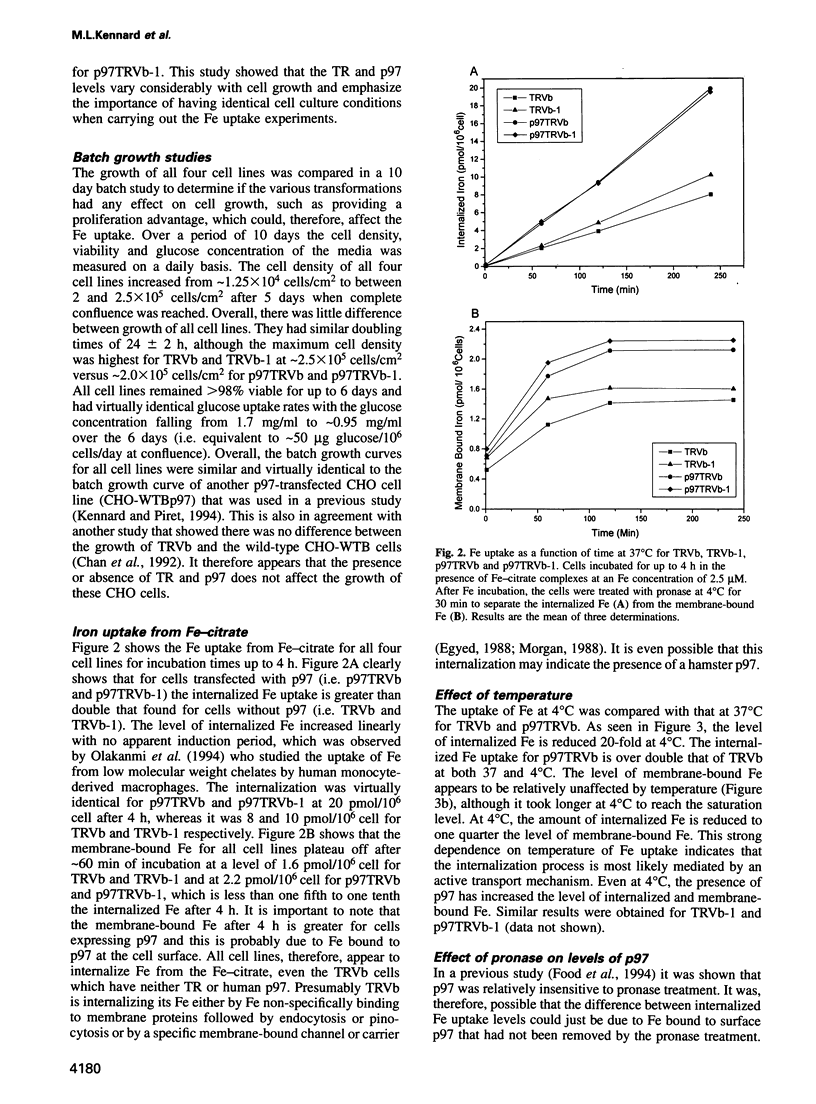
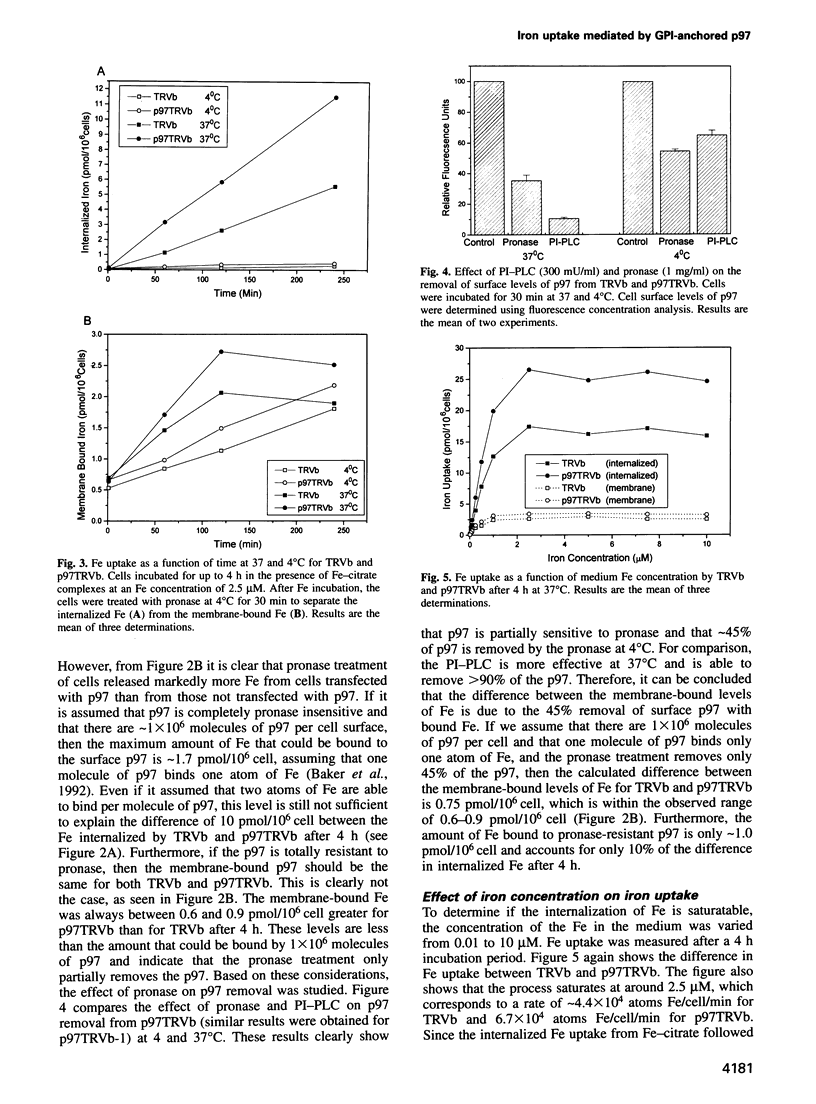
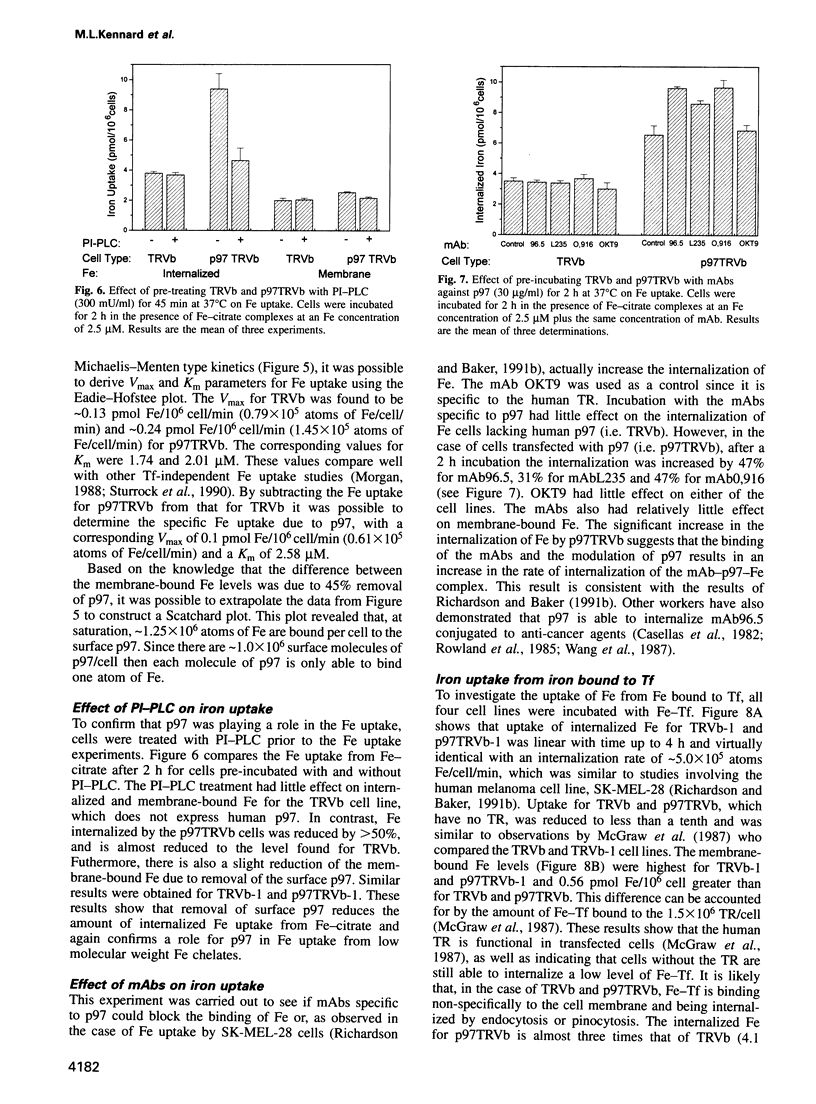
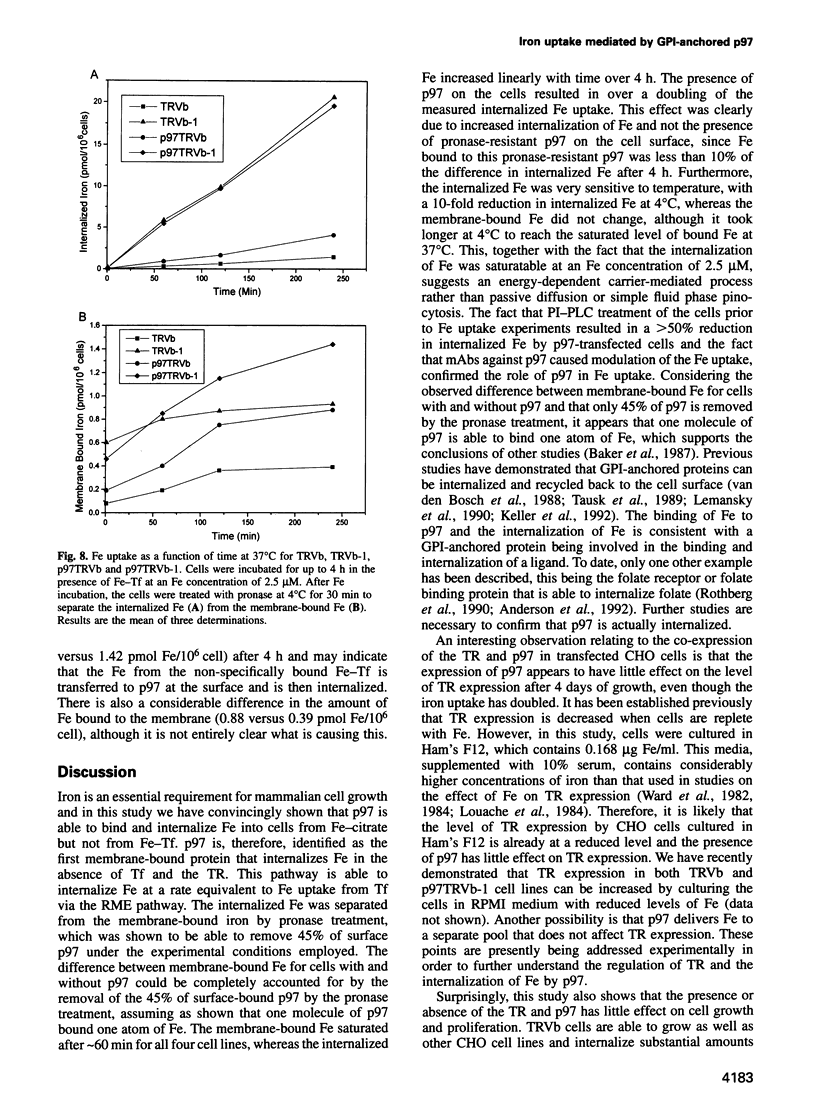
Abstract
The established process for iron uptake into mammalian cells involves transferrin and its receptor. Here, the role of the glycosyl-phosphatidylinositol (GPI)-linked transferrin homologue, melanotransferrin or p97, was studied using CHO cell lines defective in the transferrin receptor (TR) and transfected with human TR and/or human p97. The presence of p97 doubled the iron uptake, which could be explained by the binding of one atom of iron to one molecule of p97. The internalization of iron was shown to be temperature sensitive and saturated at a media iron concentration of 2.5 micrograms/ml with a Vmax of 0.1 pmol Fe/10(6) cell/min and a Km of 2.58 microM for p97. Treatment of the cells with either phosphatidylinositol-phospholipase C or monoclonal antibodies against p97 resulted in over a 50% reduction and a 47% increase in the iron uptake respectively. These data identify p97 as a unique cell surface GPI-anchored, iron binding protein involved in the transferrin-independent uptake of iron in mammals.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alemany R., Vilá M. R., Francí C., Egea G., Real F. X., Thomson T. M. Glycosyl phosphatidylinositol membrane anchoring of melanotransferrin (p97): apical compartmentalization in intestinal epithelial cells. J Cell Sci. 1993 Apr;104(Pt 4):1155–1162. doi: 10.1242/jcs.104.4.1155. [DOI] [PubMed] [Google Scholar]
- Anderson B. F., Baker H. M., Norris G. E., Rice D. W., Baker E. N. Structure of human lactoferrin: crystallographic structure analysis and refinement at 2.8 A resolution. J Mol Biol. 1989 Oct 20;209(4):711–734. doi: 10.1016/0022-2836(89)90602-5. [DOI] [PubMed] [Google Scholar]
- Anderson R. G., Kamen B. A., Rothberg K. G., Lacey S. W. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992 Jan 24;255(5043):410–411. doi: 10.1126/science.1310359. [DOI] [PubMed] [Google Scholar]
- Bailey S., Evans R. W., Garratt R. C., Gorinsky B., Hasnain S., Horsburgh C., Jhoti H., Lindley P. F., Mydin A., Sarra R. Molecular structure of serum transferrin at 3.3-A resolution. Biochemistry. 1988 Jul 26;27(15):5804–5812. doi: 10.1021/bi00415a061. [DOI] [PubMed] [Google Scholar]
- Baker E. N., Baker H. M., Smith C. A., Stebbins M. R., Kahn M., Hellström K. E., Hellström I. Human melanotransferrin (p97) has only one functional iron-binding site. FEBS Lett. 1992 Feb 24;298(2-3):215–218. doi: 10.1016/0014-5793(92)80060-t. [DOI] [PubMed] [Google Scholar]
- Basset P., Quesneau Y., Zwiller J. Iron-induced L1210 cell growth: evidence of a transferrin-independent iron transport. Cancer Res. 1986 Apr;46(4 Pt 1):1644–1647. [PubMed] [Google Scholar]
- Brissot P., Wright T. L., Ma W. L., Weisiger R. A. Efficient clearance of non-transferrin-bound iron by rat liver. Implications for hepatic iron loading in iron overload states. J Clin Invest. 1985 Oct;76(4):1463–1470. doi: 10.1172/JCI112125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. P., Hewick R. M., Hellström I., Hellström K. E., Doolittle R. F., Dreyer W. J. Human melanoma-associated antigen p97 is structurally and functionally related to transferrin. Nature. 1982 Mar 11;296(5853):171–173. doi: 10.1038/296171a0. [DOI] [PubMed] [Google Scholar]
- Brown J. P., Nishiyama K., Hellström I., Hellström K. E. Structural characterization of human melanoma-associated antigen p97 with monoclonal antibodies. J Immunol. 1981 Aug;127(2):539–546. [PubMed] [Google Scholar]
- Brown J. P., Woodbury R. G., Hart C. E., Hellström I., Hellström K. E. Quantitative analysis of melanoma-associated antigen p97 in normal and neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Jan;78(1):539–543. doi: 10.1073/pnas.78.1.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casellas P., Brown J. P., Gros O., Gros P., Hellström I., Jansen F. K., Poncelet P., Roncucci R., Vidal H., Hellström K. E. Human melanoma cells can be killed in vitro by an immunotoxin specific for melanoma-associated antigen p97. Int J Cancer. 1982 Oct 15;30(4):437–443. doi: 10.1002/ijc.2910300410. [DOI] [PubMed] [Google Scholar]
- Chan R. Y., Ponka P., Schulman H. M. Transferrin-receptor-independent but iron-dependent proliferation of variant Chinese hamster ovary cells. Exp Cell Res. 1992 Oct;202(2):326–336. doi: 10.1016/0014-4827(92)90082-j. [DOI] [PubMed] [Google Scholar]
- Conrad M. E., Umbreit J. N. A concise review: iron absorption--the mucin-mobilferrin-integrin pathway. A competitive pathway for metal absorption. Am J Hematol. 1993 Jan;42(1):67–73. doi: 10.1002/ajh.2830420114. [DOI] [PubMed] [Google Scholar]
- Dippold W. G., Lloyd K. O., Li L. T., Ikeda H., Oettgen H. F., Old L. J. Cell surface antigens of human malignant melanoma: definition of six antigenic systems with mouse monoclonal antibodies. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6114–6118. doi: 10.1073/pnas.77.10.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egyed A. Carrier mediated iron transport through erythroid cell membrane. Br J Haematol. 1988 Apr;68(4):483–486. doi: 10.1111/j.1365-2141.1988.tb04241.x. [DOI] [PubMed] [Google Scholar]
- Food M. R., Rothenberger S., Gabathuler R., Haidl I. D., Reid G., Jefferies W. A. Transport and expression in human melanomas of a transferrin-like glycosylphosphatidylinositol-anchored protein. J Biol Chem. 1994 Jan 28;269(4):3034–3040. [PubMed] [Google Scholar]
- Fuchs O., Borová J., Hradilek A., Neuwirt J. Non-transferrin donors of iron for heme synthesis in immature erythroid cells. Biochim Biophys Acta. 1988 Apr 25;969(2):158–165. doi: 10.1016/0167-4889(88)90071-7. [DOI] [PubMed] [Google Scholar]
- Gabathuler R., Reid G., Kolaitis G., Driscoll J., Jefferies W. A. Comparison of cell lines deficient in antigen presentation reveals a functional role for TAP-1 alone in antigen processing. J Exp Med. 1994 Oct 1;180(4):1415–1425. doi: 10.1084/jem.180.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garratt R. C., Jhotí H. A molecular model for the tumour-associated antigen, p97, suggests a Zn-binding function. FEBS Lett. 1992 Jun 22;305(1):55–61. doi: 10.1016/0014-5793(92)80654-y. [DOI] [PubMed] [Google Scholar]
- Grace N. D., Powell L. W. Iron storage disorders of the liver. Gastroenterology. 1974 Dec;67(6):1257–1283. [PubMed] [Google Scholar]
- Grootveld M., Bell J. D., Halliwell B., Aruoma O. I., Bomford A., Sadler P. J. Non-transferrin-bound iron in plasma or serum from patients with idiopathic hemochromatosis. Characterization by high performance liquid chromatography and nuclear magnetic resonance spectroscopy. J Biol Chem. 1989 Mar 15;264(8):4417–4422. [PubMed] [Google Scholar]
- Hellström I., Brown J. P., Hellström K. E. Melanoma-associated antigen p97 continues to be expressed after prolonged exposure of cells to specific antibody. Int J Cancer. 1983 May 15;31(5):553–555. doi: 10.1002/ijc.2910310505. [DOI] [PubMed] [Google Scholar]
- Kaplan J., Jordan I., Sturrock A. Regulation of the transferrin-independent iron transport system in cultured cells. J Biol Chem. 1991 Feb 15;266(5):2997–3004. [PubMed] [Google Scholar]
- Keller G. A., Siegel M. W., Caras I. W. Endocytosis of glycophospholipid-anchored and transmembrane forms of CD4 by different endocytic pathways. EMBO J. 1992 Mar;11(3):863–874. doi: 10.1002/j.1460-2075.1992.tb05124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., van Renswoude J., Kempf C., Rao K., Bateman J. L., Robbins A. R. Failure to release iron from transferrin in a Chinese hamster ovary cell mutant pleiotropically defective in endocytosis. J Cell Biol. 1984 Mar;98(3):1098–1101. doi: 10.1083/jcb.98.3.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvist S., Wiman K., Claesson L., Peterson P. A., Dobberstein B. Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell. 1982 May;29(1):61–69. doi: 10.1016/0092-8674(82)90090-3. [DOI] [PubMed] [Google Scholar]
- Lederman H. M., Cohen A., Lee J. W., Freedman M. H., Gelfand E. W. Deferoxamine: a reversible S-phase inhibitor of human lymphocyte proliferation. Blood. 1984 Sep;64(3):748–753. [PubMed] [Google Scholar]
- Lemansky P., Fatemi S. H., Gorican B., Meyale S., Rossero R., Tartakoff A. M. Dynamics and longevity of the glycolipid-anchored membrane protein, Thy-1. J Cell Biol. 1990 May;110(5):1525–1531. doi: 10.1083/jcb.110.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louache F., Testa U., Pelicci P., Thomopoulos P., Titeux M., Rochant H. Regulation of transferrin receptors in human hematopoietic cell lines. J Biol Chem. 1984 Sep 25;259(18):11576–11582. [PubMed] [Google Scholar]
- McGraw T. E., Greenfield L., Maxfield F. R. Functional expression of the human transferrin receptor cDNA in Chinese hamster ovary cells deficient in endogenous transferrin receptor. J Cell Biol. 1987 Jul;105(1):207–214. doi: 10.1083/jcb.105.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E. H. Membrane transport of non-transferrin-bound iron by reticulocytes. Biochim Biophys Acta. 1988 Sep 1;943(3):428–439. doi: 10.1016/0005-2736(88)90374-4. [DOI] [PubMed] [Google Scholar]
- Olakanmi O., Stokes J. B., Britigan B. E. Acquisition of iron bound to low molecular weight chelates by human monocyte-derived macrophages. J Immunol. 1994 Sep 15;153(6):2691–2703. [PubMed] [Google Scholar]
- Plowman G. D., Brown J. P., Enns C. A., Schröder J., Nikinmaa B., Sussman H. H., Hellström K. E., Hellström I. Assignment of the gene for human melanoma-associated antigen p97 to chromosome 3. Nature. 1983 May 5;303(5912):70–72. doi: 10.1038/303070a0. [DOI] [PubMed] [Google Scholar]
- Real F. X., Furukawa K. S., Mattes M. J., Gusik S. A., Cordon-Cardo C., Oettgen H. F., Old L. J., Lloyd K. O. Class 1 (unique) tumor antigens of human melanoma: identification of unique and common epitopes on a 90-kDa glycoprotein. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3965–3969. doi: 10.1073/pnas.85.11.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson D. R., Baker E. The release of iron and transferrin from the human melanoma cell. Biochim Biophys Acta. 1991 Feb 19;1091(3):294–302. doi: 10.1016/0167-4889(91)90192-z. [DOI] [PubMed] [Google Scholar]
- Richardson D. R., Baker E. The uptake of iron and transferrin by the human malignant melanoma cell. Biochim Biophys Acta. 1990 Jun 12;1053(1):1–12. doi: 10.1016/0167-4889(90)90018-9. [DOI] [PubMed] [Google Scholar]
- Richardson D. R., Baker E. Two saturable mechanisms of iron uptake from transferrin in human melanoma cells: the effect of transferrin concentration, chelators, and metabolic probes on transferrin and iron uptake. J Cell Physiol. 1994 Oct;161(1):160–168. doi: 10.1002/jcp.1041610119. [DOI] [PubMed] [Google Scholar]
- Richardson D., Baker E. The uptake of inorganic iron complexes by human melanoma cells. Biochim Biophys Acta. 1991 Jun 7;1093(1):20–28. doi: 10.1016/0167-4889(91)90133-i. [DOI] [PubMed] [Google Scholar]
- Richardson D., Baker E. Two mechanisms of iron uptake from transferrin by melanoma cells. The effect of desferrioxamine and ferric ammonium citrate. J Biol Chem. 1992 Jul 15;267(20):13972–13979. [PubMed] [Google Scholar]
- Rose T. M., Plowman G. D., Teplow D. B., Dreyer W. J., Hellström K. E., Brown J. P. Primary structure of the human melanoma-associated antigen p97 (melanotransferrin) deduced from the mRNA sequence. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1261–1265. doi: 10.1073/pnas.83.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg K. G., Ying Y. S., Kolhouse J. F., Kamen B. A., Anderson R. G. The glycophospholipid-linked folate receptor internalizes folate without entering the clathrin-coated pit endocytic pathway. J Cell Biol. 1990 Mar;110(3):637–649. doi: 10.1083/jcb.110.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland G. F., Axton C. A., Baldwin R. W., Brown J. P., Corvalan J. R., Embleton M. J., Gore V. A., Hellström I., Hellström K. E., Jacobs E. Antitumor properties of vindesine-monoclonal antibody conjugates. Cancer Immunol Immunother. 1985;19(1):1–7. doi: 10.1007/BF00199304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciot R., de Vos R., van Eyken P., van der Steen K., Moerman P., Desmet V. J. In situ localization of melanotransferrin (melanoma-associated antigen P97) in human liver. A light- and electronmicroscopic immunohistochemical study. Liver. 1989 Apr;9(2):110–119. doi: 10.1111/j.1600-0676.1989.tb00387.x. [DOI] [PubMed] [Google Scholar]
- Seligman P. A., Kovar J., Schleicher R. B., Gelfand E. W. Transferrin-independent iron uptake supports B lymphocyte growth. Blood. 1991 Sep 15;78(6):1526–1531. [PubMed] [Google Scholar]
- Smith L. H., Jr Overview of hemochromatosis. West J Med. 1990 Sep;153(3):296–308. [PMC free article] [PubMed] [Google Scholar]
- Sturrock A., Alexander J., Lamb J., Craven C. M., Kaplan J. Characterization of a transferrin-independent uptake system for iron in HeLa cells. J Biol Chem. 1990 Feb 25;265(6):3139–3145. [PubMed] [Google Scholar]
- Tausk F., Fey M., Gigli I. Endocytosis and shedding of the decay accelerating factor on human polymorphonuclear cells. J Immunol. 1989 Nov 15;143(10):3295–3302. [PubMed] [Google Scholar]
- Thorstensen K., Romslo I. The role of transferrin in the mechanism of cellular iron uptake. Biochem J. 1990 Oct 1;271(1):1–9. doi: 10.1042/bj2710001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B. S., Lumanglas A. L., Silva J., Ruszala-Mallon V., Durr F. E. Internalization and re-expression of antigens of human melanoma cells following exposure to monoclonal antibody. Cell Immunol. 1987 Apr 15;106(1):12–21. doi: 10.1016/0008-8749(87)90145-6. [DOI] [PubMed] [Google Scholar]
- Ward J. H., Jordan I., Kushner J. P., Kaplan J. Heme regulation of HeLa cell transferrin receptor number. J Biol Chem. 1984 Nov 10;259(21):13235–13240. [PubMed] [Google Scholar]
- Ward J. H., Kushner J. P., Kaplan J. Regulation of HeLa cell transferrin receptors. J Biol Chem. 1982 Sep 10;257(17):10317–10323. [PubMed] [Google Scholar]
- Woodbury R. G., Brown J. P., Loop S. M., Hellström K. E., Hellström I. Analysis of normal neoplastic human tissues for the tumor-associated protein p97. Int J Cancer. 1981 Feb 15;27(2):145–149. doi: 10.1002/ijc.2910270204. [DOI] [PubMed] [Google Scholar]
- Wright T. L., Brissot P., Ma W. L., Weisiger R. A. Characterization of non-transferrin-bound iron clearance by rat liver. J Biol Chem. 1986 Aug 15;261(23):10909–10914. [PubMed] [Google Scholar]
- Young S. P., Aisen P. Transferrin receptors and the uptake and release of iron by isolated hepatocytes. Hepatology. 1981 Mar-Apr;1(2):114–119. doi: 10.1002/hep.1840010205. [DOI] [PubMed] [Google Scholar]
- van den Bosch R. A., du Maine A. P., Geuze H. J., van der Ende A., Strous G. J. Recycling of 5'-nucleotidase in a rat hepatoma cell line. EMBO J. 1988 Nov;7(11):3345–3351. doi: 10.1002/j.1460-2075.1988.tb03206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]