Abstract
This mini-review summarizes the history of cathinone and its synthesized derivatives from early records to the present day, including the appearance of synthetic cathinones in the drug combination known as bath salts. Bath salts may consist of one compound (MDPV) or combinations of MDPV and one or more other synthetic cathinones, which may also appear alone without MDPV. We briefly review recent in vitro studies of bath salts components alone or in combination, focusing on pharmacological and biophysical studies. Finally we summarize new data from in vivo procedures that characterize the abuse-related neurochemical and behavioral effects of synthetic cathinones in rats.
Chemical Phylogeny
The following narrative traces some of the initial key initial developments and findings leading up to the class of agents now termed “synthetic cathinones”. Cathinone is a naturally-occurring stimulant found in the khat plant. The “synthetic cathinones” are analogs of cathinone. According to Alles et al. (Alles, Fairchild et al. 1961) the first written record of khat use was in the 14th century, and khat use continues to this day. The fresh leaves of the shrub Catha edulis are chewed or occasionally brewed as a tea in the Arabian Peninsula and in certain regions of Eastern Africa for their central stimulant effects. The leaves, or concoctions prepared there from, are known by a variety of names depending on specific geographic location (e.g. khat, k'at, kat, kath, gat, miraa, qat, Abyssinian tea, Arabian tea, Somali tea). Millions of people in these regions use khat regularly.
In the past, khat use was, generally, a fairly localized problem, but in recent years it has become more widespread. For example, in 2006, federal authorities in New York indicted several people that brought more than 25 tons of khat into the United States, and in 2011 a trafficking ring was broken up in northern Virginia that involved 5 tons of khat (DEA 2006; ICE 2012). The League of Nations considered khat use in the 1930s, and the United Nations World Health Organization considered it again in the 1960s and later in the 1970s (Document 1979). Thus khat use and abuse have long been recognized as a problem of international dimensions.
The norepinephrine optical isomer (+)norpseudoephedrine (now termed cathine; see Figure 1 for structure) was first isolated from the khat plant in 1930 (Wolfes 1930). It was independently isolated by others (Wolfes 1930; Alles, Fairchild et al. 1961; Ristic and Thomas 1962), and the former investigators found cathine to be a mild central stimulant. However, shortly afterward it was demonstrated that cathine was not as potent a central stimulant as ‘khat extract’ (Friebel and Brilla 1963). This led to speculation that khat might contain other stimulant component(s). In 1975, a UN working group isolated (−)α-aminopropiophenone from fresh khat leaves and termed the substance cathinone (Document 1975) (see Figure 1). In 1979-1980, the UN made available to groups of investigators synthetic (±)cathinone and its individual optical isomers. It might be noted that, initially, the term cathinone was reserved for the naturally-occuring (−)cathinone (hence, some of the early literature might be confusing); today, cathinone refers to the racemate unless an isomer is specifically identified.
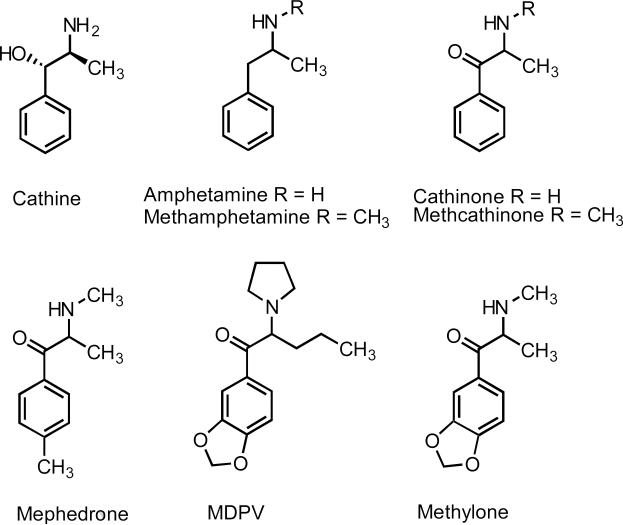
Figure 1.
The structural relationships between cathine, amphetamine, methamphetamine, cathinone, methcathinone, mephedrone, MDPV, and methylone (MDMC).
Several studies showed that (−)cathinone is more potent than (±)cathinone or cathine as a locomotor stimulant in rodents (Knoll 1979; Rosecrans, Campbell et al. 1979; Yanagita 1979; Glennon 1981). As with its structural cousin, amphetamine, (−)cathinone produced hyperthermia in rabbits that could be blocked by the dopamine (DA) antagonist haloperidol (Kalix 1980). Interestingly, van der Schoot et al. had investigated the hyperthermia effects (rabbit) and locomotor actions (mice) of a large number of random amphetamine analogs nearly 20 years earlier (van der Schoot, Ariens et al. 1962) Specific optical isomers were not indicated and it must be assumed that racemates were examined. What we now know as cathinone was coincidentally shown to be a locomotor stimulant in 1962.
Various other studies over the ensuing years concluded that, like amphetamine, cathinone and several related cathinone analogs are DA releasing agents (Kalix 1986; Glennon, Yousif et al. 1987), that both optical isomers of cathinone substitute for training drug in rats trained to discriminate (+)amphetamine from vehicle, (and that stimulus generalization could be blocked by haloperidol (Glennon, Schechter et al. 1984), and that cathinone itself could be used as a training drug in drug discrimination studies using rats as subjects (Glennon, Schechter et al. 1984; Glennon, Young et al. 1984; Schechter and Glennon 1985). In these, and other, investigations S(−)cathinone was found more potent that R(+)cathinone just as S(+)amphetamine is more potent than R(−)amphetamine.
Several investigators referred to cathinone as a “naturally-occurring amphetamine”, but some in the pharmacological community argued that the more potent optical isomer of amphetamine is the (+)-isomer whereas the more potent isomer of cathinone is the (−)-isomer. The counter-argument was that the more potent isomer of both agents possesses an S absolute configuration. Because one of the few structural alterations of the amphetamine molecule that resulted in increased stimulant potency is N-monomethylation (i.e., methamphetamine), the N-monomethyl analog of cathinone was prepared, evaluated, and termed methcathinone (Glennon, Yousif et al. 1987). Methcathinone might be considered the first “synthetic cathinone”. Methcathinone was found at least as potent as methamphetamine as a locomotor stimulant, as a DA releasing agent, and in tests of stimulus generalization using rats trained to discriminate (+)amphetamine from saline vehicle; S(−)methcathinone was found to be more potent than its R(+)enantiomer (Glennon, Young et al. 1995). Rats were subsequently trained to discriminate S(−)methcathinone from vehicle and the stimulus was potently blocked by haloperidol (Dal Cason 1997). All evidence suggested that S(−)methcathinone was potent central stimulant acting through a dopaminergic mechanism.
The substance now known as methcathinone, though not called by this name at the time, was synthesized by Roger Adams and his students in 1928 (Hyde, Browning et al. 1928), later patented by Parke-Davis as an analeptic agent (L'Italien Y.J., Park H. et al. 1957) and examined by van der Schoot et al. (van der Schoot, Ariens et al. 1962) as a locomotor stimulant. According to a USSR Interior Ministry report released in 1989, ephedrone – now realized as being equivalent to methcathinone – “surfaced [in the former Soviet Union] for the first time in 1982” (Savenko, Semkin et al. 1989). However, this information was not widely available until 1991 (Zhingel, Dovensky et al. 1990). Hence, it might be said that methcathinone has been re-discovered several times over.
Another early synthetic cathinone was methylenedioxymethcathinone (MDMC, methylone) (see Figure 1 for structure). The agent was independently prepared by two groups of investigators in the mid-1990s (Jacob and Shulgin 1996; Dal Cason 1997), and found to be about six times less potent than racemic methcathinone in tests of stimulus generalization using (+)amphetamine-trained rats (Dal Cason 1997)
Relatively little interest was shown in cathinone analogs until 2010 when Iversen submitted a report to the UK Home Office entitled “Consideration of the Cathinones” (Iversen 2010a; Iversen 2010b). Indeed, mephedrone use in the UK and Europe preceded MDPV use in the US. At about this time, bath salts were becoming a problem in the US, leading in 2011 to Schedule I status of these compounds (Fig. 1). Since then attention has been paid to drug combinations known as “bath salts”, which may include methylenedioxypyrolovalerone (MDPV), mephedrone, and methylone (MDMC) either alone, in combination with one another, or combined with other chemically related or unrelated drugs (Spiller, Ryan et al. 2011; Shanks, Dahn et al. 2012; Marinetti and Antonides 2013).
Mephedrone is the para-methyl analog of methcathinone or the beta-keto analog of para-methylmethamphetamine (a relatively weak central stimulant with known abuse properties); methylone (MDMC) was described above. MDPV seemingly came from nowhere. The first report on the possible abuse of MDPV appeared in 2007 (Fuwa, Fukumori et al. 2007). However, nearly 40 years earlier Boehringer Ingelheim specifically patented what is now known as MDPV (and several related analogs) as a central stimulants (Köppe, Ludwig et al. 1969).
The prevalence of synthetic cathinones on the clandestine market continues to increase and a recent report indicates that at least 44 synthetic cathinones have now been encountered (UNODC 2013). At this rate, it is nearly impossible to study this ever-growing list of compounds one agent at a time. A different strategy is called for – the identification of structure-activity relationships and pharmacophores for the actions of these agents. This strategy might allow the action, mechanism of action, and potency of synthetic cathinones to be forecast in advance of their appearance.
Physiology and Biophysics
With a chemical understanding of cathinone and methcathinone well in hand, attention turned toward the action of newer synthetic cathinones and drug combinations in vitro and in vivo. Recent work has focused on pharmacological profiles of synthetic cathinones as they interact with monoamine transporters and receptors (Simmler, Buser et al. 2013). Here we review mechanistic studies of synthetic cathinones, particularly those that may be found in bath salts drugs (Spiller, Ryan et al. 2011; Shanks, Dahn et al. 2012; Marinetti and Antonides 2013) as they interact with the dopamine transporter in vitro (Cameron, Kolanos et al. 2013a; Cameron, Kolanos et al. 2013b). The following section focuses on in vivo studies. To set the stage, we summarize earlier studies of AMPH or METH acting on the dopamine transporter (DAT), which are structural antecedents of cathinone and synthetic cathinones (Figure 1). Ingram et al. (2002) noted that in addition to serving as reuptake transporters DATs elicit large, channel-like currents. Using whole-cell patch clamp, they showed that substrates of DAT including DA and AMPH increase the firing rate of dopaminergic neurons maintained in tissue culture. To explain this unexpected result, Ingram et al. suggested that AMPH-induced currents through DAT were responsible for the modulation of neuronal excitability:
“Thus, in addition to clearing extracellular DA, our results suggest that the currents associated with DAT modulate excitability and may regulate release of neurotransmitter from midbrain DA neurons. We propose an alternative hypothesis of DAT-dependent somatodendritic release: substrates activate excitatory DAT-mediated currents, depolarize the cell, and augment Ca2+-dependent vesicular release of DA from midbrain DA neurons.” (Ingram, Prasad et al. 2002)
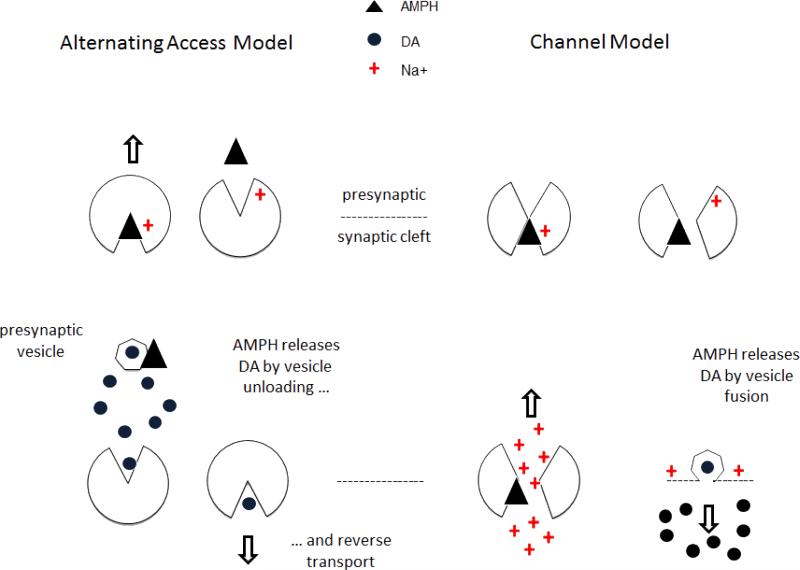
Thus the channel-like currents Ingram et al. observed regulate DA release from dopaminergic neurons. In that case, the question arises: how does AMPH release DA? Does AMPH-induced DA release occur by depolarization-induced Ca++ influx and vesicle fusion, as Ingram et al. suggest? Sulzer and Galli summarize the widely held view that DAT transports AMPH into the presynaptic terminal, where AMPH releases DA from synaptic vesicles. DAT then transports cytoplasmic DA into the cleft by DAT reverse transport; (Sulzer and Galli 2003). The prevailing view that AMPH principally redistributes DA from synaptic vesicles to the cytosol whence DAT exports it has been adequately reviewed; a corollary of this theory postulates that AMPH-depleted vesicles would modulate further DA release (Sulzer, Chen et al. 1995; Sulzer, Sonders et al. 2005). Non-vesicular transmitter release is well supported by other work (Westerink, Tuntler et al. 1987; Westerink, Damsma et al. 1989; Florin, Kuczenski et al. 1995); however, the view of non-vesicular release as an exclusive mechanism for drug-induced transmitter release has been challenged recently (see below).
The alternative view is that AMPH, METH, etc., hijack the normal DA release mechanism in which action potentials (APs) release DA via membrane depolarization: namely, APs open voltage-gated Ca++ channels located near docked vesicles causing them to fuse and release DA. Because AMPH-DAT interactions generate currents that depolarize membranes, AMPH increases the probability of Ca++ entry, vesicle fusion, and DA release. Action potentials per se may be unnecessary; a local depolarization near vesicles could induce Ca++ entry. Indeed, sustained depolarization might actually inhibit AP firing. This alternative mechanism for drug-induced DA release requires a departure from the traditional model of Na-coupled, DA transport (the fixed stoichiometry, alternating access model) and relies on a different model of co-transport (the flux coupled channel model). Figure 2 summarizes these two models. Supporting the later view are the electrical currents mediated by hDAT expressed in Xenopus laevis oocytes and HEK cells exposed to DA, METH, a known hDAT stimulant and DA releaser, mephedrone MEPH, methylenedioxypyrovalerone (MDPV) or cocaine, a known hDAT inhibitor (Cameron, Kolanos et al. 2013a; Cameron, Kolanos et al. 2013b). Pyrovalerone and its analogs (pyrovalerone, naphyrone, MDPV) are a unique chemical grouping, because of their differences from other cathinone analogs, (pyrrolidine ring and 2- carbon extended chain from amine nitrogen. Earlier supporting work was been reviewed (DeFelice and Goswami 2007; Naftalin and De Felice 2011)
The flux coupled channel model
METH, MEPH, and other DAT substrates induce an inward depolarizing current in resting cells (−60 mV), therefore acting as excitatory substrates and contributing to DA release. MDPV on the other hand is a DAT blocker. Substrates and blockers both elevate external neurotransmitter, but by different mechanisms. METH, MEPH, etc. enter the presynaptic terminal; indeed substantial literature exists that proposes cytosolic amphetamines directly involved in DA release (Sulzer, Maidment et al. 1993; Sulzer, Chen et al. 1995; Sulzer, Sonders et al. 2005; Sulzer 2012). An excitatory substrate implies that in addition to proposed chemical effects of METH, MEPH, etc., these drugs have an electrophysiological effect that could itself promote neurotransmitter release (Ingram, Prasad et al. 2002; Carvelli, Blakely et al. 2008; Cameron, Kolanos et al. 2013a; Cameron, Kolanos et al. 2013b). Structurally analogous MDPV, however, induces an outward hyperpolarizing current under similar conditions and therefore acting as an inhibitory, non-substrate blocker. In this regard MDPV is similar to cocaine (Baumann, Partilla et al. 2013; Simmler, Buser et al. 2013; Cameron, Kolanos et al. 2013a; Cameron, Kolanos et al. 2013b). Two potential components of bath salts, MEPH and/or MDPV, thus produce opposite effects at DAT, analogous in some respects to the effects of METH and cocaine, respectively. In the electrophysiological assay, MEPH is nearly as potent as METH; however, MDPV is 35X more potent than cocaine and its effect as a blocker lasts much longer. Furthermore, when applied in combination, MEPH exhibits faster kinetics than MDPV; namely, the MEPH depolarizing current occurs seconds before the slower MDPV hyperpolarizing current in the in vitro analysis. Bath salts are not a defined combination of drugs; however, it is noteworthy that those containing MEPH (or a similar synthetic cathinone) and MDPV might be expected initially to release DA and subsequently to prevent its reuptake. When MEPH and MDPV act simultaneously on the human dopamine transporter expressed in vitro, MEPH acts before MDPV (Cameron, Kolanos et al. 2013b).
The channel mode of dopamine transporters is thus crucial to understand how AMPH-like drugs including synthetic cathinones can affect the integration of inputs to dopaminergic neurons to modify excitability, activate voltage-gated channels, and regulate dopaminergic signaling (Ingram, Prasad et al. 2002; Carvelli, Blakely et al. 2008). The powerful positive feedback mechanism of AMPH-like drugs on dopaminergic neurons may generalize to drugs that interact with serotonin or norepinephrine transporters. Conversely, transporter blockers such as cocaine, MDPV, or fluoxetine may have a negative feedback on endogenous or induced currents mediated via transporters, thus lowering the probability of transmitter release. The contribution of transporters to the electrical profile of neurons depends on a more complete picture of synapses than we have at present. In particular, the setting of transporters in the context of voltage-gated ion channels and receptors would be essential to understand fully the contribution of transporter currents to neurotransmitter release. Evidence is growing, however, that AMPH (and by inference other AMPH-like drugs such as those that may be found in bath salts (Spiller, Ryan et al. 2011; Shanks, Dahn et al. 2012; Marinetti and Antonides 2013) may release DA via vesicle fusion mechanisms (Ramsson, Howard et al. ; Daberkow, Brown et al. 2013) thus challenging what has become the traditional view of reverse DAT transport.
In summary, in vitro electrophysiology suggests that if bath salts contain both DA releasers (such as mephedrone, methylone, etc.) and the reuptake inhibitor (MDPV) as can be the case (Spiller, Ryan et al. 2011; Shanks, Dahn et al. 2012; Marinetti and Antonides 2013), such a combination could act synergistically on DAT (Cameron, Kolanos et al. 2013b). Although we cannot extrapolate these in vitro data to in vitro situations, it is tempting to speculate that a synergistic combination of bath salts components may help explain the powerful effect of bath salts abusers in some cases.
Neuropharmacology
In vivo studies
To complement in vitro characterization of novel cathinone derivatives in electrophysiological assays of transporter function, our program is also using two in vivo procedures to phenotype abuse-related neurochemical and behavioral effects of these compounds in rats. First, in vivo microdialysis is being used to examine drug effects on DA and 5HT release in nucleus accumbens. Previous studies have already described differential effects of “bath salts” compounds on nucleus accumbens DA and 5HT release, and preferential release of DA>5HT appears to correlate with higher abuse potential (Baumann, Partilla et al. 2012; Baumann, Ayestas et al. 2012; Baumann, Partilla et al. 2013). Our own studies with this procedure will not be discussed further here because data are not yet published, but our initial findings with prototype compounds are consistent with published literature and provide a basis for interpreting results from future studies with novel compounds. Second, intracranial self-stimulation is being used as an operant behavioral procedure to examine expression of DA-mediated abuse-related effects and 5HT-mediated abuse-limiting effects of novel compounds. Conceivably, the net effect of a drug regardless of its mechanism to elevate extracellular DA, may reside in clearance rates and duration as parameters driving psycho-stimulation and abuse liability. The remainder of this section will focus on published results with methcathinone derivatives and related compounds on ICSS in rats.
Intracranial self-stimulation (ICSS) is the term for a family of behavioral procedures in which subjects are first equipped with intracranial electrodes that target brain reward areas and then trained to emit an operant response (e.g. pressing a response lever) to receive pulses of direct brain stimulation (Carlezon and Chartoff 2007; Vlachou and Markou 2011). Drug effects on rates of ICSS responding can then be evaluated. Our laboratory uses a so-called “frequency-rate” procedure, in which the frequency of brain stimulation is varied across a range of values (1.75-2.2 log Hz in 0.05 log increments) to generate a range of increasing ICSS response rates during each daily session. Examples of control ICSS frequency-rate curves are show by the “vehicle” data (open circles) in Figures 3 and 4. Thus, under control conditions, low frequencies of brain stimulation maintain low ICSS rates, and higher brain stimulation frequencies maintain higher rates that increase to a plateau at the highest frequencies.
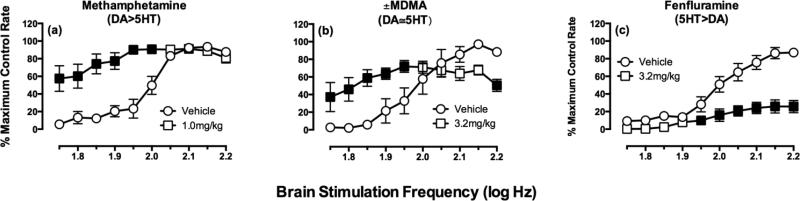
Figure 3.
Effects of methamphetamine, MDMA and fenfluramine on ICSS in rats. Abscissae: Frequency of brain stimulation. Ordinates: Rate of intracranial self-stimulation expresses as Percent Maximum Control Rate. Filled points show rates of ICSS significantly different from vehicle rates as determined by 2-way ANOVA followed by the Holm-Sidak post hoc test (p<0.05). All points show mean data from 5-6 rats. Data adapted from (Bauer, Banks et al. 2013). Complete dose-effect and time-course data with each drug are reported in the original manuscript.
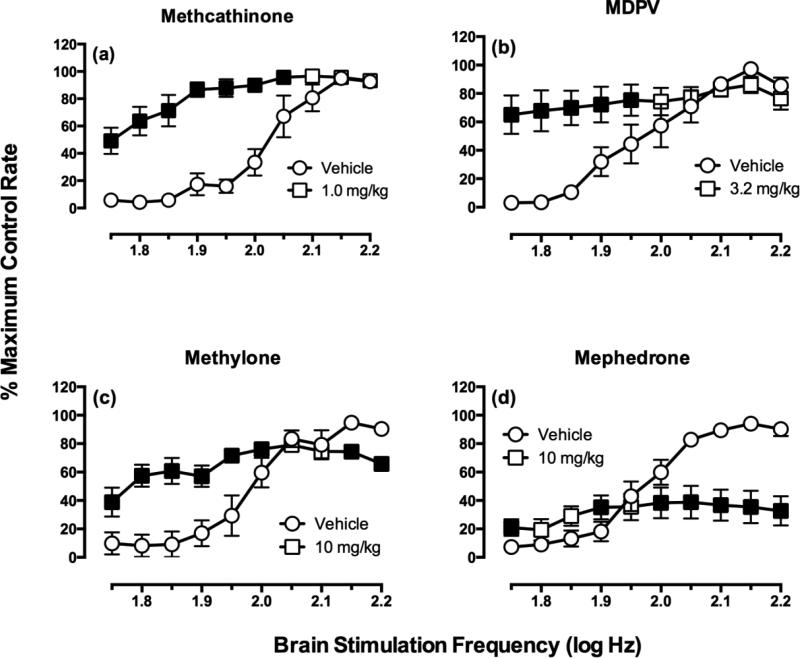
Figure 4.
Effects of synthetic cathinones on ICSS in rats. All details are as in Figure 2. Data adapted from (Bonano, Glennon et al. 2013). Fig 4 illustrates only predominate patterns. Complete dose-effect and time-course data are reported in the original paper.
Drugs of abuse often increase or “facilitate” rates of ICSS, and consequently, facilitation of ICSS is often interpreted as evidence of an abuse-related drug effect (Carlezon and Chartoff 2007; Vlachou and Markou 2011). In frequency-rate ICSS procedures, this facilitation is expressed as a leftward/upward shift in the frequency-rate curve and an increase in low ICSS response rates maintained by low brain stimulation frequencies. An example is shown for methamphetamine in Figure 3a. Conversely, drugs that lack abuse potential often fail to facilitate ICSS and instead produced only a decrease in higher rates of ICSS maintained by higher brain stimulation frequencies, such as the effects shown for fenfluramine in Figure 3c. Finally, drugs such as methylenedioxymethamphetamine (MDMA, ecstasy), which have intermediate levels of abuse liability, produce mixed effects on ICSS that include both facilitation of low ICSS rates maintained by low brain stimulation frequencies and simultaneous depression of high ICSS rates maintained by high brain stimulation frequencies (see Figure 3b). Importantly, studies with these and eight other monoamine releasers found that profiles of drug effects on ICSS correlated with expression of reinforcing drug effects in a nonhuman primate assay of drug self-administration, which has a far more established role than ICSS in preclinical abuse liability testing (Bauer, Banks et al. 2013). Taken together, these data suggest that abuse liability of novel drugs can be inferred in part from expression of abuse-related ICSS facilitation and abuse-limiting ICSS depression in frequency-rate ICSS procedures.
For monoamine releasers and monoamine uptake inhibitors, the behavioral profile of drug effects on ICSS can be related not only to other preclinical assays of abuse liability, but also to in vitro pharmacological effects on DA, 5HT and NE transporters (Bauer, Banks et al. 2013; Rosenberg, Carroll et al. 2013). For example, Figure 3 shows effects produced by three monoamine releasers that vary in their selectivity to release DA vs. 5HT. Thus, methamphetamine selectively releases DA>5HT, and it produces primarily ICSS facilitation. Conversely, fenfluramine selectively releases 5HT>DA and produces primarily ICSS depression. Lastly, MDMA is roughly equipotent to release DA and 5HT, and it produces a mixed-profile that includes both ICSS facilitation and depression. More extensive work with these and eight other monoamine releasers demonstrated that ICSS facilitation correlated with DA vs. 5HT selectivity (Bauer, Banks et al. 2013), suggesting that drug effects on ICSS can also be used to draw inferences about pharmacological selectivity of novel stimulants to act on DA vs. 5HT transporters. The role of norepinephrine in mediating these effects of monoamine releasers has been difficult to examine because available drugs have similar potencies to act at both DA and 5HT transporters. DA-selective uptake inhibitors facilitate ICSS, whereas NE-selective and 5HT-selective uptake inhibitors depress ICSS (Rosenberg, Carroll et al. 2013) However, it should be noted that NET inhibition of MDMA-induced effects can modulate psychoactive reactions of MDMA in human subjects (Hysek, Simmler et al. 2012).
We have begun to apply these principles to the study of cathinone derivatives. For example, Figure 4 shows illustrative data with high doses of methcathinone and of the three recently scheduled drugs methylenedioxypyrovalerone (MDPV), methylone and mephedrone (Bonano, Glennon et al. 2013). Methcathinone is the ß-ketone analog of methamphetamine, and like methamphetamine, methcathinone selectively promotes release of DA>5HT (Cozzi, Brandt et al. 2013) and produces robust facilitation of ICSS without ICSS depression (Figure 4a). MDPV is a monoamine uptake inhibitor rather than a releaser, but it selectively blocks uptake of DA>5HT (Baumann, Ayestas et al. 2012) and like other DA-selective uptake inhibitors (Rosenberg, Carroll et al. 2013), it also produces robust facilitation of ICSS without ICSS depression (Figure 4b). These data are consistent with high abuse liability for MDPV. By contrast, methylone (the ß-ketone derivative of MDMA) and mephedrone are both monoamine releasers with similar potencies to promote release of DA and 5HT (Baumann, Partilla et al. 2012), and consistent with this mixed pharmacological profile, both drugs produce mixed profiles of both ICSS facilitation and ICSS depression (Figure 4c,d). These results suggest that methylone and mephedrone support drug abuse patterns similar to MDMA, but evaluation of that prediction would require more longitudinal data. ICSS can only predict abuse potential. Future studies are designed to build on these results and examine structure-activity determinants of abuse-related behavioral effects produced by cathinone derivatives.
Figure 2a.
Comparison of two current models of the dopamine transporter. In the alternating access model (left), AMPH enters the presynaptic cytosol via DAT and releases AMPH from vesicles. DAT exports the cytosolic DA via reverse transport through DAT. In the channel model (right) AMPH opens DAT and AMPH and Na+ rush in through a channel-like pore. The inward depolarizing current causes vesicular fusion and DA release.
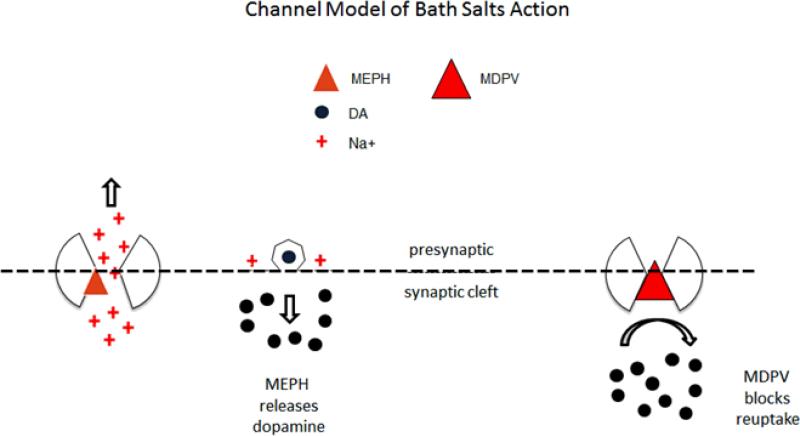
Figure 2b.
Bath salts contain synthetic cathinones, for example, a DAT substrate such as mephedrone (MEPH), a DAT blocker, MDPV, or both. Assume that MEPH and MDPV were both present. By hypotheses: MEPH opens DAT, induces a depolarizing current, stimulates vesicle fusion, and causes DA release (left). MDPV is slower acting than MEPH and prevents reuptake of DA (right).
Acknowledgements
This work is supported by NIH NIDA 5R01DA033930. The authors also wish to thank K.N. Cameron, E. Solis, R. Kolanos, M. Banks, C. Bauer, and J. Bonano for their contributions to this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abadie J, Abbott BP, et al. Directional Limits on Persistent Gravitational Waves Using LIGO S5 Science Data. Phys Rev Lett. 2011;107(27):271102. doi: 10.1103/PhysRevLett.107.271102. [DOI] [PubMed] [Google Scholar]
- Alles GA, Fairchild MD, et al. Chemical pharmacology of Catha edulis. Journal of medicinal and pharmaceutical chemistry. 1961;3:323–352. doi: 10.1021/jm50015a010. [DOI] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, et al. Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. British journal of pharmacology. 2013;168(4):850–862. doi: 10.1111/j.1476-5381.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann M,H, Partilla JS, et al. Powerful Cocaine-Like Actions of 3,4- Methylenedioxypyrovalerone (MDPV), a Principal Constituent of Psychoactive 'Bath Salts' Products. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.204. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr., et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37(5):1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MAJ, et al. The Designer Methcathinone Analogs, Mephedrone and Methylone, are Substrates for Monoamine Transporters in Brain Tissue. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, et al. Powerful cocaine-like actions of 3,4- Methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive 'bath salts' products. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38(4):552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano J, Glennon RA, et al. Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology. 2013 doi: 10.1007/s00213-013-3223-5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron KN, Kolanos R, et al. Bath salts components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter. British journal of pharmacology. 2013b;168:1750–1757. doi: 10.1111/bph.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron KN, Kolanos R, et al. Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of “bath salts,” produce opposite effects at the human dopamine transporter. Psychopharmacology. 2013a;227:493–499. doi: 10.1007/s00213-013-2967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2(11):2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Carvelli L, Blakely RD, et al. Dopamine transporter/syntaxin 1A interactions regulate transporter channel activity and dopaminergic synaptic transmission. Proc Natl Acad Sci U S A. 2008;105(37):14192–14197. doi: 10.1073/pnas.0802214105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzi NV, Brandt SD, et al. Pharmacological examination of trifluoromethyl ring-substituted methcathinone analogs. European journal of pharmacology. 2013;699(1-3):180–187. doi: 10.1016/j.ejphar.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daberkow DP, Brown HD, et al. Amphetamine paradoxically augments exocytotic dopamine release and phasic dopamine signals. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(2):452–463. doi: 10.1523/JNEUROSCI.2136-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Cason TAY, R., Glennon RA. Cathinone: An Investigation of Several N-Alkyl and Methylenedioxy-Substituted Analogs. Pharmacol., Biochem. Behav. 1997;58:1109–1116. doi: 10.1016/s0091-3057(97)00323-7. [DOI] [PubMed] [Google Scholar]
- DEA Operation Somalia Express: Largest Khat Enforcement Eve. News Release. 2006 http://www.justice.gov/dea/pubs/pressrel/pr072606p.html.
- DeFelice LJ, Goswami T. Transporters as channels. Annu Rev Physiol. 2007;69:87–112. doi: 10.1146/annurev.physiol.69.031905.164816. [DOI] [PubMed] [Google Scholar]
- Document UN. III Investigations on the phenylalkylamine fraction. MNAR; GE: 1975. Studies on the chemical composition of khat. pp. 75–12624. [Google Scholar]
- Document UN. The botany and chemistry of khat. MNAR; GE: 1979. pp. 79–10365. [Google Scholar]
- Florin SM, Kuczenski R, et al. Effects of reserpine on extracellular caudate dopamine and hippocampus norepinephrine responses to amphetamine and cocaine: mechanistic and behavioral considerations. The Journal of pharmacology and experimental therapeutics. 1995;274(1):231–241. [PubMed] [Google Scholar]
- Friebel H, Brilla R. Über den zentralerregenden Wirkstoff der frischen Blätter und Zweigspitzen von Catha edulis. Naturwissenschaften. 1963;50:354–355. [Google Scholar]
- Fuwa T, Fukumori N, et al. Microdialysis study of drug effects on central nervous system: Changes of dopamine levels in mice striatum after oral administration of methylenedioxypyrovalerone. Ann. Rep. Tokyo Metr. Inst. P. H. 2007;58:287–292. [Google Scholar]
- Glennon RA, Schechter MD, et al. Discriminative stimulus properties of S(−)- and R(+)-cathinone, (+)cathine, and several structural modifications. Pharmacol. Biochem. Behav. 1984;21:1–3. doi: 10.1016/0091-3057(84)90121-7. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, et al. Structure-activity studies on amphetamine analogs using drug discrimination methodology. Pharmacol Biochem Behav. 1984;21(6):895–901. doi: 10.1016/s0091-3057(84)80071-4. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, et al. Methcathinone (“CAT”): An enantiomeric potency comparison. Pharmacol. Biochem. Behav. 1995;50:601–606. doi: 10.1016/0091-3057(94)00348-3. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Yousif M, et al. Methcathinone: a new and potent amphetamine-like agent. Pharmacol. Biochem. Behav. 1987;26:547. doi: 10.1016/0091-3057(87)90164-x. [DOI] [PubMed] [Google Scholar]
- Glennon RAS,D. The effect of cathinone and several related derivatives on locomotor activity. Res Commun. Subst. Abuse. 1981;2:186–191. [Google Scholar]
- Hyde JF, Browning E, et al. Synthetis Homologs of d,l-Ephedrine. J. Am. Chem. Soc. 1928;50:2287–2292. [Google Scholar]
- Hysek CM, Simmler LD, et al. Duloxetine inhibits effects of MDMA (“ecstasy”) in vitro and in humans in a randomized placebo-controlled laboratory study. PloS one. 2012;7(5):e36476. doi: 10.1371/journal.pone.0036476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICE Thirteen members of international khat trafficking ring convicted. News Release. 2012 http://www.justice.gov/dea/pubs/pressrel/pr072606p.html. [Google Scholar]
- Ingram SL, Prasad BM, et al. Dopamine transporter-mediated conductances increase excitability of midbrain dopamine neurons. Nat Neurosci. 2002;5(10):971–978. doi: 10.1038/nn920. [DOI] [PubMed] [Google Scholar]
- Iversen L. Consideration of the cathinones, Advisory Council on the Misuse of Drugs (ACMD). 2010a.
- Iversen LE. A report submitted to the Home Secretary of the UK.” Consideration of the cathinones. Advisory Council on the Misuse of Drugs March. 2010b;31:2010. [Google Scholar]
- Jacob P, Shulgin AT. Novel N-substituted 2-amino-3’,4’-methylenedioxy- propiophenone. 1996 WO Patent 9639133.
- Kalix P. A constituent of khat leaves with amphetamine-like releasing properties. Eur. J. Pharmacol. 1980;68:213–215. doi: 10.1016/0014-2999(80)90326-x. [DOI] [PubMed] [Google Scholar]
- Kalix PG,RA. Further Evidence for an Amphetamine-Like Mechanism of Action of the Alkaloid Cathinone. Biochem. Pharmacol. 1986;35:3015–3019. doi: 10.1016/0006-2952(86)90380-1. [DOI] [PubMed] [Google Scholar]
- Knoll J. Studies on the central effects of (−)cathinone. NIDA, Research Monograph. 1979;27:322–323. [PubMed] [Google Scholar]
- Köppe H, Ludwig G, et al. 1-(3’,4’-Methylenedioxy-phenyl-2-pyrrlidino alkanones-(1). 1969 US Patent 3,478,050.
- L'Italien YJ, Park H, et al. Methylaminopropiophenone compounds and methods for producing the same. 1957 US Patent 2,802,865.
- Marinetti LJ, Antonides HM. Analysis of synthetic cathinones commonly found in bath salts in human performance and postmortem toxicology: method development, drug distribution and interpretation of results. Journal of analytical toxicology. 2013;37(3):135–146. doi: 10.1093/jat/bks136. [DOI] [PubMed] [Google Scholar]
- Naftalin RJ, De Felice LJ. Comprehensive Biophysics. Elsevier Press; 2011. Transporters and Co-transporters in Theory and Practice to appear in (published by ). [Google Scholar]
- Ramsson E, Howard C, et al. High doses of amphetamine augment, rather than disrupt, exocytotic dopamine release in the dorsal and ventral striatum of the anesthetized rat. J Neurochem. 119:1162–1172. doi: 10.1111/j.1471-4159.2011.07407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic S, Thomas A. On the constituents of Catha edulis. Archiv Pharm. 1962;295:524–525. doi: 10.1002/ardp.19622950709. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA, Campbell OL, et al. Discriminative stimulus and neurochemical mechanism of cathinone: A preliminary study. NIDA Research Monograph. 1979;27:328–329. [PubMed] [Google Scholar]
- Rosenberg M, Carroll FI, et al. Effects of monoamine reuptake inhibitors in assays of acute pain-stimulated and pain-depressed behavior in rats. J Pain. 2013 doi: 10.1016/j.jpain.2012.11.006. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savenko VG, Semkin EP, et al. Expert examination of narcotic substances obtained from ephedrine. U. M. o. t. Interior, All-Union Scientific Research Institute Report. 1989:1–22. [Google Scholar]
- Schechter MD, Glennon RA. Cathinone, cocaine and methamphetamine: Similarity of behavioral effects. Pharmacol. Biochem. Behav. 1985;22:913–916. doi: 10.1016/0091-3057(85)90295-3. [DOI] [PubMed] [Google Scholar]
- Shanks KG, Dahn T, et al. Analysis of first and second generation legal highs for synthetic cannabinoids and synthetic stimulants by ultra-performance liquid chromatography and time of flight mass spectrometry. Journal of analytical toxicology. 2012;36(6):360–371. doi: 10.1093/jat/bks047. [DOI] [PubMed] [Google Scholar]
- Simmler L, Buser T, et al. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013 doi: 10.1111/j.1476-5381.2012.02145.x. doi: 10.1111/j.1476-5381.2012.02145.x: (1-24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, et al. Pharmacological characterization of designer cathinones in vitro. British journal of pharmacology. 2013;168(2):458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller H, Ryan M, et al. Clinical experience with and analytical confi rmation of “ bath salts” and “ legal highs” (synthetic cathinones) in the United States. Clinical Toxicology. 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, et al. Clinical experience with and analytical confirmation of ‘bath salts’ and ‘legal highs’ (synthetic cathinones) in the United States. Clin. Toxicol. 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- Sulzer D. How Addictive Drugs Disrupt Presynaptic Dopamine Neurotransmission. Neuron. 2012;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Chen TK, et al. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15(5 Pt 2):4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Galli A. Dopamine transport currents are promoted from curiosity to physiology. Trends Neurosci. 2003;26(4):173–176. doi: 10.1016/S0166-2236(03)00063-8. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Maidment NT, et al. Amphetamine and other weak bases act to promote reverse transport of dopamine in ventral midbrain neurons. J Neurochem. 1993;60(2):527–535. doi: 10.1111/j.1471-4159.1993.tb03181.x. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, et al. Mechanisms of neurotransmitter release by amphetamines: a review. Progress in neurobiology. 2005;75(6):406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- UNODC . The challenge of new psychoactive substances. United Nations Office on Drugs and Crime; Vienna, Austria: 2013. pp. 1–108. [Google Scholar]
- van der Schoot JB, Ariens EJ, et al. Phenylisopropylamine derivatives, structure and action. Arzneimittelforschung. 1962;12:902–907. [PubMed] [Google Scholar]
- Vlachou S, Markou A, editors. Animal Models of Drug Addiction. Humana Press; New York: 2011. Intracranial self-stimulation. [Google Scholar]
- Westerink BH, Damsma G, et al. Effect of ouabain applied by intrastriatal microdialysis on the in vivo release of dopamine, acetylcholine, and amino acids in the brain of conscious rats. Journal of neurochemistry. 1989;52(3):705–712. doi: 10.1111/j.1471-4159.1989.tb02512.x. [DOI] [PubMed] [Google Scholar]
- Westerink BH, Tuntler J, et al. The use of tetrodotoxin for the characterization of drug-enhanced dopamine release in conscious rats studied by brain dialysis. Naunyn-Schmiedeberg's archives of pharmacology. 1987;336(5):502–507. doi: 10.1007/BF00169306. [DOI] [PubMed] [Google Scholar]
- Wolfes O. Über das Vorkommen von d-nor-iso-Ephedrin in Catha edulis. Archiv Pharm. 1930;268:81–83. [Google Scholar]
- Yanagita T. Studies on cathinones: Cardiovascular and behavioral effects in rats ans self-administration experiment in Rhesus monkeys. N. R. Monograph. 1979;27:326–327. [PubMed] [Google Scholar]
- Zhingel KY, Dovensky W, et al. Ephedrone: 2-Methylamino-1-phenylpropan-1- one (Jeff). J Forensic Sci. 1990;36:915–922. [Google Scholar]