Abstract
Pain is a highly personal experience that varies substantially among individuals. In search of an anatomical correlate of pain sensitivity we used voxel-based morphometry (VBM) to investigate the relationship between grey matter density across the whole brain and inter-individual differences in pain sensitivity in 116 healthy volunteers (62 females, 54 males). Structural MRI and psychophysical data from 10 previous fMRI studies were used. Age, sex, unpleasantness ratings, scanner sequence, and sensory testing location were added to the model as covariates. Regression analysis of grey matter density across the whole brain and thermal pain intensity ratings at 49°C revealed a significant inverse relationship between pain sensitivity and grey matter density in bilateral regions of the posterior cingulate cortex, precuneus, intraparietal sulcus, and inferior parietal lobule. Unilateral regions of the left primary somatosensory cortex also exhibited this inverse relationship. No regions exhibited a positive relationship to pain sensitivity. These structural variations occurred in areas associated with the default mode network, attentional direction and shifting, as well as somatosensory processing. These findings underscore the potential importance of processes related to default mode thought and attention in shaping individual differences in pain sensitivity and indicate that pain sensitivity can potentially be predicted on the basis of brain structure.
Introduction
Pain is a multidimensional sensory experience involving interactions between sensory, cognitive, affective, and genetic factors [9; 36; 45; 53]. The multi-factorial nature of pain produces wide-ranging variability in pain sensitivity and responsiveness to treatment [10; 36]. Functional neuroimaging has revealed pain-intensity related brain activations in the thalamus, anterior cingulate cortex (ACC), insula, primary (SI), and secondary somatosensory (SII) cortices [11; 12; 16; 48]. Several of these regions exhibit activity that is positively associated with subjective pain ratings across individuals [11]. Other regions within the posterior parietal cortex such as the intraparietal sulcus (IPS) and inferior parietal lobule (IPL) may also contribute to individual differences in pain sensitivity by directing attention to painful stimuli [37; 47]. In addition, experimental pain causes deactivations in the medial prefrontal cortex (mPFC), posterior cingulate cortex (PCC), and precuneus (PCu) [12; 34; 59], whereas individuals with chronic pain show reduced deactivation in these locations [5]. These areas constitute part of the default mode network (DMN) [52], an area of increasing importance in pain research.
Structural studies suggest that pain can cause short and long-term morphologic changes in the brain. Employing voxel-based morphometry (VBM), Teutsch and colleagues (2008) determined that eight consecutive days of experimentally-induced noxious stimulation significantly increased grey matter volume in regions involved in processing of nociceptive information, such as the mid-cingulate and somatosensory cortex [67]. One year later these differences were no longer detectable, suggesting that pain-related structural changes can be reversed under the absence of noxious stimulation. Additionally, chronic pain causes grey matter changes in pain associated brain areas. The locations and direction of changes varies widely across differing chronic pain conditions [3; 20; 27; 43; 55; 58; 70].
Differences in grey matter may be reflective of neural processes contributing to the construction and modulation of pain in healthy individuals. A recent study by Erpelding and colleagues found a correlation between cold pain thresholds and cortical thickness in SI, and heat pain thresholds and cortical thickness in SI, posterior midcingulate cortex, and orbitofrontal cortex [19]. These findings highlight an important relationship between pain thresholds and cortical thickness. However, the relationship between suprathreshold differences in pain sensitivity and regional grey matter remains unknown. Elucidation of this relationship may provide novel insights into brain mechanisms contributing to individual differences in pain sensitivity. In order to address this question, we executed a VBM analysis to determine whether grey matter density (GMD) is associated with individual differences in pain sensitivity, as defined by pain intensity ratings to suprathreshold stimuli. Importantly, because pain related differences in brain morphology vary in direction and across regions, we examined both positive and negative relationships between grey matter density and individual differences in pain sensitivity across the entire brain, in a fashion unconstrained by a priori hypotheses.
Materials and Methods
Subjects
Structural data were collected from 10 previous functional magnetic resonance imaging pain studies [11; 28; 37; 38; 40; 46; 51; 64; 71; 72]. All of these studies involved noxious heat stimuli with maximum temperatures of 49°C. If a subject participated in more than one study, data from only their earliest study was used. Psychophysical data and structural brain images from 116 healthy volunteers (62 females, 54 males) ranging in age from 20–75 with a mean age of 30 ± 11 were used (See Table 1). The distribution of ethnicities includes 7 African Americans, 8 Asians, 94 Caucasians, 3 Hispanics, 1 Indian, and 3 multi-ethnic. Exclusion criteria included 1) existing chronic pain conditions, 2) current opioid use, 3) psychiatric disorders, 4) current psychiatric drug use, and 5) pregnancy. In all studies, subjects gave written informed consent stating 1) they understood that they would experience painful heat stimulation, 2) that the experimental procedures were clearly explained, and 3) that they could withdraw at any time without prejudice. Wake Forest University School of Medicine Institutional Review Board approved all study procedures.
Table 1.
Subject demographics and statistics across all studies
| Original Study | Data Collection Year | Subjects Used | Gender | Ethnicity | Age (yrs) Mean ± SD | Training/Testing Interval (days) Mean ± SD | |
|---|---|---|---|---|---|---|---|
| 1 | Coghill et al., 2003 | 2000 | 17 | F/7 M/10 | C/17 | 26 ± 5 | 2 ± 1 |
| 2 | Hadsel et al., 2006 | 2005 | 8 | F/3 M/6 | C/4 AA/1 A/1 H/2 | 25 ± 2 | 8 ± 5 |
| 3 | Martucci et al., 2006 | 2006 | 17 | F/11 M/6 | C/14 AA/2 A/1 | 27 ± 3 | 8 ± 12 |
| 4 | Starr et al., 2011 | 2007 | 13 | F/6 M/7 | C/13 | 59 ± 10 | - |
| 5 | Lobanov et al., 2013 | 2009 | 16 | F/8 M/8 | C/13 AA/1 A/1 H/1 | 26 ± 4 | 4 ± 2 |
| 6 | Zeidan et al., 2011 | 2010 | 10 | F/6 M/4 | C/8 A/1 M/1 | 27 ± 3 | 3 ± 2 |
| 7 | Quevedo and Coghill, in Preparation | 2010 | 3 | F/2 M/1 | C/3 | 27 ± 3 | 22 ± 1 |
| 8 | Nahman-Averbuch et al., 2012 | 2011 | 9 | F/6 M/3 | C/6 AA/2 A/1 | 25 ± 3 | 10 ± 5 |
| 9 | Zeidan et al., 2012 | 2011 | 10 | F/5 M/5 | C/9 A/1 | 27 ± 4 | 2 ± 2 |
| 10 | Lobanov et al., 2013 | 2011 | 13 | F/8 M/5 | C/7 AA/1 A/2 M/2 1/1 | 26 ± 2 | 4 ± 3 |
| Total | n/a | 116 | F/62 M/54 | C/94 AA/7 A/8 H/3 M/3 1/1 | 30 ± 11 | 5.44 ± 6.81 |
- = data not available; SD = standard deviation; F = female; M = male; C = Caucasian; AA = African American; A = Asian; H = Hispanic; M = multi-ethnic; I = Indian
Psychophysical Data Collection
Psychophysical assessment and MRI scanning sessions occurred during 2 independent experimental sessions (Table 1). Heat stimuli (35, 43, 44, 45, 46, 47, 48, 49°C) were applied to the ventral forearm (n=57) or posterior aspect of the lower leg (n=59) using a 16 × 16 mm TSA II thermal stimulator (Medoc, Ramat Yishai, Israel), with 35°C serving as baseline. Due to the retrospective nature of the study, data on the side of stimulation is unavailable. Stimulus temperatures were delivered with rise and fall rates of 6°C/s with a plateau duration of 5 seconds and a minimum interval of 30 seconds between stimuli. Stimulus temperatures were applied in 4 blocks, with each block consisting of all 8-stimulus temperatures (32 total). The thermal probe was moved to a different location following termination of each stimulus to prevent effects of sensitization or habituation. Subjects rated pain intensity and pain unpleasantness on a scale of 0–10 (where 0 is “no pain” or “not at all unpleasant” and 10 is “most intense pain imaginable” or “most unpleasant imaginable”) using a 15-cm plastic visual analogue scale (Paresian Novelty) [49; 50]. Subjects were instructed to only provide a rating for painful stimuli; therefore, if the stimulus was not perceived as painful, subjects provided a zero for both pain intensity and unpleasantness. The average of pain intensity responses to the four 49°C stimuli was used as an index of each subject's pain sensitivity.
MRI Acquisition
Structural images were obtained on a GE Healthcare 1.5 Tesla MRI Scanner using similar high-resolution T1 weighted anatomical sequences shown in Table 2. Importantly, MRI scans were acquired in a different session than collection of psychophysical data (Table 1). All structural scans except those from Zeidan and colleagues 2011 (n=10) were obtained prior to the fMRI-testing paradigm. These 10 scans were obtained after 1 fMRI series that included noxious heat stimulation and 1 fMRI series that involved neutral stimulation.
Table 2.
Structural scan sequences across all studies
| Original Study | Sequence | TI (ms) | TR (ms) | Flip Angle (°) | TE (ms) | Matrix Size | Slice Thicknes* (mm) | Sections | In-plane resolution (mm) | FOV (cm) |
|---|---|---|---|---|---|---|---|---|---|---|
| Coghill et al., 2003 | 3D spoiled gradient-echo | 600 | 9.10 | 20 | 1.98 | 256 × 192 | 1.5 | 124 | 0.9375 × 0.9375 | 24 |
| Hadsel et al., 2006 | 3D spoiled gradient-echo | 600 | 8.59 | 20 | 1.88 | 256 × 196 | 1.5 | 124 | 0.9375 × 0.9375 | 24 |
| Martucci et al., 2006 | 3D spoiled gradient-echo | 600 | 8.59 | 20 | 1.88 | 256 × 196 | 1.5 | 124 | 0.9375 × 0.9375 | 24 |
| Starr et al., 2011 | 3D spoiled gradient-echo | 600 | 9.10 | 20 | 1.98 | 256 × 196 | 1.5 | 124 | 0.9375 × 0.9375 | 24 |
| Lobanov et al., 2013 | BRAVO | 600 | 11.41 | 12 | 4.77 | 240 × 240 | 1.5 | 160 | 0.9375 × 0.9375 | 24 |
| Zeidan et al., 2011 | 3D spoiled gradient-echo | 600 | 11.42 | 12 | 4.76 | 240 × 240 | 1.0 | 164 | 0.9375 × 0.9375 | 24 |
| Quevedo and Coghill, in Preparation | 3D spoiled gradient-echo | 600 | 9.10 | 20 | 1.98 | 256 × 196 | 1.5 | 124 | 0.9375 × 0.9375 | 24 |
| Nahman-Averbuch et al., 2012 | BRAVO | 600 | 11.46 | 12 | 4.73 | 240 × 240 | 1.5 | 0.9375 × 0.9375 | 24 | |
| Zeidan et al., 2012 | BRAVO | 600 | 11.49 | 12 | 4.74 | 256 × 256 | 1.0 | 156 | 0.9375 × 0.9375 | 24 |
| Lobanov et al., 2013 | BRAVO | 600 | 11.49 | 12 | 4.74 | 240 × 240 | 1.0 | 156 | 0.9375 × 0.9375 | 24 |
TI = inversion time; TR = repetition time; TE = echo time; FOV = field of view;
no gap between sections
Image processing and statistical analysis
VBM methods
Structural data were analyzed with FSL-VBM v1.1 http://www.fmrib.ox.ac.uk/fsl/fslvbm/index.html; Oxford University, Oxford, UK), a voxel-based morphometry analysis tool [4; 23; 61]. First, structural brain images were extracted from surrounding tissue, using BET [60]. Next, tissue type segmentation of grey matter (GM), white matter (WM), and cerebrospinal fluid (CSF) was completed with FAST4 [73]. The segmented grey matter images were aligned to Montreal Neurological Institute (MNI) 152 standard space using a 12 parameter affine transformation accomplished with FLIRT [32; 33]. The post registration images were averaged to create a study specific template for subsequent non-linear registration of GM images using FNIRT [1; 2]. The GM images were concatenated into a 4D image and corrected for local expansion and contraction by dividing by the Jacobian of the warp field. The modulated images were smoothed with an isotropic Gaussian kernel with a sigma of 4mm (full width at half-maximum = 9mm).
A regression analysis was performed using a general linear model (GLM) to examine the relationship between grey matter differences across the whole brain and pain sensitivity (VAS intensity ratings of 49°C). Age, sex, unpleasantness ratings, scanner sequence, and sensory testing location were added as covariates. Permutation-based non-parametric testing (10,000 permutations) was used to evaluate this relationship in a voxel-wise fashion. Threshold free cluster enhancement (TFCE) was utilized to define significant clusters [62]. A family wise error (FWE) corrected p-value of p < 0.05 was applied to correct for multiple comparisons and identify clusters exhibiting a significant relationship between GMD and pain sensitivity.
Psychophysical statistics
GMD can differ substantially as a function of sex, age, and ethnicity [8; 22; 23]. Accordingly, the relationships between pain sensitivity and sex, age, and ethnicity were examined. Psychophysical data and age relationships were assessed via regression analysis. Pain sensitivity between sexes was assessed using a Student's t-test. One-way ANOVA's were used to test for differences in pain sensitivity that were related to ethnicity and the study from which the data were derived. In addition, Student's t-tests were performed to ensure that pain sensitivity was not differentially distributed between the two broad types of scanning sequences (BRAVO vs. SPGR) or the two sensory testing locations (arm vs. leg).
Secondary analyses to assess potential carryover effects of sensory testing on GMD
A VBM-based Student's t-test was completed to determine grey matter differences between individuals based on sensory testing location (arm vs leg). To examine possible grey matter differences related to carry over effects from the sensory testing session, a VBM-based regression analysis of grey matter density and the number of days between sensory testing and structural acquisition was preformed.
Results
Psychophysical Responses
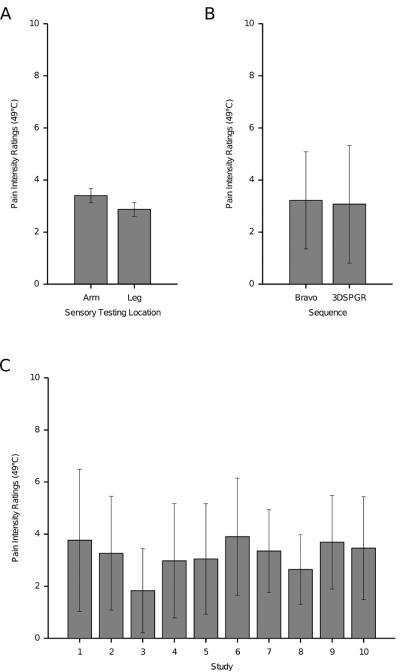
Across all subjects, mean intensity and unpleasantness ratings at 49°C were 3.13 ± 2.10 and 2.79 ± 2.12 (mean rating ± SD) respectively and were significantly related to each other (R2 = 0.66, F(1, 115) = 219.04, p < 0.001). Accordingly, pain intensity ratings were used for all subsequent analyses. No relationship was found between intensity ratings and age, (R2 = 0.00, F(1, 114) = 0.00, p = 0.98), ethnicity (F(5,110) = 1.66, p = 0.15), or sex (t(1,114) = 0.830, p = 0.40). In addition, there were no significant differences between intensity ratings and scanning sequence (t(1,114) = 0.362, p = 0.72), sensory testing location (t(1,114) = 1.362, p = 0.18), or the study from which the subjects were derived (F(9,106) = 1.25, p = 0.27) (Figure 1).
Figure 1.
Pain sensitivity differences by sensory testing location, scanning sequence, and study (mean ± SD). A, Subjects were tested for thermal heat pain sensitivity on the arm (n = 57) or leg (n = 59). Mean intensity ratings between locations did not differ significantly (p = 0.18). B, Structural images from 10 previous studies (n=116) were acquired using BRAVO (n = 48) and SPGR (n = 68) scanning sequences. Mean pain sensitivity between subjects of BRAVO and SPGR sequences did not differ significantly (p = 0.72). C, Mean intensity ratings across studies are listed according to Table 1 and did not differ significantly (p = 0.27).
Relationship between pain sensitivity and GMD
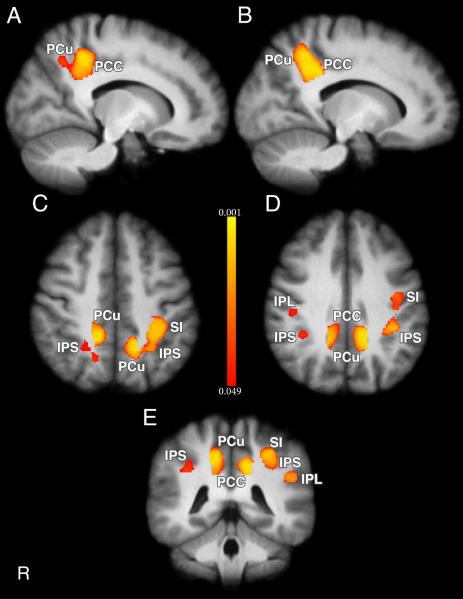
A multiple regression based VBM analysis using a whole brain search unconstrained by regions of interest determined that pain intensity ratings at 49°C were negatively related to grey matter density in bilateral PCu, (p = 0.006, corrected), bilateral PCC (p = 0.006, corrected), bilateral anterior IPS (p = 0.012, corrected), bilateral IPL (p = 0.021, corrected) and left SI (p = 0.012, corrected). Figure 2 shows significant clusters in PCu (voxels (L) = 335, (R) = 289), PCC (voxels (L) = 199, (R) = 184), IPS (voxels (L) = 408, (R) = 87), IPL (voxels (L) = 100, (R) = 51), and left SI (voxels = 417). Subjects with lower intensity ratings were found to have larger grey matter densities in these areas relative to subjects with higher intensity ratings (Figure 3). Conversely, no positive relationship was found between pain sensitivity and GMD.
Figure 2.
Differences in regional grey matter density are inversely related to pain sensitivity in healthy subjects (n=116). VBM regression analysis revealed increased grey matter in subjects reporting low VAS pain ratings at 49°C. Subjects reporting high VAS pain ratings at 49°C exhibited less grey matter. A, B, Sagittal slices showing grey matter differences in right (Coordinates: 14, −44, 42) and left (Coordinates: −14, −48, 40) PCu (voxels (R) = 289, (L) = 335; p = 0.006) and right (Coordinates: 14, −42, 36) and left (Coordinates: −14, −44, 36) PCC (voxels (R) = 184, (L) = 199; p = 0.006). C, Horizontal slice showing grey matter differences in IPS (voxels (R) = 87, Coordinates: 24, −52, 46; voxels (L) = 408, Coordinates: −34, − 42, 46; p = 0.012), PCu, and SI (voxels = 417, Coordinates: −32, −36, 46; p = 0.012). D, Horizontal slice showing grey matter differences in right IPL (voxels = 51, Coordinates: 46, −24, 34, p < 0.05), IPS, PCC, PCu, and SI. E, Coronal slice showing grey matter differences in left IPL (voxels = 100, Coordinates: −50, −42, 28, p = 0.021), IPS, PCu, PCC, and SI. Color bar values correspond to p values. IPL = inferior parietal lobule, IPS = intraparietal sulcus, PCC = posterior cingulate cortex, PCu = precuneus, SI = primary somatosensory cortex.
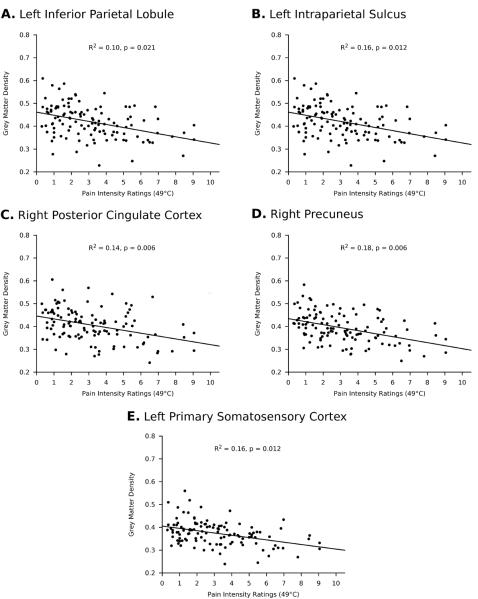
Figure 3.
Relationships between pain sensitivity and grey matter density in selected regions. Pain intensity ratings at 49°C are inversely related to grey matter density.
GMD exhibited no carryover effects from the sensory testing session
Sensory testing was completed on the arm or leg and occurred an average of 5.44 days prior to structural MRI acquisition. A between groups VBM analysis of grey matter density across the whole brain and sensory testing location (arm vs. leg) revealed no significant differences. A regression analysis of GMD across the whole brain and the number of days between sensory testing and structural scanning identified no significant relationship between GMD and time between testing and scanning.
Discussion
Using a whole brain VBM analysis, we identified an inverse relationship between pain sensitivity and GMD in the PCC, PCu, IPS, IPL, and SI. This relationship is striking since these differences in brain structure were identified an average of 5.44 days after psychophysical assessment. Accordingly, this relationship between brain structure and pain sensitivity may reflect relatively stable processes that contribute substantially to individual differences in pain sensitivity. Surprisingly, some of the regions exhibiting this relationship are deactivated during acute nociceptive stimulation and may be related to default mode processes. Others are related to top down direction of attention, as well as somatosensory processing.
DMN and Pain
The DMN consists of a collection of brain regions that are associated with self-awareness and consciousness and include the mPFC, PCC, and the PCu [52]. These regions are intrinsically active during times of rest [52], introspection [26], daydreaming [41], information gathering [25], and retrieval of episodic memories [7] and are deactivated with cognitive load [52], task performance [42], hypnosis [54], and meditation [24; 29; 72].
Our results indicate an inverse relationship between pain sensitivity and grey matter density in areas of the DMN, such that individuals who are highly sensitive to pain have less grey matter in PCC and PCu, and individuals who are least sensitive to pain have more grey matter in these areas. Areas of the DMN including the PCC and PCu have been shown to deactivate during experimental pain [12; 34; 59]. It is unclear whether structural differences in the DMN reflect a cause or a consequence of individual differences in pain sensitivity. Inter-individual differences in neural activation may result in grey matter differences, as experience is known to cause grey matter changes [17; 55; 67]. Individuals who have entrenched introspective thought patterns may have increased grey matter density in default mode areas. These introspective thought patterns may function as competitors to processes that contribute to the construction of an experience of pain, thereby resulting in relatively low pain sensitivity. For example, a recent brain imaging study by Kucyi and Colleagues found that mind wandering away from pain increased functional activations in the DMN and that structural and functional connectivity between the DMN and periaqueductal gray were associated with mind wandering away from pain [35]. Consistent with this notion, rumination about non-pain related issues may function as a competitor to the instantiation of pain. Healthy, pain-free individuals with mild sub-clinical anxiety have lower pain intensity ratings than non-anxious individuals [63].
Conversely, individuals who are easily disengaged from self-referential thought processes may have less DMN activation and decreased grey matter in default mode areas. In this case, default mode activity may not compete as efficiently with the processes generating an experience of pain. Thus, these individuals may have relatively high sensitivity to pain. Although speculative, this interpretation is consistent with findings derived from studies examining the relationship between cortical thickness and dimensions of attention including executive control, alerting, and orienting. Grey matter thickness of the PCC and PCu as well as the IPS was inversely related to the alerting response. This negative relationship was viewed as being related to reduced task motivation or a reduced ability to maintain tonic vigilance prior to presentation of warning cues [68]. Such reductions in alerting responses are consistent with a reduced ability to switch from default mode thought patterns in order to direct attention to processing external stimuli.
Posterior Parietal Cortex and Pain
Similar to the PCC and PCu, grey matter density in the posterior parietal cortex was inversely related to pain sensitivity. These changes were evident in two regions of the posterior parietal cortex: the IPS and the IPL. The IPS and IPL are involved in attentional processes related to spatial and intensity features of visual, auditory, and somatosensory modalities, including pain [14; 21; 31; 37; 39; 47; 57; 69]. Individuals with increased grey matter in IPS and IPL may be more likely to maintain top-down directed attention and not be as sensitive to the bottom up attentional demands of noxious stimuli. Consistent with this notion, intentional direction of attention away from pain has been shown to reduce pain perception [6; 15; 18]. Conversely, individuals with decreased grey matter density in IPS and IPL may be less able to regulate top-down attentional processes and therefore be more susceptible to stimuli with strong bottom-up attentional demands [68].
SI and Pain
In functional brain imaging studies, SI is frequently activated during pain and is related to perceived pain intensity, individual differences in pain sensitivity, as well as stimulus intensity [11–13]. The present investigation revealed an inverse relationship between GMD in SI and pain sensitivity to suprathreshold noxious stimuli. When pain sensitivity was determined according to heat pain thresholds, a positive relationship between cortical thickness and threshold-based sensitivity was identified in SI [19]. Thresholds and suprathreshold ratings are vastly different measures and are relatively poorly correlated within individuals [63]. Thus, differences in results from these 2 measures of pain sensitivity are not surprising. However, our findings of increased GMD in relatively insensitive individuals are consistent with the observation that repetitive exposure to suprathreshold noxious stimuli reduces pain sensitivity concomitantly with increasing grey matter volume in SI [67]. Grey matter contains both excitatory and inhibitory neurons, so a decrease in grey matter could be the result of decreased inhibitory processes. Indeed, individuals with chronic neuropathic pain show decreased grey matter volume of the somatosensory thalamus (as measured by VBM), decreased cerebral blood flow in thalamus and SI, and significantly lower thalamic GABA levels than controls [30]. Interestingly, the decreased cerebral blood flow in the thalamus was negatively correlated to pain intensity.
Limitations
Since this is a retrospective analysis of multiple data sets acquired over more than a decade, many factors could elevate variability and reduce sensitivity to more subtle changes in GMD. Although some sources of variability such as scanner sequence were incorporated into the statistical analysis, other remaining sources of variability could still somewhat diminish sensitivity. Given the variations in experimental protocols and data acquisition techniques, the identification of regional GMD differences reflecting pain sensitivity is even more striking.
Our results indicate that structural differences in areas of the DMN, IPS, IPL, and SI, are inversely related to pain sensitivity. However, more research is needed to determine the directionality of this relationship and the sources behind the grey matter differences. Although the underlying mechanism of grey matter variation is not known, there are many factors that may contribute. These include differences in blood flow, glia, neuronal cell bodies, and neuropil [17]. It is also unclear whether these differences are genetic or experience based, although it is likely both [17; 44]. Previous studies have shown that repetitive noxious heat stimulation delivered over the course of 8 daily sessions can induce changes in grey matter in healthy individuals [67]. Accordingly, we examined the possibility of carryover effects and found no relationship between grey matter density and the number of days between sensory testing and structural acquisition. Moreover, we found no effect of stimulus location (arm vs. leg) on GMD. Therefore, it is unlikely that these intensity-related grey matter differences were driven by the stimuli delivered in the psychophysical session. In addition, although data on the side of stimulation is unavailable, the absence of carryover effects indicates that the grey matter differences seen in left SI are not the result of prior stimulation.
Future research should examine whether these findings apply to other sensory modalities. A limiting factor of this study is that only thermal pain was used to test pain sensitivity. Examination of responses to other pain modalities could provide insight as to the generalizibility of the relationship between pain sensitivity and grey matter differences found here. An additional limitation of this study is that we did not assess personality traits or pain related psychological factors, such as anxiety and neuroticism, which may influence brain structure. Incorporation of such factors could further elucidate the relationship between brain anatomy and pain sensitivity.
Summary
Pain is a subjective experience with tremendous inter-individual variation. Using a global search unconstrained by a priori biases, we determined that GMD in areas associated with default mode processes, attention, as well as somatosensory processing was significantly inversely related to individual differences in pain sensitivity. Sensitivity can fluctuate over different time scales with substantial variation from one week to the next or can be influenced by developmental factors and remain altered over the course of a lifetime [56; 65; 66]. In the present investigation, the relationship between GMD and pain sensitivity was evident over a period of more than 5 days on average, and suggesting a relatively stable phenomenon. Accordingly, these kinds of morphologic differences can serve as novel predictors of pain sensitivity and provide a foundation for the development of biomarkers for diagnosis, classification, prevention, and treatment of pain.
Acknowledgments
This study was supported by the National Institutes of Health grants NS039426 and DA020168 and the US-Israel Binational Science Foundation grant 2009097.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest The authors declare no conflicts of interest.
References
- [1].Andersson JLR, Jenkinson M, Smith S. FMRIB technical report TR07JA2. 2007. Non-linear registration, aka Spatial normalisation. [Google Scholar]
- [2].Andersson JLR, Jenkinson M, Smith S. FMRIB Technical Report TR07JA1. 2007. Non-linear optimisation. [Google Scholar]
- [3].Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24(46):10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- [5].Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28(6):1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125(Pt 2):310–319. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- [7].Cabeza R, Dolcos F, Graham R, Nyberg L. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuroimage. 2002;16(2):317–330. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- [8].Chee MW, Zheng H, Goh JO, Park D, Sutton BP. Brain structure in young and old East Asians and Westerners: comparisons of structural volume and cortical thickness. J Cogn Neurosci. 2011;23(5):1065–1079. doi: 10.1162/jocn.2010.21513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Coghill RC. Individual differences in the subjective experience of pain: new insights into mechanisms and models. Headache. 2010;50(9):1531–1535. doi: 10.1111/j.1526-4610.2010.01763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Coghill RC, Eisenach J. Individual differences in pain sensitivity: implications for treatment decisions. Anesthesiology. 2003;98(6):1312–1314. doi: 10.1097/00000542-200306000-00003. [DOI] [PubMed] [Google Scholar]
- [11].Coghill RC, McHaffie JG, Yen YF. Neural correlates of interindividual differences in the subjective experience of pain. Proc Natl Acad Sci U S A. 2003;100(14):8538–8542. doi: 10.1073/pnas.1430684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82(4):1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- [13].Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, Duncan GH. Distributed processing of pain and vibration by the human brain. J Neurosci. 1994;14(7):4095–4108. doi: 10.1523/JNEUROSCI.14-07-04095.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. J Cogn Neurosci. 2002;14(3):508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- [15].Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ. Functional MRI of pain-and attention-related activations in the human cingulate cortex. J Neurophysiol. 1997;77(6):3370–3380. doi: 10.1152/jn.1997.77.6.3370. [DOI] [PubMed] [Google Scholar]
- [16].Derbyshire SW, Jones AK, Gyulai F, Clark S, Townsend D, Firestone LL. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain. 1997;73(3):431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- [17].Draganski B, May A. Training-induced structural changes in the adult human brain. Behavioural brain research. 2008;192(1):137–142. doi: 10.1016/j.bbr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- [18].Erpelding N, Davis KD. Neural underpinnings of behavioural strategies that prioritize either cognitive task performance or pain. Pain. 2013 doi: 10.1016/j.pain.2013.06.030. [DOI] [PubMed] [Google Scholar]
- [19].Erpelding N, Moayedi M, Davis KD. Cortical thickness correlates of pain and temperature sensitivity. Pain. 2012;153(8):1602–1609. doi: 10.1016/j.pain.2012.03.012. [DOI] [PubMed] [Google Scholar]
- [20].Farmer MA, Chanda ML, Parks EL, Baliki MN, Apkarian AV, Schaeffer AJ. Brain functional and anatomical changes in chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2011;186(1):117–124. doi: 10.1016/j.juro.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Giesbrecht B, Woldorff MG, Song AW, Mangun GR. Neural mechanisms of top-down control during spatial and feature attention. Neuroimage. 2003;19(3):496–512. doi: 10.1016/s1053-8119(03)00162-9. [DOI] [PubMed] [Google Scholar]
- [22].Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr., Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral cortex. 2001;11(6):490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- [23].Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- [24].Grant JA, Courtemanche J, Rainville P. A non-elaborative mental stance and decoupling of executive and pain-related cortices predicts low pain sensitivity in Zen meditators. Pain. 2011;152(1):150–156. doi: 10.1016/j.pain.2010.10.006. [DOI] [PubMed] [Google Scholar]
- [25].Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- [27].Gwilym SE, Filippini N, Douaud G, Carr AJ, Tracey I. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: a longitudinal voxel-based morphometric study. Arthritis Rheum. 2010;62(10):2930–2940. doi: 10.1002/art.27585. [DOI] [PubMed] [Google Scholar]
- [28].Hadsel MS, Quevedo A, Oshiro Y, Kraft RA, Coghill RC. fMRI studies of pain: Not all baseline periods are equal. Soc Neurosci Abstr. 2006;32(445):14. [Google Scholar]
- [29].Hasenkamp W, Wilson-Mendenhall CD, Duncan E, Barsalou LW. Mind wandering and attention during focused meditation: a fine-grained temporal analysis of fluctuating cognitive states. Neuroimage. 2012;59(1):750–760. doi: 10.1016/j.neuroimage.2011.07.008. [DOI] [PubMed] [Google Scholar]
- [30].Henderson LA, Peck CC, Petersen ET, Rae CD, Youssef AM, Reeves JM, Wilcox SL, Akhter R, Murray GM, Gustin SM. Chronic pain: lost inhibition? J Neurosci. 2013;33(17):7574–7582. doi: 10.1523/JNEUROSCI.0174-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nature neuroscience. 2000;3(3):284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- [32].Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- [33].Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- [34].Kong J, Loggia ML, Zyloney C, Tu P, Laviolette P, Gollub RL. Exploring the brain in pain: activations, deactivations and their relation. Pain. 2010;148(2):257–267. doi: 10.1016/j.pain.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kucyi A, Salomons TV, Davis KD. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci U S A. 2013;110(46):18692–18697. doi: 10.1073/pnas.1312902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lacroix-Fralish ML, Mogil JS. Progress in genetic studies of pain and analgesia. Annu Rev Pharmacol Toxicol. 2009;49:97–121. doi: 10.1146/annurev-pharmtox-061008-103222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lobanov OV, Quevedo AS, Hadsel MS, Kraft RA, Coghill RC. Frontoparietal mechanisms supporting attention to location and intensity of painful stimuli. Pain. 2013 doi: 10.1016/j.pain.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lobanov OV, Zeidan F, McHaffie JG, Kraft RA, Coghill RC. From cue to meaning: Brain mechanisms supporting the construction of expectations of pain. Pain. 2013 doi: 10.1016/j.pain.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Macaluso E, Frith CD, Driver J. Supramodal effects of covert spatial orienting triggered by visual or tactile events. J Cogn Neurosci. 2002;14(3):389–401. doi: 10.1162/089892902317361912. [DOI] [PubMed] [Google Scholar]
- [40].Martucci KT, Coghill RC, Kraft RA. A new spin on chronic pain imaging: Quantitative perfusion imaging using arterial spin labeling MRI. Soc Neurosci Abstr. 2006;32(445):22. [Google Scholar]
- [41].Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15(3):394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- [43].Moayedi M, Weissman-Fogel I, Salomons TV, Crawley AP, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. Abnormal gray matter aging in chronic pain patients. Brain research. 2012;1456:82–93. doi: 10.1016/j.brainres.2012.03.040. [DOI] [PubMed] [Google Scholar]
- [44].Muotri AR, Gage FH. Generation of neuronal variability and complexity. Nature. 2006;441(7097):1087–1093. doi: 10.1038/nature04959. [DOI] [PubMed] [Google Scholar]
- [45].Muralidharan A, Smith MT. Pain, analgesia and genetics. J Pharm Pharmacol. 2011;63(11):1387–1400. doi: 10.1111/j.2042-7158.2011.01340.x. [DOI] [PubMed] [Google Scholar]
- [46].Nahman-Averbuch H, Martucci KT, Granovsky Y, Weissman-Fogel I, Yarnitsky D, Coghill RC. 14th World Congress on Pain. Different patterns of brain activation during conditioned pain modulation and offset analgesia. in Press. [Google Scholar]
- [47].Oshiro Y, Quevedo AS, McHaffie JG, Kraft RA, Coghill RC. Brain mechanisms supporting spatial discrimination of pain. J Neurosci. 2007;27(13):3388–3394. doi: 10.1523/JNEUROSCI.5128-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Porro CA, Cettolo V, Francescato MP, Baraldi P. Temporal and intensity coding of pain in human cortex. J Neurophysiol. 1998;80(6):3312–3320. doi: 10.1152/jn.1998.80.6.3312. [DOI] [PubMed] [Google Scholar]
- [49].Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994;56(2):217–226. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]
- [50].Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- [51].Quevedo A. CR. Cognitive factors modulating the cutaneous radiation of pain. in Preparation. [Google Scholar]
- [52].Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rainville P. Brain mechanisms of pain affect and pain modulation. Curr Opin Neurobiol. 2002;12(2):195–204. doi: 10.1016/s0959-4388(02)00313-6. [DOI] [PubMed] [Google Scholar]
- [54].Rainville P, Hofbauer RK, Paus T, Duncan GH, Bushnell MC, Price DD. Cerebral mechanisms of hypnotic induction and suggestion. J Cogn Neurosci. 1999;11(1):110–125. doi: 10.1162/089892999563175. [DOI] [PubMed] [Google Scholar]
- [55].Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29(44):13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rosier EM, Iadarola MJ, Coghill RC. Reproducibility of pain measurement and pain perception. Pain. 2002;98(1–2):205–216. doi: 10.1016/s0304-3959(02)00048-9. [DOI] [PubMed] [Google Scholar]
- [57].Salmi J, Rinne T, Koistinen S, Salonen O, Alho K. Brain networks of bottom-up triggered and top-down controlled shifting of auditory attention. Brain research. 2009;1286:155–164. doi: 10.1016/j.brainres.2009.06.083. [DOI] [PubMed] [Google Scholar]
- [58].Schmidt-Wilcke T, Hierlmeier S, Leinisch E. Altered regional brain morphology in patients with chronic facial pain. Headache. 2010;50(8):1278–1285. doi: 10.1111/j.1526-4610.2010.01637.x. [DOI] [PubMed] [Google Scholar]
- [59].Seminowicz DA, Davis KD. Pain enhances functional connectivity of a brain network evoked by performance of a cognitive task. J Neurophysiol. 2007;97(5):3651–3659. doi: 10.1152/jn.01210.2006. [DOI] [PubMed] [Google Scholar]
- [60].Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- [62].Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- [63].Starr CJ, Houle TT, Coghill RC. Psychological and sensory predictors of experimental thermal pain: a multifactorial model. J Pain. 2010;11(12):1394–1402. doi: 10.1016/j.jpain.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, McHaffie JG, Coghill RC. The contribution of the putamen to sensory aspects of pain: insights from structural connectivity and brain lesions. Brain. 2011;134(Pt 7):1987–2004. doi: 10.1093/brain/awr117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Sternberg WF, Scorr L, Smith LD, Ridgway CG, Stout M. Long-term effects of neonatal surgery on adulthood pain behavior. Pain. 2005;113(3):347–353. doi: 10.1016/j.pain.2004.11.013. [DOI] [PubMed] [Google Scholar]
- [66].Taddio A, Katz J, Ilersich AL, Koren G. Effect of neonatal circumcision on pain response during subsequent routine vaccination. Lancet. 1997;349(9052):599–603. doi: 10.1016/S0140-6736(96)10316-0. [DOI] [PubMed] [Google Scholar]
- [67].Teutsch S, Herken W, Bingel U, Schoell E, May A. Changes in brain gray matter due to repetitive painful stimulation. Neuroimage. 2008;42(2):845–849. doi: 10.1016/j.neuroimage.2008.05.044. [DOI] [PubMed] [Google Scholar]
- [68].Westlye LT, Grydeland H, Walhovd KB, Fjell AM. Associations between regional cortical thickness and attentional networks as measured by the attention network test. Cerebral cortex. 2011;21(2):345–356. doi: 10.1093/cercor/bhq101. [DOI] [PubMed] [Google Scholar]
- [69].Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM. Transient neural activity in human parietal cortex during spatial attention shifts. Nature neuroscience. 2002;5(10):995–1002. doi: 10.1038/nn921. [DOI] [PubMed] [Google Scholar]
- [70].Younger JW, Shen YF, Goddard G, Mackey SC. Chronic myofascial temporomandibular pain is associated with neural abnormalities in the trigeminal and limbic systems. Pain. 2010;149(2):222–228. doi: 10.1016/j.pain.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zeidan F, Lobonov OV, Kraft RA, Coghill RC. 14th World Congress on Pain. 2012. Brain mechanisms supporting discordance between expected and experienced pain. [Google Scholar]
- [72].Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci. 2011;31(14):5540–5548. doi: 10.1523/JNEUROSCI.5791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]