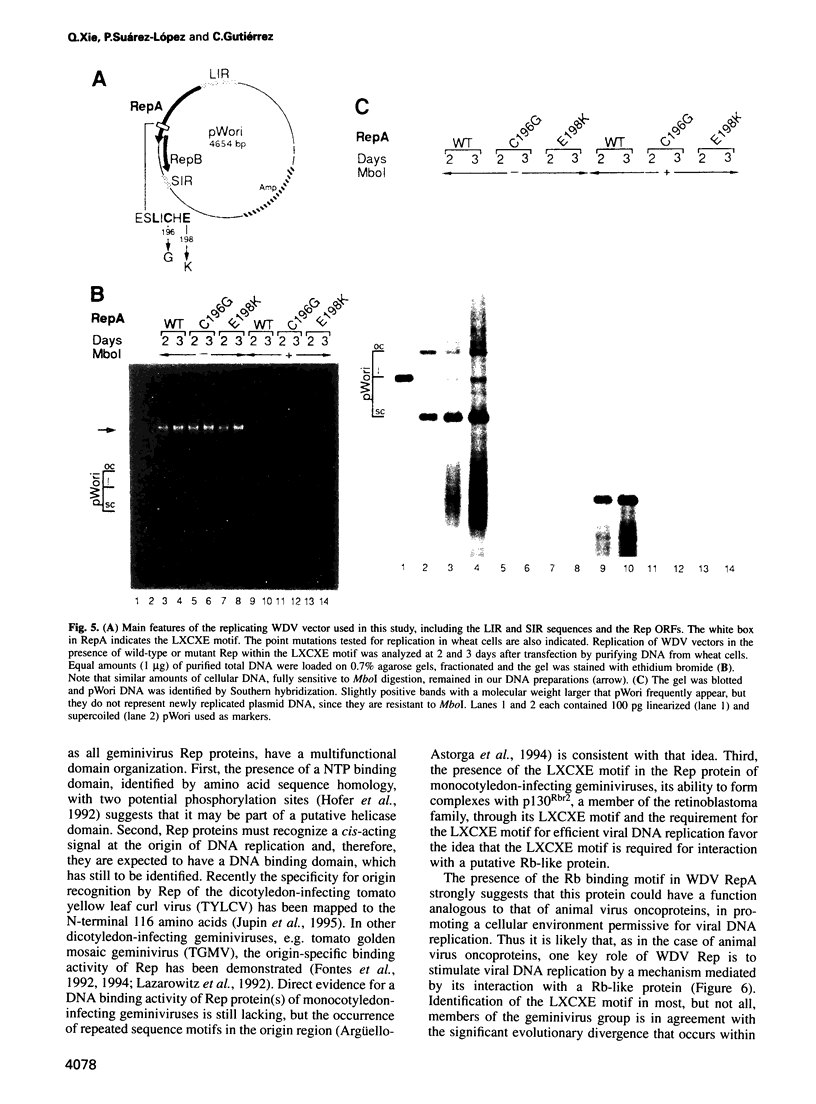
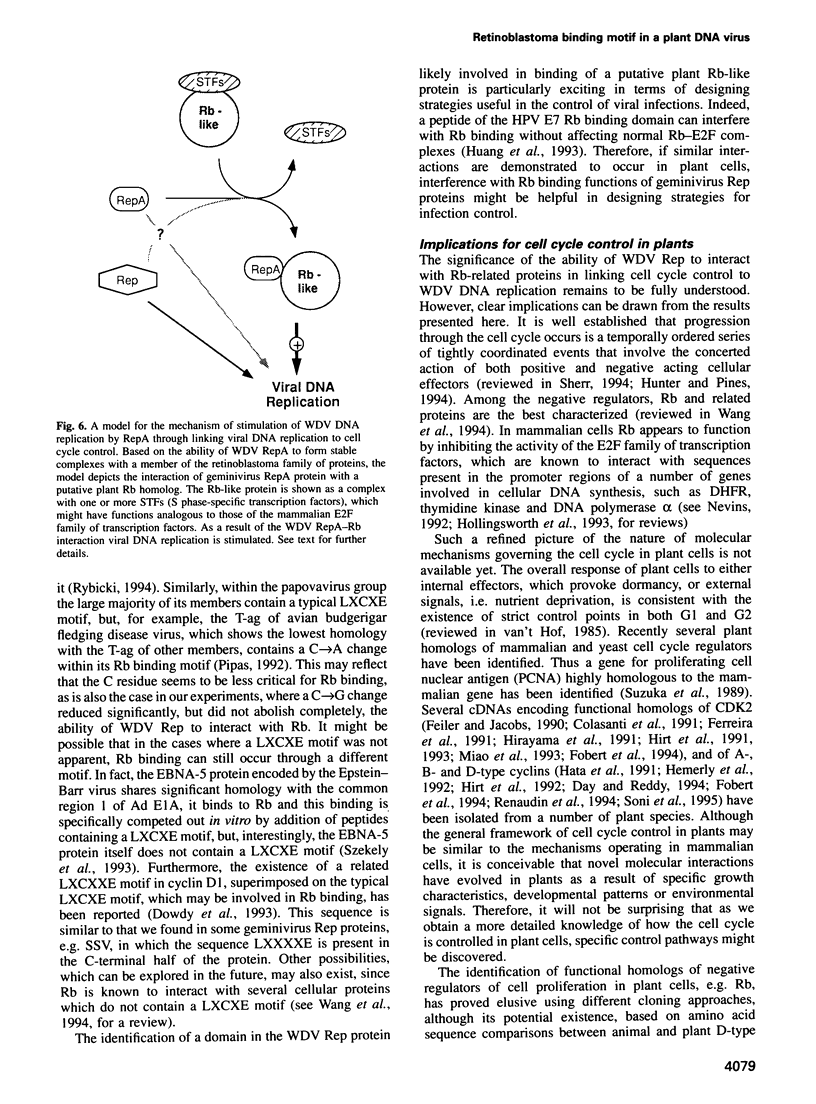
Abstract
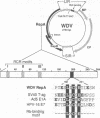
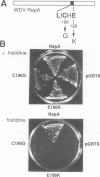
Geminiviruses are plant DNA viruses with small genomes whose replication, except for the viral replication protein (Rep), depends on host proteins and, in this respect, are analogous to animal DNA tumor viruses, e.g. SV40. The mechanism by which these animal viruses create a cellular environment permissive for viral DNA replication involves the binding of a virally encoded oncoprotein, through its LXCXE motif, to the retinoblastoma protein (Rb). We have identified such a LXCXE motif in the Rep protein of wheat dwarf geminivirus (WDV) and we show its functional importance during viral DNA replication. Using a yeast two-hybrid system we have demonstrated that WDV Rep forms stable complexes with p130Rbr2, a member of the Rb family of proteins, and single amino acid changes within the LXCXE motif abolish the ability of WDV Rep to bind to p130Rbr2. The LXCXE motif is conserved in other members of the same geminivirus subgroup. The presence of an intact Rb binding motif is required for efficient WDV DNA replication in cultured wheat cells, strongly suggesting that one of the functions of WDV Rep may be the linking between viral and cellular DNA replication cycles. Our results point to the existence of a Rb-like protein(s) in plant cells playing regulatory roles during the cell cycle.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accotto G. P., Mullineaux P. M., Brown S. C., Marie D. Digitaria streak geminivirus replicative forms are abundant in S-phase nuclei of infected cells. Virology. 1993 Jul;195(1):257–259. doi: 10.1006/viro.1993.1369. [DOI] [PubMed] [Google Scholar]
- Andersen M. T., Richardson K. A., Harbison S. A., Morris B. A. Nucleotide sequence of the geminivirus chloris striate mosaic virus. Virology. 1988 Jun;164(2):443–449. doi: 10.1016/0042-6822(88)90558-2. [DOI] [PubMed] [Google Scholar]
- Argüello-Astorga G. R., Guevara-González R. G., Herrera-Estrella L. R., Rivera-Bustamante R. F. Geminivirus replication origins have a group-specific organization of iterative elements: a model for replication. Virology. 1994 Aug 15;203(1):90–100. doi: 10.1006/viro.1994.1458. [DOI] [PubMed] [Google Scholar]
- Bartel P., Chien C. T., Sternglanz R., Fields S. Elimination of false positives that arise in using the two-hybrid system. Biotechniques. 1993 Jun;14(6):920–924. [PubMed] [Google Scholar]
- Breeden L., Nasmyth K. Regulation of the yeast HO gene. Cold Spring Harb Symp Quant Biol. 1985;50:643–650. doi: 10.1101/sqb.1985.050.01.078. [DOI] [PubMed] [Google Scholar]
- Briddon R. W., Lunness P., Chamberlin L. C., Pinner M. S., Brundish H., Markham P. G. The nucleotide sequence of an infectious insect-transmissible clone of the geminivirus Panicum streak virus. J Gen Virol. 1992 May;73(Pt 5):1041–1047. doi: 10.1099/0022-1317-73-5-1041. [DOI] [PubMed] [Google Scholar]
- Chatani M., Matsumoto Y., Mizuta H., Ikegami M., Boulton M. I., Davies J. W. The nucleotide sequence and genome structure of the geminivirus miscanthus streak virus. J Gen Virol. 1991 Oct;72(Pt 10):2325–2331. doi: 10.1099/0022-1317-72-10-2325. [DOI] [PubMed] [Google Scholar]
- Cherington V., Brown M., Paucha E., St Louis J., Spiegelman B. M., Roberts T. M. Separation of simian virus 40 large-T-antigen-transforming and origin-binding functions from the ability to block differentiation. Mol Cell Biol. 1988 Mar;8(3):1380–1384. doi: 10.1128/mcb.8.3.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobrinik D., Whyte P., Peeper D. S., Jacks T., Weinberg R. A. Cell cycle-specific association of E2F with the p130 E1A-binding protein. Genes Dev. 1993 Dec;7(12A):2392–2404. doi: 10.1101/gad.7.12a.2392. [DOI] [PubMed] [Google Scholar]
- Colasanti J., Tyers M., Sundaresan V. Isolation and characterization of cDNA clones encoding a functional p34cdc2 homologue from Zea mays. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3377–3381. doi: 10.1073/pnas.88.8.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. W., Stanley J. Geminivirus genes and vectors. Trends Genet. 1989 Mar;5(3):77–81. doi: 10.1016/0168-9525(89)90030-9. [DOI] [PubMed] [Google Scholar]
- Day I. S., Reddy A. S. Cloning of a family of cyclins from Arabidopsis thaliana. Biochim Biophys Acta. 1994 May 17;1218(1):115–118. doi: 10.1016/0167-4781(94)90112-0. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- Donson J., Accotto G. P., Boulton M. I., Mullineaux P. M., Davies J. W. The nucleotide sequence of a geminivirus from Digitaria sanguinalis. Virology. 1987 Nov;161(1):160–169. doi: 10.1016/0042-6822(87)90182-6. [DOI] [PubMed] [Google Scholar]
- Dowdy S. F., Hinds P. W., Louie K., Reed S. I., Arnold A., Weinberg R. A. Physical interaction of the retinoblastoma protein with human D cyclins. Cell. 1993 May 7;73(3):499–511. doi: 10.1016/0092-8674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Sluss H. K., Sherr C. J., Matsushime H., Kato J., Livingston D. M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993 May 7;73(3):487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- Fanning E., Knippers R. Structure and function of simian virus 40 large tumor antigen. Annu Rev Biochem. 1992;61:55–85. doi: 10.1146/annurev.bi.61.070192.000415. [DOI] [PubMed] [Google Scholar]
- Feiler H. S., Jacobs T. W. Cell division in higher plants: a cdc2 gene, its 34-kDa product, and histone H1 kinase activity in pea. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5397–5401. doi: 10.1073/pnas.87.14.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feilotter H. E., Hannon G. J., Ruddell C. J., Beach D. Construction of an improved host strain for two hybrid screening. Nucleic Acids Res. 1994 Apr 25;22(8):1502–1503. doi: 10.1093/nar/22.8.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira P. C., Hemerly A. S., Villarroel R., Van Montagu M., Inzé D. The Arabidopsis functional homolog of the p34cdc2 protein kinase. Plant Cell. 1991 May;3(5):531–540. doi: 10.1105/tpc.3.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989 Jul 20;340(6230):245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Fobert P. R., Coen E. S., Murphy G. J., Doonan J. H. Patterns of cell division revealed by transcriptional regulation of genes during the cell cycle in plants. EMBO J. 1994 Feb 1;13(3):616–624. doi: 10.1002/j.1460-2075.1994.tb06299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes E. P., Eagle P. A., Sipe P. S., Luckow V. A., Hanley-Bowdoin L. Interaction between a geminivirus replication protein and origin DNA is essential for viral replication. J Biol Chem. 1994 Mar 18;269(11):8459–8465. [PubMed] [Google Scholar]
- Fontes E. P., Luckow V. A., Hanley-Bowdoin L. A geminivirus replication protein is a sequence-specific DNA binding protein. Plant Cell. 1992 May;4(5):597–608. doi: 10.1105/tpc.4.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon G. J., Demetrick D., Beach D. Isolation of the Rb-related p130 through its interaction with CDK2 and cyclins. Genes Dev. 1993 Dec;7(12A):2378–2391. doi: 10.1101/gad.7.12a.2378. [DOI] [PubMed] [Google Scholar]
- Hata S., Kouchi H., Suzuka I., Ishii T. Isolation and characterization of cDNA clones for plant cyclins. EMBO J. 1991 Sep;10(9):2681–2688. doi: 10.1002/j.1460-2075.1991.tb07811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemerly A., Bergounioux C., Van Montagu M., Inzé D., Ferreira P. Genes regulating the plant cell cycle: isolation of a mitotic-like cyclin from Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3295–3299. doi: 10.1073/pnas.89.8.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyraud-Nitschke F., Schumacher S., Laufs J., Schaefer S., Schell J., Gronenborn B. Determination of the origin cleavage and joining domain of geminivirus Rep proteins. Nucleic Acids Res. 1995 Mar 25;23(6):910–916. doi: 10.1093/nar/23.6.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama T., Imajuku Y., Anai T., Matsui M., Oka A. Identification of two cell-cycle-controlling cdc2 gene homologs in Arabidopsis thaliana. Gene. 1991 Sep 15;105(2):159–165. doi: 10.1016/0378-1119(91)90146-3. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hirt H., Mink M., Pfosser M., Bögre L., Györgyey J., Jonak C., Gartner A., Dudits D., Heberle-Bors E. Alfalfa cyclins: differential expression during the cell cycle and in plant organs. Plant Cell. 1992 Dec;4(12):1531–1538. doi: 10.1105/tpc.4.12.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt H., Páy A., Bögre L., Meskiene I., Heberle-Bors E. cdc2MsB, a cognate cdc2 gene from alfalfa, complements the G1/S but not the G2/M transition of budding yeast cdc28 mutants. Plant J. 1993 Jul;4(1):61–69. doi: 10.1046/j.1365-313x.1993.04010061.x. [DOI] [PubMed] [Google Scholar]
- Hirt H., Páy A., Györgyey J., Bakó L., Németh K., Bögre L., Schweyen R. J., Heberle-Bors E., Dudits D. Complementation of a yeast cell cycle mutant by an alfalfa cDNA encoding a protein kinase homologous to p34cdc2. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1636–1640. doi: 10.1073/pnas.88.5.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer J. M., Dekker E. L., Reynolds H. V., Woolston C. J., Cox B. S., Mullineaux P. M. Coordinate regulation of replication and virion sense gene expression in wheat dwarf virus. Plant Cell. 1992 Feb;4(2):213–223. doi: 10.1105/tpc.4.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth R. E., Jr, Chen P. L., Lee W. H. Integration of cell cycle control with transcriptional regulation by the retinoblastoma protein. Curr Opin Cell Biol. 1993 Apr;5(2):194–200. doi: 10.1016/0955-0674(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Huang P. S., Patrick D. R., Edwards G., Goodhart P. J., Huber H. E., Miles L., Garsky V. M., Oliff A., Heimbrook D. C. Protein domains governing interactions between E2F, the retinoblastoma gene product, and human papillomavirus type 16 E7 protein. Mol Cell Biol. 1993 Feb;13(2):953–960. doi: 10.1128/mcb.13.2.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes F. L., Rybicki E. P., Kirby R. Complete nucleotide sequence of sugarcane streak Monogeminivirus. Arch Virol. 1993;132(1-2):171–182. doi: 10.1007/BF01309851. [DOI] [PubMed] [Google Scholar]
- Hunter T., Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994 Nov 18;79(4):573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Ilyina T. V., Koonin E. V. Conserved sequence motifs in the initiator proteins for rolling circle DNA replication encoded by diverse replicons from eubacteria, eucaryotes and archaebacteria. Nucleic Acids Res. 1992 Jul 11;20(13):3279–3285. doi: 10.1093/nar/20.13.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupin I., Hericourt F., Benz B., Gronenborn B. DNA replication specificity of TYLCV geminivirus is mediated by the amino-terminal 116 amino acids of the Rep protein. FEBS Lett. 1995 Apr 3;362(2):116–120. doi: 10.1016/0014-5793(95)00221-t. [DOI] [PubMed] [Google Scholar]
- Kammann M., Schalk H. J., Matzeit V., Schaefer S., Schell J., Gronenborn B. DNA replication of wheat dwarf virus, a geminivirus, requires two cis-acting signals. Virology. 1991 Oct;184(2):786–790. doi: 10.1016/0042-6822(91)90453-i. [DOI] [PubMed] [Google Scholar]
- Koonin E. V., Ilyina T. V. Geminivirus replication proteins are related to prokaryotic plasmid rolling circle DNA replication initiator proteins. J Gen Virol. 1992 Oct;73(Pt 10):2763–2766. doi: 10.1099/0022-1317-73-10-2763. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Wu L. C., Rogers S. G., Elmer J. S. Sequence-specific interaction with the viral AL1 protein identifies a geminivirus DNA replication origin. Plant Cell. 1992 Jul;4(7):799–809. doi: 10.1105/tpc.4.7.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie J. W., Loewenstein P. M., Green M. R., Green M. Functional domains of adenovirus type 5 E1a proteins. Cell. 1987 Sep 25;50(7):1091–1100. doi: 10.1016/0092-8674(87)90175-9. [DOI] [PubMed] [Google Scholar]
- Ludlow J. W. Interactions between SV40 large-tumor antigen and the growth suppressor proteins pRB and p53. FASEB J. 1993 Jul;7(10):866–871. doi: 10.1096/fasebj.7.10.8344486. [DOI] [PubMed] [Google Scholar]
- Miao G. H., Hong Z., Verma D. P. Two functional soybean genes encoding p34cdc2 protein kinases are regulated by different plant developmental pathways. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):943–947. doi: 10.1073/pnas.90.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran E. A region of SV40 large T antigen can substitute for a transforming domain of the adenovirus E1A products. Nature. 1988 Jul 14;334(6178):168–170. doi: 10.1038/334168a0. [DOI] [PubMed] [Google Scholar]
- Moran E. Interaction of adenoviral proteins with pRB and p53. FASEB J. 1993 Jul;7(10):880–885. doi: 10.1096/fasebj.7.10.8344487. [DOI] [PubMed] [Google Scholar]
- Moran E., Zerler B., Harrison T. M., Mathews M. B. Identification of separate domains in the adenovirus E1A gene for immortalization activity and the activation of virus early genes. Mol Cell Biol. 1986 Oct;6(10):3470–3480. doi: 10.1128/mcb.6.10.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris-Krsinich B. A., Mullineaux P. M., Donson J., Boulton M. I., Markham P. G., Short M. N., Davies J. W. Bidirectional transcription of maize streak virus DNA and identification of the coat protein gene. Nucleic Acids Res. 1985 Oct 25;13(20):7237–7256. doi: 10.1093/nar/13.20.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris B. A., Richardson K. A., Haley A., Zhan X., Thomas J. E. The nucleotide sequence of the infectious cloned DNA component of tobacco yellow dwarf virus reveals features of geminiviruses infecting monocotyledonous plants. Virology. 1992 Apr;187(2):633–642. doi: 10.1016/0042-6822(92)90466-3. [DOI] [PubMed] [Google Scholar]
- Mullineaux P. M., Donson J., Morris-Krsinich B. A., Boulton M. I., Davies J. W. The nucleotide sequence of maize streak virus DNA. EMBO J. 1984 Dec 20;3(13):3063–3068. doi: 10.1002/j.1460-2075.1984.tb02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992 Oct 16;258(5081):424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- Perl A., Kless H., Blumenthal A., Galili G., Galun E. Improvement of plant regeneration and GUS expression in scutellar wheat calli by optimization of culture conditions and DNA-microprojectile delivery procedures. Mol Gen Genet. 1992 Nov;235(2-3):279–284. doi: 10.1007/BF00279371. [DOI] [PubMed] [Google Scholar]
- Perrin S., Gilliland G. Site-specific mutagenesis using asymmetric polymerase chain reaction and a single mutant primer. Nucleic Acids Res. 1990 Dec 25;18(24):7433–7438. doi: 10.1093/nar/18.24.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picksley S. M., Lane D. P. p53 and Rb: their cellular roles. Curr Opin Cell Biol. 1994 Dec;6(6):853–858. doi: 10.1016/0955-0674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Pipas J. M. Common and unique features of T antigens encoded by the polyomavirus group. J Virol. 1992 Jul;66(7):3979–3985. doi: 10.1128/jvi.66.7.3979-3985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudin J. P., Colasanti J., Rime H., Yuan Z., Sundaresan V. Cloning of four cyclins from maize indicates that higher plants have three structurally distinct groups of mitotic cyclins. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7375–7379. doi: 10.1073/pnas.91.15.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybicki E. P. A phylogenetic and evolutionary justification for three genera of Geminiviridae. Arch Virol. 1994;139(1-2):49–77. doi: 10.1007/BF01309454. [DOI] [PubMed] [Google Scholar]
- Sanford J. C., Smith F. D., Russell J. A. Optimizing the biolistic process for different biological applications. Methods Enzymol. 1993;217:483–509. doi: 10.1016/0076-6879(93)17086-k. [DOI] [PubMed] [Google Scholar]
- Saunders K., Lucy A., Stanley J. DNA forms of the geminivirus African cassava mosaic virus consistent with a rolling circle mechanism of replication. Nucleic Acids Res. 1991 May 11;19(9):2325–2330. doi: 10.1093/nar/19.9.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalk H. J., Matzeit V., Schiller B., Schell J., Gronenborn B. Wheat dwarf virus, a geminivirus of graminaceous plants needs splicing for replication. EMBO J. 1989 Feb;8(2):359–364. doi: 10.1002/j.1460-2075.1989.tb03385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl R. H., Gietz R. D. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989 Dec;16(5-6):339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- Smith D. H., Ziff E. B. The amino-terminal region of the adenovirus serotype 5 E1a protein performs two separate functions when expressed in primary baby rat kidney cells. Mol Cell Biol. 1988 Sep;8(9):3882–3890. doi: 10.1128/mcb.8.9.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni R., Carmichael J. P., Shah Z. H., Murray J. A. A family of cyclin D homologs from plants differentially controlled by growth regulators and containing the conserved retinoblastoma protein interaction motif. Plant Cell. 1995 Jan;7(1):85–103. doi: 10.1105/tpc.7.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger C., Doonan J. Cell division in plants. Curr Opin Cell Biol. 1993 Apr;5(2):226–231. doi: 10.1016/0955-0674(93)90107-2. [DOI] [PubMed] [Google Scholar]
- Stanley J. Analysis of African cassava mosaic virus recombinants suggests strand nicking occurs within the conserved nonanucleotide motif during the initiation of rolling circle DNA replication. Virology. 1995 Jan 10;206(1):707–712. doi: 10.1016/s0042-6822(95)80093-x. [DOI] [PubMed] [Google Scholar]
- Stenger D. C., Revington G. N., Stevenson M. C., Bisaro D. M. Replicational release of geminivirus genomes from tandemly repeated copies: evidence for rolling-circle replication of a plant viral DNA. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8029–8033. doi: 10.1073/pnas.88.18.8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuka I., Daidoji H., Matsuoka M., Kadowaki K., Takasaki Y., Nakane P. K., Moriuchi T. Gene for proliferating-cell nuclear antigen (DNA polymerase delta auxiliary protein) is present in both mammalian and higher plant genomes. Proc Natl Acad Sci U S A. 1989 May;86(9):3189–3193. doi: 10.1073/pnas.86.9.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-López P., Martínez-Salas E., Hernández P., Gutiérrez C. Bent DNA in the large intergenic region of wheat dwarf geminivirus. Virology. 1995 Apr 1;208(1):303–311. doi: 10.1006/viro.1995.1153. [DOI] [PubMed] [Google Scholar]
- Szekely L., Selivanova G., Magnusson K. P., Klein G., Wiman K. G. EBNA-5, an Epstein-Barr virus-encoded nuclear antigen, binds to the retinoblastoma and p53 proteins. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5455–5459. doi: 10.1073/pnas.90.12.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend R., Stanley J., Curson S. J., Short M. N. Major polyadenylated transcripts of cassava latent virus and location of the gene encoding coat protein. EMBO J. 1985 Jan;4(1):33–37. doi: 10.1002/j.1460-2075.1985.tb02313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden K. Interactions of human papillomavirus transforming proteins with the products of tumor suppressor genes. FASEB J. 1993 Jul;7(10):872–879. doi: 10.1096/fasebj.7.10.8393818. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Knudsen E. S., Welch P. J. The retinoblastoma tumor suppressor protein. Adv Cancer Res. 1994;64:25–85. doi: 10.1016/s0065-230x(08)60834-9. [DOI] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]