrete auditory experiences, the noise created a sensory landscape that decoupled stimulus co-activation and co-variance. Although a near-normal complement of visual–auditory SC neurons developed, the vast majority could not engage in multisensory integration, revealing that visual–auditory co-activation was insufficient for this purpose. That experience with co-varying stimuli is required for multisensory maturation is consistent with the role of the SC in detecting and locating biologically significant events, but it also seems likely that this is a general requirement for multisensory maturation throughout the brain.
Keywords: cat, cross-modal, hearing, vision
Introduction
The role of superior colliculus (SC) neurons in detecting and orienting to environmental events is facilitated by their access to visual, auditory and somatosensory information. In cat, ‘visual–auditory’ is the most common multisensory type, but all can integrate their cross-modal inputs to enhance responses, thereby increasing the salience of the event and its likelihood of initiating overt responses (Grossberg et al., 1997; Diederich & Colonius, 2004; Stein & Stanford, 2008).
But multisensory integration capabilities are not present at birth– multisensory neurons appear gradually (Stein et al., 1973), and their integration capacity develops only after months of sensory experience (Stein, 2012). The idea that Hebbian principles and their derivatives (Young et al., 2007; Butts & Kanold, 2010; Tsui et al., 2010; Feldman, 2012) guide this development is supported by results from dark-reared animals, whose visual–auditory neurons develop but are unable to integrate visual–auditory inputs (Wallace et al., 2004; Wallace & Stein, 2007; Royal et al., 2010; Yu et al., 2010). By removing one relevant input, dark-rearing precludes engaging associative learning principles.
However, beyond revealing a general requirement for cross-modal exposure, previous experiments provide little mechanistic insight into the experiential requirements for visual–auditory integration. Recent studies speak more directly to the associative learning criteria that may apply. Specifically, multisensory integration capabilities do not develop after exposure to stochastically independent activation of the relevant sensory channels even though the unisensory systems advance to maturation (Xu et al., 2012). They do develop if those cross-modal stimuli are synchronized and co-localized, thereby promoting both strong co-activation and spatiotemporal co-variance of the sensory-specific inputs. If this process is, as assumed, based on statistical learning (Von Neumann, 1958), the requisite associative links would be strengthened only if the co-active inputs co-vary, because only then are the different signals mutually informative. If one of the inputs were always active it would be uninformative under all conditions. Thus, despite the fact that co-activation would occur frequently it would be insufficient to reinforce the associative links between sensory-specific channels necessary to provide the impetus for this maturation. Unfortunately, previous studies have been unable to distinguish between these possibilities, prompting the present studies to provide greater insight into the mechanisms driving multisensory development by rearing animals in omnidirectional sound (i.e. noise). This provides a sensory landscape in which there is co-activation of visual (normal, and discrete) and auditory (ambient, and diffuse) inputs, but in which experience with spatially and/or temporally discrete auditory events is masked, thereby eliminating cross-modal co-variance. This condition has effectively been used to study the development of auditory representations in rat cortex (Chang & Merzenich, 2003) and barn owl optic tectum (Efrati & Gutfreund, 2011).
By design, the rearing condition does not deprive the brain of visual or auditory stimulation nor, by extension, of visual and auditory pre-synaptic input to SC neurons. However, imposing uncorrelated noise on one sensory-specific channel (i.e. auditory) obviates a key requirement of statistical learning rules, allowing us to examine a key prediction – that it is not the co-activation of sensory-specific input channels per se but the co-variance between them that must be experienced for SC multisensory integration to develop.
Materials and methods
Protocols were in accordance with the NIH Guide for the Care and Use of Laboratory Animals Eighth Edition (NRC 2011). They were approved by the Animal Care and Use Committee of Wake Forest School of Medicine, an Association for the Assessment and Accreditation of Laboratory Animal Care International-accredited institution.
Experimental groups
Four control (‘normal’) and three experimental (‘noise-reared’) adult cats from two litters were used for the main portion of this study. A third cohort of three noise-reared cats was studied acutely at 3 months of age to identify any early effects of the experimental condition that might otherwise have gone unnoticed. All animals were mixed-breed domestic cats, and the normal group was reared in a normal husbandry facility. The noise-reared cats were housed from < 1 week after birth in a room in which continuous omnidirectional broadband noise was provided by speakers (n = 5) located on all sides and above their pen. They were removed only for surgical and electrophysiological recording procedures. The broadband noise (provided by a noise generator) was effective in blocking human perception of most other auditory cues and has been used extensively for this purpose in animal studies (Chang & Merzenich, 2003; Efrati & Gutfreund, 2011). The mothers and kittens were accommodated to the background noise by gradually raising its amplitude from 55 to 80 dB over a 7-day period. This period corresponds to that in which auditory-responsive neurons first appear in the SC (i.e. at 5 days postnatally) and are gradually increasing in incidence, and before the appearance of visual–auditory neurons (Stein et al., 1973). Thus, no neurons capable of visual–auditory integration would develop before or during the accommodation procedure (see also, Wallace & Stein, 1997). The background noise stimulus was maintained thereafter at 80 dB and monitored continuously. In all other relevant respects, the housing environment was the same as for animals in the comparator control group. No differences were apparent in the health, activity level, stress or weight of noise-reared and control cats. Substantial efforts were made to preclude any confounding auditory experiences during transfer to and from the recording room by giving initial anesthetic and sedation drugs to animals in their home cage, fitting them with ear plugs during transport (and recovery) and maintaining anesthesia throughout experimentation.
Surgical and electrophysiological recording procedures
Surgery
One day prior to surgery, and again on the day of surgery, animals were given a single dose of dexamethasone sodium phosphate (1 mg/kg, i.m.) to reduce the likelihood of edema. On the day of surgery the anesthetic ketamine hydrochloride (20 mg/kg, i.m.) with acepromazine maleate (0.1 mg/kg, i.m.) was administered to animals in their home cage before transport. They were then transported to the surgical suite, intubated for the induction (2–4%) and maintenance (1.5–2%) of gaseous (isoflurane) anesthesia during surgery, and placed in a stereotaxic frame. During surgery expiratory CO2, blood pressure and heart rate were continuously monitored using a digital vital signs monitor (VetSpecs VSM7), and body temperature was maintained at ~37–38°C with a heating pad. A stainless steel recording chamber was placed over a craniotomy to provide access to the SC and attached to the skull with stainless steel screws and dental acrylic (McHaffie & Stein, 1983). After surgery, animals were administered analgesics (buprenorphine 0.005–0.01 mg/kg, i.m., b.i.d. for 3 days), antibiotics (ceftriaxone, 20 mg/kg, i.m., b.i.d. for 7 days) and a dexamethasone ‘taper’ (0.5 mg/kg, i.m., b.i.d. for 3 days; 0.25 mg/kg, i.m., b.i.d. on day 4 and s.i.d. on day 5).
Electrophysiological recording
Weekly recording sessions began after at least 7 days of post-surgical recovery. Again, the animal was administered ketamine hydrochloride (20 mg/kg, i.m.) with acepromazine maleate (0.1 mg/kg, i.m.) in the home cage, transported to the experimental room, intubated and ventilated mechanically. It lay in a recumbent position and its head was fixed in orientation by two horizontal stainless steel bars attached to the recording chamber, thereby avoiding the induction of any wounds or pressure points. Respiratory rate and volume were adjusted to keep the end-tidal CO2 at ~4.0%. Expiratory CO2, heart rate and blood pressure were monitored continuously to assess and, if necessary, adjust depth of anesthesia. Neuromuscular blockade was induced with an injection of pancuronium bromide (0.1 mg/kg, i.v.) to preclude movement artifacts, prevent ocular drift and fix the pinnae in place. The pupil of the eye contralateral to the SC under study was dilated with 1% atropine sulfate, and a contact lens focused the eye on a tangent screen in front of the animal. The optic disc of that eye was mapped on the screen and an opaque lens occluded the other eye. Anesthesia, paralysis and hydration were maintained by intravenous infusion of ketamine hydrochloride (5–10 mg/kg/h), pancuronium bromide (0.05 mg/kg/h) and 5% dextrose in sterile saline (2.4–3.6 mL/h). Body temperature was maintained at 37–38°C.
Conventional methods were used for single-neuron electrophysiological recording (Meredith & Stein, 1983; Yu et al., 2009, 2010; Xu et al., 2012). A glass-coated tungsten electrode (tip diameter: 1–3 μm, impedance: 1–3 MΩ at 1 kHz) was lowered to the surface of the SC and then advanced by a hydraulic microdrive to search for single neurons in its multisensory (i.e. deep) layers. Single neurons were studied when they were clearly isolated (i.e. action potential amplitude was at least three times the level of background noise). Single neuron activity was recorded, amplified and routed to an oscilloscope, audio monitor and computer for on-line and off-line analyses.
At the end of the recording session, the animal was injected with 40–50 mL of saline subcutaneously to ensure postoperative hydration, and anesthesia and neuromuscular blockade were terminated. The animal was removed from the head-holder, the endotracheal tube and intravenous lines were removed, and it was returned to its home cage in the noise room once stable respiration and locomotion returned.
Procedures for acute recordings in 3-month-old animals were substantially the same as those described above for the adults albeit their head wells were implanted on the day of recording, they were not treated with dexamethasone and were terminated at the end of the recording session (200 mg/kg sodium pentobarbital, i.v.).
During experimentation, visual search stimuli consisted of a moving or flashed bar of light (75 cd/m2) back-projected from an LC 4445 Philips projector onto the tangent screen. Auditory search stimuli consisted of 60–65 dB broadband (20–20 000 Hz) noise bursts and tones delivered via one of 15 stationary speakers positioned around the animal on a hoop whose axis of rotation was in line with the animal’s interaural axis. Somatosensory search stimuli consisted of tapping and brushing the hair and skin, as well as manual manipulation of deep tissue and movement of joints. However, visual–auditory neurons were of primary concern for quantitative study and, when such a neuron was identified, its receptive fields were mapped using conventional methods (Meredith & Stein, 1986) and transferred to standardized representations of visual and auditory space (Stein & Meredith, 1993). The unisensory and multisensory responses of these neurons were examined and quantified using randomly interleaved modality-specific and cross-modal stimulus pairs at inter-trial intervals of 5–7 s, 20 trials per condition. The auditory stimuli consisted of brief (100–200 ms) bursts of the broadband noise of varying intensity (55–70 dB) presented against an ambient background of 51.4 dB. Visual stimuli (100–200 ms duration) were rectangular bars of light (6° × 2°) of varying intensity (1.1–13.5 cd/m2) presented against a uniformly dark background (0.86 cd/m2) and moved in the most effective direction and speed for the neuron under study. For cross-modal tests the visual and auditory stimuli were presented within their respective receptive fields and in close spatial and temporal register. In normal animals, this cross-modal stimulus produces a multisensory response that is enhanced relative to that elicited by the best component stimulus (Meredith & Stein, 1983). To identify stimulus intensity values for quantitatively testing a given neuron, a brief series of preliminary tests were conducted in which stimulus intensities were systematically manipulated to quantify its dynamic range. To maximize the integrated multisensory product of the neuron under study, weakly effective individual modality-specific component stimuli were chosen (Kadunce et al., 1997; Perrault et al., 2005; Stanford et al., 2005).
When time permitted, the spatial tuning within a neuron’s receptive fields was examined by varying the location in azimuth of a modality-specific stimulus. For examination of visual spatial response profiles, a rectangular moving bar (4° × 1°) was used. Its intensity was 16.5 cd/m2 presented against the uniformly dark background and it was moved at 60°/s through a tightly restricted region (3°) of the visual receptive field. For examination of auditory spatial response profiles, a broadband noise burst 10 dB above threshold was presented multiple times from each of the hoop-mounted speakers in a randomly interleaved fashion. The mean number of impulses evoked by 20 repetitions of each stimulus was used to construct a spatial response profile for each modality. In circumstances in which receptive fields contracted during development to become approximately Gaussian, the tuning profile quantification was improved by fits with Gaussian functions. These made it possible to assess the receptive field center, and the alignment of two receptive fields could be quantified as a t-score:
where Xa and Xv are the locations of the peaks of the Gaussian fits to the auditory spatial and visual spatial tuning profiles, and σa and σv are the standard deviations. The lower the t value, the higher the degree of visual–auditory spatial register.
Three subsets of neurons were subjected to additional testing to examine specific response properties. One subset was tested both with simultaneously presented pairs of visual–visual stimuli (within-modal tests to examine unisensory integration) and pairs of visual–auditory stimuli (cross-modal tests to examine multisensory integration). To evaluate the integrative profiles of these neurons, three stimulus effectiveness levels spanning a neuron’s dynamic range were included. In the two other subsets, the spatial and temporal properties of the stimuli were manipulated to determine whether noise-rearing had substantially altered the spatial and temporal principles of multisensory integration rather than precluding its maturation.
Impulse times were recorded for each trial with 1-ms resolution and analysed off-line. The response window was defined using a geometric algorithm based on the cumulative impulse count as described in earlier studies (Rowland et al., 2007). The mean spontaneous firing rate for each condition was calculated in the 500-ms window preceding the stimulus. The magnitude of each response was identified as the mean number of impulses occurring in the response window minus the expected number based on the spontaneous firing rate. The mean response to the stimulus combination was then statistically compared with the response to the most effective single-modality component stimulus (t test, P < 0.05). Multisensory enhancement, the most reliable metric for multisensory integration (Kadunce et al., 1997), was defined as a significant increase in the number of impulses to the combined (cross-modal) stimuli compared with that to the most effective of its individual (modality-specific) component stimuli. The magnitude of multisensory response enhancement (ME) was calculated by the following formula: ME = [(CM − SMmax)/SMmax] × 100, where CM represents the mean magnitude of the multisensory response, and SMmax represents the magnitude of the response evoked by the more effective modality-specific stimulus (Meredith & Stein, 1983). These evaluations were restricted to those samples in which the two unisensory response trains overlapped (i.e. two independent unisensory responses could not be discerned). In cases where enhancement was compared across multiple efficacy levels, including cases where response levels were very low, a contrast index was used in place of ME for stability. The formula for the contrast index is: CME = (CM − SMmax)/(CM + SMmax). The contrast index ranges from −1 to 1, with values near 1 indicating that the multisensory response is far greater than the best unisensory response, and values near −1 indicating the opposite.
Data were compared statistically to determine significant differences using SPSS 11.5, t tests, Wilcoxon’s signed rank test, paired t-test, Mann–Whitney rank sum test, and χ2 tests where appropriate. The criterion for statistical significance was P < 0.05.
Results
A total of 231 visual–auditory neurons in the cat SC were examined in the present study. The receptive fields of 134 neurons in noise-reared animals and 97 in normally reared animals were mapped, and their unisensory and multisensory response properties were quantitatively evaluated. Ninety of the former and 51 of the latter neurons were held for sufficient periods of time to also quantitatively evaluate their responses to stimulation of multiple receptive field sites.
Convergence patterns
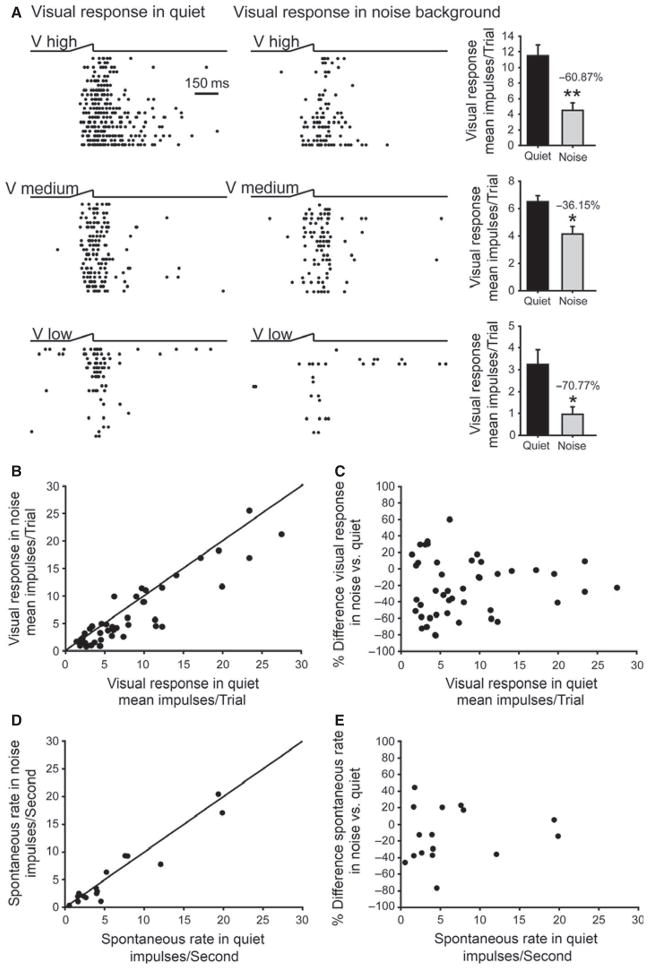
The modality convergence patterns noted in the noise-reared animals were not significantly different from those in the normally reared animals (χ2 test, d.f. = 6, χ2 = 4.819, P = 0.567; Fig. 1), revealing that the rearing condition had little effect on how the various modalities accessed these multisensory neurons. Indeed, visual responses continued to be evoked, albeit the auditory background had a tonic inhibitory influence (Fig. 2) as would be expected based on the large inhibitory surrounds of auditory-responsive SC neurons (see Kadunce et al., 1997).
Fig. 1.
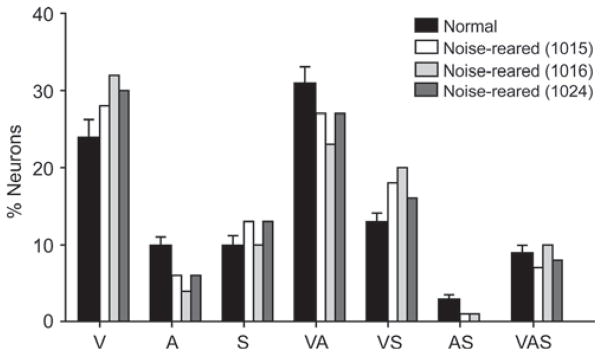
The modality-convergence patterns in the SC were similar in noise-reared and normally reared animals. The histogram shows the similar distributions of subtypes of unisensory and multisensory neurons in the SC of normally reared and noise-reared animals. Black bars (plus SEM represented by vertical lines) indicate the average distribution in normal animals, white and gray bars indicate the distribution in each of the noise-reared animals (1015; 1016; 1024). V, visual; A, auditory; S, somatosensory; VA, visual– auditory; VS, visual–somatosensory; AS, auditory–somatosensory; VAS, visual–auditory–somatosensory. The patterns were not significantly different in noise-reared and normally reared animals.
Fig. 2.
Visual responses in an omnidirectional noise-reared animal were depressed by background noise. (A) The visual responses of a visual–auditory neuron at three visual stimulus intensities (high, 3.47 cd/m2; medium, 1.44 cd/m2; low, 0.93 cd/m2) in the presence and absence of background noise equivalent to that in the rearing condition. Note the response depression at all three levels of stimulus effectiveness. (B) Comparison of the responses of a population of neurons to a visual stimulus in the noise background versus the same stimulus in quiet. Visual responses were significantly depressed by the background noise and most fell below the diagonal line of equality (Wilcoxon signed rank test, P ≤0.001, n = 48). (C) The percentage difference in each neuron between its visual response in noise and in quiet. (D) Comparison of the spontaneous activity recorded from each neuron in the presence of the background noise or in quiet. Spontaneous activity was slightly, albeit not significantly, depressed by the omnidirectional background noise stimulus (paired t-test, P = 0.194, n = 16). (E) The percentage difference between the spontaneous rates of each neuron in noise and in quiet.
The rearing condition also seemed to have little effect on the overall incidence of sensory-responsive neurons, as the mean distance between two adjacent sensory-responsive neurons along an electrode penetrations (240.92 ± 122.51 μm) did not differ significantly from that obtained in normally reared animals (228.81 ± 115.57 μm; t-test, P = 0.23).
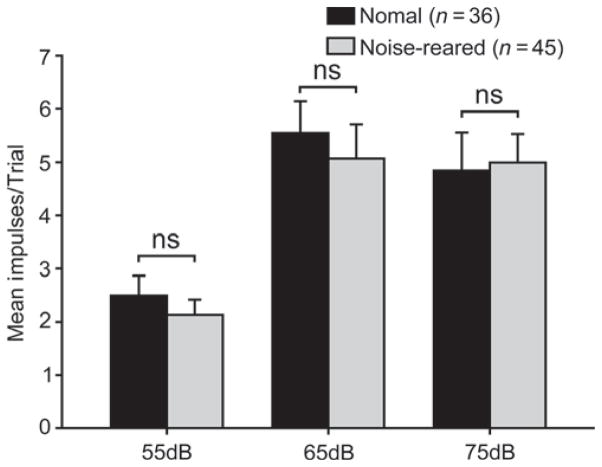
To evaluate whether the noise-rearing had an effect on the robustness with which multisensory neurons responded to acoustic stimuli, comparisons were made between the auditory responses to three stimulus intensities (55, 65 and 75 dB, against a background of 51.4 dB) in normal (n = 36) and noise-reared (n = 45) SC neurons. No significant differences from normal response magnitudes were noted at any intensity (Mann–Whitney rank sum test, 55 dB: P = 0.433; 65 dB: P = 0.239; 75 dB: P = 0.527, Fig. 3). However, there were abnormalities in the auditory receptive fields of these neurons.
Fig. 3.
Auditory response magnitudes were not altered by noise-rearing. Shown are the mean numbers of impulses per trial for each of the three acoustic intensities tested in normal and noise-reared animals. Error bars, SEM; ns, non-significant (P > 0.05).
Effects on receptive field organization
In normally reared animals, multisensory SC neurons exhibit topographically overlapping visual, auditory and somatosensory representations that are constructed from inputs derived from multiple sources. These inputs converge in ways that yield a variety of modality convergence patterns, with the visual–auditory neuron having the highest incidence (Stein & Meredith, 1993).
Neonatal SC neurons have very large visual and auditory receptive fields, and both normally show substantial contraction over time (Wallace & Stein, 1997). During this process, the receptive fields of individual neurons consolidate into spatial register with one another, thereby establishing the overall alignment of these sensory representations. The noise-rearing condition did not appear to alter the contraction of the visual receptive fields of multisensory neurons, as their mean diameters were similar to those in the normal population (normal, mean ± SD = 44.36 ± 10.51°, n = 97; noise-reared, mean ± SD = 43.05 ± 12.23°, n = 134 Mann–Whitney rank sum test, P = 0.233). Furthermore, as is generally the case in normally reared animals, multisensory (i.e. deep layer) neurons had visual receptive fields that remained in good topographic register with those of overlying unisensory superficial layer neurons that were recorded in the same electrode penetration. However, the auditory receptive fields of many multisensory neurons appeared anomalous, typically because they contracted but failed to align properly with their visual receptive field counterpart (‘contracted group’), or alternatively because they failed to contract at all and remained omnidirectional, responding to stimuli everywhere in space (see below). These findings are illustrated in Fig. 4 and contrast with the typical example from a normally reared animal (Fig. 4A).
Fig. 4.
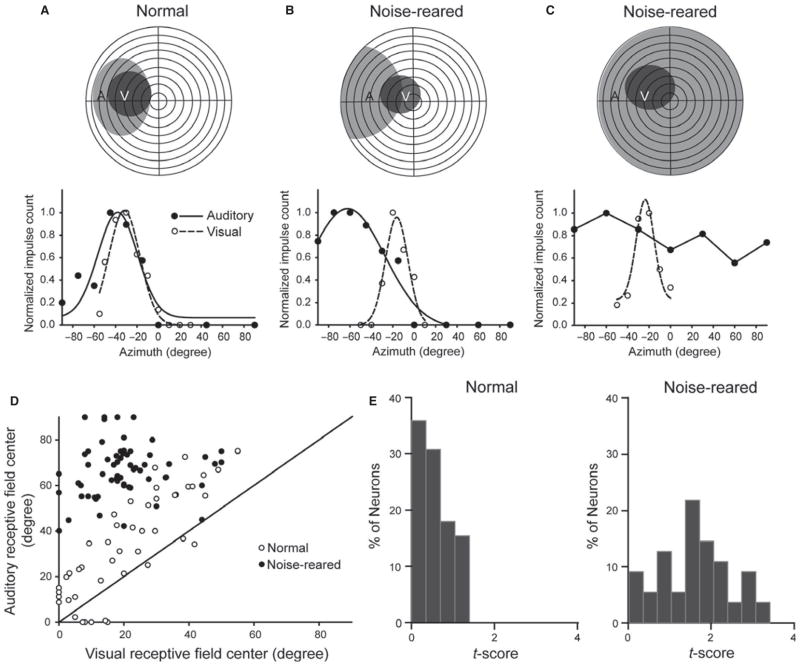
Auditory receptive fields and visual–auditory spatial register were abnormal in noise-reared animals. (A) An illustrative example of a visual–auditory neuron from a normal animal. At the top are shown the neuron’s visual (V) and auditory (A) receptive fields plotted on a schematic of visual–auditory space. In this flattened depiction of space, the horizontal and vertical lines depict meridians, and each concentric circle represents 10° of sensory space. The neuron’s response profile to a visual (dashed line) and auditory (solid line) stimulus at different azimuths is shown below. Responses were normalized to give the proportion of the maximum response at each location, ranging from 0 (locations yielding no impulses) to 1 (locations yielding the maximum number of impulses). Note the overlapping receptive fields and parallel visual–auditory spatial response profiles of this neuron. (B,C) Two neurons from a noise-reared animal. The example on the left (B) reflects the majority of the population, showing poor visual–auditory register and a lack of parallelism in the visual and auditory response profiles. The example on the right (C) illustrates a significant minority of neurons with very large (i.e. omnidirectional) and broadly tuned auditory receptive fields. (D) A plot of the auditory–visual center displacements in normal and noise-reared animals. The solid diagonal line represents the line of unity. There is little overlap among the normally reared and noise-reared populations due to the peripheral shift of nearly all the auditory receptive fields in noise-reared animals. (E) Histograms showing the rightward shift in receptive field alignment t scores (see text) in noise-reared animals relative to normal animals, reflecting lower spatial correspondence in their visual–auditory spatial tuning profiles.
As noted above, the majority (72%, 96/134) of visual–auditory neurons in noise-reared animals showed contracted auditory receptive fields with poor auditory–visual alignment. An example of such a neuron is shown in Fig. 4B. Across samples in this large subgroup, auditory receptive field sizes were not significantly different from normal (normal diameter: mean ± SD = 72.75 ± 25.87°, n = 88; noise-reared: mean ± SD = 67.57 ± 29.24°, n = 96; Mann–Whitney Rank Sum test, P = 0.167). A quantitative evaluation conducted on a subset of neurons with contracted auditory receptive fields (62 from noise-reared animals, 47 from normally reared animals) revealed that, in the noise-reared animals, the auditory receptive field center was displaced peripheral to its visual counterpart. This displacement significantly (Mann–Whitney rank sum test, P ≤ 0.001) exaggerated the normal trend (Middlebrooks & Knudsen, 1984; Kadunce et al., 2001). Thus, the mean absolute distance between the auditory and visual receptive field centers was expanded from 15.42 ± 8.71° in the normal sample by threefold, to 46.05 ± 15.76° in the noise-reared group. This additional displacement disrupted the correlation between visual and auditory receptive field centers (Fig. 4D) so that this significant correlation in the normally reared group (slope = 1.26, intercept = 6.28, R2 = 0.69, F = 99.62, P < 0.001) was absent in the noise-reared group (slope = 0.14, intercept = 63.82, R2 = 0.023, F = 1.41, P = 0.24). To further quantify these findings, receptive fields were fit with Gaussian functions and their misalignment calculated as t-scores (see Methods), with higher t-scores indicating greater misalignment. Normal receptive fields averaged a misalignment t-score of 0.58 with a heavily skewed distribution (Fig. 4E, left) that reveals an average auditory–visual displacement tending toward smaller values. In the contracted noise-reared group there was a significantly larger (Mann–Whitney rank sum test, P ≤0.001) average misalignment t-score of 1.60, around which the population was roughly symmetric (Fig. 4E, right), indicating little bias towards smaller values.
The remaining minority (28%, 38/134) of noise-reared neurons appeared omnidirectional, effectively responding to auditory stimuli at every spatial location without any apparent spatial tuning (see example in Fig. 4C). These auditory receptive fields had mean diameters that were at least 250% greater than those of contracted receptive fields, a rarity (i.e. 9% incidence) in the normal SC population, but typical of neonates and not uncommon in dark-reared animals (see Wallace & Stein, 1997; Yu et al., 2010).
Effects on multisensory integration
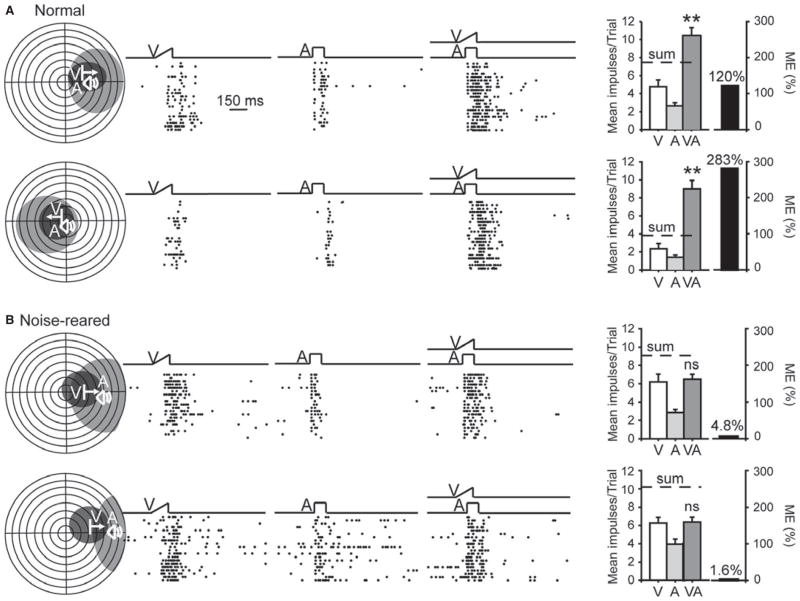
Of particular interest in the present study was whether diminishing an animal’s opportunity for experiencing discrete, spatiotemporally concordant visual–auditory events via noise-rearing would affect the development of SC multisensory integration capabilities. To maximize the probability that this capability, if present, would be exposed by the testing paradigm, individual neurons were tested with multiple combinations of visual and auditory component stimuli. This test battery always included weakly effective component stimuli that normally elicit the most proportionally enhanced multisensory responses (Stein et al., 2009). In response to these tests, 77.3% (75/97) of the SC neurons in the normally reared animals studied here could be categorized as showing multisensory integration capabilities, a number similar to the incidence reported in earlier studies (Jiang et al., 2001; Alvarado et al., 2007a). Representative examples of their unisensory and multisensory responses are shown in Fig. 5A. In each of these examples, responses to the spatiotemporally concordant cross-modal stimuli were significantly larger than those to the most effective of its component stimuli, and also exceeded the sum of these unisensory responses. This has been shown to be a common response profile elicited by weakly effective stimuli, and is progressively less commonly elicited by combinations of increasingly more effective stimuli (Stanford et al., 2005), a relationship that was also noted here.
Fig. 5.
Most visual–auditory neurons in noise-reared animals failed to develop multisensory integration capability. (A) At the left are the receptive fields of representative multisensory neurons from normally eared controls. In the middle are the raster displays illustrating responses to visual (V), auditory (A), and cross-modal (VA) stimuli. Each dot in a raster represents a single impulse and each row (ordered bottom-to-top) a single trial. Note the increase in the discharges to the cross-modal stimulus, which exceeds each component response. At the right are summary bar graphs that illustrate the mean number of impulses elicited in each stimulus condition, and the magnitude of multisensory response enhancement (ME). In these cases the multisensory response exceeded the sum of impulses in the two unisensory responses. (B) The same conventions are used here in neurons from noise-reared animals. Note the absence of multisensory integration in the two neurons (ns, not significant, P > 0.05). This was typical of 81.3% of the neurons tested. **P < 0.001.
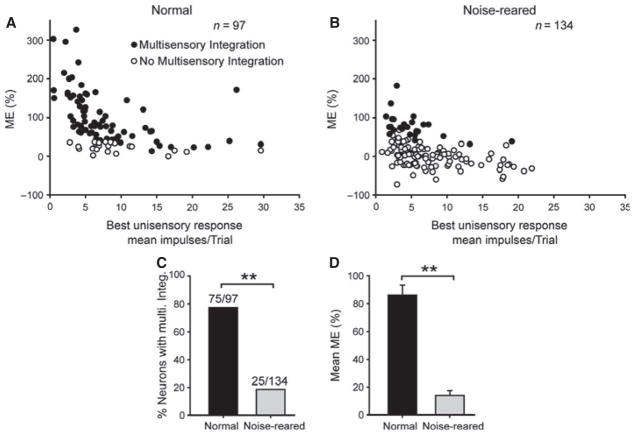
However, using the same stimulus parameters, the majority (81.3%, 109/134) of neurons in noise-reared animals failed to show multisensory integration capabilities; that is, their (multisensory) responses to the spatially concordant cross-modal stimulus were not significantly greater than their (unisensory) responses to the most effective of its component stimuli. Indeed, sometimes (9.7%, 13/134) the multisensory response was significantly (P < 0.05) lower than the largest of the two unisensory responses, a response depression usually elicited by spatially discordant stimuli (Stein & Meredith, 1993). A minority (18.7% (25/134) of neurons in these animals did show multisensory enhancement capabilities. Examples of these neurons are shown in Fig. 5B. Population comparisons between the normal and noise-reared neurons are shown in Fig. 6. This population analysis revealed that the incidence of significant multisensory enhancement (χ2 test, d.f. = 1, χ2 = 76.504, P ≤ 0.001) and its mean magnitude (i.e. ME) were significantly lower in the noise-reared than in the normally reared control animals (Mann–Whitney rank sum test, P ≤ 0.001; normal, mean ± SEM = 86.12 ± 7.00%, n = 97; noise-reared, 13.88 ± 3.55%, n = 134). There were no significant differences in the incidence of significant multisensory enhancement and the mean ME between the populations of neurons with contracted and non-contracted auditory receptive fields in the noise-reared animals (incidence: χ2 test, d.f. = 1, P = 0.434, ME: Mann–Whitney rank sum test, P = 0.702). The incidence of such neurons was similar in each of the animals (23, 17 and 15%) as was their mean MEs (17.72 ± 7.28, 10.40 ± 4.78 and 14.12 ± 6.38%).
Fig. 6.
Multisensory integration was rare and yielded less response enhancement in noise-reared versus normally reared animals. (A,B) ME is plotted for each neuron as a function of the best (largest) unisensory response. Although the typically high proportion of neurons (n, number of neurons) in normally reared animals exhibited multisensory integration capabilities, few neurons in the noise-reared animals did. (C,D) These group differences are also evident in summary bar graphs which compare the incidences of multisensory integration, and the mean ME magnitudes (normally reared control, black bar; noise-reared, gray bar). These group differences characterized each of the animals studied. Conventions are the same as in previous figures.
Testing alternative spatial and temporal relationships
Despite the apparent absence of multisensory integration in the majority of neurons in the noise-reared cohort it was possible that SC neurons had developed abnormal governing principles such that only anomalously configured cross-modal stimuli (e.g. those out of spatial and/or temporal alignment) would be integrated to produce response enhancement. Such an outcome has been observed in animals reared in atypical circumstances in which consistent spatially disparate visual–auditory experience led to misaligned visual–auditory receptive fields (Wallace & Stein, 2007) and required spatially disparate cross-modal stimuli to initiate multisensory integration. Such effects might have been missed here using the previous testing paradigm.
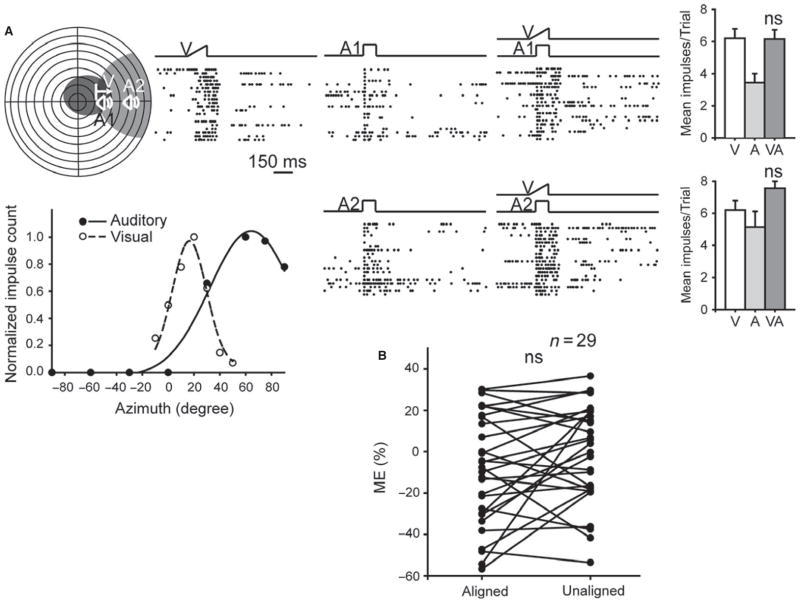
To examine the possibility that a different spatial relationship was required for multisensory integration, a subset of neurons that exhibited misaligned receptive fields (n = 29) were tested with two stimulus configurations: one in which the stimuli were aligned in space and located within the overlapping portion of their receptive fields, and another in which they were misaligned in space but positioned in the most effective regions of their respective receptive fields. An example is shown in Fig. 7A. In neither configuration did the cross-modal combination result in multisensory integration, and this was the characteristic result among the population of neurons examined (see Fig. 7B). ME was not significantly different when the visual and auditory stimuli were in spatial congruence (−8.69 ± 4.96%) or disparate and in their respective receptive field centers (−0.78 ± 4.41%) (paired t test, P = 0.08).
Fig. 7.
Manipulating cross-modal spatial relationships did not reveal multisensory integration in noise-reared animals. (A) Although some excitability differences were noted with cross-modal pairings at different receptive field positions in this representative example (i.e. aligned in space, or aligned by comparable receptive field positions), all tests failed to generate multisensory integration. (B) This was the case in the population of neurons studied, as shown by the absence of a statistical difference (ns) between the MEs in the two alignment conditions. Conventions are the same as in previous figures.
To examine the possibility that the neurons required an atypical temporal relationship between the cross-modal stimuli for the expression of multisensory integration, the visual–auditory stimulus onset asynchrony (SOA) was systematically manipulated in 15 neurons, some with misaligned (n = 11) and some with aligned (n = 4) receptive fields. In normally reared animals there is typically an increase in ME as the SOA approaches the optimum (Meredith et al., 1987; Xu et al., 2012). As can be seen for the normal exemplar neurons (Fig. 8A), and across the normal population (Fig. 8B), the maximum ME is observed when the visual stimulus precedes the auditory by 100 ms (V100A). However, no multisensory integration was seen at any of the SOAs tested in noise-reared animals, as illustrated by the exemplar in Fig. 8A and the population analysis in Fig. 8B.
Fig. 8.
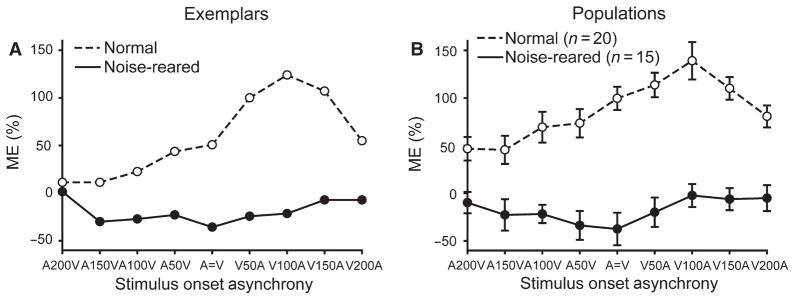
Manipulating cross-modal temporal relationships did not reveal multisensory integration in SC neurons of noise-reared animals. (A) Data from one exemplar neuron in each condition. (B) Population data. In both graphs ME was plotted as a function of SOA. Note that high MEs were apparent in the normal exemplar neuron and in the normal population, with both showing the presence of a ‘best’ SOA (V 100 ms before A). In contrast, neurons in noise-reared animals had MEs at or below 0, and had no clear best SOA.
Comparison with unisensory integration
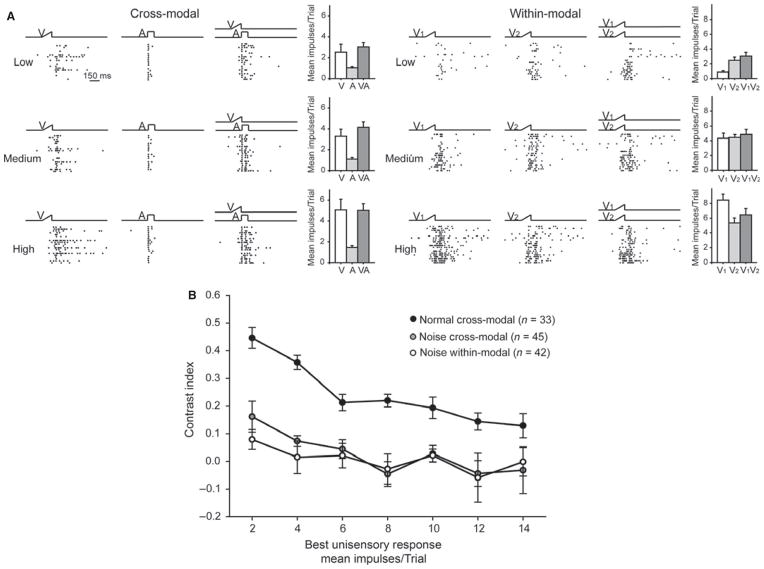
Previous findings (e.g. see Alvarado et al., 2007a,b) showed that SC multisensory neurons had no response enhancement: they yielded response magnitudes to a spatiotemporally concordant within-modal stimulus (i.e. unisensory integration) that were equal to or less than those evoked by the best component stimulus. They also did so regardless of the efficacy of the component stimuli. Thus, unisensory and multisensory integration produced distinctly different products. The present result shows that noise-rearing eliminated this unisensory–multisensory integration distinction, as shown by the exemplar neuron and the population data provided in Fig. 9.
Fig. 9.
Responses of multisensory neurons to pairs of cross-modal and within-modal stimuli were similar in noise-reared animals. (A) On the left are shown unisensory and multisensory responses of a neuron from a noise-reared animal that were elicited by visual stimuli of low, medium and high effectiveness (the auditory response was insensitive to stimulus intensity, see text). Note the absence of response enhancement to the cross-modal stimulus pair at any level of stimulus effectiveness. This is typical of responses to pairs of within-modal stimuli as shown by the example on the right. (B) Population data of responses to these stimulus configurations are displayed using the contrast index (+ 1 and −1 indicate responses greater or less than those to the most effective component stimulus – the best unisensory response). Noise, neurons from noise-reared animals; normal, neurons from normally reared animals. Other conventions are the same as in previous figures. Note that in the noise-reared condition responses to cross-modal stimuli overlapped those from within-modal stimuli, and were far lower than expected based on the function from the normally reared condition.
Evaluation of early development
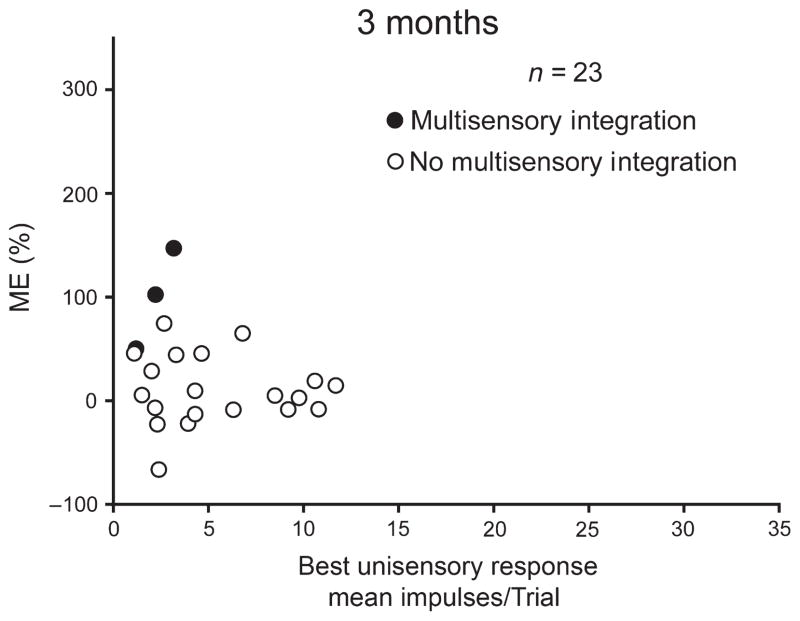
The absence of multisensory integration capabilities in these neurons suggests that this capability never developed. Although this seems a reasonable assumption, some SC properties may develop and then be lost in the absence of relevant experience (e.g. see Carrasco et al., 2005). To examine this possibility in the current context, tests of multisensory integration were conducted on three kittens at 3 months of age, the approximate time at which the adult-like proportion of neurons capable of multisensory integration is normally achieved (Wallace & Stein, 1997; Stein & Stanford, 2008). A total of 23 visual–auditory neurons were examined, and their auditory spatial tuning properties and multisensory response properties were quantitatively evaluated in the same manner as described above for their adult counterparts. The auditory receptive fields of each of these neurons encompassed the entire region of auditory space and were comparable to a subset of those observed in animals allowed to mature to adulthood (see Fig. 4C). A total of 13% (3/23) of them showed weak multisensory integration capabilities, a proportion that is similar to the 18.7% found in the noise-reared adult animals described above. These values are substantially lower than the 77.3% found here in normally reared adult controls and the 70% observed in 3-month-old normally reared kittens by Wallace & Stein (1997) (Fig. 10). Thus, the adult deficits appear to have been present earlier in life, and multisensory integration does not appear to have developed at this stage only to be later attenuated or ‘degraded’ by the rearing condition.
Fig. 10.
The failure to develop multisensory integration capabilities was evident at 3 months of age. Conventions are the same as in previous figures.
Discussion
The present findings show that masking patterned auditory stimulation by rearing animals in omnidirectional noise precludes the maturation of visual–auditory multisensory integration in SC neurons. This indicates that the perturbations in a single sensory modality, such as those induced by constant environmental noise (or congenital cataract, see Putzar et al., 2010) can have far-reaching deleterious consequences on the brain’s ability to use information from multiple senses in concert.
Noise-rearing minimized experience with spatiotemporally co-variant visual and auditory cues while still providing opportunities for concurrent stimulation of their sensory-specific input channels. By decoupling these two factors, data derived from the present paradigm add a key insight to prior studies in which these factors were coupled (Yu et al., 2010; Xu et al., 2012). Co-activation of sensory-specific input channels is insufficient for developing SC multisensory integration capabilities, in contrast to what would have been expected if this process were based on encoding cross-modal signal correlations (i.e. ‘fire together, wire together’, see Senn, 2002). Instead, co-variance appears to be the critical factor. Its potency in this context is apparent in its ability to render even such impoverished stimuli as flashes of light and broad-band noise bursts effective in instantiating these capabilities in neonates and naive adults (Yu et al., 2010; Xu et al., 2012).
The precise mechanisms by which co-variant cross-modal statistics drive the development of SC multisensory integration are not known; however, the present findings are generally consistent with models based on information theoretic principles in which associative learning is sensitive to conditional relationships between input signals (Hebb, 1949). Cues that are ‘always off’ or ‘always on’ are equally uninformative and not predictive of the occurrence of other cues; thus, they should not drive the formation of any associations. In fact, learning in normal environments would be highly unstable if every instance of co-activation altered the processing rules because complex circumstances are replete with experiences in which two signals are concurrent but unrelated. Co-variance among signals, on the other hand, is statistically less likely to be spurious or due to noise (Kording et al., 2007). By being preferentially sensitive to cross-modal co-variance, associative principles for the development of multisensory integration can selectively associate cues that have a common source.
The distinction between co-activation and co-variance provides important insight into the mechanistic requirements for multisensory learning and development. Learning algorithms based on signal co-variance necessitate a more sophisticated mechanism for implementation than those based on co-activation alone (Senn, 2002). Of particular interest in the present context are algorithms in which temporal correlation between pre- and post-synaptic activity is a critical constraint for synaptic modification (Senn et al., 2001; Feldman, 2012). This prerequisite for synapse strengthening means that stable connections will be formed by pre- and post-synaptic events that bear a causal relationship to one another (Hebb, 1949; Senn et al., 2001). It is readily apparent that normal cross-modal experiences, such as those derived from events providing both visual and auditory cues, are characterized by the spatiotemporal concordance of the stimuli. They should increase the likelihood that post-synaptic activity follows pre-synaptic activity by virtue of the temporal coincidence of converging sensory-specific inputs. In contrast, diffuse auditory stimulation, to the extent that it produces reliable pre- and post-synaptic activity within the SC, would contribute ‘noise’, effectively weakening the temporal correlation between pre- and post-synaptic events consequent to combined visual and auditory stimulation. From the perspective of statistical learning, the reduced ability to attribute a post-synaptic event specifically to a cross-modal pre-synaptic event would diminish the likelihood of synapse strengthening and therefore the formation of the cross-modal association necessary for multisensory integration. This scenario could even destabilize existing synapses by de-correlating presynaptic and postsynaptic activity (Gerstner et al., 1996; Markram & Tsodyks, 1996).
In a normal rearing environment, cross-modal cues that derive from a common source will co-vary in both space and time. It is important to note that the noise-rearing condition disrupted both the spatial and the temporal correlation of visual and auditory events by providing a continuous and spatially diffuse auditory stimulus. The few neurons that did show the development of integration capabilities might be attributable to imperfect masking in the rearing environment or, alternatively, a very marginal capability of spatiotemporally discrete stimuli from only one modality (i.e. visual) of a cross-modal pair to ‘parse’ omnipresent, omnidirectional, stimulation in the other (i.e. auditory) into a set of cross-modal events for this purpose. However, the present findings do not preclude the possibility that some, or even many, SC neurons would have developed some form of integrative capacity (though not necessarily normal) had auditory experiences been discrete in only one of the spatial or temporal domains. Events that are temporally discrete but spatially continuous cannot inform a localization decision, but can inform detection of synchronized events. Analogously, events that are spatially discrete but temporally continuous will help little in detecting events, but could be of substantial value to their localization. Given that the primary role of the SC is in detecting and localizing events so that appropriate orientation responses can be initiated, both capabilities are of importance to its function. Thus, additional experimentation is necessary to evaluate the extent to which the maturation of multisensory integration capabilities, which enhance both these SC functions, depends on experience with each of these stimulus features.
The failure of visual–auditory neurons in noise-reared animals to develop multisensory integration capabilities rendered them similar in appearance to those in normally reared adult animals following ablation, or during reversible deactivation, of the cortico-collicular inputs from association cortex (Jiang et al., 2001; Alvarado et al., 2009). Whether this similarity is superficial or reflective of a common etiology – i.e. whether the cortico-collicular projection is specifically compromised by noise-rearing – is unknown. Yet, ablation of association cortex not only disrupts multisensory integration in visual–auditory SC neurons, but also disrupts the alignment of auditory– visual receptive fields so that they appear very similar to those observed here (Jiang et al., 2006). This similarity, coupled with the impact of this rearing condition on other regions of auditory-responsive cortex (Chang & Merzenich, 2003), suggests that the corticocollicular projection is likely to be vulnerable to noise-rearing. Indeed, its vulnerability to disruptions in sensory input is also apparent from dark-rearing experiments, wherein its failure to facilitate SC multisensory integration is coupled with the development of a non-selective enhancement of the excitability of SC neurons to all sensory inputs (Yu et al., 2013). Such a cortical influence is atypical, and in order to craft its normal selective effect over the multisensory responses of SC neurons it must not only be active during periods of cross-modal exposure, but as noted here, it must also have available information about the co-variance of the two sensory inputs. These results provide insight into the brain’s requirement for the kinds of cross-modal experiences that are effective in realizing its normal multisensory potential, and they have obvious implications for the developmental trajectory of neonatal brains whose abilities to properly segregate the spatiotemporal structure of environmental events have been compromised.
Acknowledgments
This research was supported by NIH grants EY016716 and NS036916. We thank Nancy London for technical assistance.
Abbreviations
- ME
multisensory response enhancement
- SC
superior colliculus
- SOA
stimulus onset asynchrony
References
- Alvarado JC, Stanford TR, Vaughan JW, Stein BE. Cortex mediates multisensory but not unisensory integration in superior colliculus. J Neurosci. 2007a;27:12775–12786. doi: 10.1523/JNEUROSCI.3524-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado JC, Vaughan JW, Stanford TR, Stein BE. Multi-sensory versus unisensory integration: contrasting modes in the superior colliculus. J Neurophysiol. 2007b;97:3193–3205. doi: 10.1152/jn.00018.2007. [DOI] [PubMed] [Google Scholar]
- Alvarado JC, Stanford TR, Rowland BA, Vaughan JW, Stein BE. Multisensory integration in the superior colliculus requires synergy among corticocollicular inputs. J Neurosci. 2009;29:6580–6592. doi: 10.1523/JNEUROSCI.0525-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts DA, Kanold PO. The applicability of spike time dependent plasticity to development. Front Synaptic Neurosci. 2010;2:30. doi: 10.3389/fnsyn.2010.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco MM, Razak KA, Pallas SL. Visual experience is necessary for maintenance but not development of receptive fields in superior colliculus. J Neurophysiol. 2005;94:1962–1970. doi: 10.1152/jn.00166.2005. [DOI] [PubMed] [Google Scholar]
- Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science. 2003;300:498–502. doi: 10.1126/science.1082163. [DOI] [PubMed] [Google Scholar]
- Diederich A, Colonius H. Bimodal and trimodal multisensory enhancement: effects of stimulus onset and intensity on reaction time. Percept Psychophys. 2004;66:1388–1404. doi: 10.3758/bf03195006. [DOI] [PubMed] [Google Scholar]
- Efrati A, Gutfreund Y. Early life exposure to noise alters the representation of auditory localization cues in the auditory space map of the barn owl. J Neurophysiol. 2011;105:2522–2535. doi: 10.1152/jn.00078.2011. [DOI] [PubMed] [Google Scholar]
- Feldman DE. The spike-timing dependence of plasticity. Neuron. 2012;75:556–571. doi: 10.1016/j.neuron.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner W, Kempter R, van Hemmen JL, Wagner H. A neuronal learning rule for sub-millisecond temporal coding. Nature. 1996;383:76– 81. doi: 10.1038/383076a0. [DOI] [PubMed] [Google Scholar]
- Grossberg S, Roberts K, Aguilar M, Bullock D. A neural model of multimodal adaptive saccadic eye movement control by superior colliculus. J Neurosci. 1997;17:9706–9725. doi: 10.1523/JNEUROSCI.17-24-09706.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior; a Neuropsychological Theory. Wiley; New York: 1949. [Google Scholar]
- Jiang W, Wallace MT, Jiang H, Vaughan JW, Stein BE. Two cortical areas mediate multisensory integration in superior colliculus neurons. J Neurophysiol. 2001;85:506–522. doi: 10.1152/jn.2001.85.2.506. [DOI] [PubMed] [Google Scholar]
- Jiang W, Jiang H, Stein BE. Neonatal cortical ablation disrupts multisensory development in superior colliculus. J Neurophysiol. 2006;95:1380–1396. doi: 10.1152/jn.00880.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadunce DC, Vaughan JW, Wallace MT, Benedek G, Stein BE. Mechanisms of within- and cross-modality suppression in the superior colliculus. J Neurophysiol. 1997;78:2834–2847. doi: 10.1152/jn.1997.78.6.2834. [DOI] [PubMed] [Google Scholar]
- Kadunce DC, Vaughan JW, Wallace MT, Stein BE. The influence of visual and auditory receptive field organization on multisensory integration in the superior colliculus. Exp Brain Res. 2001;139:303–310. doi: 10.1007/s002210100772. [DOI] [PubMed] [Google Scholar]
- Kording KP, Beierholm U, Ma WJ, Quartz S, Tenenbaum JB, Shams L. Causal inference in multisensory perception. PLoS ONE. 2007;2:e943. doi: 10.1371/journal.pone.0000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Tsodyks M. Redistribution of synaptic efficacy between neocortical pyramidal neurons. Nature. 1996;382:807–810. doi: 10.1038/382807a0. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Stein BE. A chronic headholder minimizing facial obstructions. Brain Res Bull. 1983;10:859–860. doi: 10.1016/0361-9230(83)90220-4. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Interactions among converging sensory inputs in the superior colliculus. Science. 1983;221:389–391. doi: 10.1126/science.6867718. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Spatial factors determine the activity of multisensory neurons in cat superior colliculus. Brain Res. 1986;365:350–354. doi: 10.1016/0006-8993(86)91648-3. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Nemitz JW, Stein BE. Determinants of multisensory integration in superior colliculus neurons. I. Temporal factors. J Neurosci. 1987;7:3215–3229. doi: 10.1523/JNEUROSCI.07-10-03215.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrooks JC, Knudsen EI. A neural code for auditory space in the cat’s superior colliculus. J Neurosci. 1984;4:2621–2634. doi: 10.1523/JNEUROSCI.04-10-02621.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault TJ, Jr, Vaughan JW, Stein BE, Wallace MT. Superior colliculus neurons use distinct operational modes in the integration of multisensory stimuli. J Neurophysiol. 2005;93:2575–2586. doi: 10.1152/jn.00926.2004. [DOI] [PubMed] [Google Scholar]
- Putzar L, Hotting K, Roder B. Early visual deprivation affects the developments of face recognition and of audio-visual speech perception. Restor Neurol Neurosci. 2010;28:251–257. doi: 10.3233/RNN-2010-0526. [DOI] [PubMed] [Google Scholar]
- Rowland BA, Quessy S, Stanford TR, Stein BE. Multisensory integration shortens physiological response latencies. J Neurosci. 2007;27:5879–5884. doi: 10.1523/JNEUROSCI.4986-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal DW, Krueger J, Fister MC, Wallace MT. Adult plasticity of spatiotemporal receptive fields of multisensory superior colliculus neurons following early visual deprivation. Restor Neurol Neurosci. 2010;28:259–270. doi: 10.3233/RNN-2010-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn W. Beyond spike timing: the role of nonlinear plasticity and unreliable synapses. Biol Cybern. 2002;87:344–355. doi: 10.1007/s00422-002-0350-1. [DOI] [PubMed] [Google Scholar]
- Senn W, Markram H, Tsodyks M. An algorithm for modifying neurotransmitter release probability based on pre- and postsynaptic spike timing. Neural Comput. 2001;13:35–67. doi: 10.1162/089976601300014628. [DOI] [PubMed] [Google Scholar]
- Stanford TR, Quessy S, Stein BE. Evaluating the operational underlying multisensory integration in the cat superior colliculus. J Neurosci. 2005;25:6499–6508. doi: 10.1523/JNEUROSCI.5095-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BE. Early experience affects the development of multisensory integration in single neurons of the superior colliculus. In: Stein BE, editor. The New Handbook of Multisensory Processing. MIT Press; Cambridge, MA: 2012. pp. 589–606. [Google Scholar]
- Stein BE, Meredith MA. The Merging of the Senses. MIT Press; Cambridge, MA: 1993. [Google Scholar]
- Stein BE, Stanford TR. Multisensory integration: current issues from the perspective of the single neuron. Nat Rev Neurosci. 2008;9:255– 266. doi: 10.1038/nrn2331. [DOI] [PubMed] [Google Scholar]
- Stein BE, Labos E, Kruger L. Sequence of changes in properties of neurons of superior colliculus of the kitten during maturation. J Neurophysiol. 1973;36:667–679. doi: 10.1152/jn.1973.36.4.667. [DOI] [PubMed] [Google Scholar]
- Stein BE, Stanford TR, Ramachandran R, Perrault TJ, Jr, Rowland BA. Challenges in quantifying multisensory integration: alternative criteria, models, and inverse effectiveness. Exp Brain Res. 2009;198:113–126. doi: 10.1007/s00221-009-1880-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui J, Schwartz N, Ruthazer ESA. Developmental sensitive period for spike timing-dependent plasticity in the retinotectal projection. Front Synaptic Neurosci. 2010;2:13. doi: 10.3389/fnsyn.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Neumann J. The Computer and the Brain. Yale University Press; New Haven, CT: 1958. [Google Scholar]
- Wallace MT, Stein BE. Development of multisensory neurons and multisensory integration in cat superior colliculus. J Neurosci. 1997;17:2429–2444. doi: 10.1523/JNEUROSCI.17-07-02429.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. Early experience determines how the senses will interact. J Neurophysiol. 2007;97:921–926. doi: 10.1152/jn.00497.2006. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Perrault TJ, Jr, Hairston WD, Stein BE. Visual experience is necessary for the development of multisensory integration. J Neurosci. 2004;24:9580–9584. doi: 10.1523/JNEUROSCI.2535-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Yu L, Rowland BA, Stanford TR, Stein BE. Incorporating cross-modal statistics in the development and maintenance of multisensory integration. J Neurosci. 2012;32:2287–2298. doi: 10.1523/JNEUROSCI.4304-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JM, Waleszczyk WJ, Wang C, Calford MB, Dreher B, Obermayer K. Cortical reorganization consistent with spike timing-but not correlation-dependent plasticity. Nat Neurosci. 2007;10:887–895. doi: 10.1038/nn1913. [DOI] [PubMed] [Google Scholar]
- Yu L, Stein BE, Rowland BA. Adult plasticity in multisensory neurons: short-term experience-dependent changes in the superior colliculus. J Neurosci. 2009;29:15910–15922. doi: 10.1523/JNEUROSCI.4041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Rowland BA, Stein BE. Initiating the development of multisensory integration by manipulating sensory experience. J Neurosci. 2010;30:4904–4913. doi: 10.1523/JNEUROSCI.5575-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Xu J, Rowland BA, Stein BE. Development of cortical influences on superior colliculus multisensory neurons: Effects of dark-rearing. Eur J Neurosci. 2013;37:1594–1601. doi: 10.1111/ejn.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]