Abstract
In zebrafish, cranial sensory circuits form by 4 days post-fertilization. We used a forward genetic screen to identify genes involved in the formation of these circuits. In one mutant allele, sl23, axons arising from the epibranchial sensory ganglia do not form their stereotypical terminal fields in the hindbrain. These embryos also had small eyes and deformed jaws, suggesting a pleiotropic effect. Using positional cloning, a 20-nucleotide deletion in the carbamoyl-phosphate-synthetase2-aspartate-transcarbamylase-dihydroorotase (cad) gene was found. Injection of a CAD morpholino phenocopied the mutant and mutants were rescued by injection of cad RNA. Cad activity is required for pyrimidine biosynthesis, and thus is a prerequisite for nucleic acid production and UDP-dependent protein glycosylation. Perturbation of nucleic acid biosynthesis can result in cell death. sl23 mutants did not exhibit elevated cell death, or gross morphological changes, in their hindbrains. To determine if defective protein glycosylation was involved in the aberrant targeting of sensory axons, we treated wild type embryos with tunicamycin, which blocks N-linked protein glycosylation. Interference with glycosylation via tunicamycin treatment mimicked the sl23 phenotype. Loss of cad reveals a critical role for protein glycosylation in cranial sensory circuit formation.
Keywords: epibranchial, ganglia, zebrafish, axon guidance, glycosylation
1. INTRODUCTION
Survival of an organism is dependent upon the correct wiring of its neural circuits. In vertebrates, the cranial sensory circuits are vital, as they provide essential information from a wide variety of stimuli and organs, e.g., pain, touch and temperature from the head/throat, taste, blood pH and arterial pressure, and organ distension originating from thoracic and abdominal viscera. Many of the cranial nerves that carry afferent sensory information also contain efferent motor axons, and all cranial nerves contain glial elements. We have previously shown in zebrafish that sensory axon pathfinding from the epibranchial ganglia to the hindbrain involves interactions with both branchiomotor axons and peripheral glia and occurs during the first four days post-fertilization (dpf) (Cox et al., 2011). The mechanisms underlying these cell-cell interactions are poorly understood.
Very little is known about the gene networks responsible for establishing the connectivities of these circuits. However, the zebrafish offers the opportunity to identify genes involved in developmental processes, in an unbiased manner, using forward genetic screens (Driever et al., 1996). We have taken advantage of this attribute by engineering a transgenic zebrafish line Tg(p2xr3.2:gfp) that expresses eGFP (green fluorescent protein) in nearly all peripheral sensory neurons, beginning in embryogenesis and continuing into adulthood (Kucenas et al., 2006). The eGFP fills the cell body together with its processes, allowing for visualization of the limbs of sensory circuits as they form and are maintained in the fish. Using this line, we carried out an ethylnitrosourea-based forward screen to identify genes involved in cranial sensory circuit formation. In this paper, we describe one mutant allele, sl23, in which the terminal fields of the central projections of the facial (gVII), glossopharyngeal (gIX) and vagal (gX) ganglia do not form properly. Using positional cloning techniques, we have identified the sl23 mutation as a deletion within the gene encoding the carbamoyl-phosphate-synthetase2-aspartate trancarbamylase-dihydroorotase (Cad) enzyme, which results in a null protein. This enzyme is responsible for the rate limiting step in the pyrimidine biosynthesis pathway and is essential for the production of the UDP-sugars required for protein glycosylation (Jones, 1980). The results presented here indicate that defective protein glycosylation plays a major role in the sensory axon malformations seen in these mutants, although defects in nucleic acid synthesis leading to perturbations in cell cycle and cell death may also contribute to the observed phenotype.
2. MATERIALS AND METHODS
2.1 Maintenance of fish
Fish were kept on a 14-hr day, 10-hr night schedule at a constant 28.5 °C with feeding done twice daily. All animal husbandry was carried out as described by Westerfield (Westerfield, 2000). Embryos were staged according to hours post-fertilization (hpf) and morphological criteria (Kimmel et al., 1995). Embryos used for microscopy were treated with 0.003% phenylthiourea to reduce pigmentation. The Tg(p2xr3.2:eGFP) line has been previously described (Kucenas et al., 2009; Kucenas et al., 2006) and Tg(isl1:eGFP) (Higashijima et al., 2000) fish were a gift from H. Okamoto.
2.2 Imaging of embryos/larvae
Epifluorescent microscopy was carried out on embryos/larvae that were anesthetized with 0.01% Tricaine (ethyl 3-aminobenzoate methanesulfonate salt) in fish water and transferred to a 96-well plate. Images were obtained using a Nikon TE200 inverted microscope equipped with a CoolSNAP HQ digital camera. MetaMorph software (Universal Imaging Corp) was used to acquire and process images. Cropping and rotating of images was carried out using Adobe Photoshop. For confocal microscopy, embryos/larvae were embedded in 1 % low-melting point agarose containing 0.01% Tricaine. Imaging was performed with an Olympus FV1000 MPE using a 20×/0.95 water immersion objective. Images collected in the z-dimension were collapsed into one maximal intensity projection or were rendered into 3-dimensions using either Olympus Fluoview or NIH ImageJ software. Final brightness and/or contrast values of images were adjusted in Adobe Photoshop CS3.
2.3 Alcian blue staining
5 dpf larvae were fixed overnight at 4°C in 4 % paraformaldehyde/PBS. They were then dehydrated with sequential incubations in 50% ethanol and then 100% ethanol. Embryos were incubated overnight in 0.1% Alcian blue solution in 70% ethanol/30% acetic acid, neutralized in saturated sodium borate solution for 2 hours, digested in trypsin (2 mg/ml in 30% saturated borate) at 37°C for 1 hour and then bleached in 5% hydrogen peroxide/1% KOH for 2–3. They were then washed with PBS and fixed in 4% paraformaldehyde/PBS at 4°C overnight. For imaging, embryos were embedded in 1% agarose and viewed using an Olympus BX60 upright microscope equipped with an Olympus DP71 digital camera. Olympus software was used to acquire and process images. Cropping and rotating of images was done in Adobe Photoshop.
2.4 Embryo injections
RNA or morpholino oligonucleotide (MO) was injected into single-cell embryos using a Picospritzer III (General Valve Corporation, Fairfield, NJ) attached to a broken glass capillary. Full length Cad cDNA was subcloned into pCR-Blunt (Invitrogen) and linearized with Spe I. Capped RNA was transcribed in vitro using the T7 Amplicap kit (Epicentre). The RNA was dissolved in diethylpyrocarbonate-treated distilled H2O and mixed 1:1 with injection buffer (0.1 M KCl, 20 mM HEPES (pH 7.4) and 0.01% Phenol Red). A 1mM solution of Cad morpholino (TAAAGATGCCATTTTCAGCGACATG) (Willer et al., 2005), which overlaps the start codon, was heated at 65 °C for 10 minutes, to ensure it was in solution. The solution was then diluted to 500 µM with distilled H2O and mixed 1:1 with injection buffer, to give a 250 µM solution (~2.5 ng/nl).
2.5 Positional cloning
Standard mapping methods using bulk segregant and meiotic recombination analysis of microsatellite markers (Green et al., 2009) were used to map the sl23 mutation to chromosome 20. Total RNA was then extracted from mutant and wild type 4 dpf larvae and cDNA synthesized with Superscript III reverse transcriptase (Invitrogen). Sequences corresponding to candidate genes were then obtained by PCR and sequenced using gene specific primers.
2.6 Immunohistochemistry
Embryos/larvae were fixed in 4% paraformaldehyde/PBS at 4°C overnight and then washed 3× for 5 min in PBS-1% TritonX-100 (PBT). The larvae were then treated with 0.25% trypsin in PBS on ice for 10–15 min and then washed 3× for 10 min in PBT. After incubation in a blocking solution (2% normal goat serum/1% DMSO/2 mg/ml BSA in PBT) for one hour at room temperature, the embryos were incubated with mouse anti-acetylated tubulin (1:1000) (Sigma), mouse anti-zrf-1 (1:500) (ZIRC) or rabbit anti-phospho-histone 3 (1:750) (Santa Cruz) antibody overnight at 4 °C. Embryos were then washed in PBT for 3 hours with at least 4 changes and then incubated with rabbit anti-mouse or goat anti-rabbit Alexa 568-conjugated secondary antibodies (1:1000) (Invitrogen) overnight at 4 °C. Embryos were then washed extensively and maintained in PBS at 4 °C for up to a week. Images were obtained by confocal microscopy as described above.
2.7 Acridine orange assay
Live dechorionated embryos are incubated in a 2 µg/ml solution of acridine orange (Sigma) in PBS for 20 minutes at room temperature. Embryos are then washed 4 × 1 minutes in fish water and then visualized using epifluorescence.
2.8 Tunicamycin treatment
Tunicamycin (Sigma) was dissolved in fish water at a stock concentration of 1 mg/ml in DMSO. Embryos at the 16 hpf stage were placed into a 96 well plate and the fish water replaced with new water containing tunicamycin at a final concentration of 1 µg/ml. Embryos were kept in drug for 24 hpf, at which time they were washed and imaged by epifluorescence microscopy.
3. RESULTS AND DISCUSSION
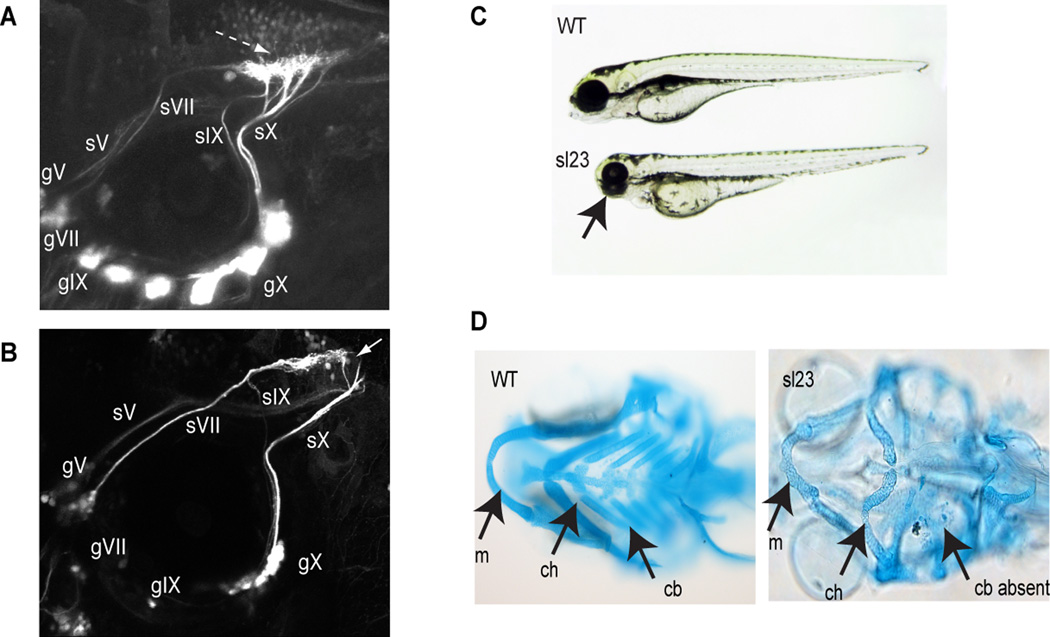
We have carried out a forward genetic screen to identify genes involved in branchiomeric sensory axon pathfinding, and have identified a number of mutant lines that displayed perturbations in sensory circuits. One of these alleles, sl23, exhibited defective circuit formation of the epibranchial afferent nerves (Figure 1). When live larvae were examined using confocal microscopy, all epibranchial ganglia were found in their stereotypical positions around the ventral margins of the otic vesicle, although the facial (gVII), glossopharyngeal (gIX) and vagal (gX) ganglia appeared reduced in size when compared to wild-type Tg(p2xr3.2:gfp) larvae (Figure 1A and B). The sensory axons from the VIIth and IXth ganglia projected to the hindbrain following their normal trajectories; however, once in the CNS, perturbations in afferent axon pathfinding within the hindbrain were observed, with the epibranchial axons unable to form the stereotypical hindbrain plexus seen in wild type embryos (n=1062 mutants) (Figure 1B). In some cases, the IXth axons are very faint and difficult to observe, as there appears to be less axons in the mutant compared to wild type and their trajectory take them close to the axons of the Xth. The sensory axons of the Xth do not defasciculate into their typical branching pattern as they course alongside the external surface of the hindbrain, but instead remain as one or a few bundles as they enter the CNS. Again, their terminal fields in the hindbrain are malformed.
Figure 1. The sl23 allele affects cranial sensory circuit formation.
(A) Confocal image of control wild type fish showing normal pattern of cranial sensory ganglia (CSG) projections. Dashed arrow shows normal hindbrain plexus, where the sVII, sIX and sX nerves terminate. sV, sVII, sIX and sX designate the axons of their respective sensory ganglia (gV, gVII, gIX, gX). (B) CSG projections in sl23 mutants. Note apparent decreased size of ganglia compared to the wild type. Of special note is the absence of any well-defined hindbrain plexus (white arrow), despite the sensory axons pathfinding to the correct region of the hindbrain. (C) Brightfield image of 4 dpf wild type and sl23 mutant larvae show that the sl23 allele is pleiotropic in nature. Notice that the mutant exhibits reductions in the size of the head, eyes and jaws (arrow). (D) Ventral views of Alcian blue stained pharyngeal cartilages of 5 dpf wild type and sl23 mutant larvae. Note the shortened Meckels cartilage (m), deformed ceratohyal cartilage (ch) and absence of ceratobranchial cartilages (cb) in the sl23 mutant.
In addition to the epibranchial nerve defects, sl23 homozygotes possessed other developmental dysmorphisms: they were smaller than their wild-type clutch mates, had smaller eyes (Figure 1C) and died between 8 and 10 days post-fertilization. In addition, their pharyngeal cartilages were malformed, with inverted and reduced ceratohyals, deformed Meckels cartilages and a loss of ceratobranchials (Figure 1D). Pigment formation was not affected. Together, these findings suggested that the mutated gene in sl23 is not specific to axon guidance, but instead functions in a pleiotropic manner across many cell types.
3.1 sl23 is a truncated CAD
Genetic analysis demonstrated that the sl23 mutation was recessive and possessed a Mendelian inheritance pattern: on average, mutants comprised 29 ± 2% of larvae in a clutch (n= 908, from 6 clutches). Using positional cloning, we localized the sl23 lesion to a region of chromosome 20 (20:38,670,000–39,330,000) that contains all or parts of the mpv17, trim54, cad, ift172, msra, rcan and reps1 genes (Fig. 2A). Sequencing of candidate genes in this critical interval revealed a 20 bp deletion in exon 5 of the carbamoyl-phosphate synthetase 2-aspartate transcarbamylase-dihydroorotase (cad) gene (Figure 2B). Although insertions and deletions are not common in zebrafish ENU screens, they have been reported in other systems (Watson et al., 1998). This gene encodes a multifunctional 2,230 amino acid protein (Figure 2C) whose activities comprise the first three steps in de novo pyrimidine biosynthesis (Sigoillot et al., 2002) and which is responsible for the rate-limiting step in this pathway. The observed deletion excises base pairs 510–528 (corresponding to aa 170–175 of the wild-type protein), resulting in a frame-shift into an alternative reading frame that contains a stop codon 105 bp downstream of the deletion (Figure 2B). The protein predicted to arise from this allele ends in the middle of the glutamine amidotransferase domain upstream of the CPSase A, CPSase B, dihydroorotase and aspartate transcarbamylase domains (Figure 2C) and thus is likely a null mutation. A different mutant allele of Cad, perplexed (Plxa52), has been described and it displays the same eye and jaw phenotypes of sl23. (Willer et al., 2005). Other mutant alleles of Cad have been reported in two zebrafish insertional mutagenesis screens, but these have not been characterized (Amsterdam et al., 2004; Wang et al., 2007). Willer and colleagues (Willer et al., 2005) partially rescued their mutant phenotype using injections of either orotic acid or uridine into the yolk. Such injections in our hands were unable produce a convincing rescue (data not shown); this could be due to differences in either the alleles and/or the strain backgrounds. We next performed RNA rescue experiments using full length capped RNA encoding wild-type Cad. Injection of this RNA into embryos from sl23+/− incrosses (n= 388) resulted in a 40% reduction in the number of mutant larvae detected at 4 dpf when compared to controls (Figure 2E), confirming that the phenotype arises from the defective cad gene. To further confirm that the deletion in cad was responsible for the observed phenotype, we injected a previously characterized morpholino targeting the start codon of cad (CAD MO3) (Willer et al., 2005) into Tg(p2rx3.2:gfp) embryos. At 4 dpf, larvae injected with CAD MO3 (250 µM) demonstrated a phenotype that replicated that seen in the sl23−/− larvae: misrouted sensory axons of the VIIth, IXth and Xth ganglia, with an example shown in Figure 2D. A total of 33 embryos were injected and 29 (88%) of these showed defects (p<0.0001, Fisher’s Chi-square test). The morphants also displayed deformed jaws and small eyes, as observed in the mutants (data not shown).
Figure 2. Identification of a defective cad allele as the gene responsible for the sl23 phenotype.

(A) Critical interval for the sl23 locus on chromosome 20, cad gene is in red. Arrows denote direction of transcription, numbers above map coordinates represent the number of recombinants out of 652 mutant larvae. (B) Alignment of the nucleotide sequences from exon 5 of the wild type and sl23 alleles: the missing 20 nucleotides are shown as dashes. The predicted frame shift encodes a novel amino acid sequence (shown in red) containing a stop codon. (C) Diagram of domains in the wildtype protein and the predicted null protein, showing the position of the deletion in sl23. (D) Rescue of the sl23 phenotype after injection of full-length wild-type Cad RNA into clutches of sl23+/− incrosses, supporting the mutant CAD gene as responsible for the sl23 phenotype. The data are shown as a bar graph (p≤0.1, one tail t-test). (E) Epifluorescent image of Tg(p2xr3.2:eGFP) injected with 250 µM Cad ATG morpholino. As seen in sl23 mutants, the central projections of the VII, IX and X sensory nerves are malformed.
Homozygous mutants cannot use zygotic Cad to synthesize dihydroorotate, the precursor of UMP, which in turn is the precursor of CTP, TTP, and UTP. Defects in the metabolism of these nucleotides leads to perturbations in DNA and RNA synthesis, and to alterations in post-translational protein modifications due to changes in UDP-sugar metabolism. However, the ability of sl23−/− embryos to develop for several days, before dying at around 8 days, suggests maternally derived stores of Cad and/or pyrimidine nucleotides are present early but become greatly diminished during early stages of development. This is supported from a previous study (Willer et al., 2005) demonstrating the presence of maternal cad mRNA in zebrafish embryos prior to zygotic gene expression. It is obvious, however, that this maternal contribution is not sufficient to enable correct sensory circuit formation in the mutants. Our next question was whether these circuit defects resulted from disturbances in DNA/RNA synthesis and/or protein glycosylation due to lack of Cad.
3.2 Loss of CAD does not result in gross perturbation of hindbrain organization
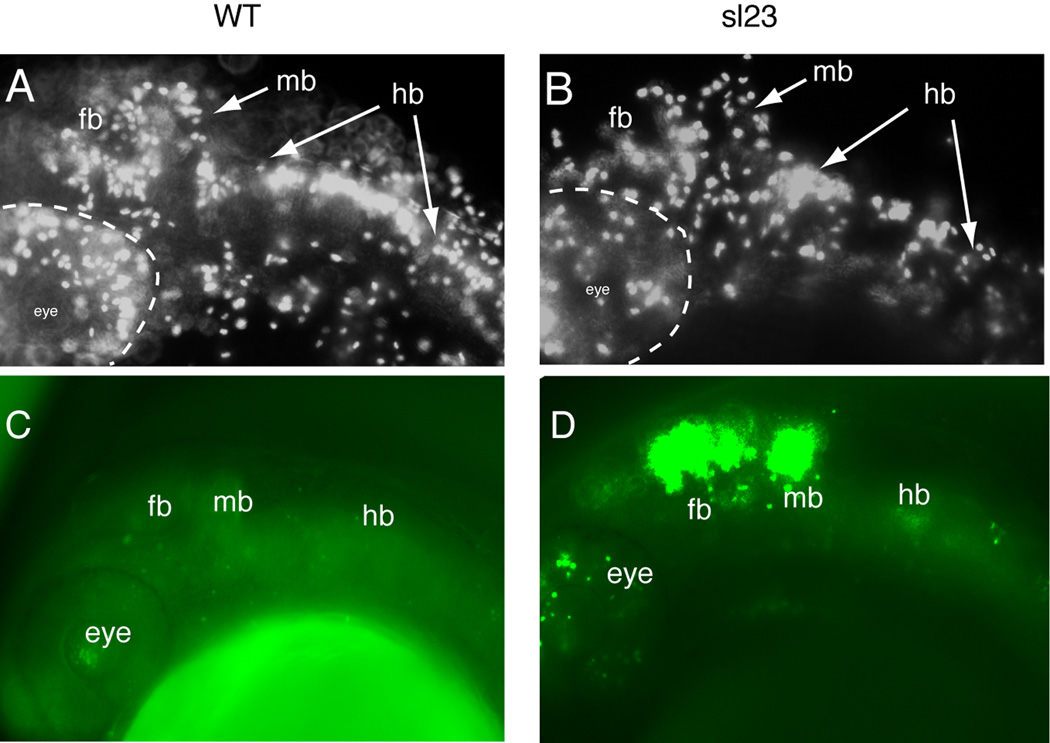
There are three steps in establishing the connections of the epibranchial neurons with their CNS targets: extension of the axon to the correct hindbrain entry zone, the continued growth of the axon to the appropriate region of the hindbrain, and the formation of synapses within the target regions (Cox et al., 2011). In the sl23 mutants, the epibranchial afferents find their way to their hindbrain target region in rhombomeres 6–8, demonstrating that the first two steps in connectivity are not affected by the loss of Cad. However, the afferents do not form the prototypical arborized terminal field, or plexus, in the hindbrain as seen in wild-type siblings (Figure 1B). Disruption of Cad activity results in defective nucleic acid biosynthesis, causing perturbations in cell cycle kinetics leading to alterations in proliferation and/or cell death. Phospho-histone 3 is a useful marker for cells in the G2 phase of mitosis (Tapia et al., 2006). Immunostaining of 30 hpf sl23−/− embryos revealed a decrease in hindbrain labeling when compared to controls (Figure 3 A,B). This decrease could be due to perturbations in cell cycle, loss of cells through apoptosis or a combination of the two. Acridine orange staining, used to identify dead/dying cells in vivo, revealed minimal cell death in the caudal hindbrain at 24 hpf, even though elevated cell loss was detected in the regions of the optic tectum and midbrain/cerebellum (Figure 3C and D). At 48 hpf, there was still no detectable increase in hindbrain cell death in the mutants (data not shown). Together, these data suggest that the decrease in mitotic cells within the hindbrain is not a result of elevated cell death, but instead is most likely due to alterations in cell cycle kinetics: this is consistent with a previous report describing a cad mutant in zebrafish (Willer et al., 2005).
Figure 3. sl23 mutants show alterations in mitotic and apoptotic activities.
Anti-phospho-histone 3 staining of wild-type (WT) (A) and sl23 mutants (B) showed a decreased labeling throughout the head of the mutants, suggesting a reduction in the numbers of mitotically active cells. Acridine orange staining of dead cells in 24 hpf WT embryos (C) and sl23 mutant embryos (D) shows elevated cell death in the head of sl23 mutants, especially in the eye, forebrain and midbrain. Staining is much less in the hindbrain (hb).
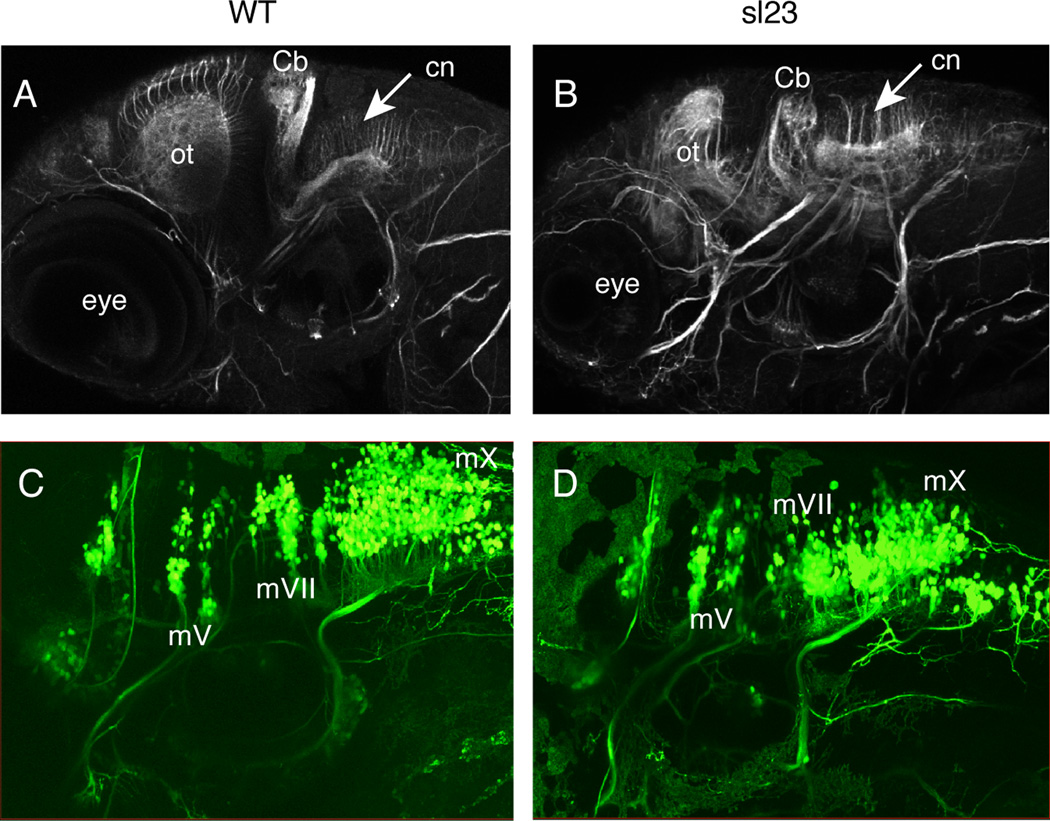
Acetylated tubulin immunostaining at 4 dpf in control fish (Figure 4A) revealed the normal distribution of commissural axons in the hindbrain. In mutants (Figure 4B) the commissural axons appear slightly disorganized and shortened, but no gross defects in hindbrain organization were observed. In contrast, the optic tectum and midbrain/cerebellum, areas where increased cell death did occur, showed clear disruptions in their architecture (Figure 4B). Further support for proper hindbrain organization in the mutants derives from three sets of studies. In the first, we investigated the branchiomotor neuronal nuclei using the Tg(isl:gfp) transgenic line. In sl23 mutant embryos, the isl:gfp transgene reveals that these nuclei developed normally, with their positions and projections indistinguishable from their wild-type siblings (Figure 4C and D). In the second set of experiments, immunostaining of 30 hpf embryos using zrf-1 to delineate rhombomere boundaries (Trevarrow et al., 1990) did not show any differences between mutants and their wild-type siblings (n=100 total embryos examined, data not shown). Finally, 48 hpf mutant embryos exhibited stereotypical escape behavior when a touch stimulus was applied to their heads (n=10/10 mutants), demonstrating that the trigeminal sensory and reticulospinal motor circuits were intact and functional. These findings suggest that the aberrant terminal fields of the epibranchial axons in the mutant are not caused by a major disruption of hindbrain architecture, and therefore we next tested if altered protein glycosylation played a role in the observed phenotype.
Figure 4. The hindbrain does not appear to be grossly affected in sl23 mutants.
Anti-acetylated tubulin immunostaining of 4 dpf WT (A) and sl23 mutant (B) larvae shows decreased size and structure of the optic tectum (ot) and cerebellum (Cb). Acetylated tubulin staining in the hindbrain of sl23 mutants appears to be almost normal, with the commissural neurons (cn) extending their axons dorsally, similar to the wildtype. There are no gross differences in the peripheral nerves between the sl23 mutants and WT. Confocal images of branchiomotor nerves at 4 dpf in WT (C) and sl23 mutant (D) larvae expressing the isl:gfp transgene show that the segmental organization of the motor nuclei in the hindbrain appears normal in sl23 compared to WT. The IXth and Xth motor nuclei in sl23 are a little compressed rostral to caudal, but their projections are normal. mV, Vth cranial motor nerve; mVII, VIIth cranial motor nerve; mX, Xth cranial motor nerve.
3.3 Disruption of glycosylation alters cranial sensory circuit formation
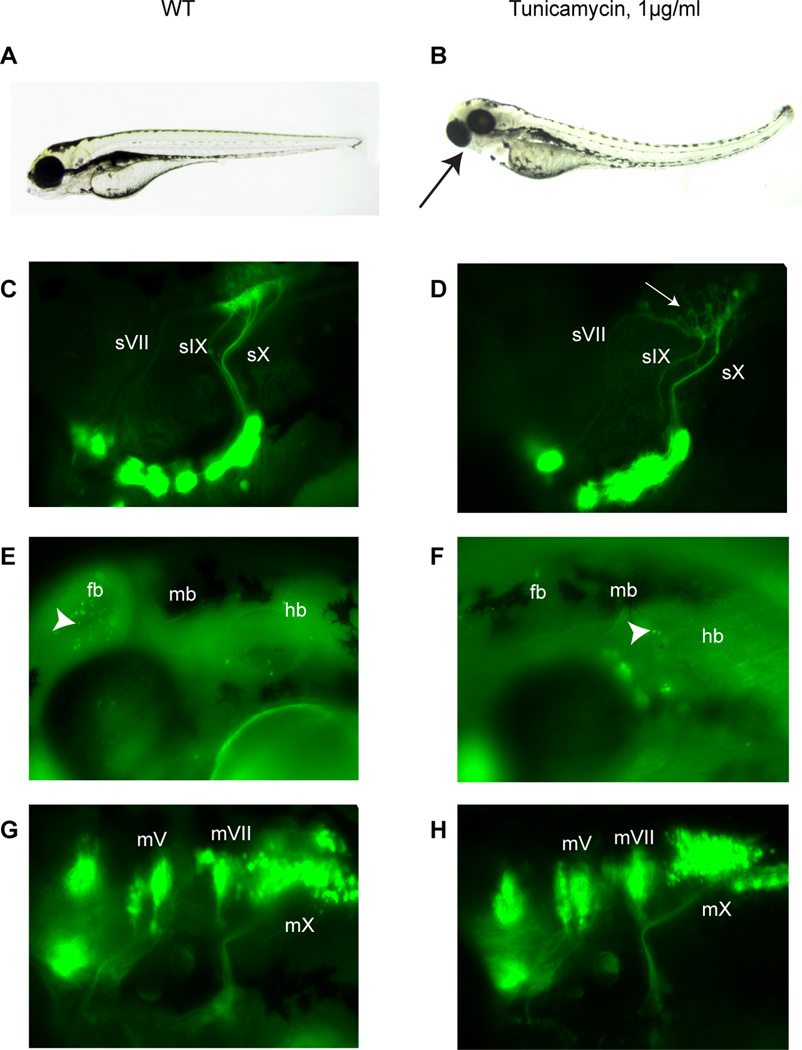
Pyrimidines are required for the synthesis of UDP-sugars, which are essential for protein glycosylation. The absence of this post-translational modification has been found to alter the targeting of proteins to the membrane or the secretory pathway (Helenius, 1994; Torres et al., 1998), and therefore can have profound effects on molecules critical for normal cellular function. In the plxa52 mutant, Willer and colleagues (Willer et al., 2005), using thymidylate synthase mutants, found that defective retinal differentiation arose from a lack of UDP-dependent glycosylation. To test whether defective glycosylation might be involved in the sl23 afferent circuit phenotype, we treated wild-type Tg(p2xr3.2:gfp) fish with tunicamycin (1 µg/ml). This compound inhibits the enzyme dolichyl-phosphate (UDP-N-acetylglucosamine) transferase, which is required for N-linked protein glycosylation in the endoplasmic reticulum (Heifetz et al., 1979). Wild-type embryos were treated from 16–40 hpf (a timeframe before the formation of the epibranchial sensory circuits). The treated embryos (Figure 5B) had malformed jaw cartilages and small eyes reminiscent of the sl23 phenotype. As can be seen in Figure 5D (compared to untreated Figure 5C), the VIIth, IXth and Xth nerves find their way to the hindbrain, but the sensory plexus fails to form correctly, similar to the sl23 mutant phenotype. As with the sl23 mutants, the Vth sensory axons were unaffected. One potential complication with the use of tunicamycin is the induction of the unfolded protein response (UPR), brought about by misfolding of proteins in the ER due to the lack of N-linked glycosylation (Travers et al., 2000). The UPR can lead to apoptosis of the affected cell (Travers et al., 2000), and thus loss of cells could account for the tunicamycin phenotype. This possibility was assessed by acridine orange staining of embryos treated with tunicamycin (1 mg/ml) from 16–40 hpf: this treatment regimen did not result in an appreciable increase in cell death compared to controls (Figure 5E,F), indicating that the dose of tunicamycin used did not cause a substantial UPR-induced apoptosis. Since previous work has shown that the sensory circuit formation depends upon the presence of branchiomotor neurons in the hindbrain (Cox et al., 2011), and the bmn are unaffected in the sl23 mutants (Figure 4D), we wanted to demonstrate that the tunicamycin effects were not due simply to perturbations in the bmn. Treatment of Tg(isl:gfp) with tunicamycin at 16 hpf, a time before the formation of the VIIth, IXth and Xth bmn (Cox et al., 2011), revealed that inhibition of N-linked glycosylation after that developmental time point did not cause a defect in either their organization in the hindbrain or their projections to the periphery (Figure 5G,H).
Figure 5. sl23 phenotype is mimicked by tunicamycin treatment of wild type larvae.
Brightfield images of 4 dpf control (A) and treated (tunicamycin at 1µg/ml from 16–40 hpf) larvae (B). Black arrow in panel B indicates small eyes and deformed jaws in tunicamycin treated larvae at 4 dpf. Epifluorescent images of cranial sensory ganglia and their projections at 4 dpf in control (C), and tunicamycin treated Tg(p2rx3.2:gfp) larvae (D). White arrow indicates the malformed plexus. sV, Vth cranial sensory nerve; sVII, VIIth cranial sensory nerve; sIX, IXth cranial sensory nerve; sX, Xth cranial sensory nerve. Acridine orange staining of dead cells in the head of 48 hpf control (E) and tunicamycin treated (1µg/ml from 16–40 hpf) (F) embryos shows that the concentration of tunicamycin used did not increase cell death in the hindbrain. Arrowheads in both panels indicate Dead cells revealed by staining. fb, forebrain; mb, midbrain; hb, hindbrain. Epifluorescent images of branchiomotor nerves at 4 dpf in Tg(isl:gfp) (G) and tunicamycin-treated Tg(isl:gfp) (H) larvae. The segmental organization of the motor nuclei in the hindbrain appears normal in treated larvae. All views lateral, anterior to the left. mV, Vth cranial motor nerve; mVII, VIIth cranial motor nerve; mX, Xth cranial motor nerve.
sl23 was identified in a forward genetic screen to find mutants with defective peripheral sensory circuit formation. A small deletion in the gene encoding Cad, the rate-limiting enzyme in pyrimidine synthesis, was found to be the lesion responsible for the sl23 phenotype. Our findings suggest that the defect in terminal fields of the epibranchial sensory axons in the hindbrain arise from alterations in protein glycosylation brought about by loss of Cad activity and thus further supports N-linked glycosylation as having an important role in the formation of sensory circuits in the vertebrate head.
Supplementary Material
Highlights.
sensory axon terminations in the hindbrain are disrupted in a cad null mutant
tunicamycin mimics the cad null phenotype
protein glycosylation is critical for sensory circuit formation
ACKNOWLEDGMENTS
We would like to thank Rachael Sheridan for help with the fish husbandry, Dr. Bruce Appel for providing ENU-mutagenized males to cross with our transgenic line, Dr. H. Okamoto for the gift of his Tg(isl:gfp) fish line and Ryan McAdow for help in the positional cloning experiments. This work was supported by NIH grants to S.L.J. (GM56988) and M.M.V. (NS060074).
Abbreviations
- cad
carbamoyl-phosphate synthetase 2-aspartate transcarbamylase-dihydroorotase
- sl23
Tg(p2xr3.2:gfp)sl23
- plxa52
perplexed mutant
- UPR
unfolded protein response
- bmn
branchiomotor neurons
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jane A. Cox, Email: coxj@slu.edu.
Angela LaMora, Email: angie_lamora@hotmail.com.
Stephen L. Johnson, Email: sjohnson@genetics.wustl.edu.
Mark M. Voigt, Email: voigtm@slu.edu.
REFERENCES
- Amsterdam A, Nissen RM, Sun Z, Swindell EC, Farrington S, Hopkins N. Identification of 315 genes essential for early zebrafish development. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:12792–12797. doi: 10.1073/pnas.0403929101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JA, Lamora A, Johnson SL, Voigt MM. Diverse mechanisms for assembly of branchiomeric nerves. Dev Biol. 2011;357:305–317. doi: 10.1016/j.ydbio.2011.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever W, Solnica-Krezel L, Schier AF, Neuhauss SC, Malicki J, Stemple DL, Stainier DY, Zwartkruis F, Abdelilah S, Rangini Z, Belak J, Boggs C. A genetic screen for mutations affecting embryogenesis in zebrafish. Development. 1996;123:37–46. doi: 10.1242/dev.123.1.37. [DOI] [PubMed] [Google Scholar]
- Green J, Taylor JJ, Hindes A, Johnson SL, Goldsmith MI. A gain of function mutation causing skeletal overgrowth in the rapunzel mutant. Developmental Biology. 2009;334:224–234. doi: 10.1016/j.ydbio.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifetz A, Keenan RW, Elbein AD. Mechanism of action of tunicamycin on the UDP-GlcNAc:dolichyl-phosphate Glc-NAc-1-phosphate transferase. Biochemistry. 1979;18:2186–2192. doi: 10.1021/bi00578a008. [DOI] [PubMed] [Google Scholar]
- Helenius A. How N-linked oligosaccharides affect glycoprotein folding in the endoplasmic reticulum. Mol Biol Cell. 1994;5:253–265. doi: 10.1091/mbc.5.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashijima S, Hotta Y, Okamoto H. Visualization of cranial motor neurons in live transgenic zebrafish expressing green fluorescent protein under the control of the islet-1 promoter/enhancer. J Neurosci. 2000;20:206–218. doi: 10.1523/JNEUROSCI.20-01-00206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ME. Pyrimidine nucleotide biosynthesis in animals: genes, enzymes, and regulation of UMP biosynthesis. Annual review of biochemistry. 1980;49:253–279. doi: 10.1146/annurev.bi.49.070180.001345. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kucenas S, Cox JA, Soto F, Lamora A, Voigt MM. Ectodermal P2X receptor function plays a pivotal role in craniofacial development of the zebrafish. Purinergic Signal. 2009;5:395–407. doi: 10.1007/s11302-009-9165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucenas S, Soto F, Cox JA, Voigt MM. Selective labeling of central and peripheral sensory neurons in the developing zebrafish using P2X(3) receptor subunit transgenes. Neuroscience. 2006 doi: 10.1016/j.neuroscience.2005.11.058. [DOI] [PubMed] [Google Scholar]
- Sigoillot FD, Evans DR, Guy HI. Autophosphorylation of the mammalian multifunctional protein that initiates de novo pyrimidine biosynthesis. The Journal of biological chemistry. 2002;277:24809–24817. doi: 10.1074/jbc.M203512200. [DOI] [PubMed] [Google Scholar]
- Tapia C, Kutzner H, Mentzel T, Savic S, Baumhoer D, Glatz K. Two mitosis-specific antibodies, MPM-2 and phospho-histone H3 (Ser28), allow rapid and precise determination of mitotic activity. The American journal of surgical pathology. 2006;30:83–89. doi: 10.1097/01.pas.0000183572.94140.43. [DOI] [PubMed] [Google Scholar]
- Torres GE, Egan TM, Voigt MM. N-Linked glycosylation is essential for the functional expression of the recombinant P2X2 receptor. Biochemistry. 1998;37:14845–14851. doi: 10.1021/bi981209g. [DOI] [PubMed] [Google Scholar]
- Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101:249–258. doi: 10.1016/s0092-8674(00)80835-1. [DOI] [PubMed] [Google Scholar]
- Trevarrow B, Marks DL, Kimmel CB. Organization of hindbrain segments in the zebrafish embryo. Neuron. 1990;4:669–679. doi: 10.1016/0896-6273(90)90194-k. [DOI] [PubMed] [Google Scholar]
- Wang D, Jao LE, Zheng N, Dolan K, Ivey J, Zonies S, Wu X, Wu K, Yang H, Meng Q, Zhu Z, Zhang B, Lin S, Burgess SM. Efficient genome-wide mutagenesis of zebrafish genes by retroviral insertions. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12428–12433. doi: 10.1073/pnas.0705502104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DE, Cunningham ML, Tindall KR. Spontaneous and ENU-induced mutation spectra at the cII locus in Big Blue Rat2 embryonic fibroblasts. Mutagenesis. 1998;13:487–497. doi: 10.1093/mutage/13.5.487. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. Eugene, OR: University of Oregon; 2000. [Google Scholar]
- Willer GB, Lee VM, Gregg RG, Link BA. Analysis of the Zebrafish perplexed mutation reveals tissue-specific roles for de novo pyrimidine synthesis during development. Genetics. 2005;170:1827–1837. doi: 10.1534/genetics.105.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.