Abstract
Originally synthesized for research purposes, indole- and pyrrole-derived synthetic cannabinoids are the most common psychoactive compounds contained in abused products marketed as “spice” or “herbal incense.” While CB1 and CB2 receptor affinities are available for most of these research chemicals, in vivo pharmacological data are sparse. In mice, cannabinoids produce a characteristic profile of dose-dependent effects: antinociception, hypothermia, catalepsy and suppression of locomotion. In combination with receptor binding data, this tetrad battery has been useful in evaluation of the relationship between the structural features of synthetic cannabinoids and their in vivo cannabimimetic activity. Here, published tetrad studies are reviewed and additional in vivo data on synthetic cannabinoids are presented. Overall, the best predictor of likely cannabimimetic effects in the tetrad tests was good CB1 receptor affinity. Further, retention of good CB1 affinity and in vivo activity was observed across a wide array of structural manipulations of substituents of the prototypic aminoalkylindole molecule WIN55,212-2, including substitution of an alkyl for the morpholino group, replacement of an indole core with a pyrrole or phenylpyrrole, substitution of a phenylacetyl or tetramethylcyclopropyl group for JWH-018’s naphthoyl, and halogenation of the naphthoyl group. This flexibility of cannabinoid ligand-receptor interactions has been a particular challenge for forensic scientists who have struggled to identify and regulate each new compound as it has appeared on the drug market. One of the most pressing future research needs is determination of the extent to which the pharmacology of these synthetic cannabinoids may differ from those of classical cannabinoids.
Keywords: alkylindoles, aromatic stacking, cannabinoids, herbal marijuana, indoles, JWH-018, pyrroles, review, spice, synthetic cannabinoids
Introduction
The use of marijuana and other constituents of the cannabis plant (e.g., hashish) for medicinal and religious purposes can be traced back to ancient China. Yet, intensive scientific interest in marijuana awaited development of appropriate tools, the first of which arrived in 1964 when Dr. Raphael Mechoulam and colleagues isolated and identified Δ9-tetrahydrocannabinol (Δ9-THC) as the primary psychoactive substituent of the marijuana plant (Gaoni and Mechoulam, 1964). Continuing his work in the emerging field of cannabinoid science, Professor Mechoulam founded a lab that served as an incubator for several foundational discoveries that occurred later in the 1980’s and 1990’s. During these formative years, major research findings included discovery of the endocannabinoid system (Devane, et al., 1992; Hanus, et al., 2001; Mechoulam, et al., 1995), identification and cloning of the CB1 and CB2 subtypes of cannabinoid receptor (Devane, et al., 1988; Matsuda, et al., 1990; Munro, et al., 1993), and synthesis of selective antagonists for each of these receptors (Rinaldi-Carmona, et al., 1994; Rinaldi-Carmona, et al., 1998). Although cannabinoid agonists used in initial in vivo research studies were phytocannabinoids such as Δ9-THC and cannabidiol (Compton, et al., 1990; Martin, et al., 1981), these plant-derived cannabinoids soon shared the research arena with synthetic cannabinoids, including the novel classes of bicyclic cannabinoids (e.g., CP55,940; Little, et al., 1988) and the aminoalkylindoles (e.g., WIN55,212-2; Compton, et al., 1992), developed by scientists at Pfizer and Sterling-Winthrop, respectively, as well as the endocannabinoids (e.g., anandamide and 2-arachidonoylglycerol; Devane, et al., 1992; Mechoulam, et al., 1995).
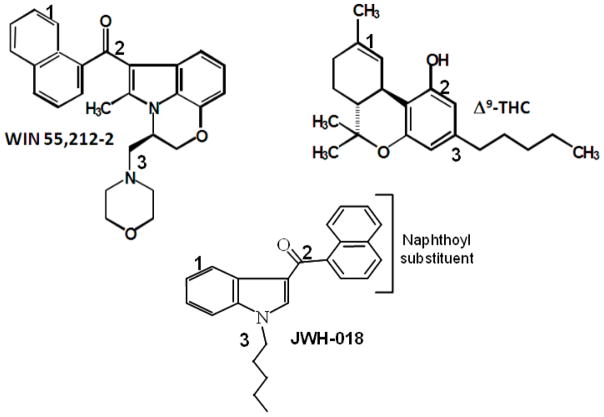
The synthetic cannabinoids, which were later hijacked for use in designer drugs labeled as “herbal incense,” were originally synthesized in this milieu (Huffman, et al., 1994). JWH-018 [1-pentyl-3-(1-naphthoyl)indole], one of the first such compound identified in a forensic sample (Lindigkeit, et al., 2009), and other structurally related synthetic cannabinoids were created as part of a research program directed by Dr. John Huffman at Clemson University, Clemson, South Carolina. In effort to determine how such structurally diverse molecules as Δ9-THC and WIN55,212-2 could fit into the same receptor, Dr. Huffman considered the then popular 3-point attachment model of cannabinoid ligand-receptor interaction (Thomas, et al., 1991) and hypothesized that the attachment location for the morpholino group of WIN would correspond with the pentyl side chain of Δ9-THC (Figure 1). To test this hypothesis, he synthesized a series of indole- and pyrrole-derived cannabinoids and elicited the assistance of Dr. Billy Martin and colleagues at Virginia Commonwealth University, Richmond, Virginia to evaluate them. Dr. Martin’s structure-activity relationship (SAR) analysis of synthetic cannabinoids used a two-step approach to measure binding affinity at CB1 and CB2 receptors and to assess the cannabinoids in a tetrad of in vivo tests in which cannabinoid agonists produce a characteristic profile of effects in mice, including suppression of motor activity, antinociception, hypothermia and catalepsy (Martin, et al., 1991). This mini-review focuses on summarizing the findings of published SAR studies which examined the in vivo activity of indole- and pyrrole-derived cannabinoids. In addition, new in vivo data are presented for structurally related indoles and pyrroles. These unpublished data were collected in the same lab and under the same experimental parameters used for the previously published data (Wiley, et al., 2012a; Wiley, et al., 2012b; Wiley, et al., 1998). Animals used in these experiments were cared for in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Virginia Commonwealth University (Richmond, Virginia) and the ‘Guide For The Care And Use Of Animals’ (National Research Council, 1996). Not covered in this review are manuscripts that present only binding and/or functional in vitro data. The interested reader is referred to several excellent reviews that have been written on this topic (Huffman, 1999; Huffman, 2009; Huffman and Padgett, 2005; Manera, et al., 2008). In addition, other manuscripts in this volume review separate aspects of the in vivo and behavioral pharmacology of this class of cannabinoids, including their discriminative stimulus effects (Järbe and Gifford) and their liability for producing tolerance and dependence (Lichtman et al..).
Figure 1.
Historical 3-point attachment model used to develop JWH-018. An overlay of WIN55,212-2, Δ9-THC and JWH-018 was hypothesized, in which each cannabinoid would attach to the CB1 receptor at the 3 locations specified in the figure.
WIN55,212-2
WIN55,212-2 represents the prototypic aminoalkylindole cannabinoid which inspired the idea for synthetic indole cannabinoids (Huffman, et al., 1994). Coincidentally, the enantiomers of WIN55,212 and Δ9-THC with cannabinoid activity are polar opposites: (+)-WIN55,212 (designated WIN55,212-2) and (−)-Δ9-THC. (+)-Δ9-THC and the (−)-enantiomer, (−)-WIN55,212 (designated as WIN55,212-3) are not active in the tetrad test battery in mice (Compton, et al., 1992; Martin, et al., 1991). While Δ9-THC binds with approximately equal affinity to both identified cannabinoid receptors (CB1 Ki = 41 nM, CB2 Ki = 36), WIN55,212-2 has better affinity for CB2 (Ki = 0.28 nM) vs CB1 (Ki = 1.89 nM) receptors (Showalter, et al., 1996). Δ9-THC and WIN55,212-2 also differ in their in vitro efficacy at the CB1 receptor, with Δ9-THC acting as a partial agonist in functional assays such as [35S]GTPγS binding and WIN55,212-2 acting as a full agonist (Breivogel and Childers, 2000); however, both compounds show approximately equal efficacy in the mouse tetrad tests. In the present study, a full dose-effect curve determination was conducted with WIN55,212-2 in the tetrad battery (Figure 2) and serves as a comparison for other novel synthetic indole and pyrrole cannabinoids shown in Tables 1–4. Results are similar to those that have been obtained previously with WIN55,212-2 (Compton, et al., 1992), Δ9-THC (Martin, et al., 1991), and a variety of other psychoactive cannabinoid agonists (Compton, et al., 1993). At lower doses, WIN55,212-2 (present study; Figure 2, panel A) may increase locomotion, whereas at higher doses cannabinoid agonists, including WIN55,212-2 (present study; Compton, et al., 1992), reliably and dose-dependently suppress locomotor activity (Martin, et al., 1991). Maximum suppression typically approaches 100%. WIN55,212-2 also produced robust antinociceptive effects (100% maximum possible effect at higher doses; Figure 2, panel B) and substantial decreases in rectal temperature (−6 °C is typical at higher doses; Figure 2, panel C). WIN55,212-2 increases catalepsy in the mouse ring test, with efficacy ranging from 60 – 100% (Figure 2, panel D). Acute Δ9-THC produces similar biphasic locomotor effects, decreases in rectal temperature, and increases in catalepsy (Martin, et al., 1991; Sañudo-Peña, et al., 2000; Wiley, et al., 2008). ED50s for the effects of WIN55,212-2 in these tests are provided in Table 1.
Figure 2.
Effects of WIN55,212-2 on spontaneous activity (panel A), antinociception (panel B), rectal temperature (panel C), and catalepsy (panel D) in adult male ICR mice. Spontaneous activity was measured as total number of photocell beam interruptions during a 10-min session and is shown as % inhibition of activity of the vehicle group. Antinociception in a tail flick assay is expressed as the percent maximum possible effect (MPE) using a 10-s maximum test latency as follows: [(test−control)/(10−control)]×100. Rectal temperature values are expressed as the difference between control temperature (before injection) and temperature following drug administration (Δ°C). During assessment for catalepsy, the total amount of time (in s) that the mouse remained motionless on the ring apparatus (except for breathing and whisker movement) was measured and was used as an indication of catalepsy-like behavior. This value was divided by 300 s and multiplied by 100 to obtain percent immobility, as shown in the figure. Values represent the mean (± SEM) of 5–6 mice per group. The typical maximal effect observed for each measure is shown in the box at the upper left of each panel. ED50s for data presented in these panels are provided in Table 1.
Table 1.
Cannabinoid receptor binding and in vivo effects of indoles with structural variations in the side chain and indole substituent

| ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound | R | R′ | Affinities (nM) | In Vivo Tests* | ||||
| CB1 | CB2 | SA | %MPE | RT | RI | |||
| WIN55,212-2** | morpholino | H | 1.9 ± 1a | 0.28 ± 0.16a | 87% (10) | 100% (10) | −5.0 (10) | 87% (10) |
|
| ||||||||
| 1.3 | 0.3 | 0.6 | 2.2 | |||||
|
| ||||||||
| JWH-446 | propyl | Br | 3322 ± 430 | 2011 ± 239 | 83% (30) | 76% (30) | −3.6 (30) | 64% (30) |
| JWH-443 | butyl | Br | 364 ± 14 | 332 ± 84 | ND | ND | ND | ND |
| JWH-444 | pentyl | Br | 145 ± 8 | 207 ± 5 | 79% (30) | 100% (30) | −5.0 (30) | 76% (30) |
| JWH-445 | hexyl | Br | 197 ± 14 | 499 ± 117 | ND | ND | ND | ND |
|
| ||||||||
| JWH-453 | propyl | I | 1967 ± 478 | 778 ± 50 | inactive (30) | inactive (30) | inactive (30) | inactive (30) |
| JWH-455 | butyl | I | 686 ± 48 | 722 ± 157 | 79% (30) | 68% (30) | −3.3 (30) | inactive (30) |
| JWH-452 | pentyl | I | 178 ± 6 | 193 ± 19 | 74% (30) | 62% (30) | −5.4 (30) | 45% (30) |
| JWH-454 | hexyl | I | 152 ± 10 | 364 ± 15 | 66% (30) | inactive (30) | −2.5 (30) | ND |
SA = spontaneous activity, %MPE = % maximum possible antinociceptive effect, RT = rectal temperature, RI = ring immobility. ND = not determined. Single dose tests are indicated by magnitude of effect and dose tested (mg/kg, in parentheses). “Inactive” refers to doses that produced less than 30% of the maximal cannabimimetic effect for the measure (see Figure 2).
Values for in vivo tests for WIN55,212-2 were calculated based on data shown in Figure 2 and are presented for comparison and for illustration of the system used for data presented in all tables. Numbers in top row exemplify testing of a single 10 mg/kg dose; values on the bottom row show ED50s for dose-effect data presented in Figure 2.
Table 4.
Cannabinoid receptor binding and in vivo effects of miscellaneous indoles
| Compound | Structure | Affinities (nM) | In Vivo Tests* | ||||
|---|---|---|---|---|---|---|---|
| CB1 | CB2 | SA | %MPE | RT | RI | ||
| JWH-001 |

|
> 10,000 | ND | inactive (100) | inactive (100) | inactive (100) | inactive (100) |
| JWH-002 |
|
> 10,000 | ND | inactive (100) | inactive (100) | inactive (100) | ND |
| JWH-317 |

|
101 ± 1 | 257 ± 3 | 89% (30) | 100% (30) | −5.5 (30) | ND |
SA = spontaneous activity, %MPE = % maximum possible antinociceptive effect, RT = rectal temperature, RI = ring immobility. ND = not determined. Single dose tests are indicated by magnitude of effect and dose tested (mg/kg, in parentheses). “Inactive” refers to doses that produced less than 30% of the maximal cannabimimetic effect for the measure (see Figure 2).
Indoles
Substitutions for morpholino group
The initial series of indole-derived cannabinoids that were used to evaluate the hypothesis that the morpholino group of the aminoalkylindoles and the C3 side chain of Δ9-THC occupied similar spatial domains within the CB1 receptor were a series of indole compounds in which an alkyl group was substituted for the oxazine and morpholino substituents of WIN55,212-2. One of the primary findings of this study was that a cyclic amino group at this position was unnecessary for psychoactivity. Substitution of a n-pentyl group resulted in a compound (JWH-018; Figure 1) with good CB1 receptor affinity (Ki = 9 nM) and correspondingly good potency in the tetrad tests (Wiley, et al., 1998). Further, affinity and potency varied systematically with the length of the carbon chain (position R1 of naphthoylindole structure in Figure 3), with optimal activity from n-butyl to n-hexyl and absence or reduction of receptor binding at shorter or longer carbon chains. This finding is consistent with the results of a structure-activity analysis of classical and bicyclic cannabinoids, in which length and branching of the C3 carbon chain of Δ8-THC and CP55,940 analogs dramatically affected CB1 affinity and in vivo potency (Compton, et al., 1993). Fluoropentyl substitution also resulted in an active compound (AM-2201) that has been found in “herbal incense” products (Denooz, et al., 2013; Logan, et al., 2012). CB1 receptor recognition and in vivo activity were also retained with some cyclic substitutions (cyclohexylethyl and cyclopropylmethyl), but not with others (2-phenylethyl), albeit none of these compounds were as potent as WIN55,212-2 or JWH-018 (Wiley, et al., 1998). Of the compounds tested in this early study, the most potent compounds (JWH-073, JWH-018, JWH-019, and AM-2201: n-butyl, n-pentyl, n-hexyl, and n-fluorpentyl substitutions, respectively) were detected in samples purchased in 2011 in the state of North Carolina in the United States (Cox, et al., 2012), suggesting that manufacturers of these now illicit products may have been aware of these initial findings.
Figure 3.
Templates for major structural classes of synthetic cannabinoids discussed in this review: naphthoylindoles, naphthoylpyrroles, 1-pentyl-3-phenylacetylindoles and tetramethylcyclopropyl ketone indoles. Additional structural templates are shown in the tables.
Addition of substituents to indole group
The n-alkyl indoles described above were unmethylated at the 2-indole position (position R2 of naphthoylindole structure in Figure 3). A parallel set synthesized during the same time period contained a 2-methylindole substituent. While 2-methylation did not affect the overall pattern of results (i.e., optimal affinity and potency with n-butyl to n-hexyl chain lengths), absolute affinities and potencies the methylated n-butyl to n-hexyl analogs were 2- to 4-fold less than those of the unmethylated compounds (Wiley, et al., 1998). Further, 2-methylation appeared to result in a shift in the SAR profile such that the methylated n-propyl was more active and the n-heptyl compound was less active than the corresponding unmethylated compounds. These results are consistent with those described for the aminoalkylindole analogs of WIN55,212-2, in that the 2-methylated compound, WIN53,365, showed 2-fold less affinity for CB1 receptors than did its unmethylated counterpart, WIN55,225 (Dutta, et al., 1997).
The effects of a series of compounds with C-5 halogenation (Br and I) of the indole group on binding and in vivo activity are presented in Table 1. Compared to compounds with n-alkyl groups of comparable lengths, these halogenated indoles exhibited greatly decreased affinity for the CB1 receptor. For example, JWH-455 (Ki = 686 nM) showed 77-fold less CB1 receptor affinity than did JWH-073 (Ki = 8.9 nM), the non-halogenated n-butyl analog. As with the non-halogenated series, optimal chain length was n-pentyl (Ki = 145 and 178 for the Br analog JWH-444 and the I analog JWH-452, respectively). Unlike the original non-halogenated compounds, however, none of the analogs in Table 1 showed < 100 nM CB1 receptor affinity. Consequently, a single 30 mg/kg intravenous dose of each compound was assessed in vivo (where available supply allowed). Although one compound (JWH-453) showed poor CB1 receptor affinity and was not active in any of the tests, all of the other tested compounds exhibited activity or partial activity in one or more test. Given the poor CB1 receptor binding affinity of some of the compounds (e.g., JWH-446 and JWH-455), these results are somewhat surprising. One possibility is that these compounds may be metabolized to active metabolites, as has been demonstrated for other indole-derived cannabinoids (Brents et al., 2012). On the other hand, the tetrad tests are not entirely selective for cannabinoids (Wiley and Martin, 2003), suggesting that non-cannabinoid receptor actions may have played a role in the effects of these compounds at the 30 mg/kg probe dose. Assessment of the ability of rimonabant to reverse the effects would be necessary to determine whether the observed effects were CB1 receptor mediated.
Pyrroles
Naphthoylpyrroles
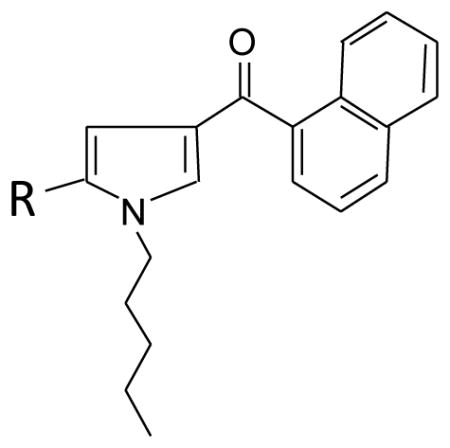
Conversion of the original series of naphthoylindoles to naphthoylpyrroles resulted in compounds with greatly reduced CB1 receptor affinities and in vivo potencies (Wiley, et al., 1998). Compounds with chain lengths of n-methyl to n-propyl (position R1 of naphthoylpyrrole structure in Figure 3) did not bind to the CB1 receptor (Ki > 10,000 nM). Whereas lengthening the chain to n-butyl or longer improved affinity, only the n-pentyl pyrrole (JWH-030) had reasonable affinity (Ki = 87 nM), although its potency in at least two of the in vivo tests (temperature and ring immobility) remained low (> 70 μM/kg). Perhaps not surprisingly, these naphthoylpyrroles are not commonly detected in forensic samples, unlike the comparable naphthoylindole analogs (e.g., JWH-018, JWH-073), albeit there are exceptions: e.g., 3-naphthoyl-n-pentylpyrrole (JWH-030) has been detected in samples obtained over the internet (Uchiyama, et al., 2013).
Phenylpyrroles
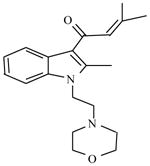
Although the 3-point attachment model formed the original basis of the creation of indole-derived cannabinoids, exploration of their SAR led to revision of the model in favor of the hypothesis that interaction of these new cannabinoids with the CB1 receptor might involve stacking of their aromatic naphthoyl and indole substituents (Huffman, et al., 2003; Reggio, et al., 1998). Later synthesis of a series of phenylpyrroles and evaluation of CB1 receptor binding data was supportive of this aromatic stacking hypothesis (Huffman, et al., 2006). Table 2 presents unpublished results of in vivo tetrad tests with some of these compounds. Due to limited supplies, a full dose-effect curve could not be determined for all of the compounds. Hence, potencies could not be estimated; however, several observations may be made based upon these data. First, with the exception of JWH-309 (which could not be tested at an adequate dose due to limited supply), all of the compounds were fully or partially active in one or more of the tetrad tests. Second, these results are generally consistent with the excellent CB1 receptor binding affinities of the majority of the compounds. JWH-363 had the worst binding affinity (Ki=245 nM) and showed the least activity in vivo (at 30 mg/kg i.v., inactive in two tests and minimal activity in the other two). In contrast, JWH-370 had the best binding affinity (Ki=5.6 nM) and was fully active in all four tests at a dose of 10 mg/kg, i.v. Third, both the nature and the position of the phenyl substituent affected in vivo activity. For example, compounds with 2- or 3-phenyl substituents (JWH-370, JWH-346, JWH-365, JWH-367, and JWH-307) were fully active in the complete tetrad whereas compounds with 4-phenyl substituents (JWH-364 and JWH-371) were inactive in at least one of the tests, despite reasonable CB1 binding affinities. Trifluoromethylphenyl substitution (JWH-372 and JWH-363) resulted in decreased activity in one or more of the tests, as compared to compounds with other 2- or 3-phenyl substituents. Together, these results demonstrate overall concordance of in vivo activity and good CB1 receptor binding affinities for the compounds and are supportive of the aromatic stacking hypothesis.
Table 2.
Cannabinoid receptor binding and in vivo effects of 2-aryl-4-(1-naphthoyl)-N-pentyl-pyrroles
| |||||||
|---|---|---|---|---|---|---|---|
| Compound | R | Affinities (nM) a | In VivoTests* | ||||
| CB1 | CB2 | SA | %MPE | RT | RI | ||
| JWH-309 | 1-naphthyl | 41 ± 3 | 49 ± 7 | inactive (3) | inactive (3) | inactive (3) | ND |
| JWH-366 | 3-pyridyl | 191 ± 12 | 24 ± 1 | 91% (30) | 86% (30) | −4.25 (30) | 44% (30) |
|
| |||||||
| JWH-370 | 2-methylphenyl | 5.6 ± 0.4 | 4.0 ± 0.5 | 93% (10) | 100% (10) | −6.2 (10) | 94% (10) |
| JWH-346 | 3-methylphenyl | 67 ± 6 | 39 ± 2 | 1.5 | 1.3 | 2.6 | ND |
|
| |||||||
| JWH-365 | 2-ethylphenyl | 17 ± 1 | 3.4 ± 0.2 | 86% (10) | 95% (10) | −5.1 (10) | 48% (10) |
| JWH-364 | 4-ethylphenyl | 34 ± 3 | 29 ± 1 | inactive (30) | 76% (30) | −5.1 (10) | 48% (10) |
|
| |||||||
| JWH-371 | 4-butylphenyl | 42 ± 1 | 64 ± 2 | 76% (30) | 74% (30) | −2.7 (30) | inactive (30) |
| JWH-367 | 3-methoxyphenyl | 53 ± 2 | 23 ± 1 | 83% (30) | 100% (30) | −5.0 (30) | 58% (30) |
|
| |||||||
| JWH-307 | 2-fluorophenyl | 7.7 ± 1.8 | 3.3 ± 0.2 | 0.42 | 0.53 | 0.96 | ND |
| JWH-308 | 4-fluorophenyl | 41± 1 | 33 ± 2 | 2.4 | 60% (3) | −2.6 (3) | ND |
|
| |||||||
| JWH-372 | 2-trifluorophenyl | 77 ± 2 | 8.2 ± 0.2 | 63% (30) | 100% (30) | −4.6 (30) | 27% (30) |
| JWH-363 | 3-trifluorophenyl | 245 ± 5 | 71 ± 1 | 48% (30) | inactive (30) | −2.0 (30) | inactive (30) |
SA = spontaneous activity, %MPE = % maximum possible antinociceptive effect, RT = rectal temperature, RI = ring immobility. ND = not determined. Single dose tests are indicated by magnitude of effect and dose tested (mg/kg, in parentheses). “Inactive” refers to doses that produced less than 30% of the maximal cannabimimetic effect for the measure (see Figure 2). Whenever supplies allowed determination of a dose-effect curve, ED50s (mg/kg) are provided for active compounds.
Naphthoyl manipulations
Addition of substituents to naphthoyl group
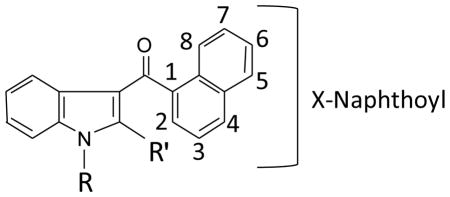
The steric and electronic effects of two types of substituents on the naphthoyl group have been examined in SAR with an in vivo component: electron withdrawing halogen substituents and electron donating methoxy. The effects of moderately electron withdrawing halogen substituents (Br, Cl, F, and I) at C-4 or C-8 (see chemical structure at the top of Table 3) on CB1 and CB2 receptor affinities and in vivo pharmacology were examined in a series of 1-alkyl-3-(1-naphthoyl)indoles (Wiley, et al., 2012b). In addition, two electroneutral structural features (the length of the N-alkyl group and the presence of a methyl at the 2-indole position) were manipulated in this series. Results observed with the two electroneutral structural manipulations were consistent with a number of previous SAR investigations of indole- and pyrrole-derived cannabinoids in which these structural manipulations produce similar patterns of alterations of CB1 and CB2 affinities and in vivo potencies, suggesting that the length of the alkyl substituent is as crucial for this cannabinoid structural motif (Wiley, et al., 1998) as it is for cannabinoids based on the tetrahydrocannabinol structural motif (Compton, et al., 1993). As found in these previous studies, 1-pentyl substitution was optimal for enhancement of CB1 receptor affinity and in vivo activity. Further, C-4 substituents showed accentuated CB1 receptor affinity and in vivo activity compared to C-8 substituents, some of which did not bind to the receptor nor produce activity in the tetrad tests. Less variability occurred across halogen groups. The authors hypothesized that steric interference with aromatic stacking was a probable cause of the decreased activity of compounds with C-8 substituents (Wiley, et al., 2012b).
Table 3.
Cannabinoid receptor binding and in vivo effects of methoxy naphthoyl indoles
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | Substituents | Affinities (nM) | In Vivo Tests* | ||||||
| R | R′ | X** | CB1 | CB2 | SA | %MPE | RT | RI | |
| JWH-391 | butyl | H | 2-OCH3 | > 10,000 | 1236 ± 103 | inactive (30) | 67% (30) | inactive (30) | inactive (30) |
| JWH-390 | butyl | CH3 | 2-OCH3 | > 10,000 | > 10,000 | stim (30) | inactive (30) | inactive (30) | inactive (30) |
| JWH-388 | pentyl | H | 2-OCH3 | 2398 ± 228 | 147 ± 8 | inactive (30) | inactive (30) | inactive (30) | inactive (30) |
| JWH-389 | pentyl | CH3 | 2-OCH3 | > 10,000 | > 10,000 | inactive (30) | inactive (30) | −2.1 (30) | inactive (30) |
|
| |||||||||
| JWH-080 | butyl | H | 4-OCH3 | 5.6 ± 1a | 2.2 ± 1.3a | 2.7 | 1.5 | 0.88 | ND |
| JWH-081 | pentyl | H | 4-OCH3 | 1.2 ± 0.03a | 12.5 ± 2.23 a | 0.06 | 0.084 | 0.06 | ND |
| JWH-082 | hexyl | H | 4-OCH3 | 5.3 ± 0.80a | 6.4 ± 0.94a | 1.7 | 0.62 | 1.1 | ND |
| JWH-083 | heptyl | H | 4-OCH3 | 106 ± 12a | 102 ± 50a | stim | 24 | 28 | ND |
|
| |||||||||
| JWH-411 | butyl | H | 6-OCH3 | 2036 ± 203 | 561 ± 54 | inactive (30) | inactive (30) | inactive (30) | inactive (30) |
| JWH-410 | butyl | CH3 | 6-OCH3 | 2682 ± 397 | 322 ± 46 | 64% (30) | 75% (30) | −2.7 (30) | 33% (30) |
| JWH-408 | pentyl | H | 6-OCH3 | 190 ± 12 | 91 ± 3 | 80% (30) | 96% (30) | −4.3 (30) | 62% (30) |
| JWH-409 | pentyl | CH3 | 6-OCH3 | 746 ± 77 | 232 ± 20 | 83% (30) | 42% (30) | −3.9 (30) | 38% (30) |
SA = spontaneous activity, %MPE = % maximum possible antinociceptive effect, RT = rectal temperature, RI = ring immobility. ND = not determined. Stim = stimulation of locomotor activity of at least 10% above vehicle levels (i.e., −10% inhibition of activity). Single dose tests are indicated by magnitude of effect and dose tested (mg/kg, in parentheses). “Inactive” refers to doses that produced less than 30% of the maximal cannabimimetic effect for the measure (see Figure 2). Whenever supplies allowed determination of a dose-effect curve, ED50s (mg/kg) are provided for active compounds.
X indicates substituent and its position on the naphthoyl group.
The effects of methoxy substitution on the naphthoyl group appear to support this hypothesis (Table 3; Aung, et al., 2000). Although halogen and methoxy substituents are electronic opposites (electron withdrawing vs. electron donating, respectively), C-4 substitution of either type of substituent resulted in compounds with the best CB1 receptor affinities and in vivo activity, as compared to substitution at other positions. Unlike substituents at other positions on the naphthoyl, the rotation of C-4 substituents is less hindered and thereby, less likely to interfere with optimal aromatic stacking, which has been shown to be important for cannabinoid receptor recognition (Huffman, et al., 2003; Reggio, et al., 1998). Consistent with this idea, three of the four compounds (JWH-389 JWH-390, and JWH-391) with C-2 methoxy subsituents did not bind to the CB1 receptor. Further, none of the C-2 compounds were active in any of the tetrad tests at doses up to 30 mg/kg i.v. (Table 3). While compounds with C-6 methoxy substituents showed slightly increased activity across tests, none of these compounds were fully active in all four tests. In addition, as in previous studies, the length of the 1-alkyl and presence or absence of 2-methylation also contributed to affinity for the cannabinoid receptors and activity in the tetrad tests (Table 3). Together, these results suggest that steric effects play a stronger role in determining the nature of CB1 receptor affinity and in vivo activity than did electronic effects.
Substitution for naphthoyl group
Because the naphthoyl group itself is involved in aromatic stacking of indole- and pyrrole-derived cannabinoids described thus far in this review, its manipulation would be expected to produce profound changes in CB1 receptor recognition and in vivo activity and, indeed, it does. Deletion of the naphthoyl (JWH-001) or substitution of a non-cyclic group (JWH-002) eliminates both CB1 receptor binding affinity and in vivo activity (Table 4; Huffman, et al., 1994). The number of aromatic constitutents also may play a role in interaction with the CB1 receptor and resultant pharmacological activity in the tetrad tests. For example, WIN56,098, an aminoalkylindole analog with anthracene (3 aromatic ring) substitution for the naphthalene, does not bind to the CB1 receptor nor is it active in vivo in the tetrad tests (Compton, et al., 1992). In contrast, substitution of a two-ringed group (methyl-benzodioxole) results in a 3,4-meylenedioxyphenylacetylindole (JWH-317) that binds to CB1 receptors with moderate affinity (Ki=101 nM) and is active with near maximal efficacy in suppressing locomotor activity and producing antinociception and hypothermia (Table 4). Decreasing the number of aromatic rings on the non-indole side of the carbonyl to one, as in a series of 1-pentyl-3-phenylacetylindoles (Figure 3), attenuated CB1 receptor affinities and reduced in vivo potencies compared to 1-pentyl-3-naphthoylindole congeners (Wiley, et al., 2012a). The major structural manipulations in this series included the type of substituent (i.e., unsubstituted, methyl, methoxy, chloro, bromo, and fluoro) and the position of the substituent on the phenyl ring (i.e., 2-, 3- or 4-position). Of these manipulations, the most critical factor affecting in vivo potency was the position of the substituent. Whereas compounds with 2- and 3-phenylacetyl substituents were efficacious with good potencies, 4-substituents resulted in compounds that had poor potency or were inactive. Interestingly, this pattern also occurred for indole-derived cannabinoids with a 1-morpholinomethtyl substituent instead of a 1-pentyl (Compton, et al., 1992). For example, pravadoline (WIN48,098), one of Sterling-Winthrop’s original lead compounds in the aminoalkylindole series (Haubrich, et al., 1990), has a 4-methoxyphenyl substituent in place of the naphthoyl. Pravadoline, and a similar analog without 2-methylation, do not bind to the CB1 receptor and are not active in the tetrad tests (Compton, et al., 1992). This pattern of results suggests that steric influences are as important for aminoalkylindoles and 1-pentyl-3-phenylacetylindoles as they are for the halogenated 1-alkyl-3-(1-naphthoyl)indoles (Wiley, et al., 2012b).
Recently, we completed assessment of two tetramethylcyclopropyl ketone indoles, UR-144 [(1-pentyl-1H-indol-3-yl)-(2,2,3,3-tetramethylcyclopropyl)methanone] and XLR-11 [(1-(5-fluoropentyl)-1H-indol-3-yl)-(2,2,3,3-tetramethylcyclopropyl)methanone]. In these compounds, a tetramethylcyclopropyl group is substituted for the naphthoyl of the parent 1-pentyl-3-naphthoylindole (Figure 3). In addition, XLR-11 has a fluoro group at the terminal end of the 1-pentyl (position R1 of tetramethylcyclopropyl ketone indole structure in Figure 3). These compounds are similar to those contained in a series of compounds synthesized by Abbott Laboratories (Frost, et al., 2010; Frost, et al., 2008), suggesting that manufacturers of “herbal incense” products have used multiple sources of information in their quest for novel psychoactive cannabinoids. While the Abbott compounds were CB2 receptor selective, many also possessed significant affinity for the CB1 receptor. As other compounds (e.g., JWH-018, AM-2201) have been banned, UR-144 and XLR-11 have started appearing in samples of “herbal incense” sold over the internet (Kavanagh, et al., 2013; Uchiyama, et al., 2013). Both compounds have good CB1 and CB2 receptor binding affinities and produced the full complement of cannabimimetic effects in the tetrad tests in mice, with potencies several-fold greater than Δ9-THC (Wiley et al., 2013). Given that attenuation of cannabinoid activity was observed with the 1-pentyl-3-phenylacetylindoles (JWH-205 and JWH-167, with and without 2-methylation, respectively), this result is rather surprising and suggests that the nature of the cyclic substituent (e.g., phenyl vs. tetramethylcyclopropyl), as well as their number, contribute to receptor recognition and in vivo potency. Both the phenylacetylindoles and the Abbott compounds have shown up in “herbal incense” products.
Conclusions
Originally synthesized for research purposes, indole- and pyrrole-derived synthetic cannabinoids are the most commonly identified psychoactive chemicals contained in products marketed as “herbal incense.” Although hundreds of these cannabinoids have been evaluated for their CB1 and CB2 receptor affinities (Aung, et al., 2000; Huffman, et al., 2006; Huffman, et al., 2003; Huffman, et al., 2005a; Huffman, et al., 2005b; Lainton, et al., 1995; Manera, et al., 2008), most have never been tested in animals before they were discovered in products confiscated from human users. The purpose of this mini-review was to summarize the findings of SAR studies in which the in vivo activity of these cannabinoids was examined. Based upon the collective data, good CB1 affinity was the best predictor of the propensity of a given compound to produce cannabimimetic effects in the tetrad tests in mice, suggesting that these effects are mediated by CB1 receptor activation, as has also been shown for traditional and bicyclic cannabinoids (Compton, et al., 1996; Compton, et al., 1993). Consistent with this idea, reversal of psychoactive effects of indole-derived cannabinoids has been demonstrated in nonhuman primates, rats and mice (Ginsburg, et al., 2012; Järbe, et al., 2011; Wiebelhaus, et al., 2012). Another overall observation is that good CB1 affinity and its associated cannabimimetic in vivo activity were retained across a surprisingly wide array of structural manipulations of substituents of the prototypic WIN55,212-2 molecule, including substitution of an alkyl for the WIN55,212-2 morpholino group, replacement of an indole core with a pyrrole or phenylpyrrole, substitution of a phenylacetyl or tetramethylcyclopropyl group for JWH-018’s naphthoyl, and halogenation of the naphthoyl group. This flexibility of cannabinoid ligand-receptor interactions has severely undermined the efforts of forensic scientists who have struggled to identify and regulate each new compound as it has appeared in marketed products (Grabenauer, et al., 2012; Vardakou, et al., 2010). Further complicating characterization of this class of abused cannabinoids is the fact that their affinities for novel cannabinoid or noncannabinoid receptors and the role that these receptors may play in mediating or modulating their pharmacological effects are largely unknown. For example, the indole structure of many of these compounds suggests that they may interact with one or more serotonin receptors. Given that the focus of extant studies has been examination of the effects of these compounds in assays specifically designed to detect cannabinoid activity, perhaps the most pressing future research need is determination of the extent to which the pharmacology of these compounds may differ from those of classical cannabinoids.
Acknowledgments
Preparation of this manuscript was supported by National Institute on Drug Abuse (NIDA) grants DA-031988 and DA-03672; NIDA had no further role in the writing of the review or in the decision to submit the paper for publication.
Footnotes
Conflict of interest statement
The authors do not have any real or perceived conflicts of interest with regard to the data presented in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aung MM, Griffin G, Huffman JW, Wu M, Keel C, Yang B, Showalter VM, Abood ME, Martin BR. Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB(1) and CB(2) receptor binding. Drug Alcohol Depend. 2000;60:133–40. doi: 10.1016/s0376-8716(99)00152-0. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR. Cannabinoid agonist signal transduction in rat brain: comparison of cannabinoid agonists in receptor binding, G-protein activation, and adenylyl cyclase inhibition. J Pharmacol Exp Ther. 2000;295:328–36. [PubMed] [Google Scholar]
- Brents LK, Gallus-Zawada A, Radominska-Pandya A, Vasiljevik T, Prisinzano TE, Fantegrossi WE, Moran JH, Prather PL. Monohydroxylated metabolites of the K2 synthetic cannabinoid JWH-073 retain intermediate to high cannabinoid 1 receptor (CB1R) affinity and exhibit neutral antagonist to partial agonist activity. Biochem Pharmacol. 2012;83:952–61. doi: 10.1016/j.bcp.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton DR, Aceto MD, Lowe J, Martin BR. In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A): inhibition of delta 9-tetrahydrocannabinol-induced responses and apparent agonist activity. J Pharmacol Exp Ther. 1996;277:586–94. [PubMed] [Google Scholar]
- Compton DR, Gold LH, Ward SJ, Balster RL, Martin BR. Aminoalkylindole analogs: cannabimimetic activity of a class of compounds structurally distinct from delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1992;263:1118–26. [PubMed] [Google Scholar]
- Compton DR, Rice KC, De Costa BR, Razdan RK, Melvin LS, Johnson MR, Martin BR. Cannabinoid structure-activity relationships: Correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther. 1993;265:218–26. [PubMed] [Google Scholar]
- Compton DR, Little PJ, Martin BR, Gilman JW, Saha JK, Jorapur VS, Sard HP, Razdan RK. Synthesis and pharmacological evaluation of amino, azido, and nitrogen mustard analogues of 10-substituted cannabidiol and 11- or 12-substituted delta 8-tetrahydrocannabinol. J Med Chem. 1990;33:1437–43. doi: 10.1021/jm00167a025. [DOI] [PubMed] [Google Scholar]
- Cox AO, Daw RC, Mason MD, Grabenauer M, Pande PG, Davis KH, Wiley JL, Stout PR, Thomas BF, Huffman JW. Use of SPME-HS-GC-MS for the analysis of herbal products containing synthetic cannabinoids. J Anal Toxicol. 2012;36:293–302. doi: 10.1093/jat/bks025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denooz R, Vanheugen JC, Frederich M, de Tullio P, Charlier C. Identification and structural elucidation of four cannabimimetic compounds (RCS-4, AM-2201, JWH-203 and JWH-210) in seized products. J Anal Toxicol. 2013;37:56–63. doi: 10.1093/jat/bks095. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–13. [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–9. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Dutta AK, Ryan W, Thomas BF, Singer M, Compton DR, Martin BR, Razdan RK. Synthesis, pharmacology, and molecular modeling of novel 4-alkyloxy indole derivatives related to cannabimimetic aminoalkyl indoles (AAIs) Bioorg Med Chem. 1997;5:1591–600. doi: 10.1016/s0968-0896(97)00111-9. [DOI] [PubMed] [Google Scholar]
- Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, El-Kouhen OF, Yao BB, Hsieh GC, Pai M, Zhu CZ, Chandran P, Meyer MD. Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity. J Med Chem. 2010;53:295–315. doi: 10.1021/jm901214q. [DOI] [PubMed] [Google Scholar]
- Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, El-Kouhen OF, Miller LN, Li L, Yao BB, Hsieh GC, Pai M, Zhu CZ, Chandran P, Meyer MD. Indol-3-yl-tetramethylcyclopropyl ketones: effects of indole ring substitution on CB2 cannabinoid receptor activity. J Med Chem. 2008;51:1904–12. doi: 10.1021/jm7011613. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Amer Chem Soc. 1964;86:1646–7. [Google Scholar]
- Ginsburg BC, Schulze DR, Hruba L, McMahon LR. JWH-018 and JWH-073: Delta-tetrahydrocannabinol-like discriminative stimulus effects in monkeys. J Pharmacol Exp Ther. 2012;340:37–45. doi: 10.1124/jpet.111.187757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenauer M, Krol WL, Wiley JL, Thomas BF. Analysis of synthetic cannabinoids using high-resolution mass spectrometry and mass defect filtering: implications for nontargeted screening of designer drugs. Anal Chem. 2012;84:5574–81. doi: 10.1021/ac300509h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, Kustanovich I, Mechoulam R. 2-Arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci USA. 2001;98:3662–5. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubrich DR, Ward SJ, Baizman E, Bell MR, Bradford J, Ferrari R, Miller M, Perrone M, Pierson AK, Saelens JK, et al. Pharmacology of pravadoline: a new analgesic agent. J Pharmacol Exp Ther. 1990;255:511–22. [PubMed] [Google Scholar]
- Huffman JW. Cannabimimetic indoles, pyrroles and indenes. Curr Med Chem. 1999;6:705–20. [PubMed] [Google Scholar]
- Huffman JW. Cannabimimetic indoles, pyrroles, and indenes: Structure-activity relationships and receptor interactions. In: Reggio PH, editor. The cannabinoid receptors. New York: Humana Press; 2009. pp. 49–94. [Google Scholar]
- Huffman JW, Padgett LW. Recent developments in the medicinal chemistry of cannabimimetic indoles, pyrroles and indenes. Curr Med Chem. 2005;12:1395–411. doi: 10.2174/0929867054020864. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Dai D, Martin BR, Compton DR. Design, synthesis and pharmacology of cannabimimetic indoles. Bioorg Med Chem Lett. 1994;4:563–6. [Google Scholar]
- Huffman JW, Padgett LW, Isherwood ML, Wiley JL, Martin BR. 1-Alkyl-2-aryl-4-(1-naphthoyl)pyrroles: New high affinity ligands for the cannabinoid CB(1) and CB(2) receptors. Bioorg Med Chem Lett. 2006;16:5432–5. doi: 10.1016/j.bmcl.2006.07.051. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Mabon R, Wu MJ, Lu J, Hart R, Hurst DP, Reggio PH, Wiley JL, Martin BR. 3-Indolyl-1-naphthylmethanes: new cannabimimetic indoles provide evidence for aromatic stacking interactions with the CB(1) cannabinoid receptor. Bioorg Med Chem. 2003;11:539–49. doi: 10.1016/s0968-0896(02)00451-0. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Szklennik PV, Almond A, Bushell K, Selley DE, He H, Cassidy MP, Wiley JL, Martin BR. 1-Pentyl-3-phenylacetylindoles, a new class of cannabimimetic indoles. Bioorg Med Chem Lett. 2005a;15:4110–3. doi: 10.1016/j.bmcl.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Zengin G, Wu MJ, Lu J, Hynd G, Bushell K, Thompson AL, Bushell S, Tartal C, Hurst DP, Reggio PH, Selley DE, Cassidy MP, Wiley JL, Martin BR. Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB(1) and CB(2) receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB(2) receptor agonists. Bioorg Med Chem. 2005b;13:89–112. doi: 10.1016/j.bmc.2004.09.050. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Deng H, Vadivel SK, Makriyannis A. Cannabinergic aminoalkylindoles, including AM678=JWH018 found in ‘Spice’, examined using drug (Delta9-tetrahydrocannabinol) discrimination for rats. Behav Pharmacol. 2011;22:498–507. doi: 10.1097/FBP.0b013e328349fbd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh P, Grigoryev A, Savchuk S, Mikhura I, Formanovsky A. UR-144 in products sold via the Internet: Identification of related compounds and characterization of pyrolysis products. Drug Test Anal. 2013 doi: 10.1002/dta.1456. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Lainton JAH, Huffman JW, Martin BR, Compton DR. 1-Alkyl-3-(1-naphthoyl)pyrroles: A new cannabinoid class. Tetrahedron Lett. 1995;36:1401–4. [Google Scholar]
- Lindigkeit R, Boehme A, Eiserloh I, Luebbecke M, Wiggermann M, Ernst L, Beuerle T. Spice: a never ending story? Forensic Sci Int. 2009;191:58–63. doi: 10.1016/j.forsciint.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Little PJ, Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J Pharmacol Exp Ther. 1988;247:1046–51. [PubMed] [Google Scholar]
- Logan BK, Reinhold LE, Xu A, Diamond FX. Identification of synthetic cannabinoids in herbal incense blends in the United States. J Forensic Sci. 2012;57:1168–80. doi: 10.1111/j.1556-4029.2012.02207.x. [DOI] [PubMed] [Google Scholar]
- Manera C, Tuccinardi T, Martinelli A. Indoles and related compounds as cannabinoid ligands. Mini Rev Med Chem. 2008;8:370–87. doi: 10.2174/138955708783955935. [DOI] [PubMed] [Google Scholar]
- Martin BR, Balster RL, Razdan RK, Harris LS, Dewey WL. Behavioral comparisons of the stereoisomers of tetrahydrocannabinols. Life Sci. 1981;29:565–74. doi: 10.1016/0024-3205(81)90434-3. [DOI] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, Johnson MR, Melvin LS, Mechoulam R, Ward SJ. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav. 1991;40:471–8. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–4. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–4. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, D.C: National Academy Press; 1996. [Google Scholar]
- Reggio PH, Basu-Dutt S, Barnett-Norris J, Castro MT, Hurst DP, Seltzman HH, Roche MJ, Gilliam AF, Thomas BF, Stevenson LA, Pertwee RG, Abood ME. The bioactive conformation of aminoalkylindoles at the cannabinoid CB1 and CB2 receptors: insights gained from (E)- and (Z)-naphthylidene indenes. J Med Chem. 1998;41:5177–87. doi: 10.1021/jm9801197. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Néliat G, Caput D, Ferrara P, Soubrié P, Brelière JC, Le Fur G. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–4. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Millan J, Defrocq J, Casellas P, Congy C, Oustric D, Sarran M, Bouaboula M, Calandra B, Portier M, Shire D, Breliere J, Le Fur G. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther. 1998;284:644–50. [PubMed] [Google Scholar]
- Sañudo-Peña MC, Romero J, Seale GE, Fernandez-Ruiz JJ, Walker JM. Activational role of cannabinoids on movement. Eur J Pharmacol. 2000;391:269–74. doi: 10.1016/s0014-2999(00)00044-3. [DOI] [PubMed] [Google Scholar]
- Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther. 1996;278:989–99. [PubMed] [Google Scholar]
- Thomas BF, Compton DR, Martin BR, Semus SF. Modeling the cannabinoid receptor: a three-dimensional quantitative structure-activity analysis. Mol Pharmacol. 1991;40:656–65. [PubMed] [Google Scholar]
- Uchiyama N, Kawamura M, Kikura-Hanajiri R, Goda Y. URB-754: A new class of designer drug and 12 synthetic cannabinoids detected in illegal products. Forensic Sci Int. 2013;227:21–32. doi: 10.1016/j.forsciint.2012.08.047. [DOI] [PubMed] [Google Scholar]
- Vardakou I, Pistos C, Spiliopoulou C. Spice drugs as a new trend: mode of action, identification and legislation. Toxicol Lett. 2010;197:157–62. doi: 10.1016/j.toxlet.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Wiebelhaus JM, Poklis JL, Poklis A, Vann RE, Lichtman AH, Wise LE. Inhalation exposure to smoke from synthetic “marijuana” produces potent cannabimimetic effects in mice. Drug Alcohol Depend. 2012;126:316–23. doi: 10.1016/j.drugalcdep.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Martin BR. Cannabinoid pharmacological properties common to other centrally acting drugs. Eur J Pharmacol. 2003;471:185–93. doi: 10.1016/s0014-2999(03)01856-9. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Evans RL, Grainger DB, Nicholson KL. Age-dependent differences in sensitivity and sensitization to cannabinoids and ‘club drugs’ in male adolescent and adult rats. Addict Biol. 2008;13:277–86. doi: 10.1111/j.1369-1600.2007.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Lefever TW, Grabenauer M, Moore KN, Thomas BF. Cannabinoids in disguise: Δ9-Tetrahydrocannabinol-like effects of tetramethylcyclopropyl ketone indoles. Neuropharmacology. 2013;75:145–54. doi: 10.1016/j.neuropharm.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Martin BR, Huffman JW. 1-Pentyl-3-phenylacetylindoles and JWH-018 share in vivo cannabinoid profiles in mice. Drug Alcohol Depend. 2012a;123:148–53. doi: 10.1016/j.drugalcdep.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Smith VJ, Chen J, Martin BR, Huffman JW. Synthesis and pharmacology of 1-alkyl-3-(1-naphthoyl)indoles: steric and electronic effects of 4- and 8-halogenated naphthoyl substituents. Bioorg Med Chem. 2012b;20:2067–81. doi: 10.1016/j.bmc.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Compton DR, Dai D, Lainton JA, Phillips M, Huffman JW, Martin BR. Structure-activity relationships of indole- and pyrrole-derived cannabinoids. J Pharmacol Exp Ther. 1998;285:995–1004. [PubMed] [Google Scholar]