Abstract
Airway inflammation has a pathophysiological role in asthma. Eosinophils, which are commonly increased in asthmatic airways, express eosinophil peroxidase and thereby produce hypobromite and bromotyrosine. Bromotyrosine is believed to be a specific marker for eosinophil activity, but developing an antibody against monobromotyrosine, the predominate brominated tyrosine residue found in vivo has proven difficult. We evaluated whether a 3-bromobenozoic acid hapten antigen produced antibodies that recognized halogenated tyrosine residues. Studies with small-molecule inhibitors or brominated or chlorinated protein suggested that a mouse monoclonal antibody (BTK-94C) selectively bound free and protein mono- and dibromotyrosine and, to a lesser degree, chlorotyrosine, and thus was designated a general halotyrosine antibody. We evaluated if this antibody had potential for characterizing human asthma using an enzyme-linked immunosorbent assay (ELISA) microarray platform to examine the halogenation of 23 proteins in three independent sets of sputum samples (52 samples total). In 15 healthy control or asthmatic subjects, ICAM, PDGF and RANTES had greater proportional amounts of halogenation in asthmatic subjects and the halogenation signal was associated with the severity of exercise-induced airway hyperresponsiveness. In 17 severe asthma patients treated with placebo or mepolizumab to suppress eosinophils, drug-related decreases in halogenation were observed with p values ranging from 0.006 to 0.11 for these 3 proteins. Analysis of 20 subjects that either had neutrophilic asthma or were healthy controls demonstrated a broad increase in halotyrosine (possibly chlorotyrosine) in neutrophilic asthmatics. Overall, these results suggest that an ELISA utilizing BTK-94C could prove useful for assessing airway inflammation in asthma patients.
1. Introduction
Asthma is a common disease that affects about 23 million adults in the United States (Pleis et al. 2010). Eosinophil and neutrophil activities in the airways contribute to the pathophysiology of asthma (Nakagome et al. 2012; Rosi et al. 2006). Eosinophil markers have shown promise as indices of airway inflammation (Wolthers 2003). Bromotyrosine protein modifications are increased in asthma patients as the result of eosinophil peroxidase (EPO), an abundant granule protein that produces the brominating agent, hypobromous acid (HOBr). There is clear evidence that brominated proteins are useful markers for airway eosinophil activity (Aldridge et al. 2002; Hawkins and Davies 2005; Wu et al. 2000). Total protein levels of 3-bromotyrosine and 3,5-dibromotyrosine are significantly elevated about 3-fold in airways of asthmatics compared to healthy controls and are similarly suppressed in asthma patients by corticosteroid therapy (Aldridge et al. 2002; van Dalen et al. 2009). More substantial increases in tyrosine bromination are observed during asthma exacerbations.
Studies on brominated proteins in asthma have been limited by a lack of a rapid, simple method to monitor halotyrosine levels in biofluids. Previous attempts have failed to produce a useful antibody that recognizes 3-bromotyrosine, which is the predominant brominated tyrosine modification that occurs in vivo. For example, in 1930, Wormall reported producing rabbit antiserum against 3,5-dibromotyrosine, but did not generate a 3-bromotyrosine antibody (Wormall 1930). More recently, Kambayashi et al. (2009) and Kato et al. (2005) generated polyclonal and monoclonal antibodies against brominated proteins, but their antibodies also only reacted with 3,5-dibromotyrosine. Dibromotyrosine modifications are preferentially produced by in vitro protein bromination, especially at relatively high concentrations of HOBr (Kato et al. 2005), suggesting that past failures to produce an antibody against 3-bromotyrosine may reflect how the antigen was brominated. To bypass this potential problem, we evaluated the antigenicity of a bromotyrosine analog, 3-bromo-4-hydroxybenzoic acid, which was coupled to keyhole limpet hemocyanin (KLH). This hapten antigen produced a mouse monoclonal antibody that recognizes halogenated tyrosine residues, including mono- and di-bromotyrosine and, to a much lesser degree, chlorotyrosine residues. We used this antibody in a custom enzyme-linked immunosorbent assay (ELISA) microarray platform to analyze halogenation of individual proteins in sputum. Overall, our data suggest that this type of assay has the potential to characterize asthma.
2. Materials and methods
2.1. Materials
3-bromo-4-hydroxybenzoic acid (HBA) was from Indofine Chemical Company Inc. (Hillsborough, NJ). 3,5-dibromo-4-hydroxybenzalehyde, 3,5-dichloro-4-hydroxybenoic acid, 3,4-dihydroxybenic acid, 3-nitrotyrosine, 3-chloro-tyrosine and L-tyrosine were from Sigma- Aldrich. KLH, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), ascites conditioning reagent, Melon monoclonal antibody purification kit and EZ-link sulfo-NHS-biotin kit were from Pierce-Thermo Scientific (Rockford, IL). Sodium hypobromite solution was from Fisher Scientific, and was purchased fresh each time it was used and was used within a few days of receipt. Capture antibodies were from commercial sources, as listed in Supplemental Table S1.
2.2. Subjects
All human subjects research was originally approved by the Research Ethics Board at McMaster University, or the Internal Review Board at the University of Washington. All subjects provided written consent for their samples to be used for research. This consent was confirmed by an investigator on the research team. These human subjects protocols and approvals, including the consent forms, were subsequently reviewed and approved by the Internal Review Board at the Pacific Northwest National Laboratory prior to sample transfer and analysis.
The first set of 15 induced sputum samples were obtained from a repository of samples at the University of Washington. The asthma group consisted of persons aged 18–59 with asthma treated only with a short-acting β2-agonist as needed, a forced expiratory volume in 1 second (FEV1) >65% predicted and a ≥15% fall in FEV1 after exercise challenge (0% relative humidity, 22 °C). Subjects with an FEV1 below 65% predicted were excluded because the exercise challenge test presented a health hazard to subjects in this category. Spirometry (Miller et al. 2005) and exercise challenges (Crapo et al. 2000) were conducted in accordance with American Thoracic Society standards. Subjects were excluded if they smoked cigarettes within one year, had a ≥7 pack-year smoking history, or had used an inhaled corticosteroid, leukotriene modifier, long-acting antihistamine, cromone, or long-acting β2-agonist in the previous 30 days. Induced sputum samples from the subjects with asthma were selected based on the percentage of eosinophils in the induced sputum, with the “high” group having >2.5% eosinophils and the “low” group with <2.5% eosinophils. The control group consisted of persons 18–59 years of age with no history of asthma or atopy, baseline FEV1 ≥80% predicted, and <7% fall in FEV1 after exercise challenge.
Two other sets of sputum samples were collected at McMaster University. For the mepolizumab study, the subjects have previously been characterized and described in detail (Nair et al. 2009). These samples were analyzed with the ELISA microarrays blinded in regards to therapy. For the neutrophil asthma study, all subjects had a PC20 methacholine result of less than 4 mg/mL and a sputum total cell count of >25 million cells/g and a neutrophil differential of >80% at the time of sputum collection. These subjects were studied because they had purulent or mucopurulent sputum and had presented to the clinic with symptoms suggestive of an infective exacerbation of asthma.
2.3. Induced sputum preparation
Induced sputum was collected using hypertonic saline via an ultrasonic nebulizer for 12 min, as described previously (Hallstrand et al. 2005; Nair et al. 2009). The sputum samples were diluted with equal volumes of 0.1% (m:v) dithiothreitol and placed in a shaking water bath at 37 °C for 15 min. Total cell count was determined with a hemocytometer, and slides for differential cell counts were prepared with a cytocentrifuge. Slides were stained, and at least 400 non-squamous cells per slide were evaluated. The diluted, induced sputum supernatant sample was centrifuged at 250 g for 10 min, and the supernatant was treated with protease inhibitors. These processed sputum samples were frozen at −80 °C. At the time of ELISA microarray analysis, thawed sputum samples were centrifuged to remove particulates, and the supernatants were diluted 5-fold in 0.1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS).
2.4. Preparation of the brominated antigen, related modified proteins, and the monoclonal antibody
For the preparation of the antigen, a modified protocol of the carbodiimide method (Davis and Preston 1981) was used. Briefly, 0.12 mM 3-Br-HBA was dissolved in 2.5 ml methanol and combined with 0.75 mM EDC in 2.5 ml of 20 mM potassium phosphate buffer (pH 5.0) at room temperature for 2 min. This solution was combined with 8 ml 2.5 mg/ml KLH in 200 mM potassium phosphate buffer (pH 8.0). The bromination level was quantified by absorbance at 310 nm (Hawkins and Davies 2005).
All phases of antibody production, including immunization of mice, preparation of mouse serum, hybridoma cell lines and ascites, and antibody isotyping, were undertaken at the Washington State University Monoclonal Antibody Center (Pullman). All animal treatment protocols were approved by the Washington State University Institutional Animal Care and Use Committee (IACUC). The BTK-94C antibody was biotinylated using the EZ-Link Sulfo- NHS-LC-Biotinylation Kit (Pierce, Rockford, IL), according to the manufacturer’s protocol.
Brominated BSA was prepared using sodium hypobromite (Fisher Scientific, Pittsburg, PA), as previously reported (Hawkins and Davies 2005), using conditions that maximize the ratio of 3-bromotyrosine to 3,5-dibromotyrosine. That is, 1 ml of 10 mg BSA/ml was reacted with 200 µl of freshly prepared 20 mM sodium hypobromite (in pH 7.2 PBS) at 25 °C for 15 h. Chlorinated BSA and nitrated BSA were generated with 6% sodium hypochlorite (The Clorox Company, Oakland, CA) and peroxynitrite (Millipore Corporation, Boston, MA), as described (Hawkins and Davies 2005; Kambayashi et al. 2009).
Antibody inhibition tests were under taken with tyrosine and modified tyrosine analogs at concentrations of 1.4 µM to 1 mM. These inhibitors were incubated overnight at room temperature with 1 µg/ml of biotinylated BTK-94C antibody in PBS (plus up to 0.1% methanol). The antibody activity was then tested using protein microarray chips, as described below.
2.5. ELISA microarray analysis
For the analysis of total antigen concentrations, custom antibody microarray chips were manufactured and the sandwich ELISA microarray analysis was performed essentially as previously described (Jin and Zangar 2012). For the halotyrosine analyses, the detection antibody was biotinylated BTK-94C. In brief, the capture antibodies were printed onto the slide surface and used to concentrate a specific protein from the diluted sputum samples. Signal was then generated by incubation with the biotinylated detection antibody, biotinyltyramide signal amplification, incubation with fluorophore-tagged streptavidin, and laser microarray scanner analysis. Each sample was analyzed on 3 separate chips, and each chip contained 4 replicate spots for each capture antibody. An internal calibrant assay was included in each analysis, as described previously (Daly et al. 2010; Zangar et al. 2009).
2.6. Statistics
We estimated the inhibitor concentration that caused a 50% suppression (IC50) of the BTK94C antibody signal by fitting the data to a 3-parameter sigmoidal concentration-response curve that is commonly used in binding competition assays (DeLean et al. 1978). For the first set of samples, which were used to compare halotyrosine levels in healthy controls and asthmatics with either low or high sputum eosinophil counts, statistically significant differences between groups were identified using one-way analysis of variance and Tukey’s Honest Significant Difference method (Yandell 1997). For the other studies, which only had two study groups, group comparisons were made using a Wilcoxon ranked sum test (Hollander and Wolfe 1999). A probability (p) value of ≤0.05 was used to delineate statistical significance for all analyses. The statistical analyses were conducted using the R statistical package (R Development Team 2010).
3. Results
3.1. Antigen and monoclonal antibody preparation and testing
To prepare a 3-bromotyrosine-like antigen, we modified KLH by amidation with 3- bromo-4-hydroxy-benzoic-acid (supplemental Figure 1A). We then evaluated the ability of this artificial hapten antigen to produce antibodies that recognize a physiologically relevant brominated protein, as produced by reacting BSA with hypobromite (supplemental Figure 1B). This analysis confirmed that serum antibodies from the immunized mice bound hypobromitetreated BSA, but not unmodified BSA (data not shown).
Splenocytes of the immunized mice were used to generate hybridoma cell lines, of which 225 different hybridomas produced an antibody that reacted with the hapten-modified KLH antigen. Supernatants from these cultured cells were then individually tested for reactivity and specificity using a custom protein microarray chip that contained individual spots of modified and unmodified BSA. These tests demonstrated that an antibody, designated BTK-94C, strongly bound brominated BSA, weakly bound chlorinated BSA, but did not bind either unmodified or nitrated BSA (supplemental Figure 2). Isotyping of the BTK-94C indicated that it is an IgM antibody that is secreted as a monomeric protein in the ascites fluid (data not shown). To further characterize the specificity of this antibody, we evaluated the inhibitory characteristics of reagents that mimic tyrosine modifications. The binding of BTK- 94C to brominated BSA was strongly inhibited by both 3-bromo-4-hydroxybenzoic acid and 3,5-dibromo,4-hydroxybenzoic acid, and about a 100-fold weaker inhibition by 3-chlorotyrosine and 3,5-dichloro,4-hydroxybenzoic acid (Table 2). Binding to brominated BSA was not inhibited by intact tyrosine, 3-nitrotyrosine, or 3,4-dihydroxybenzoic acid. Thus, BTK-94C strongly recognizes mono- and di-bromotyrosine residues and has a much weaker affinity for chlorotyrosine residues.
Table 2.
Inhibition of BTK-94C binding to brominated albumin by modified tyrosine analogs.
| Chemical1 | Structure | Inhibition (IC50, µM) of BTK- 94C binding to brominated BSA2 |
|---|---|---|
| Tyr | 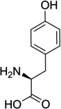 |
N.D.3 |
| 3-NO2-Tyr | 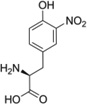 |
N.D. |
| 3-OH-HBA | 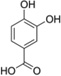 |
N.D. |
| 3-Br-HBA | 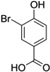 |
2 (0.3–6.3) |
| 3,5-Di-Br-HBA | 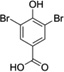 |
0.7 (0.2–1.5) |
| 3-Cl-Tyr | 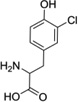 |
107.8 (70.6–145.3) |
| 3,5-Di-Cl-HBA | 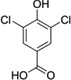 |
113.6 (53.4–550.8) |
Abbreviations: Tyr: L-Tyrosine; 3-NO2-Tyr, 3-nitrotyrosine; 3-OH-HBA, 3,4-dihydroxybenic acid; 3-Br-HBA, 3-bromo-4-hydroxybenzoic acid; 3,5-DiBr-HBA, 3,5-dibromo-4-hydroxybenzalehyde; 3-Cl-Tyr, 3-chlorotyrosine; and DiCl-HBA, 3,5-dichloro-4-hydroxybenoic acid.
The IC50 value is the median of triplicate analyses, each of which was fit to 3-parameter logistic binding curves. The 90% confidential intervals are shown are shown in parentheses.
N.D., inhibition was not detectable at any concentration examined (maximal concentration 1 mM).
3.2. Halogenated tyrosine residues are increased in induced sputum proteins from asthmatics
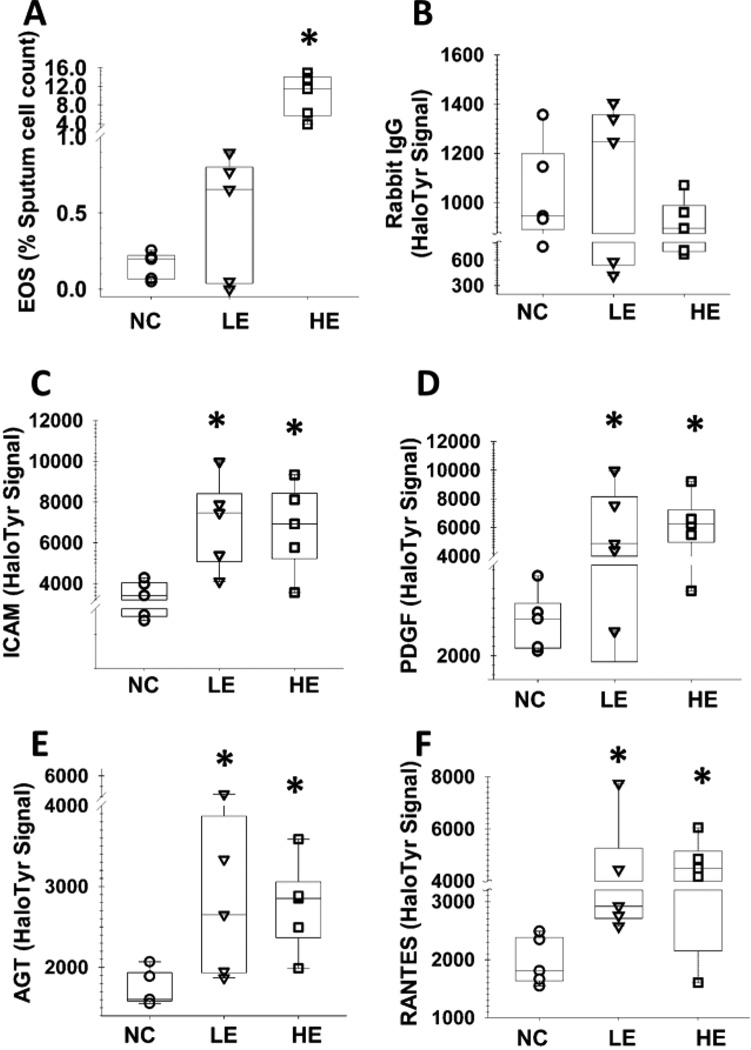
To determine if the halotyrosine antibody had the potential to characterize asthma, we assessed protein halogenation in sputum samples that were archived from three separate studies. We first analyzed induced sputum samples from healthy controls and asthma subjects that were selected based on the percentage of sputum eosinophils, with the “high” group having >2.5% eosinophils and the “low” group having <2.5% eosinophils (Supplemental Table S2). Although these asthma subjects had different levels of sputum eosinophils, they had similar baseline lung function determined by spirometry (Supplemental Table S2). Thus, these individuals are well-suited for characterizing differences in lung function that are not closely associated with sputum eosinophil counts. We analyzed 23 proteins that are potentially related to asthma (Supplemental Table S1) and, as a negative control, nonimmune rabbit IgG. The signal from this negative-control spot was very low compared to the other assay spots and did not vary between groups (Figure 1). Thus, the halotyrosine signal in the other assays was most likely associated with modified antigen (rather than non-specific protein binding to the immunoglobin) and any differential signal between the groups was likely due to differential halogenation of the targeted, captured protein. Relative to healthy controls, asthmatics had significantly greater levels of halogenation signal in four proteins: angiotensinogen (AGT), intracellular adhesion molecular 1 (ICAM), platelet-derived growth factor A (PDGF) and regulated upon activation, normal T cell expressed and secreted (RANTES) (Figure 1). Quantitative sandwich ELISAs were used to measure the total (most likely halogenated and unmodified) concentrations of these four proteins in the sputum samples using the same capture antibodies and sample incubation conditions as the halotyrosine analysis described above. Thus, the antigen binding should be the same in the prior experiment on protein halogenation and the current experiment that was designed to assess total antigen binding levels. Only sputum total AGT binding was significantly increased in asthmatics compared to the healthy controls (data not shown), suggesting that the AGT halotyrosine levels potentially could have been confounded by differences in the total AGT concentration. The AGT results were not evaluated in subsequent experiments as the relationship between AGT halogenation and asthma is vague.
Figure 1.
Individual halogenated proteins are increased in sputum from asthmatics. Sputum eosinophil (EOS) counts (A). Fluorescent signal from nonimmune rabbit immunoglobin G (IgG, panel B), which was used as a negative control; intracellular adhesion molecule (ICAM; C), platelet-derived growth factor (PDGF; D), angiotensinogen (AGT, E), and RANTES (F). The lateral lines represent the median values, and boxes are the 25th and 75th quantile’s. Data from ELISA microarray analysis of 15 clinical samples (5 per group). NC, normal controls; LE, low sputum eosinophils; HE, high sputum eosinophils. * Significantly different (p≤0.05) from NC based on ANOVA and Tukey’s test.
Although differences in halogenation were observed between asthma patients and controls, halogenation levels were similar in the high and low eosinophil groups of asthma patients. These results suggested that the halogenated protein levels may reflect changes in leukocyte activity that are not identified by eosinophil cell counts. We therefore evaluated if protein halogenation was related to lung function, as measured by bronchodilator response and airway hyperresponsiveness, as assessed by exercise-induced bronchoconstriction (EIB). Both eosinophil counts and halogenated protein levels were correlated with the bronchodilator response, but only the halogenated protein levels were associated with EIB (Table 1 and Fig. S3). For example, for the area under the FEV1 curve over the first 30 min after exercise (AUC30), eosinophil counts had an R value of 0.009 (p=0.84) but the halogenation assays had significant R values that ranged from 0.61 to 0.65 (Table 1). Similarly, for correlative analyses of the maximum fall in FEV1 after exercise (Max FEV1%), eosinophil counts had an R value of 0.09 (p=0.54), while the halogenation assays had significant R values from 0.66 (to 0.69 (p=0.006) (Table 1). Thus, halotyrosine levels in certain proteins in sputum were clearly correlated with EIB and this association was greater than for eosinophil counts.
Table 1. Correlations between sputum assays and lung function tests.
N=15 for all comparisons. Graphs of the data are shown in supplemental Figure S1.
| Sputum Assay |
Bronchodilator Response |
Exercise-Induced Bronchoconstriction | ||||
|---|---|---|---|---|---|---|
| Max FEV1 % | AUC30 | |||||
| R | p value | R | p value | R | p value | |
| EOS | 0.61 | 0.02 | 0.09 | 0.54 | 0.009 | 0.84 |
| ICAM | 0.67 | 0.006 | 0.69 | 0.005 | 0.62 | 0.01 |
| PDGF | 0.57 | 0.02 | 0.69 | 0.005 | 0.65 | 0.008 |
| RANTES | 0.64 | 0.01 | 0.66 | 0.007 | 0.61 | 0.02 |
Abbreviations: Bronchodilator Response, change in FEV1 after administration of a short-acting β2-agonist; AUC30, area under the FEV1 curve over the first 30 min after exercise (% change*min); Max FEV1 %, maximal fall in FEV1 after exercise challenge relative to baseline; EOS, sputum eosinophil counts; ICAM, halogenated intracellular adhesion molecule 1; halogenated PDGF, platelet-derived growth factor.
3.3. Halotyrosine levels are decreased when eosinophil levels are systemically suppressed
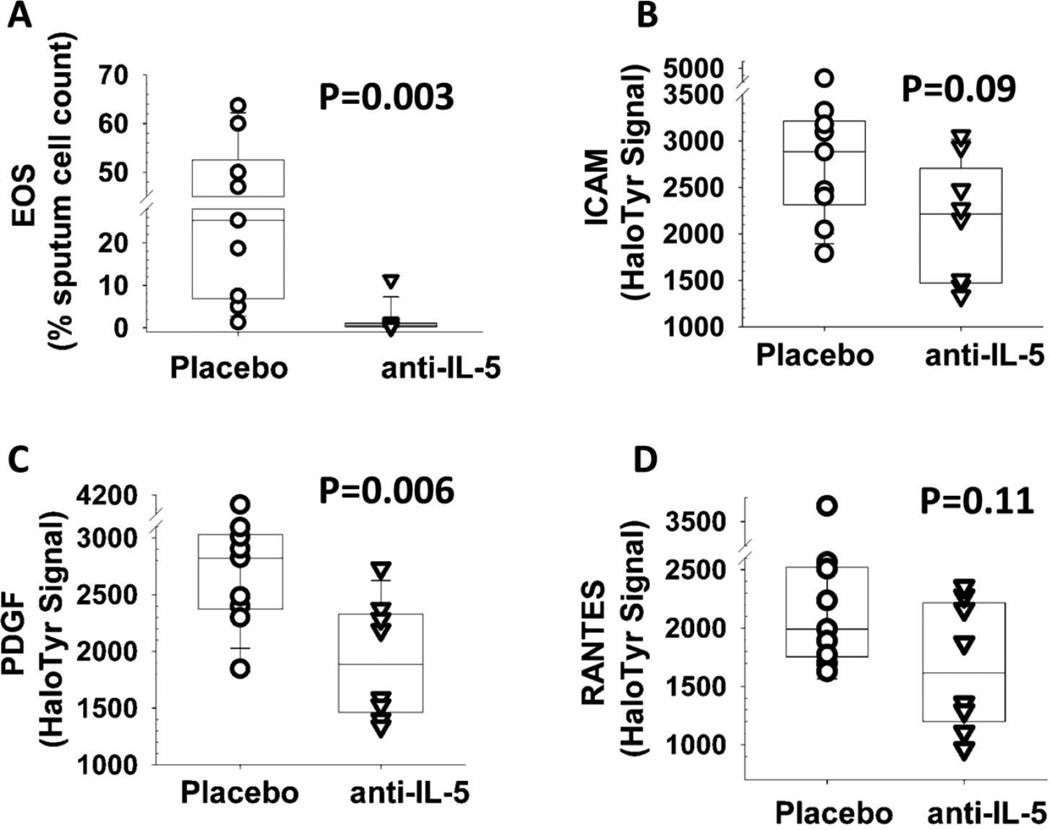
To better define the association between sputum eosinophil counts and halotyrosine levels, we measured protein halogenation in a second group of patients with asthma and sputum eosinophilia before and after treatment with mepolizumab, an interluekin-5 antibody that suppresses eosinophil production and thereby protects against eosinophilic asthma (Nair et al. 2009). The 17 patients who donated these sputum samples were randomized prior to treatment and were approximately equally divided between the mepolizumab and placebo groups. Mepolizumab administration significantly decreased halogenated PDGF (Figure 2). The halotyrosine levels in ICAM and RANTES also showed a trend towards a decrease, (p values of 0.09 and 0.11, respectively). Overall, our results suggest that the halotyrosine modifications of specific airway proteins can be used as markers of eosinophil activity, but also suggest some discordance between the sputum eosinophil counts and the levels of halotyrosine modified proteins.
Figure 2.
Changes in eosinophils (EOS, panel A), and halotyrosine levels of intracellular adhesion molecule (ICAM, B), platelet-derived growth factor (PDGF, C), and RANTES (D) in sputum samples from asthmatics after treatment with placebo (n=9) or mepolizumab (anti-IL-5, n=8). Numbers above the columns indicate the p value, as determined by a Wilcoxon’s test. For each box plot, the embedded lateral line represents the median value, and the lower and upper limits of the boxes are the 25th and 75th quantiles, respectively.
3.4. Neutrophilic asthma is associated with a broad increase in protein halogenation
The neutrophilic phenotype of asthma is defined by high counts of neutrophils, but not eosinophils, in the sputum. Chlorotyrosine but not bromotyrosine is a marker for neutrophil activity (Saude et al. 2004). Given the detectable but relatively weak affinity of BTK-94C for chlorotyrosine residues, it was unclear if this antibody could be used to assess neutrophilic asthma. We therefore evaluated halotyrosine protein levels in sputum samples from 10 neutrophilic asthma patients and 10 healthy controls. In contrast to the prior analysis, which identified four differentially modified proteins, in neutrophilic asthma, halogenation tended to increase in all 24 sputum proteins, with the signal statistically increased (p≤0.05) in 10 of the 24 proteins analyzed (supplemental Fig. S4).
4. Discussion
We developed a novel antibody that recognizes monobromotyrosine. Previous studies have used HOBr to modify proteins in an effort to develop an antigen with the same chemical adduct as produced by in vivo bromination, but the resulting antigens only produced antibodies for dibromotyrosine (Kambayashi et al. 2009; Kato et al. 2005; Wormall 1930). This unexpected result may be because in vitro bromination with HOBr predominately produces dibromotyrosine (Kato et al. 2005) suggesting that the antigen used in these previous studies was enriched with dibromotyrosine. We therefore used a hapten antigen of defined chemistry that mimics a monobromotyrosine residue. We found that a resulting monoclonal antibody, BTK-94C, binds protein modified with HOBr and that binding was inhibited by small molecules containing either mono- or di-bromo phenolic rings, such as found in brominated tyrosine residues. These studies also suggested that this antibody bound mono- and di-chlorotyrosine residues, although the affinity for bromotyrosine residues appeared to be 100-fold greater than for chlorotyrosine residues. Since other halotyrosine residues are not normally found in mammalian proteins, we consider BTK-94C to recognize these two physiological halotyrosine residues but has a preference for brominated tyrosine residues. Next, to determine if BTK-94C had potential for characterizing airway inflammation in asthma patients, we undertook a series of preliminary studies on human sputum samples. The results of these studies are discussed below in the broader context of asthma pathology.
Eosinophils and neutrophils release granule proteins when activated, and these proteins are strongly implicated in the pathogenesis of asthma. The production of hypobromite by eosinophil peroxidase and the production of hypochlorite by neutrophil myeloperoxidase leads to bromotyrosine or chlorotyrosine protein modifications, respectively. Eosinophils in induced sputum have been used to monitor asthma severity and response to therapy (Lonnkvist et al. 2001; Metso et al. 2001) but there is a discordance between eosinophil counts in either induced sputum or bronchoalveolar lavage (BAL) fluid and the number of eosinophils in airway tissue obtained by endobronchial biopsy (Grootendorst et al. 1997; Maestrelli et al. 1995; Silkoff et al. 2003). In addition, a longitudinal analysis of sputum eosinophils in 995 asthmatics found that sputum eosinophilia was commonly variable within the same patient over time (McGrath et al. 2012). In combination, these studies suggest that a single count of airway eosinophils may not fully reflect eosinophil activity in asthma. Although eosinophils in sputum must originally come from the blood stream, sputum eosinophil counts may also be a function of removal. That is, airway macrophages phagocytize eosinophils, and levels of phagocytosis are similar in asthma subjects with high and low sputum eosinophil counts (Kulkarni et al. 2010). Thus, although useful, sputum eosinophil counts may not fully characterize their activity in asthma airways.
The likelihood of a relationship between protein halogenation and airway inflammation is further supported by the correlation between EIB and protein halogenation. EIB is a measure of indirect airway hyperresponsiveness and is normally only found in asthma (Hallstrand et al. 2005). Prior studies demonstrated that EIB is associated with markers of airway inflammation including elevated levels of exhaled nitric oxide and production of cysteinyl leukotrienes (Brannan and Turton 2010; Hallstrand et al. 2005). Eosinophils play a major role in the pathogenesis of EIB along with other airway cells such as mast cells and basophils (Hallstrand et al. 2005; Mickleborough et al. 2005). In our study, EIB measurements correlated with halogenation levels of specific proteins, supporting a relationship between these markers and eosinophil activity. Eosinophils counts have been associated with the presence of EIB in prior studies (Duong et al. 2008; Yoshikawa et al. 1998) but were not found to be so in our study. The failure to observe this relationship in the current study may reflect the relatively small sample size. Even so, our results suggest there may be a stronger relationship between EIB and protein halogenation than between EIB and sputum eosinophil counts.
EPO is a cationic protein and should not diffuse far in the anionic interstitial environment. Likewise, HOBr is very reactive and bromotyrosine formation is expected to be concentrated around EPO. Overall, protein bromination is likely to occur in the immediate vicinity of activated eosinophils, although the brominated proteins may diffuse after modification. In our study, in the absence of airway neutrophilia, halogenation was clearly increased in 3 of the 24 proteins we examined. Experimentally, our goal is to saturate the capture antibodies we use, such that the measured levels of protein modifications are inherently proportional to a consistent amount of antigen. Therefore, the increase halogenation of these three proteins should be due to a change in the proportion of modified antigen and not influenced by total antigen levels. We previously found that this proportional analysis of antigen modification minimized normal human variation for nitrotyrosine levels of circulating proteins and thereby allowed us to clearly identify effects on protein nitration that were associated with tobacco smoke exposure and chronic obstructive lung disease (Jin et al. 2011). The comparable binding levels of the 3 unmodified antigens to the capture antibodies simply means that the antigens consistently saturated the capture antibodies and does not imply that the unmodified antigens are at comparable levels between individuals or groups. The three differentially halogenated proteins are known to be expressed by lung cells and we hypothesize that that the lung cells that express these proteins may be in close proximity with activated eosinophils. That is, these proteins are RANTES, which attracts eosinophils; ICAM, which binds eosinophils, and PDGF, which facilitates eosinophils degranulation. Thus, these three proteins attract, bind and activate eosinophils, functions which could facilitate an increase in halogenation.
A fourth protein, AGT, was differentially halogenated in the sputum of asthmatics relative to healthy controls. Because AGT binding to its capture antibody was also increased in the asthma subjects (i.e., the capture antibody was not fully saturated), we cannot determine if the increase in AGT halogenation was due to a relative increase in halogenation levels or was dependent on the varying amounts of captured AGT. Even so, this finding may be of significance since AGT is expressed by pulmonary cells and AGT expression has been linked to lung fibrosis, a common pathological feature of asthma that is associated with eosinophil infiltration (Aceves and Broide 2008; Uhal et al. 2012). In addition, promoter-region polymorphisms in the AGT gene are associated with altered risk of idiopathic pulmonary fibrosis (Dang et al. 2013). Since AGT expression is increased by oxidative stress (Mastruzzo et al. 2002), it may be that association between AGT protein levels we observed in asthma patients is related to oxidants produced by activated immune cells. Overall, further studies of the role of AGT in asthma may be warranted.
A role for eosinophils in protein halogenation was supported by the analysis of sputum samples from severe asthmatics that were treated with mepolizumab or placebo. In these subjects, mepolizumab systemically suppressed eosinophil counts and reduced halogenation of our marker proteins. The severe asthma subjects in this study had no eosinophil-related exacerbations after the start of mepolizumab treatment, although 90% of the randomized, placebo-control subjects experienced exacerbations over the same time period (Nair et al. 2009). Overall, the change in halogenation levels in response to mepolizumab treatment suggests that eosinophils contribute to the halotyrosine levels in specific proteins but that the suppression of protein halogenation is not as complete as observed for the sputum eosinophil counts. This partial suppression of halogenation by mepolizumab is consistent with a prior study that found that mepolizumab strongly suppresses eosinophil counts in the airways but these cells and extracellular levels of eosinophil major basic protein were not completely suppressed in the bronchial mucosa (Flood-Page et al. 2003). Thus, our results are consistent with a residual level of granulocyte activity in lung tissue after mepolizumab treatment.
In addition to eosinophils, neutrophils also can be important in asthma (Douwes et al. 2002). Neutrophils produce myeloperoxidase, an enzyme that generates hypochlorous acid and the related formation of chlorotyrosine. Purified human eosinophils and neutrophils selectively produce bromotyrosine and chlorotyrosine, respectively (Saude et al. 2004). Knock-out mice lacking either EPO or myeloperoxidase no longer produce bromotyrosine or chlorotyrosine protein modifications, respectively, in response to aerosolized allergen or infection (Brennan et al. 2002). Even so, tests with purified myeloperoxidase suggest that this enzyme can produce bromotyrosine in vitro under certain conditions (Senthilmohan and Kettle 2006). Although the BTK-94C antibody apparently has about a 100-fold greater sensitivity for bromotyrosine than chlorotyrosine (Table 2), it may be that this antibody still detected chlorotyrosine protein modifications in subjects with neutrophilic inflammation.
Compared to sputum, which can be problematic to collect in the clinic, blood is a more promising potential source of halogenated biomarkers. We used our ELISA microarray platform to analyze paired sputum and serum samples from 55 individuals who did not have asthma. Of the 24 proteins analyzed, 18 were significantly correlated (p<0.05) across individuals between the two sample types (data not shown). Interestingly, the three proteins with the best correlations between sputum and blood were AGT, PDGF and ICAM (Spearman’s R>0.5, p<10−4 for each analysis), suggesting that future studies on individual protein halogenation in blood may identify useful circulating markers of asthma.
There is a clear need for better methods to evaluate airway inflammation in asthma. The novel antibody developed here allows for the analysis of halotyrosine levels in single proteins and our results suggests that these analyses may offer unique insights into airway inflammation in asthmatics.
Supplementary Material
Acknowledgement
We thank Dr. William Davis, Washington State University, Department of Veterinary Microbiology and Pathology and director of the Monoclonal Antibody Center, for his guidance and advice in producing the monoclonal antibody.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aceves SS, Broide DH. Airway fibrosis and angiogenesis due to eosinophil trafficking in chronic asthma. Curr. Mol. Med. 2008;8:350–358. doi: 10.2174/156652408785161023. [DOI] [PubMed] [Google Scholar]
- Aldridge RE, Chan T, van Dalen CJ, Senthilmohan R, Winn M, Venge P, Town GI, Kettle AJ. Eosinophil peroxidase produces hypobromous acid in the airways of stable asthmatics. Free Radic. Biol. Med. 2002;33:847–856. doi: 10.1016/s0891-5849(02)00976-0. [DOI] [PubMed] [Google Scholar]
- Brannan JD, Turton JA. The inflammatory basis of exercise-induced bronchoconstriction. Phys. Sportsmed. 2010;38:67–73. doi: 10.3810/psm.2010.12.1827. [DOI] [PubMed] [Google Scholar]
- Brennan ML, Wu W, Fu X, Shen Z, Song W, Frost H, Vadseth C, Narine L, Lenkiewicz E, Borchers MT, Lusis AJ, Lee JJ, Lee NA, Abu-Soud HM, Ischiropoulos H, Hazen SL. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J. Biol. Chem. 2002;277:17415–17427. doi: 10.1074/jbc.M112400200. [DOI] [PubMed] [Google Scholar]
- Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, Cockcroft DW, Fish JE, Sterk PJ. Guidelines for methacholine and exercise challenge testing-1999. Am. J. Respir. Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- Daly DS, Anderson KK, Seurynck-Servoss SL, Gonzalez RM, White AM, Zangar RC. An internal calibration method for protein-array studies. Stat. Appl. Genet. Mol. Biol. 2010;9 doi: 10.2202/1544-6115.1506. Article. [DOI] [PubMed] [Google Scholar]
- Dang MT, Gu C, Klavanian JI, Jernigan KA, Friderici KH, Cui Y, Molina-Molina M, Ancochea J, Xaubet A, Uhal BD. Angiotensinogen Promoter Polymorphisms Predict Low Diffusing Capacity in US Spanish IPF Cohorts. Lung. 2013;191:353–360. doi: 10.1007/s00408-013-9476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MT, Preston JF. A simple modified carbodiimide method for conjugation of small-molecular-weight compounds to immunoglobulin G with minimal protein crosslinking. Anal. Biochem. 1981;116:402–407. doi: 10.1016/0003-2697(81)90380-8. [DOI] [PubMed] [Google Scholar]
- DeLean A, Munson PJ, Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am. J Physiol. 1978;235:E97–E102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. 2002;57:643–648. doi: 10.1136/thorax.57.7.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong M, Subbarao P, Adelroth E, Obminski G, Strinich T, Inman M, Pedersen S, O'Byrne PM. Sputum eosinophils and the response of exercise-induced bronchoconstriction to corticosteroid in asthma. Chest. 2008;133:404–411. doi: 10.1378/chest.07-2048. [DOI] [PubMed] [Google Scholar]
- Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am. J Respir. Crit Care Med. 2003;167:199–204. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]
- Grootendorst DC, Sont JK, Willems LN, Kluin-Nelemans JC, van Krieken JH, Veselic-Charvat M, Sterk PJ. Comparison of inflammatory cell counts in asthma: induced sputum vs bronchoalveolar lavage and bronchial biopsies. Clin. Exp. Allergy. 1997;27:769–779. [PubMed] [Google Scholar]
- Hallstrand TS, Moody MW, Wurfel MM, Schwartz LB, Henderson WR, Jr, Aitken ML. Inflammatory basis of exercise-induced bronchoconstriction. Am. J Respir. Crit Care Med. 2005;172:679–686. doi: 10.1164/rccm.200412-1667OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins CL, Davies MJ. The role of reactive N-bromo species and radical intermediates in hypobromous acid-induced protein oxidation. Free Radic. Biol Med. 2005;39:900–912. doi: 10.1016/j.freeradbiomed.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Hollander M, Wolfe DA. Nonparametric statistical methods. New York: John Wiley and Sons; 1999. [Google Scholar]
- Jin H, Webb-Robertson BJ, Petersen H, Tan RM, Bigelow D, Scholand MB, Hoidal JR, Pounds JG, Zangar RC. Smoking, COPD and nitrotyrosine levels of plasma proteins. Environ. Health Perspect. 2011;119:1314–1320. doi: 10.1289/ehp.1103745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Zangar RC. High-throughput, multiplexed analysis of 3-nitrotyrosine in individual proteins. Curr. Protoc. Toxicol. 2012;Chapter 17 doi: 10.1002/0471140856.tx1715s51. [DOI] [PubMed] [Google Scholar]
- Kambayashi Y, Ogino K, Takemoto K, Imagama T, Takigawa T, Kimura S, Hibino Y, Hitomi Y, Nakamura H. Preparation and Characterization of a Polyclonal Antibody against Brominated Protein. J. Clin. Biochem. Nutr. 2009;44:95–103. doi: 10.3164/jcbn.08-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Kawai Y, Morinaga H, Kondo H, Dozaki N, Kitamoto N, Osawa T. Immunogenicity of a brominated protein and successive establishment of a monoclonal antibody to dihalogenated tyrosine. Free Radic. Biol. Med. 2005;38:24–31. doi: 10.1016/j.freeradbiomed.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Kulkarni NS, Hollins F, Sutcliffe A, Saunders R, Shah S, Siddiqui S, Gupta S, Haldar P, Green R, Pavord I, Wardlaw A, Brightling CE. Eosinophil protein in airway macrophages: a novel biomarker of eosinophilic inflammation in patients with asthma. J. Allergy Clin. Immunol. 2010;126:61–69. doi: 10.1016/j.jaci.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonnkvist K, Hellman C, Lundahl J, Hallden G, Hedlin G. Eosinophil markers in blood, serum, and urine for monitoring the clinical course in childhood asthma: impact of budesonide treatment and withdrawal. J. Allergy Clin. Immunol. 2001;107:812–817. doi: 10.1067/mai.2001.114246. [DOI] [PubMed] [Google Scholar]
- Maestrelli P, Saetta M, Di SA, Calcagni PG, Turato G, Ruggieri MP, Roggeri A, Mapp CE, Fabbri LM. Comparison of leukocyte counts in sputum, bronchial biopsies, and bronchoalveolar lavage. Am. J Respir. Crit Care Med. 1995;152:1926–1931. doi: 10.1164/ajrccm.152.6.8520757. [DOI] [PubMed] [Google Scholar]
- Mastruzzo C, Crimi N, Vancheri C. Role of oxidative stress in pulmonary fibrosis. Monaldi Arch. Chest Dis. 2002;57:173–176. [PubMed] [Google Scholar]
- McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Sutherland ER, Chinchilli VM, Fahy JV. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am. J. Respir. Crit Care Med. 2012;185:612–619. doi: 10.1164/rccm.201109-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metso T, Rytila P, Peterson C, Haahtela T. Granulocyte markers in induced sputum in patients with respiratory disorders and healthy persons obtained by two sputum-processing methods. Respir. Med. 2001;95:48–55. doi: 10.1053/rmed.2000.0970. [DOI] [PubMed] [Google Scholar]
- Mickleborough TD, Lindley MR, Ray S. Dietary salt, airway inflammation, and diffusion capacity in exercise-induced asthma. Med. Sci. Sports Exerc. 2005;37:904–914. [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, Macintyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur. Respir. J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O'Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N. Engl. J Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- Nakagome K, Matsushita S, Nagata M. Neutrophilic inflammation in severe asthma. Int. Arch. Allergy Immunol. 2012;158(Suppl 1):96–102. doi: 10.1159/000337801. [DOI] [PubMed] [Google Scholar]
- Pleis JR, Ward BW, Lucas JW. Summary health statistics for U.S.adults: National Health Interview Survey, 2009. Vital Health Stat. 2010;10:1–207. [PubMed] [Google Scholar]
- R Foundation for Statistical Computing, editor. R Development Team. R. A language and environment for statistical computing. 2010. [Google Scholar]
- Rosi E, Stendardi L, Binazzi B, Scano G. Perception of airway obstruction and airway inflammation in asthma: a review. Lung. 2006;184:251–258. doi: 10.1007/s00408-005-2590-z. [DOI] [PubMed] [Google Scholar]
- Saude EJ, Lacy P, Musat-Marcu S, Mayes DC, Bagu J, Man SF, Moqbel R. NMR analysis of neutrophil activation in sputum sample from patients with cystic fibrosis. Magn. Reson. Med. 2004;52:807–814. doi: 10.1002/mrm.20242. [DOI] [PubMed] [Google Scholar]
- Senthilmohan R, Kettle AJ. Bromination and chlorination reactions of myeloperoxidase at physiological concentrations of bromide and chloride. Arch. Biochem. Biophys. 2006;445:235–244. doi: 10.1016/j.abb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Silkoff PE, Trudeau JB, Gibbs R, Wenzel S. The relationship of induced-sputum inflammatory cells to BAL and biopsy. Chest. 2003;123:371S–372S. [PubMed] [Google Scholar]
- Uhal BD, Dang MT, Li X, Abdul-Hafez A. Angiotensinogen gene transcription in pulmonary fibrosis. Int. J Pept. 2012;2012:875910. doi: 10.1155/2012/875910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dalen CJ, Aldridge RE, Chan T, Senthilmohan R, Hancox RJ, Cowan JO, Taylor DR, Town GI, Kettle AJ. Bromotyrosines in sputum proteins and treatment effects of terbutaline and budesonide in asthma. Ann. Allergy Asthma Immunol. 2009;103:348–353. doi: 10.1016/S1081-1206(10)60536-4. [DOI] [PubMed] [Google Scholar]
- Wolthers OD. Eosinophil granule proteins in the assessment of airway inflammation in pediatric bronchial asthma. Pediatr. Allergy Immunol. 2003;14:248–254. doi: 10.1034/j.1399-3038.2003.00030.x. [DOI] [PubMed] [Google Scholar]
- Wormall A. The immunological specificity of chemically altered proteins: halogenation and nitrated proteins. J Exp Med. 1930;51:295–317. doi: 10.1084/jem.51.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Samoszuk MK, Comhair SA, Thomassen MJ, Farver CF, Dweik RA, Kavuru MS, Erzurum SC, Hazen SL. Eosinophils generate brominating oxidants in allergen-induced asthma. J. Clin. Invest. 2000;105:1455–1463. doi: 10.1172/JCI9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yandell BS. Practical data analysis for designed experiments. Chapman & Hall; 1997. [Google Scholar]
- Yoshikawa T, Shoji S, Fujii T, Kanazawa H, Kudoh S, Hirata K, Yoshikawa J. Severity of exercise-induced bronchoconstriction is related to airway eosinophilic inflammation in patients with asthma. Eur. Respir. J. 1998;12:879–884. doi: 10.1183/09031936.98.12040879. [DOI] [PubMed] [Google Scholar]
- Zangar RC, Daly DS, White AM, Servoss SL, Tan RM, Collett JR. ProMAT calibrator: A tool for reducing experimental bias in antibody microarrays. J. Proteome. Res. 2009;8:3937–3943. doi: 10.1021/pr900247n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.