Abstract
Objective
To characterize the incidence, onset, management, predictors, and clinical impact of mTOR inhibitor associated noninfectious pneumonitis (NIP) on patients with metastatic renal cell carcinoma.
Patients and methods
Retrospective review of 310 patients with metastatic renal cell carcinoma who received temsirolimus and/or everolimus between 6/1/2007 and 10/1/2010. Clinical correlations were made with serial radiologic imaging. Fisher’s exact, Wilcoxon rank sum, and logistic regression analysis were performed to evaluate the association of NIP with demographic or clinical factors. Log rank and Cox proportional hazards regression analysis were used for the time-to-event analysis.
Results
NIP occurred in 6% of temsirolimus and 23% of everolimus treated patients. Symptoms included cough, dyspnea, and fever (median of two and three symptoms per patient, respectively). Median NCI-CTCAE pneumonitis grade was 2 for both groups. Older age and everolimus treatment were predictive of NIP. Patients who developed NIP had significantly longer time on treatment (median 4.1 months vs 2 months) and overall survival (OS) (median 15.4 months vs 7.4 months). NIP was a predictor of improved OS by multivariate analysis.
Conclusions
An increased incidence of NIP was observed in everolimus treated patients. Improved OS in patients who developed NIP is an intriguing finding and should be further investigated. Given the incidence, morbidity, and outcomes seen in patients on everolimus who develop NIP, management should include proactive monitoring and treatment of NIP with the goal of preserving mTOR inhibitor therapy.
Keywords: renal cell carcinoma, pneumonitis, everolimus, temsirolimus
Introduction
Temsirolimus and everolimus are inhibitors of mammalian target of rapamycin (mTOR) with proven activity in patients with advanced renal cell carcinoma (mRCC). In a randomized first-line phase III trial, temsirolimus, compared with interferon alpha, prolonged overall survival (OS) in patients with poor-prognosis mRCC.[1] In the pivotal RECORD-1 phase III trial, everolimus as compared to placebo prolonged progression-free survival (PFS) in patients with mRCC who progressed on prior tyrosine kinase inhibitor receptor therapy.[2] Both agents were approved by the US Food and Drug Administration for patients with mRCC and are recommended treatment options in consensus guidelines.[3, 4]
mTOR inhibitors are analogs of rapamycin (sirolimus) and demonstrate similar class-specific adverse effects, including rash, stomatitis, fatigue, increased risk of infection, and metabolic abnormalities.[1, 2, 5] Drug-related pneumonitis characterized by noninfectious, non-malignant infiltrates has also been reported with varying incidence among the mTOR inhibitors. In the previous phase III clinical trials, temsirolimus had a reported incidence of noninfectious pneumonitis (NIP) of 2%, and everolimus 8%.[1, 2, 6] Recent retrospective analyses indicate an incidence of NIP of 14 – 39% with the rapalogs.[7–11] The mechanism by which mTOR inhibitors induce NIP is not understood. Likewise, there is a limited account of the incidence, clinical significance, radiographic presentation, identified patient risk factors, or treatment for mTOR inhibitor-induced NIP in a non-clinical trial setting.
Herein, we describe the incidence, clinical manifestations, radiographic findings, and treatment of NIP associated with temsirolimus and everolimus in patients with mRCC treated at a large referral center. Additionally, we report patient risk factors and outcomes of mRCC patients who developed temsirolimus or everolimus related NIP.
Patients and Methods
Patient Selection
After obtaining Institutional Review Board approval, a retrospective analysis of 310 mRCC patients who received temsirolimus, everolimus, or both agents between June 2007 and October 2010 was performed at the University of Texas MD Anderson Cancer Center (UTMDACC) . Complete electronic medical records were reviewed. Patient demographics including date of birth, gender, co-morbidities, smoking history, and history of nephrectomy were captured. Race/ethnicity was categorized as Caucasian, African-American, Hispanic, Asian, and other. Tumor pathology was categorized as clear cell, papillary, sarcomatoid, and other. Drug therapy with temsirolimus or everolimus was recorded, including dose, time on treatment, and presence of prior oncologic drug therapies. Eastern Cooperative Oncology Group performance status (ECOG PS), baseline laboratory values, laboratory values at time of NIP onset, previous therapies, location of metastatic sites, and survival outcomes were captured. The development of clinical and/or radiologic NIP onset, related patient symptoms, physician management of the adverse event, and pneumonitis grading according to the National Cancer Institute Common Toxicity Criteria (NCI CTCAE) version 4.0 were assessed.[12]
Radiologic Review
The chest computed tomography (CT) scans of all patients with clinical symptoms and signs of pneumonitis identified from the above records were reviewed by a single radiologist. Available chests CTs of each patient from baseline to cessation of therapy were reviewed. One patient was excluded because the CTs obtained at an outside hospital were not available for review. The radiological signs of each chest CT were recorded. The laterality and distribution of signs were documented including nodules, linear opacities, consolidation (air-space shadowing), ground glass opacities and pleural effusions. The serial progressions of radiological signs were also documented. Instances in which radiological signs were considered to be inconsistent with the diagnosis of NIP were recorded and clinical records further reviewed to confirm or reject the radiological impression. Such situations included focal segmental or lobar consolidation and/or atelectasis, which were considered more in keeping with pneumonia, and large pleural effusions, which was considered more likely related to malignancy or cardiac failure. If such patients on clinical review were found to have responded to antibiotics or diuretics, they were considered not to have had a NIP.
The radiological signs of the CT chest scans of the remaining patients who were considered to have radiological signs of NIP were classified according to White et al.[10]
Statistical Analysis
Fisher’s exact test, Wilcoxon rank sum test, and logistic regression analysis were performed to evaluate the association between NIP and demographic or clinical factors. Variables in the univariate analysis with P value < 0.15 were included in the multivariate logistic regression analysis. The backward selection procedure was used for the model selection. Variables with P value < 0.05 were considered statistically significant
The log rank test and Cox proportional hazards regression analysis were used for the time-to-event analysis. For multivariate analysis to evaluate whether NIP was an independent prognostic factor for survival outcomes, a base model was established that included variables in the univariate analysis with P value < 0.15 (without NIP) and applied the backward selection procedure. NIP was added to the base model to evaluate whether NIP was significantly associated with the survival outcomes after adjusting for the effects of variables in the base model. SAS software 9.3 (SAS institute Inc., Cary NC) and S Plus software 8.2 (TIBCO Software Inc., Palo Alto, CA) were used for analyses.
Results
Our study population included 310 patients with mRCC and who received therapy with an mTOR inhibitor; 210 patients (68%) received temsirolimus and 100 patients (32%) received everolimus. Baseline characteristics are presented in Table 1. This patient population was predominantly male (71%) and Caucasian (74%) with a median age of 61 years (range 23–85 years). Clear-cell histology was the predominant histology (74%) and most patients had a prior nephrectomy (74%). Therapy with an mTOR inhibitor was given mainly in the second-line/salvage setting (74%) with a median number of prior therapies of two (range 1–10 therapies) and median time from initial systemic treatment to mTOR inhibitor therapy of 277 days (range 0–6747 days; 95% CI: 597.6–798.5 days).
Table 1.
Patients’ demographics and characteristics
| Characteristic | Whole population |
No NIP, n (%) | NIP, n (%) | P |
|---|---|---|---|---|
| Total | 310 | 274 (88) | 36 (12) | |
| Median age, years | 61 (23–85) | 60 (23–85) | 67 (45–80) | 0.007 |
| Male | 219 (71) | 197 (72) | 22 (61) | 0.242 |
| Caucasian | 230 (74) | 204 (74) | 26 (72) | 0.744 |
| ECOG PS | 0.49 | |||
| 0 | 34 (11) | 30 (11) | 4 (11) | |
| 1 | 152 (49) | 128 (47) | 24 (67) | |
| 2 | 90 (29) | 86 (31) | 4 (11) | |
| ≥ 3 | 34 (11) | 30 (11) | 4 (11) | |
| Pathology | 0.41 | |||
| Clear-cell | 228 (74) | 198 (72) | 30 (83) | |
| Papillary | 31 (10) | 29 (11) | 2 (6) | |
| Other | 51 (16) | 47 (17) | 4 (11) | |
| Nephrectomy | 228 (74) | 199 (73) | 29 (81) | 0.422 |
| MSKCC prognosis | 0.006 | |||
| Good | 70 (23) | 57 (21) | 13 (36) | |
| Intermediate | 108 (35) | 92 (33) | 16 (44) | |
| Poor | 132 (42) | 125 (46) | 7 (20) | |
| Median Platelets, K | 271 (39–1069) | 281 (39–1069) | 218 (91–540) | 0.007 |
| Median # metastatic sites | 2 (0–7) | 2 (0–7) | 2 (0–6) | 0.851 |
| Prior systemic therapy | 230 (74) | 198 (72) | 32 (89) | 0.041 |
| Median # prior therapy | 1 (0–10) | 1 (0–10) | 1 (0–7) | 0.331 |
| Median time from systemic therapy initiation to mTOR inhibitor, days | 277 (0–6747) | 252 (0–6747) | 440 (0–3430) | 0.048 |
| mTOR inhibitor | <0.001 | |||
| Temsirolimus | 210 (68) | 197 (72) | 13 (36) | |
| Everolimus | 100 (32) | 77 (28) | 23 (64) |
NIP developed in 36 mRCC patients (12%) treated with an mTOR inhibitor with a median time to symptom onset of 65 days (range 21–855 days; 95% CI: 43.2–188.2 days) and radiographic appearance of 62.5 days (range 35–736 days; 95% CI: 60.6–140.2 days). Thirteen patients (6%) who received temsirolimus as compared to 23 patients (23%) who received everolimus developed NIP (P<0.0001). However, the median time to symptom onset, radiographic appearance, and NCI CTCAE pneumonitis grade did not differ significantly between treatments (P=NS). By multivariate logistic regression analysis, older age (odds ratio (OR) 1.04; 95% CI: 1.004–1.078; P=0.0296) and mTOR inhibitor treatment with everolimus (OR 4.106; 95% CI: 1.96–8.595; P=0.0002) were predictive of NIP.
Patients predominantly presented with pneumonitis severity of NCI CTCAE grade 2 (n=28, 78%), followed by grade 1 (n=5, 14%) and grade 3 (n=3, 8%); no grade 4 events were noted. Cough (n=26, 72%), and dyspnea (n=26, 72%) were the most common clinical symptoms of NIP. Other symptoms included fever (n=5, 14%), hemoptysis (n=3, 8%), and chest pain (n=3, 8%). As a result of NIP, mTOR inhibitor therapy was discontinued in nine patients (25%), continued at same dose in seven patients (19%), continued at lower dose in two patients (6%), held and then resumed at lower dose in two patients (6%), and held and then resumed at same dose in one patient (3%) [Table 2]. Fifteen patients (42%) discontinued mTOR inhibitor treatment at NIP onset due to progressive disease. In addition to the discontinuation of the mTOR inhibitor, NIP interventions and treatments included systemic corticosteroid therapy (n=18, 50%), antibiotics (n=11, 31%), inhaled bronchodilators (n=5, 14%), and supplemental oxygen (n=3, 8%). Three patients with grade 3 NIP were hospitalized for management with length of hospitalization range of 3–20 days and corticosteroid therapy duration of 13–120 days. One patient death occurred during hospitalization; however, progressive disease and not NIP was attributed to the death.
Table 2.
Noninfectious pneumonitis severity by NCI Common Toxicity Criteria grade and management, (n=36).
| n = | Grade 1 | Grade 2 | Grade 3 | All grades† |
|---|---|---|---|---|
| Clinical suspicion of NIP | 5 | 28 | 3 | 36 |
| mTOR inhibitor therapy | ||||
| Discontinued* | 0 | 7 | 2 | 9 |
| Continued | 3 | 4 | 0 | 7 |
| Continued, dose reduced | 0 | 2 | 0 | 2 |
| Delayed, dose reduced | 0 | 2 | 0 | 2 |
| Delayed, dose maintained | 0 | 1 | 0 | 1 |
| NIP intervention | ||||
| Steroid initiated | 2 | 13 | 3 | 18 |
| Antibiotic initiated | 1 | 8 | 2 | 11 |
| Bronchodilator initiated | 0 | 3 | 2 | 5 |
| Oxygen supplementation | 0 | 0 | 3 | 3 |
No patients with grade 4 pneumonitis were identified.
Fifteen patients discontinued mTOR inhibitor therapy at time of NIP onset due to progressive disease.
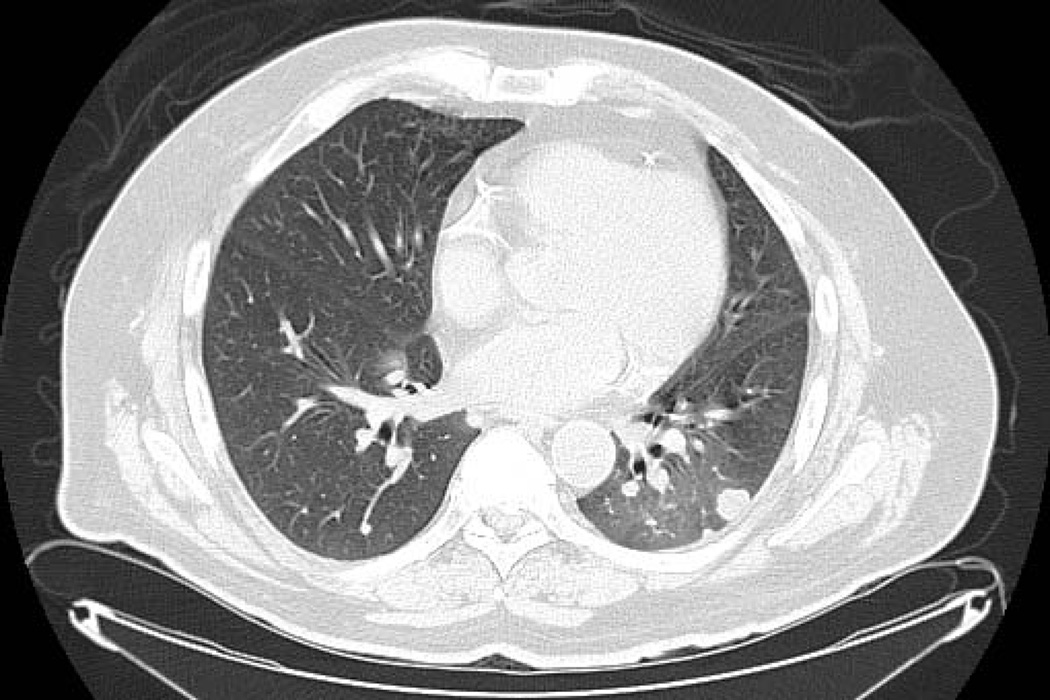
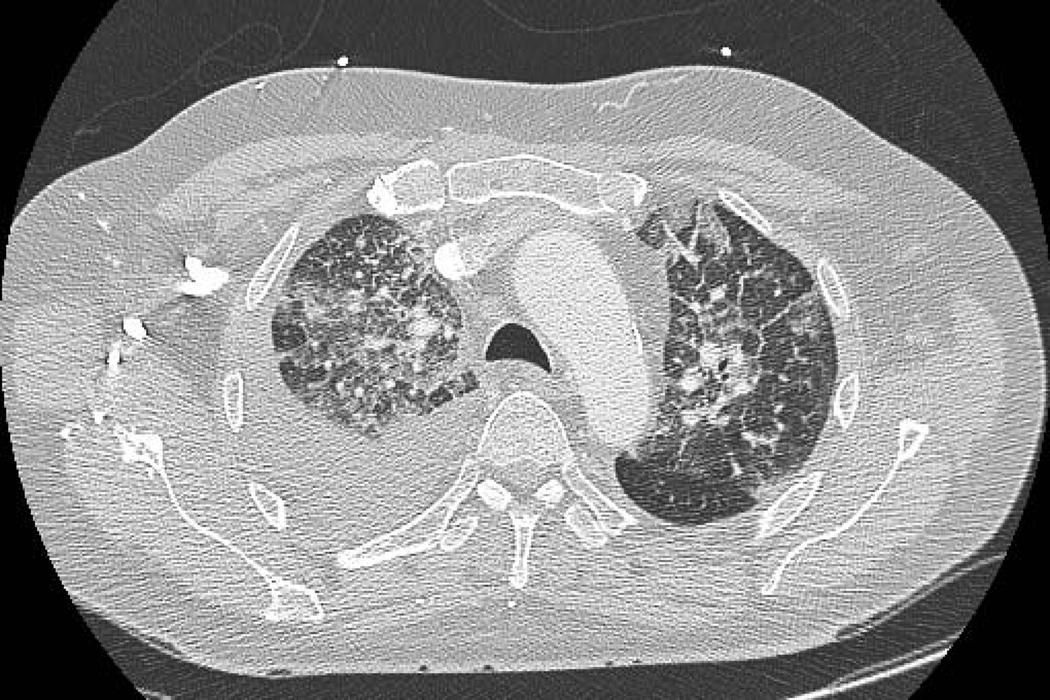
Chest CT findings demonstrated three radiologic patterns of NIP. Sixteen patients (44%) had CT findings of patchy distribution of ground-glass opacities accompanied by interlobar septal thickening [Figure 1a], 13 patients (36%) had nonspecific area with ground-glass attenuation [Figure 1b], four patients (11%) had multifocal area of airspace consolidation [Figure 1c], and three patients (8%) had a mixed radiologic pattern of multifocal airspace consolidation with patchy distribution of ground-glass attenuation accompanied by interlobar septal thickening. The predominant distribution of NIP was bilateral as seen in 32 patients (89%) as compared to four patients (11%) who had unilateral distribution.
Figure 1.
CT chest radiographic charaterization of NIP. Patchy distribution of ground-glass opacities with interlobar septal thickening (1a. Note: also right pleural effusion), nonspecific area with ground-glass attenuation (1b. Note: lung metastasis in left lower lobe), and multifocal area of airspace consolidation (1c).
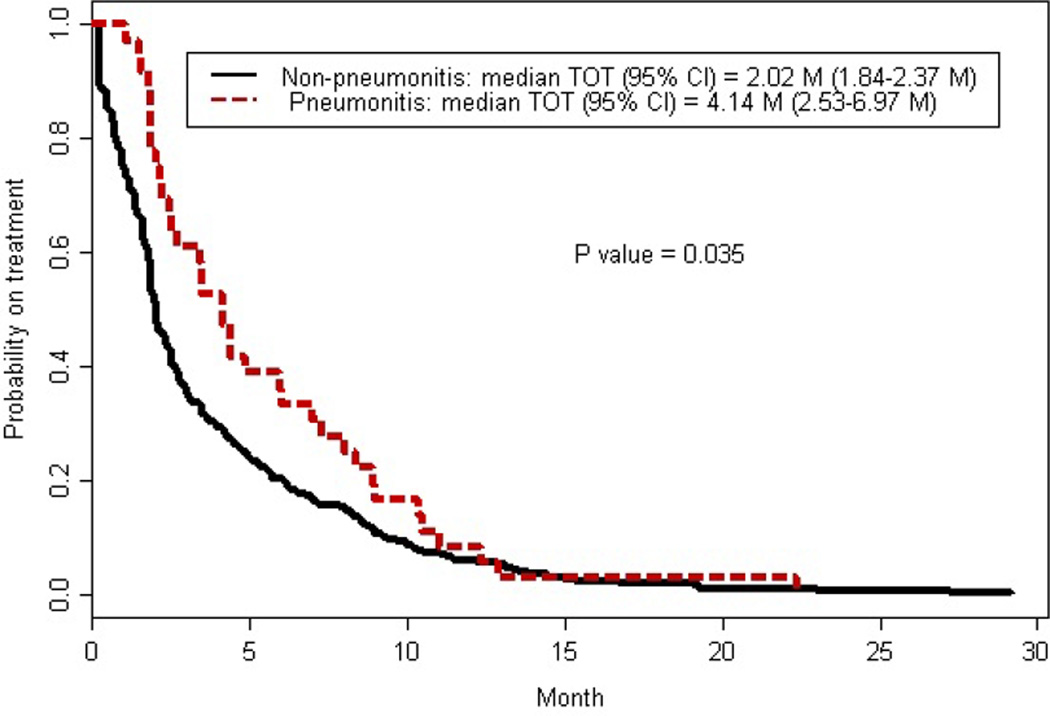
The median time on treatment, calculated as time from initiation of mTOR inhibitor therapy to discontinuation, was two months (95% CI: 1.8–2.4 months). The median time on treatment was significantly longer for patients who developed NIP (median 4.1 months; 95% CI: 2.5–7 months) compared to patients who did not (median 2 months; 95% CI: 1.8–2.4 months) (P=0.035) [Figure 2]. Patient characteristics associated with longer time on treatment by multivariate logistics regression analysis included normal albumin (hazard ratio (HR) 0.77; 95% CI: 0.6–0.9; P=0.0074), NIP (HR 0.61; 95% CI: 0.4–0.8; P=0.0065), and prior sunitinib (HR 0.45; 95% CI: 0.3–0.7; P=0.0005). Compromised ECOG PS was associated with shorter time on treatment; PS of one (HR 1.48; 95% CI: 1.01–2.2; P= 0.0445), PS of two (HR 1.55; 95% CI: 1.03–2.4; P=0.0368), PS of three (HR 2.17; 95% CI: 1.3–3.7; P=0.0043), and PS of four (HR 6.7; 95% CI: 1.5–30.9; P=0.0148).
Figure 2.
Association between noninfectious pneumonitis and Kaplan-Meier estimate for time on treatment (TOT).
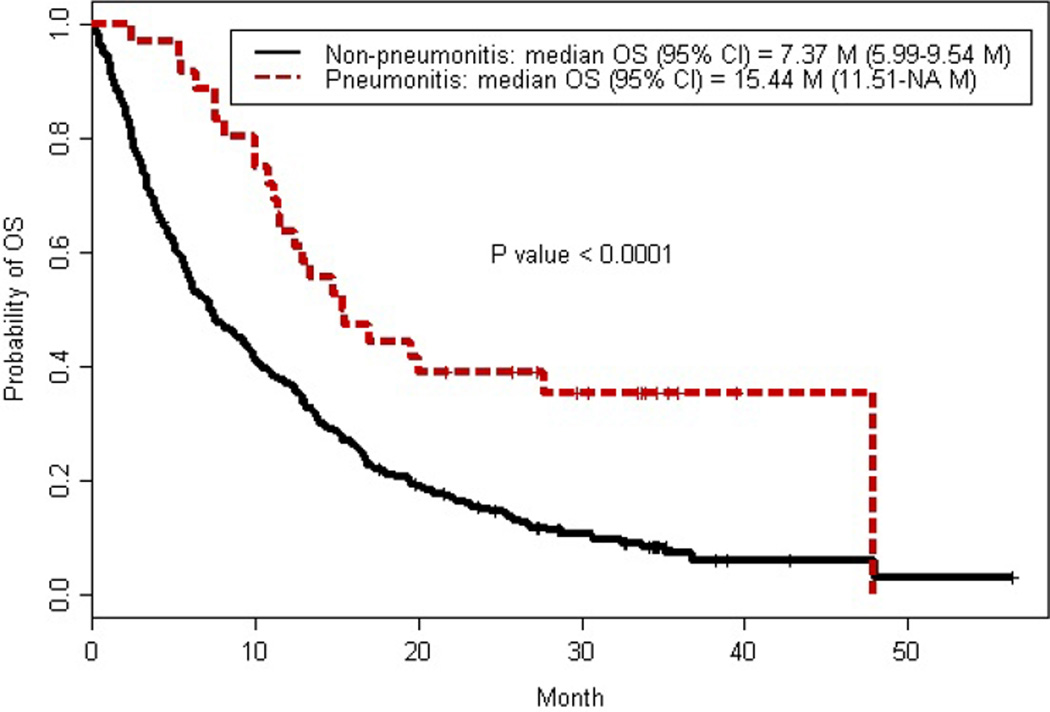
At the time of our analysis, 271 deaths (87%) were recorded with a median OS of 8.7 months (95% CI:7.2–10.4 months). Patients treated with mTOR inhibitor therapy that developed NIP had significantly longer OS (15.4 months; 95% CI: 11.5-NA months) compared to patients who did not develop NIP (7.4 months; 95% CI: 6–9.5 months) (P<0.0001) [Figure 3]. The 3-year survival rate of patients who developed NIP was 35.4% (95% CI: 22.5–55.5%) compared to 7.2% in patients without NIP (95% CI: 4.3–12.2%) (P<0.0001). Predictors of improved OS included longer time from systemic treatment initiation to mTOR inhibitor therapy (HR 0.988; 95% CI: 0.98–0.995; P=0.0013), normal albumin (HR 0.71; 95% CI: 0.56–0.89; P=0.0027), increased platelets (HR 0.999 ; 95% CI: 0.998–1; P=0.0119), prior sunitinib (HR 0.58; 95% CI: 0.35–0.96; P=0.035), and NIP (HR 0.32; 95% CI: 0.2–0.5; P<0.0001). Predictors of shorter OS included treatment with temsirolimus (HR 1.83; 95% CI: 1.33–2.54; P=0.0003), increased serum lactate dehydrogenase level (HR 1.003; 95% CI: 1.001–1.004; P=0.0026), and compromised ECOG PS; PS of one (HR 2.33; 95% CI: 1.44–3.77; P=0.0005), PS of two (HR 3.681; 95% CI: 2.22–6.11; P<0.0001), PS of three (HR 8.28; 95% CI: 4.49–15.28; P<0.0001), and PS of four (HR 11.73, 95% CI: 2.48–55.38; P=0.0019).
Figure 3.
Association between noninfectious pneumonitis and Kaplan-Meier estimate for overall survival (OS).
Discussion
mTOR inhibitors are currently FDA approved for multiple indications including mRCC, and ongoing clinical trials are evaluating this class of agents in a variety of other oncologic settings.[1, 2, 13–15] The toxicity profiles of these mTOR inhibitors are similar, and NIP is a class associated adverse event. NIP is characterized by non-specific, non-malignant inflammatory infiltrates, which may produce clinical symptoms, and without an infectious etiology.[8, 10, 16] We retrospectively identified patients with mRCC who received temsirolimus or everolimus and had clinical documentation of NIP (symptoms or radiographic interpretation by medical provider). Identified cases were independently reviewed by a radiologist for radiographic evidence of NIP that developed during treatment with mTOR inhibitor therapy.
The incidence of NIP with mTOR inhibitors in our retrospective experience was 12%, with a significantly greater incidence in patients who received everolimus. The overall incidence of NIP is similar to that previously reported in phase 3 studies in patients with mRCC and to other published retrospective experiences with mTOR inhibitor treatment in solid tumors, which is in the order of 2–39%.[1, 2, 6–11] The median time to development of clinical symptoms and radiographic manifestation of NIP were similar with a median time of 65 days to symptom onset and 62.5 days to radiographic manifestation. This slight variation, with radiographic manifestation predating clinical symptoms may serve as a predictive tool for those patients who are at an increased risk of NIP and who may need a more thorough evaluation and increased monitoring. The radiographic patterns associated with NIP in our study are similar to those reported in other reviews of mTOR inhibitor-associated NIP in oncology and solid organ transplant with ground glass opacities and parenchymal consolidation being the most common presentation.[7, 8, 10, 11, 17, 18]
The pathogenesis of mTOR inhibitor associated NIP has not yet been elucidated. Hypersensitivity mechanisms are supported by the findings of lung biopsies, bronchoalveolar lavage, and the clinical response to corticosteroids. Studies with sirolimus suggest an induced autoimmune response in which sirolimus exposes cryptic antigens that induce an immune response leading to lymphocytic alveolitis.[17] mTOR inhibitors may also induce a pro-inflammatory state as a hapten with ongoing T-cell recognition of the processed antigen complex.[18]
Recent consensus treatment recommendations have been published for the management of mTOR inhibitor associated NIP.[12, 16] In asymptomatic patients with radiologic changes only (grade 1), treatment with mTOR inhibitor therapy may continue without dose adjustment, but the patient should be followed closely for the development of clinical symptoms. A key recommendation was the subdivision of symptomatic grade 2 NIP into grades 2a and 2b, where grade 2a with slight to moderate cough is closer to grade one, and grade 2b with severe cough and dyspnea is closer to grade 3. This subdivision is important in regards to the nature and frequency of follow-up and to identify a subgroup of patients in whom treatment may be continued. In patients experiencing clinical benefit on mTOR inhibitor therapy who have mild respiratory symptoms (grade 2a), treatment may be continued with close monitoring and without a dose adjustment. If a patient presents with grade 2b NIP or worsening symptoms, then dose reductions should be considered in addition to corticosteroid therapy. For patients with severe NIP with alterations in oxygen saturation or interference with activities of daily living (grade 3) or life-threatening NIP (grade 4), inpatient admission with pulmonary evaluation and corticosteroid therapy is recommended. Patients with grade 3 NIP should have mTOR inhibitor treatment withheld until resolution to grade 1; drug should then be reinitiated at a reduced dose if patients are perceived to have a therapeutic benefit on mTOR inhibitor therapy. Therapy should be permanently discontinued for patients who experience grade 4 NIP. Empiric antibiotic therapy should be considered when an infectious etiology cannot be excluded. Our NIP treatment and management algorithm was similar to these recommendations: corticosteroid therapy, empiric antibiotic therapy, bronchoscopy when indicated, and mTOR inhibitor treatment dose reduction, interruption, or discontinuation were principal NIP management strategies. Predictors of mTOR inhibitor associated NIP are limited; in our analysis increased age and treatment with everolimus were predictive of NIP.
We recognize the limitations of our study in that we identified patients with NIP by clinical presentation and may have excluded patients with grade 1 NIP (radiographic appearance only) since we did not systematically review imaging studies of all patients treated with mTOR inhibitors. Radiographic changes consistent with NIP are not always associated with clinical symptoms. In the absence of clinical symptoms, the radiographic diagnosis of NIP likely has minimal impact on the management of patients treated with mTOR inhibitors. We sought to identify the impact of NIP as it related to mTOR inhibitor treatment and patient outcomes. Interestingly, the development of mTOR inhibitor associated NIP was correlated with improved outcomes, including time on treatment and survival, as opposed to those who did not develop NIP. In a multivariate regression analysis, NIP was a strong predictor of OS. This supports a recent published report demonstrating a relationship with mTOR inhibitor associated NIP and a trend in improved treatment outcomes. Dabydeen and colleagues reported on 46 patients with mRCC treated with either temsirolimus or everolimus.[11] Fourteen patients developed NIP on treatment, which was associated with greater disease response by RECIST. Conflicting results have been reported by White and colleagues.[10] An analysis of 37 patients who developed NIP while treated with everolimus in the RECORD 1 trial demonstrated similar PFS as compared to patients who did not develop NIP. OS was not assessed in either analysis.
Valid biomarkers to predict clinical activity and guide clinical decisions with mTOR inhibitor therapies have not been identified. Mechanism-based toxicities (MBTs) may serve as a surrogate biomarker of pharmacodynamic effect with some targeted therapies.[19, 20] Examples of MBTs with targeted therapies include rash with epidermal growth factor receptor inhibitors and hypertension or hypothyroidism with antiangiogenic therapies.[21–34] Although the pathogenesis of these MBTs has not been well characterized, they have been correlated with superior outcomes. Further studies are needed to validate mTOR inhibitor associated NIP as a predictive MBT associated with improved outcomes in patients with mRCC and in other oncologic disease settings. This question should be investigated in a prospective fashion with correlative pharmacokinetic and pharmacodynamic analyses.[35]
While our study is limited by its retrospective nature, the implications of our results are relevant to practice, in that patients with mRCC who experience NIP may be appropriately managed with supportive measures, and continued on mTOR inhibitor therapy, if anti-tumor response is achieved.[16] Patients who experience NIP do not have worse mRCC treatment related outcomes; on the contrary, in our analysis, these patients benefit the most. While more studies are needed to support this finding, it could guide mRCC therapeutic decisions and clinical monitoring. Oncologists and radiologists should be familiar with the presentation and management of mTOR inhibitor associated NIP; efforts need to be made to manage toxicities and maintain mTOR inhibitor therapy except in cases of severe NIP.
Acknowledgments
Supported in part by a Cancer Center Support Grant (CA016672) from the National Institutes of Health.
The research was supported in part by a Cancer Center Support Grant (CA016672) from the National Institutes of Health. Authors EJ and NT serve in a consultant/advisory role and receive research funding from Novartis and Pfizer.
Footnotes
Financial disclosures/disclaimers:
Bradley J. Atkinson: None
Diana H. Cauley: None
Chaan Ng: None
Randall E. Millikan: None
Lianchun Xiao: None
Paul Corn: None
Eric Jonasch: Pfizer, Novartis – consult/advisory role; Pfizer, Novartis, GSK, Aveo – research funding
Nizar M. Tannir: Pfizer, Novartis, GSK – consult/advisory role; Pfizer, Novartis, GSK – research funding
Conflicts of Interest
Authors BA, DC, CN, RM, LX, and PC have no financial relationships to disclose. We attest that we have herein disclosed any and all financial or other relationships and that all sources of financial support for this study have been disclosed and are indicated.
References
- 1.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. The New England journal of medicine. 2007 May 31;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008 Aug 9;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. [Accessed May 1, 2013];Kidney Cancer. V1. 2013 doi: 10.6004/jnccn.2009.0043. Available at http://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf. [DOI] [PubMed]
- 4.European Association of Urology. [Accessed May 1, 2013];EAU Guidelines on Renal Cell Carcinoma. 2013 Available at http://www.uroweb.org/gls/pdf/10_Renal_Cell_Carcinoma_LRV2pdf.
- 5.Soefje SA, Karnad A, Brenner AJ. Common toxicities of mammalian target of rapamycin inhibitors. Targeted oncology. 2011 Jun;6:125–129. doi: 10.1007/s11523-011-0174-9. [DOI] [PubMed] [Google Scholar]
- 6.Bellmunt J, Szczylik C, Feingold J, Strahs A, Berkenblit A. Temsirolimus safety profile and management of toxic effects in patients with advanced renal cell carcinoma and poor prognostic features. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2008 Aug;19:1387–1392. doi: 10.1093/annonc/mdn066. [DOI] [PubMed] [Google Scholar]
- 7.Maroto JP, Hudes G, Dutcher JP, et al. Drug-related pneumonitis in patients with advanced renal cell carcinoma treated with temsirolimus. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 May 1;29:1750–1756. doi: 10.1200/JCO.2010.29.2235. [DOI] [PubMed] [Google Scholar]
- 8.Duran I, Siu LL, Oza AM, et al. Characterisation of the lung toxicity of the cell cycle inhibitor temsirolimus. Eur J Cancer. 2006 Aug;42:1875–1880. doi: 10.1016/j.ejca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma : final results and analysis of prognostic factors. Cancer. 2010 Sep 15;116:4256–4265. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 10.White DA, Camus P, Endo M, et al. Noninfectious pneumonitis after everolimus therapy for advanced renal cell carcinoma. American journal of respiratory and critical care medicine. 2010 Aug 1;182:396–403. doi: 10.1164/rccm.200911-1720OC. [DOI] [PubMed] [Google Scholar]
- 11.Dabydeen DA, Jagannathan JP, Ramaiya N, et al. Pneumonitis associated with mTOR inhibitors therapy in patients with metastatic renal cell carcinoma: incidence, radiographic findings and correlation with clinical outcome. Eur J Cancer. 2012 Jul;48:1519–1524. doi: 10.1016/j.ejca.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 12.National Cancer Institute. [Accessed May 1, 2013];Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. Available at http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_403_2010-06-14_QuickReference_5x7pdf.
- 13.Dancey J. mTOR signaling and drug development in cancer. Nature reviews Clinical oncology. 2010 Apr;7:209–219. doi: 10.1038/nrclinonc.2010.21. [DOI] [PubMed] [Google Scholar]
- 14.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. The New England journal of medicine. 2012 Feb 9;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. The New England journal of medicine. 2011 Feb 10;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albiges L, Chamming's F, Duclos B, et al. Incidence and management of mTOR inhibitor-associated pneumonitis in patients with metastatic renal cell carcinoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012 Aug;23:1943–1953. doi: 10.1093/annonc/mds115. [DOI] [PubMed] [Google Scholar]
- 17.Morelon E, Stern M, Israel-Biet D, et al. Characteristics of sirolimus-associated interstitial pneumonitis in renal transplant patients. Transplantation. 2001 Sep 15;72:787–790. doi: 10.1097/00007890-200109150-00008. [DOI] [PubMed] [Google Scholar]
- 18.Pham PT, Pham PC, Danovitch GM, et al. Sirolimus-associated pulmonary toxicity. Transplantation. 2004 Apr 27;77:1215–1220. doi: 10.1097/01.tp.0000118413.92211.b6. [DOI] [PubMed] [Google Scholar]
- 19.Dancey JE, Dobbin KK, Groshen S, et al. Guidelines for the Development and Incorporation of Biomarker Studies in Early Clinical Trials of Novel Agents. Clinical Cancer Research. 2010 Mar 15;16:1745–1755. doi: 10.1158/1078-0432.CCR-09-2167. [DOI] [PubMed] [Google Scholar]
- 20.Dienstmann R, Brana I, Rodon J, Tabernero J. Toxicity as a Biomarker of Efficacy of Molecular Targeted Therapies: Focus on EGFR and VEGF Inhibiting Anticancer Drugs. Oncologist. 2011 Dec;16:1729–1740. doi: 10.1634/theoncologist.2011-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding K, Pater J, Whitehead M, Seymour L, Shepherd FA. Validation of treatment induced specific adverse effect as a predictor of treatment benefit: a case study of NCIC CTG BR21. Contemporary clinical trials. 2008 Jul;29:527–536. doi: 10.1016/j.cct.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Gatzemeier U, von Pawel J, Vynnychenko I, et al. First-cycle rash and survival in patients with advanced non-small-cell lung cancer receiving cetuximab in combination with first-line chemotherapy: a subgroup analysis of data from the FLEX phase 3 study. The lancet oncology. 2011 Jan;12:30–37. doi: 10.1016/S1470-2045(10)70278-3. [DOI] [PubMed] [Google Scholar]
- 23.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. The lancet oncology. 2010 Jan;11:21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. The New England journal of medicine. 2004 Jul 22;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 25.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007 May 1;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 26.Peeters M, Siena S, Van Cutsem E, et al. Association of progression-free survival, overall survival, and patient-reported outcomes by skin toxicity and KRAS status in patients receiving panitumumab monotherapy. Cancer. 2009 Apr 1;115:1544–1554. doi: 10.1002/cncr.24088. [DOI] [PubMed] [Google Scholar]
- 27.Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010 May 1;28:2137–2143. doi: 10.1200/JCO.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rixe O, Billemont B, Izzedine H. Hypertension as a predictive factor of Sunitinib activity. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2007 Jun;18:1117. doi: 10.1093/annonc/mdm184. [DOI] [PubMed] [Google Scholar]
- 29.Rini BI, Cohen DP, Lu DR, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. Journal of the National Cancer Institute. 2011 May 4;103:763–773. doi: 10.1093/jnci/djr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grunwald V, Fishman MN, Carducci M, et al. Clinic and Home Blood Pressure Measurements Are Reliable for Guiding Therapy in Patients with Metastatic Renal Cell Carcinoma Receiving Axitinib as First-Line Therapy. Annals of Oncology. 2012 Sep;23:268. [Google Scholar]
- 31.Rini BI, Schiller JH, Fruehauf JP, et al. Association of diastolic blood pressure (dBP) >= 90 mmHg with overall survival (OS) in patients treated with axitinib (AG-013736) Journal of Clinical Oncology. 2008 May 20;26 [Google Scholar]
- 32.Wolter P, Stefan C, Decallonne B, et al. The clinical implications of sunitinib-induced hypothyroidism: a prospective evaluation. British journal of cancer. 2008 Jul 29;99:448–454. doi: 10.1038/sj.bjc.6604497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baldazzi V, Tassi R, Lapini A, Santomaggio C, Carini M, Mazzanti R. The impact of sunitinib-induced hypothyroidism on progression-free survival of metastatic renal cancer patients: a prospective single-center study. Urologic oncology. 2012 Sep;30:704–710. doi: 10.1016/j.urolonc.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 34.Schmidinger M, Vogl UM, Bojic M, et al. Hypothyroidism in patients with renal cell carcinoma: blessing or curse? Cancer. 2011 Feb 1;117:534–544. doi: 10.1002/cncr.25422. [DOI] [PubMed] [Google Scholar]
- 35.Mandrekar SJ, Sargent DJ. Clinical trial designs for predictive biomarker validation: theoretical considerations and practical challenges. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Aug 20;27:4027–4034. doi: 10.1200/JCO.2009.22.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]