Abstract
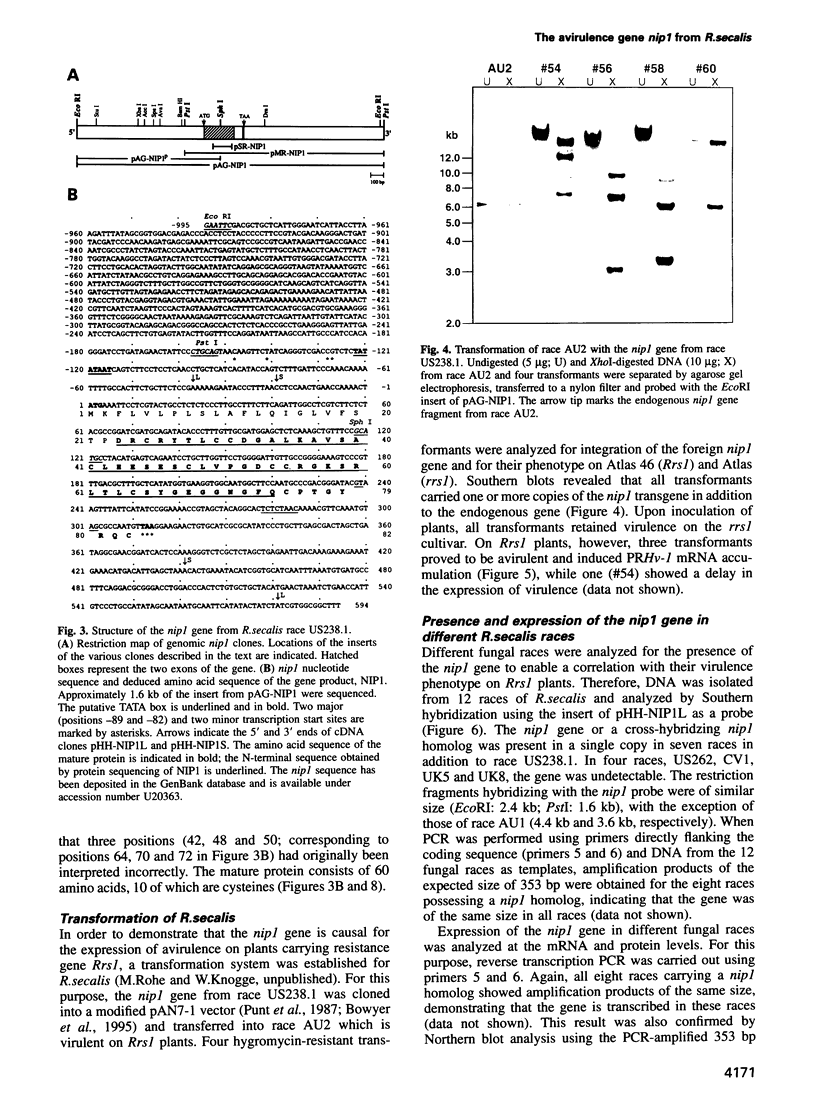
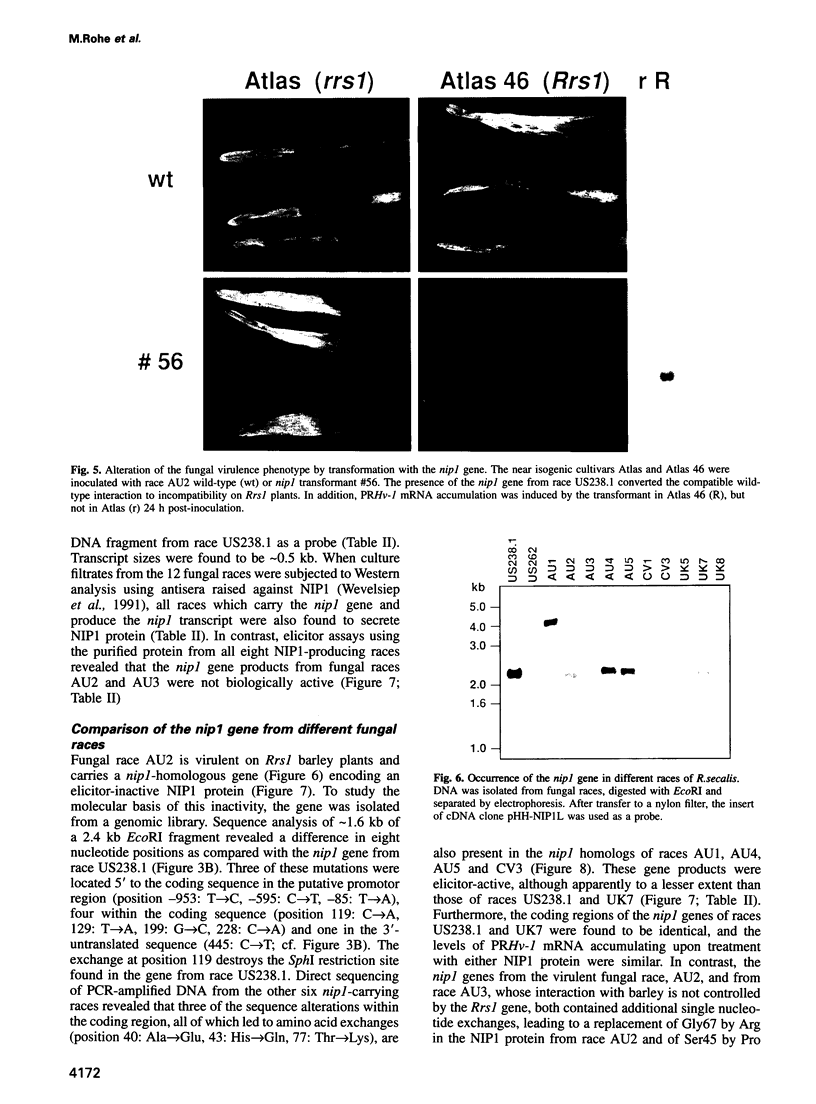
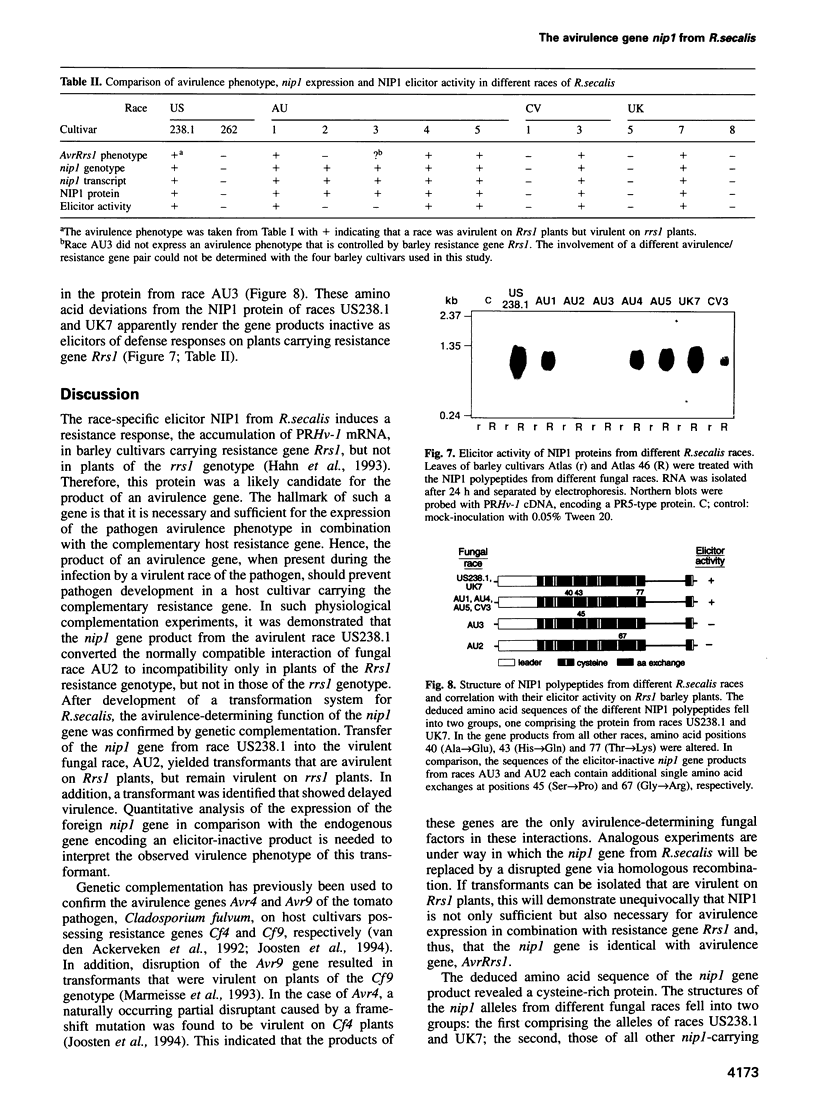
NIP1, a small phytotoxic protein secreted by the barley pathogen Rhynchosporium secalis, is a race-specific elicitor of defense responses in barley cultivars carrying the resistance gene, Rrs1. Co-inoculation employing spores from a virulent fungal race together with the NIP1 protein converted the phenotype of the interaction from compatible to incompatible only on Rrs1-containing plants. In addition, transformation of a virulent fungal race with the nip1 gene yielded avirulent transformants. This demonstrated that the protein is the product of a fungal avirulence gene. The fungal genome was found to contain a single copy of the nip1 gene. Sequence analysis of nip1 cDNA and genomic clones revealed that the gene consists of two exons and one intron. The derived amino acid sequence comprised a secretory signal peptide of 22 amino acids and a cysteine-rich mature protein of 60 amino acids. All fungal races that were avirulent on barley cultivars of the Rrs1 resistance genotype carry and express the nip1 gene and secrete an elicitor-active NIP1 polypeptide. In contrast, races lacking this gene were virulent. In addition, single nucleotide exchanges were detected in the coding region of the nip1 alleles in one virulent fungal race and in a race whose interaction with barley is not controlled by the Rrs1 gene. The resulting exchanges of single amino acids render the gene products elicitor-inactive. Thus, the R.secalis-barley interaction provides the first example of a pathosystem conforming to the gene-for-gene hypothesis in which a plant with a particular resistance gene recognizes a pathogen by a virulence factor, i.e. one of its offensive weapons. On the fungal side, in turn, recognition by the host plant is eluded by either deletion of the encoding gene or alteration of the primary structure of the gene product.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beever R. E., Dempsey G. P. Function of rodlets on the surface of fungal spores. Nature. 1978 Apr 13;272(5654):608–610. doi: 10.1038/272608a0. [DOI] [PubMed] [Google Scholar]
- Bell-Pedersen D., Dunlap J. C., Loros J. J. The Neurospora circadian clock-controlled gene, ccg-2, is allelic to eas and encodes a fungal hydrophobin required for formation of the conidial rodlet layer. Genes Dev. 1992 Dec;6(12A):2382–2394. doi: 10.1101/gad.6.12a.2382. [DOI] [PubMed] [Google Scholar]
- Bowyer P., Clarke B. R., Lunness P., Daniels M. J., Osbourn A. E. Host range of a plant pathogenic fungus determined by a saponin detoxifying enzyme. Science. 1995 Jan 20;267(5196):371–374. doi: 10.1126/science.7824933. [DOI] [PubMed] [Google Scholar]
- Carpenter C. E., Mueller R. J., Kazmierczak P., Zhang L., Villalon D. K., Van Alfen N. K. Effect of a virus on accumulation of a tissue-specific cell-surface protein of the fungus Cryphonectria (Endothia) parasitica. Mol Plant Microbe Interact. 1992 Jan-Feb;5(1):55–61. doi: 10.1094/mpmi-5-055. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Dangl J. L. The enigmatic avirulence genes of phytopathogenic bacteria. Curr Top Microbiol Immunol. 1994;192:99–118. doi: 10.1007/978-3-642-78624-2_5. [DOI] [PubMed] [Google Scholar]
- Drenth J., Low B. W., Richardson J. S., Wright C. S. The toxin-agglutinin fold. A new group of small protein structures organized around a four-disulfide core. J Biol Chem. 1980 Apr 10;255(7):2652–2655. [PubMed] [Google Scholar]
- Hahn M., Jüngling S., Knogge W. Cultivar-specific elicitation of barley defense reactions by the phytotoxic peptide NIP1 from Rhynchosporium secalis. Mol Plant Microbe Interact. 1993 Nov-Dec;6(6):745–754. doi: 10.1094/mpmi-6-745. [DOI] [PubMed] [Google Scholar]
- Joosten M. H., Cozijnsen T. J., De Wit P. J. Host resistance to a fungal tomato pathogen lost by a single base-pair change in an avirulence gene. Nature. 1994 Jan 27;367(6461):384–386. doi: 10.1038/367384a0. [DOI] [PubMed] [Google Scholar]
- Kamoun S., Klucher K. M., Coffey M. D., Tyler B. M. A gene encoding a host-specific elicitor protein of Phytophthora parasitica. Mol Plant Microbe Interact. 1993 Sep-Oct;6(5):573–581. doi: 10.1094/mpmi-6-573. [DOI] [PubMed] [Google Scholar]
- Keen N. T. Gene-for-gene complementarity in plant-pathogen interactions. Annu Rev Genet. 1990;24:447–463. doi: 10.1146/annurev.ge.24.120190.002311. [DOI] [PubMed] [Google Scholar]
- Keen N. T. The molecular biology of disease resistance. Plant Mol Biol. 1992 May;19(1):109–122. doi: 10.1007/BF00015609. [DOI] [PubMed] [Google Scholar]
- Lamb C. J. Plant disease resistance genes in signal perception and transduction. Cell. 1994 Feb 11;76(3):419–422. doi: 10.1016/0092-8674(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Logemann J., Schell J., Willmitzer L. Improved method for the isolation of RNA from plant tissues. Anal Biochem. 1987 May 15;163(1):16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Murray M. G., Thompson W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punt P. J., Oliver R. P., Dingemanse M. A., Pouwels P. H., van den Hondel C. A. Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene. 1987;56(1):117–124. doi: 10.1016/0378-1119(87)90164-8. [DOI] [PubMed] [Google Scholar]
- Rao V. B. Direct sequencing of polymerase chain reaction-amplified DNA. Anal Biochem. 1994 Jan;216(1):1–14. doi: 10.1006/abio.1994.1001. [DOI] [PubMed] [Google Scholar]
- Ricci P., Bonnet P., Huet J. C., Sallantin M., Beauvais-Cante F., Bruneteau M., Billard V., Michel G., Pernollet J. C. Structure and activity of proteins from pathogenic fungi Phytophthora eliciting necrosis and acquired resistance in tobacco. Eur J Biochem. 1989 Aug 15;183(3):555–563. doi: 10.1111/j.1432-1033.1989.tb21084.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel D., Parker J. E. Elicitor recognition and signal transduction in plant defense gene activation. Z Naturforsch C. 1990 Jun;45(6):569–575. doi: 10.1515/znc-1990-0601. [DOI] [PubMed] [Google Scholar]
- St Leger R. J., Staples R. C., Roberts D. W. Cloning and regulatory analysis of starvation-stress gene, ssgA, encoding a hydrophobin-like protein from the entomopathogenic fungus, Metarhizium anisopliae. Gene. 1992 Oct 12;120(1):119–124. doi: 10.1016/0378-1119(92)90019-l. [DOI] [PubMed] [Google Scholar]
- Stringer M. A., Timberlake W. E. Cerato-ulmin, a toxin involved in Dutch elm disease, is a fungal hydrophobin. Plant Cell. 1993 Feb;5(2):145–146. doi: 10.1105/tpc.5.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot N. J., Ebbole D. J., Hamer J. E. Identification and characterization of MPG1, a gene involved in pathogenicity from the rice blast fungus Magnaporthe grisea. Plant Cell. 1993 Nov;5(11):1575–1590. doi: 10.1105/tpc.5.11.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Ackerveken G. F., Van Kan J. A., De Wit P. J. Molecular analysis of the avirulence gene avr9 of the fungal tomato pathogen Cladosporium fulvum fully supports the gene-for-gene hypothesis. Plant J. 1992 May;2(3):359–366. doi: 10.1111/j.1365-313x.1992.00359.x. [DOI] [PubMed] [Google Scholar]
- Wessels JGH., De Vries OMH., Asgeirsdottir S. A., Schuren FHJ. Hydrophobin Genes Involved in Formation of Aerial Hyphae and Fruit Bodies in Schizophyllum. Plant Cell. 1991 Aug;3(8):793–799. doi: 10.1105/tpc.3.8.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wevelsiep L., Rupping E., Knogge W. Stimulation of Barley Plasmalemma H+-ATPase by Phytotoxic Peptides from the Fungal Pathogen Rhynchosporium secalis. Plant Physiol. 1993 Jan;101(1):297–301. doi: 10.1104/pp.101.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kan J. A., van den Ackerveken G. F., de Wit P. J. Cloning and characterization of cDNA of avirulence gene avr9 of the fungal pathogen Cladosporium fulvum, causal agent of tomato leaf mold. Mol Plant Microbe Interact. 1991 Jan-Feb;4(1):52–59. doi: 10.1094/mpmi-4-052. [DOI] [PubMed] [Google Scholar]