Abstract
Patients with Fanconi anemia (FA) and other inherited bone marrow failure syndromes (IBMFS) have extremely high risks of squamous cell head and neck and gynecologic carcinomas. In the general population, these sites are often associated with infection with human papillomaviruses (HPV), particularly HPV16 and HPV18. Our objectives were to measure the levels of HPV antibodies in unvaccinated patients, and to determine whether these patients developed and maintained similar levels of antibodies as those observed in healthy women, following vaccination with the currently licensed HPV L1 virus-like particles (VLP) vaccines. We measured anti-HPV IgG antibody levels in sera from patients, using an HPV16 or HPV18 L1 VLP enzyme-linked immunoabsorbent assay. Most unvaccinated patients did not have detectable antibody levels, except for a few women above age 25. Both FA and other IBMFS patients developed antibody levels following vaccination that were similar to those previously described in healthy women, and those levels appeared to be sustained out to 5 years after immunization. Thus, antibody responses to the HPV L1 VLP vaccine in patients with FA and other IBMFS appeared to be similar to the responses reported in the general population, implying potential efficacy against future infections with the HPV types contained in the vaccine.
Keywords: Human papillomavirus, vaccine, Fanconi anemia, dyskeratosis congenita, inherited bone marrow failure syndromes
Introduction
Patients with the inherited bone marrow failure syndrome (IBMFS) Fanconi anemia (FA) have recessive gene mutations in the FA/BRCA (Fanconi Anemia/Breast Cancer) DNA repair pathway. These patients have exceptionally high risks of cancer at all sites combined (more than 50-fold), and particularly for head and neck squamous cell carcinomas (HNSCC), and vulvar SCCs (~800- and ~3000-fold respectively compared with the general population) [1]. Dyskeratosis congenita (DC) is an IBMFS associated with aberrant telomere biology. The risks of solid tumors overall in DC are ~8-fold increased, and the risks of HNSCC are increased by ~1100-fold [1]. In the general population, approximately 70% of cervical and 40% of vulvar cancers due to high-risk human papillomavirus infections (HPV) are associated with HPV16 and HPV18 [2;3]. In addition, approximately 25% of all HNSCC, specifically more than 50% of those arising in the oropharynx versus <10% of those originating in the oral cavity, are associated with HPV infection [4-7]. Thus, a prophylactic vaccine has the potential to prevent both cervical and non-cervical HPV-related cancers [8]. Such vaccines are currently recommended in the general population for girls and boys ages 9 to 26 [9].
Data on the presence of HPV in FA-related HNSCC and vulvar SCC are controversial, with Kutler et al reporting the presence of HPV in 84% of 25 tumors (6 vulvar and 15 oral cavity) [10], while van Zeeburg et al detected HPV in 2 of 3 anogenital SCC and none of 16 HNSCC or esophageal SCC [11]. We recently examined HNSCC and vulvar SCC from FA and DC patients, and found HPV16 in only one of 4 FA-related vulvar SCC, and none of 5 FA- and 4 DC-related HNSCC [12]. Thus, it is not clear whether a prophylactic HPV vaccine might have a role in prevention of HNSCC in these IBMFS patients. However, assuming that patients with FA, DC, and other IBMFS respond well to vaccination, it is anticipated that such a vaccine would reduce the risk of cervical cancer, and of any other cancer that is due to the high-risk HPV types included in the two licensed vaccines.
There are potential concerns regarding the efficacy of vaccines like the currently-licensed HPV L1 VLPs in patients with IBMFS, since it has been suggested that some of these patients might have altered immune responses [13;14]. Although we do not generally view the majority of these patients as truly immunodeficient, the specific question of whether patients with FA or other IBMFS would respond in the same way as healthy individuals to HPV vaccines (and sustain the immunity) needs to be addressed. To answer these questions we measured HPV16/18 L1 antibody levels in sera from unvaccinated and vaccinated patients in the National Cancer Institute (NCI) IBMFS cohort.
Methods
The protocol was approved by the NCI Institutional Review Board (NCI 02-C-0052, NCT0002724, www.marrowfailure.cancer.gov). Sera were available from participants in the NCI IBMFS cohort [1]. In some cases, we used frozen sera from visits that antedated the current study, while in others sera were obtained prospectively. All patients who had reached ≥age 9 after the year 2006 when Gardasil™ (Merck) was introduced, were contacted to determine whether they had been vaccinated, the date of vaccination, and the specific vaccine, Gardasil or Cervarix™ (GlaxoSmithKline ); in cases (about 25%) where the patient or parent were not sure, the date and vaccine type were obtained from medical records or the patients’ physicians. HPV16 and HPV18 antibody levels were measured in the same serum sample from each patient.
Anti-HPV IgG antibodies were detected by an enzyme-linked immunosorbent assay (ELISA), as previously described [15;16]. Antibody levels, expressed as ELISA units, EU/mL, were calculated by interpolation of optical density values from a standard curve by averaging the calculated concentrations from all dilutions that fell within the working range of the reference curve. The seropositivity minimum cut-points were 8 EU/mL for anti-HPV16 and 7 EU/mL for anti-HPV18. Levels lower than the minimally-detectable cut points were arbitrarily assigned the lower cut-point values.
Longitudinal results in the patients were compared with published values for the geometric mean level following Gardasil vaccination in healthy women ages 18-45 years. Those data were reported in age groups 18-26, 27-35, and 36-45 years; we compared our results with the average of the reported geometric mean level in each of the same age groups obtained using an ELISA assay similar to the one used in our study [17]. Intervals from vaccination for our patients were the time from the first dose of vaccine to subsequent phlebotomy.
Results
HPV16 and 18 antibody levels were measured in sera from 145 patients with an IBMFS (Table 1): 131 patients were unvaccinated, and 23 were vaccinated individuals (9 were initially included in the unvaccinated group). The samples prior to and following vaccination were available from 8 FA and 1 DC patients. Sera from 4 patients were obtained prior to completion of the full recommended series of 3 doses. One with FA reported only one dose, and one each with FA, DC and SDS reported only two doses. Serial longitudinal samples post-vaccination were available from 3 FA and 1 DC patients among the 23 who had been vaccinated. Twenty-two subjects received Gardasil; one FA patient received Cervarix.
Table 1.
Characteristics of Study Participants
| Syndrome | FA | DBA | DC | SDS | TAR |
|---|---|---|---|---|---|
| Total Patients | 38 | 42 | 56 | 7 | 2 |
| Unvaccinated Number | 34 | 39 | 50 | 6 | 2 |
| Male:Female Ratio | 17:17 | 25:14 | 39:11 | 3:3 | 1:1 |
| Age at Serum Collectionb | 18 (9-57) | 15 (3-58) | 19 (2-69) | 22 (13-42) | 14, 22 |
| Vaccinated Numbera | 12 | 3 | 7 | 1 | 0 |
| Male:Female Ratio | 5:7 | 0:3 | 1:6 | 0:1 | 1:1 |
| Age at Serum Collectionb | 22 (12-59) | 17 (16-20) | 18 (13-26) | 22 | - |
| Age at Vaccinationb | 18 (9-57) | 14 (13-18) | 15 (12-21) | 20 | - |
| Interval from Vaccinationc | 2.6 (0.3-5) | 2.5 (2.3-2.5) | 3.8 (0.3-5.4) | 2 | - |
22 of the 23 patients received Gardasil; 1 received Cervarix.
8 FA and 1 DC were studied both prior to and following vaccination.
Age at serum collection, or at vaccination, in years. Data are medians and ranges.
Interval from first vaccination dose to subsequent phlebotomy in years, medians and ranges.
Fanconi Anemia
We measured HPV antibody levels in sera from 38 FA patients, 34 unvaccinated and 12 following vaccination (8 patients provided samples prior to and following immunization) (Table 1). The male and female numbers were equal. The unvaccinated patients ranged in age from 3 to 58, while the vaccinated samples were from patients from 12 to 59 years of age; the median age at vaccination was 18 years, and the median interval from the first vaccination dose to serum sampling was 2.6 years.
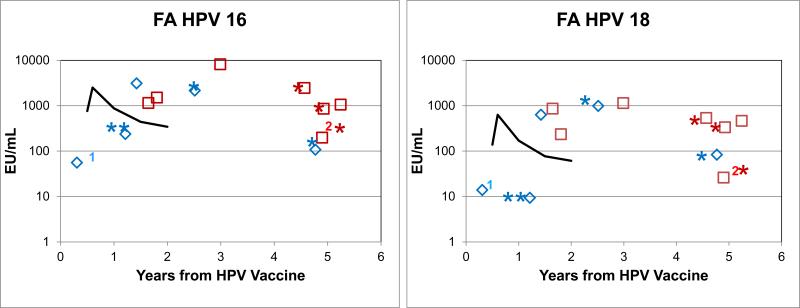
Thirty of 34 unvaccinated FA patients had antibody levels that were below the level of sensitivity of the assay. Detectable levels of HPV16 (≥100 EU/mL) were seen in 4 FA patients, and of HPV18 (between 10 and 100 EU/mL) in 3 of these 4; all 4 were married females, and were among the 9 females who were ≥18 at the time of study (Fig. 1A). No unvaccinated males, including 6 who were at least 18 years old, had detectable levels. These results suggest that unvaccinated patients in the NCI FA cohort serologically resembled the unvaccinated general population.
Figure 1A. HPV16 and HPV18 antibody levels in unvaccinated FA patients.
The same serum samples were used for both antibody assays. Left, HPV16. Right, HPV18. Those with HPV16 antibody levels above 8 or HPV18 above 7 EU/mL (the detection limits) were married females older than 25 years of age. All other females and all males had undetectable levels. Red squares= females. Blue diamonds=males. Y-axis is log of level in EU/mL.
Eleven of the 12 vaccinated FA patients received Gardasil, 7 of whom had elevated antibody levels to both HPV16 and 18 at 2-5 years after vaccination (6 were at more than 4 years from vaccination) (Fig. 1B). Ten patients developed HPV16 antibody levels above 100 EU/mL, and HPV18 levels above 25 EU/mL. A 13 year-old male who received Gardasil vaccine and whose serum was obtained 3 months after the first immunization dose (at the time he belatedly received his second dose) had an HPV16 level of only 56 EU/mL, and HPV18 of only 14 EU/mL. Another male Gardasil recipient whose serum was drawn 12 years after bone marrow transplant did not have detectable antibody levels to HPV18 at 1.2 years after vaccination, but did have an HPV16 level of 238 EU/mL. The one patient who received Cervarix had titers to HPV16 and HPV18 of 1500 and 235 respectively, similar to Gardasil-related serological responses in the general population. Longitudinal studies in 3 patients showed antibody levels to HPV16 and 18 at up to 5 years that were above the published geometric mean levels for healthy women (Fig. 1C). These data indicate that the majority of the FA patients in this study had antibody responses to immunization with HPV vaccines of generally similar magnitude to those reported in healthy women between ages 18 and 45 using an ELISA assay similar to the one used in our study [17].
Figure 1B. HPV antibody levels following the first dose of HPV vaccine in FA patients.
*Previous bone marrow transplant (BMT). The solid line shows the geometric mean levels in females in the general population immunized with Gardasil, averaged over the 3 published age groups [17]. One male had low but detectable levels 3 months after his first dose. **One male 12 yrs post-BMT and 1.2 yrs post-vaccination had low HPV18 levels (~8 EU/mL); the other patients had levels in the range seen in post-vaccination healthy women. “1, 2” indicate patients who received only one or two vaccine doses.
Figure 1C. Serial HPV antibody levels following vaccination of three FA patients.
Blue squares, patient 1: 6 and 8 years after BMT. Red and green squares, patients 2 and 3, no BMT. Antibody levels persisted beyond 5 years after vaccination, which is longer than the published values for healthy women vaccinated with Gardasil, using an HPV L1 VLP ELISA assay.
Other IBMFS
We studied 97 patients with an IBMFS other than FA: 42 DBA, 56 DC, 7 SDS, and 2 thrombocytopenia with absent radii (TAR) (Table 1). There were more males than females with DBA or DC. The ages at the time of collection of sera, vaccination, and intervals after vaccination were in the same range for the non-FA as for the FA subjects. However, only 3 DBA and 7 DC were vaccinated, a significantly lower proportion than among the FA patients (p = 0.01 and 0.03, respectively).
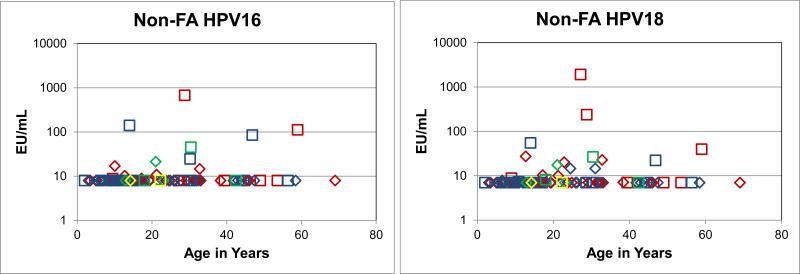
HPV16 antibodies were detected in 4 DC and 1 SDS unvaccinated males at between 10 and 21 EU/mL; and 3 DBA, 2 DC, and 1 SDS unvaccinated females at between 25 and 678 EU/mL (Fig. 2A). The same 5 males plus 2 DBA and 1 more DC had slightly elevated antibody levels to HPV18, but all were below 30 EU/mL. The 6 unvaccinated females with elevated HPV16 levels also had elevated HPV18 levels, from 22 to 1910 EU/mL. Five of the 6 females were older than 25 years of age, indicating that in general the unvaccinated non-FA IBMFS patients serologically resembled those with FA.
Figure 2A. HPV16 and HPV18 antibody levels in unvaccinated non-FA patients.
The same serum samples were used for both antibody assays. Squares, females. Diamonds, males. Blue, DBA. Red, DC. Green, SDS. Yellow, TAR. Those with HPV16 levels above 8 EU/mL or HPV18 above 7 EU/mL were generally older than 20 years of age.
All 11 vaccinated non-FA patients received Gardasil. The majority had HPV16 and 18 antibody levels at or above the lower level of published geometric mean levels for healthy women that received the vaccine (Fig. 2B). The only vaccinated female with SDS had relatively low levels at 2 years post-vaccination, 94 and 23 EU/mL for HPV16 and 18 respectively when compared to other non-FA patients. Longitudinal measurements in one DC male indicated stable antibody levels to both viruses at up to 4 years following vaccination; the levels were within the published range for healthy women (Fig. 2C). Thus, the non-FA patients had responses to immunization with the HPV vaccine that in general resembled those of healthy women [17].
Figure 2B. HPV antibody levels following vaccination of non-FA patients.
The solid line shows the geometric mean levels in normal females immunized with Gardasil, averaged over the 3 published age groups [17]. All patients had levels in the range described in post-vaccination healthy women. “2” indicates patients who received only two vaccine doses.
Figure 2C. Serial HPV antibody levels following vaccination of one male with DC.
Antibody levels persisted beyond 4 years after vaccination.
Discussion
Patients with FA as well as with other types of IBMFS appeared to be in general similar to each other and to resemble the titers and durability reported in healthy women with regard to antibody responses to HPV16 and 18 following vaccination. All of the patients remained seropositive for HPV-16 and the majority for HPV-18 up to 5 years after vaccination, with antibody levels higher than the ones previously reported in unvaccinated, naturally infected healthy individuals [17]. These data suggest that patients with any IBMFS, including FA, can be successfully immunized with the current HPV vaccines. Our data are consistent with the premise that the HPV vaccine should prevent HPV16- and HPV18-related infection and, therefore, may reduce the risk of cervical and perhaps vulvar and other anogenital cancers associated with HPV16 or HPV18, in young uninfected women with an IBMFS [18]. However, the vaccine is unlikely to reduce non-HPV associated HNSCC either in the general population or in patients with an IBMFS.
There are several limitations to our study. Despite enrolling nearly 150 participants, the number of subjects with each syndrome was small. Information about vaccination and the dates for each dose were based on self-report by the patient or parent, although those where the family member was unclear about details were documented from local physician records. We did not have serum samples from a concurrently-evaluated healthy control group, and there are insufficient published comparative data on antibody levels ≥2 years beyond immunization in healthy individuals of similar age and sex who have received Gardasil, and in whom the serological assays were identical to ours.
Despite these limitations, our study benefitted from the inclusion of all of the rare IBMFS. This provided an internal control, using sera from the same cohort as the FA patients, assayed in the same laboratory, at the same time, with the same reagents. If the FA patients failed to achieve and sustain normal antibody levels, they would have differed from the non-FA patients, and they did not.
Our study has addressed concerns regarding the immunogenicity of the currently licensed HPV vaccines in patients with FA. The only FA patients with measurable antibody levels prior to immunization were married women over age 25. The antibody levels following immunization were in general in the same range as in healthy women, supporting the ability of FA patients to develop antibodies to HPV16 and HPV18 following vaccination. The most logical interpretation is that FA patients and those with the other IBMFS are similar to healthy women in their response to HPV vaccination. Along with our previously-published data in which we found that HNSCC tumors in FA and DC patients generally did not contain HPV DNA [12], we can conservatively conclude that vaccination with HPV, while recommended as good pediatric practice as prophylaxis for HPV-related cervical cancer, may not in itself reduce the very high risk of HNSCC in these patients, since HPV may not be of etiologic importance in those tumors.
Highlights.
Patients with Fanconi anemia (FA) are at very high risk for head and neck and gynecologic squamous cell carcinomas; cancers that in the general population may be associated with infection with HPV. Since some of these patients may be immunodeficient, we measured HPV L1 antibodies in unvaccinated and vaccinated FA patients. The antibody levels in the former were in the reported range for healthy women. And, the post-vaccination levels were in the reported range for vaccinated healthy women, and remained there for up to five years.
Acknowledgments
We are extremely grateful to all subjects for their enthusiastic participation in the IBMFS study. We thank Drs Mark H. Greene and Mark H. Schiffman, National Cancer Institute, for their careful critiquing of the manuscript.
This research was supported in part by the Intramural Research Program of the National Institutes of Health and the National Cancer Institute (B.P.A., N.G., and S.A.S.), and by contracts N02-CP-91026, N02-CP-11019 and HHSN261200655001C with Westat, Incorporated. The project was funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E (L.A.P. and Y.P.) The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Alter BP, Giri N, Savage SA, et al. Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br J Haematol. 2010 Jul;150(2):179–88. doi: 10.1111/j.1365-2141.2010.08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munoz N, Bosch FX, Castellsague X, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004 Aug 20;111(2):278–85. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- 3.Forman D, de MC, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012 Nov 20;30(Suppl 5):F12–F23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 4.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007 Sep 8;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 5.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005 Feb;14(2):467–75. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 6.Chaturvedi AK. Epidemiology and clinical aspects of HPV in head and neck cancers. Head Neck Pathol. 2012 Jul;6(Suppl 1):S16–S24. doi: 10.1007/s12105-012-0377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lingen MW, Xiao W, Schmidt A, et al. Low etiologic fraction for high-risk human papillomavirus in oral cavity squamous cell carcinomas. Oral Oncol. 2012 Jul 27; doi: 10.1016/j.oraloncology.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008 Nov 15;113(10 Suppl):3036–46. doi: 10.1002/cncr.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kutler DI, Auerbach AD, Satagopan J, et al. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg. 2003;129:106–12. doi: 10.1001/archotol.129.1.106. [DOI] [PubMed] [Google Scholar]
- 10.van Zeeburg HJ, Snijders PJ, Wu T, et al. Clinical and molecular characteristics of squamous cell carcinomas from Fanconi anemia patients. J Natl Cancer Inst. 2008 Nov 19;100(22):1649–53. doi: 10.1093/jnci/djn366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alter BP, Giri N, Savage SA, Quint WG, de Koning MN, Schiffman M. Squamous cell carcinomas in patients with Fanconi anemia and dyskeratosis congenita: A search for human papillomavirus. Int J Cancer. 2013 Sep 15;133(6):1513–5. doi: 10.1002/ijc.28157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.HPV vaccine recommendations. Pediatrics. 2012 Mar;129(3):602–5. doi: 10.1542/peds.2011-3865. [DOI] [PubMed] [Google Scholar]
- 13.Fagerlie SR, Bagby GC. Immune defects in fanconi anemia. Crit Rev Immunol. 2006;26(1):81–96. doi: 10.1615/critrevimmunol.v26.i1.40. [DOI] [PubMed] [Google Scholar]
- 14.Khan S, Pereira J, Darbyshire PJ, et al. Do ribosomopathies explain some cases of common variable immunodeficiency? Clin Exp Immunol. 2011 Jan;163(1):96–103. doi: 10.1111/j.1365-2249.2010.04280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dauner JG, Pan Y, Hildesheim A, Harro C, Pinto LA. Characterization of the HPV-specific memory B cell and systemic antibody responses in women receiving an unadjuvanted HPV16 L1 VLP vaccine. Vaccine. 2010 Jul 26;28(33):5407–13. doi: 10.1016/j.vaccine.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dessy FJ, Giannini SL, Bougelet CA, et al. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin. 2008 Nov;4(6):425–34. doi: 10.4161/hv.4.6.6912. [DOI] [PubMed] [Google Scholar]
- 17.Einstein MH, Baron M, Levin MJ, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 vaccine and HPV-6/11/16/18 vaccine: Follow-up from Months 12-24 in a Phase III randomized study of healthy women aged 18-45 years. Hum Vaccin. 2011 Dec 1;7(12):1343–58. doi: 10.4161/hv.7.12.18281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012 Nov 20;30(Suppl 5):F123–F138. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]