Abstract
Purpose
Sudden cardiac death (SCD) is an important cause of mortality in the adult population. Height has been associated with cardiac hypertrophy and an increased risk of arrhythmias, but also with decreased risk of coronary heart disease, suggesting a complex association with SCD.
Methods
We examined the association of adult height with the risk of physician-adjudicated SCD in two large population-based cohorts: the Cardiovascular Health Study (CHS) and the Atherosclerosis Risk in Communities (ARIC) study.
Results
Over an average follow-up time of 11.7 years in CHS, there were 199 (3.6%) cases of SCD among 5,556 participants. In ARIC, over 12.6 years, there were 227 (1.5%) cases of SCD among 15,633 participants. In both cohorts, there was a trend towards decreased SCD with taller height. In fixed effects meta-analysis, the pooled hazard ratio per 10 cm of height was 0.84 (95%CI 0.73, 0.98, p=0.03). The association of increased height with lower risk of SCD was slightly attenuated after inclusion of risk factors associated with height, such as hypertension and left ventricular hypertrophy. The association appeared stronger among men than women in both cohorts.
Conclusion
In two population-based prospective cohorts of different ages, greater height was associated with lower risk of SCD.
MeSH Key Words: Body height, risk factors, death, sudden, cardiac
Sudden cardiac death (SCD), with an estimated incidence of between 180,000 and 450,000 cases in the United States(1) and a global incidence of 4–5 million people per year(2), is a major public health issue. Although SCD can result from multiple pathological processes, the major cause for SCD is ventricular tachyarrhythmias, including ventricular tachycardia and fibrillation. Left ventricular (LV) mass, which has been associated with risk of both ventricular fibrillation, and SCD(3, 4), is also associated with increased height(5). Based on this association, many investigators have suggested the need to index LV mass to body stature, although the specific manner of this adjustment remains uncertain(6). Implicit in these adjustments is the concept that height itself is not associated with SCD, such that by adjusting for it, one is able to more clearly identify the ‘pathological’ increase in LV mass. However, if height itself is associated with SCD, then depending upon the nature of this association, such an adjustment might be counterproductive. Indeed, we recently demonstrated this phenomenon for the association of height with atrial fibrillation(7).
Analysis of the causes of SCD is complicated by the multiple pathological mechanisms that can lead to ventricular tachyarrhythmias (and other causes of SCD, such as asystole). Myocardial ischemia can cause ventricular fibrillation itself. This mechanism is well-described, with approximately 80% of cases of SCD(8) being associated with coronary artery disease. To complicate matters, height is inversely associated with risk of coronary heart disease (CHD)(9), emphasizing that the overall association of height and SCD, with potentially both adverse (via LV mass) and beneficial (via CHD) pathways, requires formal study.
To address these issues, we examined this association in two large prospective cohort studies that span a range of ages and include formal adjudication of cases of SCD.
MATERIALS AND METHODS
In both cohorts, all study procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975, as revised in 2004.
The Atherosclerotic Risk in Communities (ARIC) study
The ARIC Study(10) is a multicenter prospective cohort study investigating the etiology of atherosclerotic disease in a middle-aged biracial population. Participants at baseline (1987–1989) included 15,792 men and women aged 45–64, recruited from 4 communities in the US: Forsyth County, NC; Jackson, MS (African Americans only); the northwest suburbs of Minneapolis, MN; and Washington County, MD.
The ARIC Study protocol was approved by the institutional review board of each participating center. After obtaining written informed consent, participants underwent a baseline clinical examination and were re-examined in 1990–92, 1993–95, and 1996–98. Of the original study population, 159 subjects were excluded due to missing data, leaving an analysis size of 15,633 participants.
Risk factors examined in this analysis were ascertained at the baseline examination and were followed through December 31, 2001. Participants reported information on smoking status, education, history of cardiovascular disease, use of medications, and underwent examination that included standard height and weight measurements. Other prevalent risk factors examined included diabetes mellitus, resting blood pressure or use of anti-hypertensives, prevalent heart failure (HF)(11), coronary heart disease (CHD)(11), and stroke.
Participants underwent a standard supine digitally recorded 12-lead electrocardiogram with classification according to the Minnesota Code(12). Left ventricular hypertrophy (LVH) was defined electrocardiographically based on Cornell criteria(13).
Determination of SCD in ARIC
All participants were contacted annually by phone and all hospitalizations and deaths in the previous year were identified. For deaths, we obtained death certificates. If the death occurred out-of-hospital, we also sought next of kin interviews and physician, coroner, and autopsy information about the death. To classify SCD, all events classified as having fatal CHD (definite fatal myocardial infarction, definite fatal CHD, or possible fatal CHD, in- and out-of-hospital) were reviewed again and adjudicated by a committee of physicians, funded through the Johns Hopkins University Donald W. Reynolds Cardiovascular Research Center. SCD was defined as a sudden pulseless condition from a cardiac origin in a previously stable individual. After review of data available, cases were classified as ‘definite’ sudden arrhythmic death, ‘possible’ sudden arrhythmic death, ‘not’ sudden arrhythmic death, or unclassifiable.
The Cardiovascular Health Study (CHS)
The design and objectives of the Cardiovascular Health Study have been previously described(14). In brief, CHS is a longitudinal study of men and women aged 65 years or older, randomly selected from Medicare lists in Pittsburgh, PA; Forsyth County, NC; Sacramento County, CA; and Washington County, MD. The original cohort of 5201 participants was enrolled in 1989–1990; a second cohort of 687 African Americans was recruited in 1992–1993. Except where specified otherwise, both cohorts were used in this analysis, providing a total of 5888 participants. The institutional review board at each center approved the study, and each participant gave informed consent.
The baseline examination included a standardized questionnaire assessing a variety of risk factors, including smoking, alcohol intake, history of stroke, coronary heart disease, and heart failure, self-reported health status, and medication use on enrollment. Methods of determining prevalent cardiovascular disease were previously validated.(15) The examination included measurements of standing height, weight, and seated blood pressure (measured with a random-zero sphygmomanometer)(15), as well as a resting 12-lead ECG.
Of the initial 5888 individuals in the study population, we excluded 332 participants missing data on height, leaving a total of 5556 individuals for the analysis (Table 1).
Table 1.
Baseline Characteristics
| CHS | ARIC | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Total (N=5541) | Women (N=3183) | Men (N=2358) | Total (N=15,633) | Women (N= 8630) | Men (N=7003) | |
| Age (yrs) | 72.8±5.6 | 72.5±5.5 | 73.2±5.7 | 54.2±5.8 | 53.8±5.7 | 54.6±5.8 |
| African American | 837 (15%) | 521 (16%) | 316 (13%) | 4181 (27%) | 2594 (30%) | (23%) |
| BMI (kg/m2) | 26.7±4.7 | 26.9±5.3 | 26.5±3.8 | 27.7±5.4 | 27.9±6.1 | 27.5±4.2 |
| Standing Height (cm) | 165.0±9.5 | 158.9±6.3 | 173.1±6.6 | 168.5±9.3 | 162.3±6.0 | 176.1±6.6 |
| Waist Circumference (cm) | 94.6±13.2 | 92.2±14.4 | 97.7±10.4 | 97.0±13.9 | 95.4±15.7 | 99.1±11.1 |
| Treated HTN | 2608(47%) | 1556(49%) | 1052(45%) | 5446(35%) | 3043(35%) | 2403(34%) |
| Diabetes | 901 (16%) | 456 (14%) | 445 (19%) | 1856 | 1016 | 840 (12%) |
| Prevalent CHF | 243 (4%) | 130 (4%) | 113 (5%) | 743 (5%) | 516 (6%) | 227 (3%) |
| Prevalent CHD | 1068(19%) | 494 (16%) | 574 (24%) | 762 (5%) | 189 (2%) | 573 (8%) |
| Prior CVA | 229 (4%) | 102 (3%) | 127 (5%) | 283 (2%) | 161 (2%) | 122 (2%) |
| Current Cigarette smoking | 647 (12%) | 395 (12%) | 252 (11%) | 4088(26%) | 2145(25%) | 1943(28%) |
| Former Cigarette | 2315(42%) | 965(30%) | 1350(57%) | 5038(32%) | 1932(22%) | 3106(44%) |
| LVH on ECG | 274 (5%) | 158 (5%) | 116 (5%) | 346 (2%) | 195 (2%) | 151 (2%) |
| Resting heart rate (bpm) | 68.0±11.2 | 69.1±10.8 | 66.4±11.5 | 66.7±10.4 | 68.0±10.1 | 65.1±10.4 |
For measurements, values displayed are mean±standard deviation.
Participants were contacted every 6 months for follow-up, alternating between a telephone interview and a clinic visit for the first 10 years, and by telephone interview only thereafter. Participants were followed from baseline until June 30, 2006, or death from other causes. The maximum follow-up was 16 years (median 12.5 years).
Determination of SCD in CHS
Death certificates, inpatient records, nursing home or hospice records, physician questionnaires, interviews with next-of-kin, and autopsy reports, where available, were reviewed to determine the cause of death. SCD was defined as a sudden pulseless condition, presumed to be due to a cardiac arrhythmia, in a previously stable individual that occurred out of the hospital or in the emergency room. For unwitnessed deaths, the participant must have been seen within 24 hours of the arrest in a stable condition and without evidence of a noncardiac cause of cardiac arrest. SCD cases could not be under hospice or nursing home care or have a life-threatening noncardiac comorbidity.
Analysis
Analyses were performed separately for each cohort, and then combined in a pre-specified meta-analysis. We examined definite SCD as the primary endpoint, with total SCD (includes both definite and possible) used as validation. For individual cohort analyses, a baseline model was employed using Cox proportional hazards regression with adjustment for age, sex, race, study location, smoking status and highest level of education achieved. If death during follow-up was due to other causes than SCD, the individual was censored at that time. We chose to include smoking status given the likelihood that achieved height and smoking status are markers of early life socioeconomic status(16). A second model was examined with inclusion of potential mediators (risk factors) of SCD potentially influenced by height, and included waist circumference, hypertension, resting heart rate, diabetes, prevalent heart failure, stroke, or coronary heart disease (CHD), and left ventricular hypertrophy as defined by ECG criteria(12, 17). These analyses were also repeated with stratification by sex and race. We tested multiplicative interactions between height and sex, prevalent CHD, and race. In a sub-analysis, we examined any incident non-fatal CHD as a time-varying covariate in the two models, with and without inclusion of prevalent CHD as a covariate (Note: In the model without prevalent CHD as a separate covariate, prevalent CHD is coded as a 1 for incident non-fatal CHD). We separately examined non-linear associations of height with risk in each cohort but found none and hence we report risks per 10-centimeter increments in height.
All CHS analysis was performed using the R statistical package(18) and statistical analysis of ARIC data was performed using SAS version 9.2 (SAS Institute Inc., Cary, NC). Fixed-effects meta-analysis was performed using the regression coefficients (natural log of the hazard ratio) and standard errors for each cohort, using Stata 11.2 (StataCorp, LP). We examined heterogeneity between the studies with the I2 statistic. All authors had access to the final manuscript.
RESULTS
Baseline characteristics of the two cohorts are shown in Table 1. The CHS cohort was older (mean age in CHS was 72.8 years vs. 54.2 years in ARIC) and generally had a greater prevalence of CHD, hypertension, and stroke, than the ARIC cohort.
ARIC
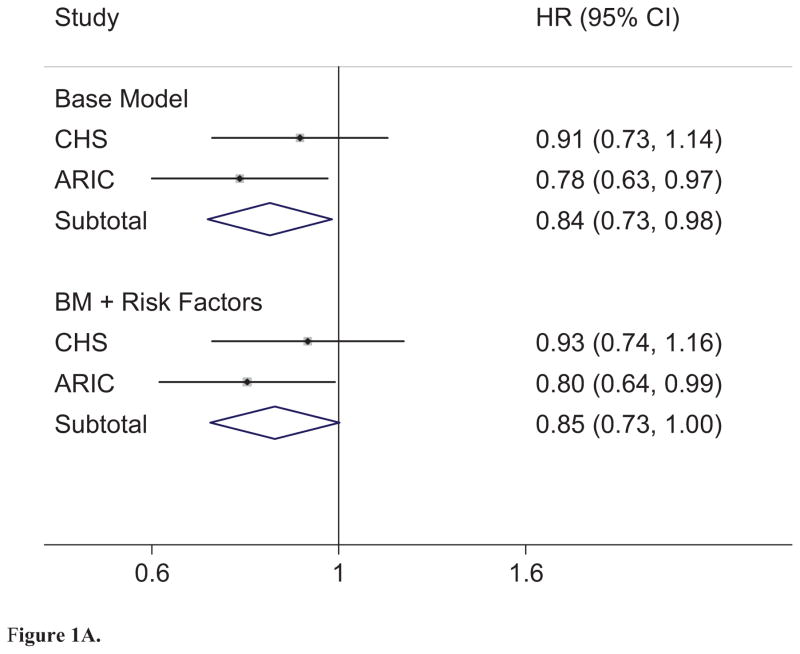
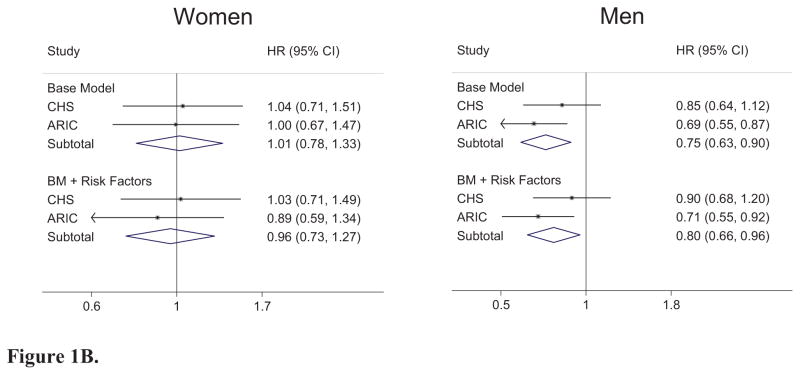
In the ARIC cohort, we documented 276 cases of SCD, of which 227 cases were defined as definite SCD, 157 in men and 70 in women, during a mean follow-up time of 12.6 (Standard deviation 2.5 years). The crude incidence rate of definite SCD per 1000 person-years was 1.2 overall, and 0.6 for women and 1.8 for men. Height was inversely associated with the risk of SCD in ARIC (Figure 1A), an association that appeared to be related mainly to lower risk in men (Figure 1B). Adjustment for risk factors attenuated the observed risks only minimally. The result was similar for total SCD (Base model: HRoverall 0.79, 95%CI 0.65, 0.95, p = 0.01; HRwomen 0.89, 95%CI 0.63, 1.26, p=0.51; HRmen 0.72, 95%CI 0.57, 0.90, p<0.01. Adjusted for Risk factors: HRoverall 0.78, 95%CI 0.64, 0.95, p=0.01; HRwomen 0.85, 95%CI 0.60, 1.21, p=0.36; HRmen 0.74, 95%CI 0.58, 0.94, p=0.01).
Figure 1.
Figure 1A. Combined Meta-analysis. Combined Meta-Analysis. Base Model (BM): Adjusted for age, sex, race, study location, Smoking status (current, former, and never), and education. Risk Factors: Waist Circumference, HTN, resting heart rate (bpm), diabetes, prevalent heart failure, stroke, or coronary heart disease, and ECG-defined LVH. (Baseline Meta-analysis I2 = 0.0%, p = 0.34; BL + Risk Factors Meta-analysis I2 = 0.0%, p = 0.36). Note: Hazard ratio (HR) defined per 10cm of height.
Figure 1B. Sex-stratified Meta-analysis. Base Model (BM): Adjusted for age, race, study location, Smoking status (current, former, and never), and education. Risk Factors: Waist circumference, HTN, resting heart rate (bpm), diabetes, prevalent heart failure, stroke, or coronary heart disease, and ECG-defined LVH. (Women: Baseline Meta-analysis I2 = 0.0%, p = 0.86; BL + Risk Factors Meta-analysis I2 = 0.0%, p = 0.61; Men: Baseline Meta-analysis I2 = 15.0%, p = 0.28; BL + Risk Factors Meta-analysis I2 = 36.6%, p = 0.21). ). Note: Hazard ratio (HR) defined per 10cm of height.
CHS
In CHS, there were 319 cases of SCD, of which 199 were identified as definite SCD—123 in men and 76 in women—after a mean follow-up time of 11.7 (Standard deviation 4.9 years). The crude incidence rate of definite SCD per 1000 person-years was 2.9 overall; 1.9 in women and 4.9 in men. In CHS, we observed a trend toward an inverse association found between height and definite SCD (Figure 1A) that was again stronger in men (Figure 1B), although the association was not statistically significant. This result was also similar for total SCD (Base model: HRoverall 0.86, 95%CI 0.71, 1.03, p = 0.11; HRwomen 0.92, 95%CI 0.68, 1.24, p=0.58; HRmen 0.83, 95%CI 0.65, 1.05, p=0.11. Adjusted for Risk factors: HRoverall 0.87, 95%CI 0.72, 1.04, p = 0.13; HRwomen 0.90, 95%CI 0.66, 1.22, p=0.49; HRmen 0.87, 95%CI 0.68, 1.10, p=0.24).
Combined analyses
In meta-analysis, taller height was significantly associated with lower risk of definite SCD overall (Figure 1A) with a 16% decrease in overall risk per 10 cm increase in height. This effect was significant only in men, where increased height was associated with a 20–25% decreased risk of definite SCD (Figure 1B). The overall association of height with definite SCD was minimally attenuated with inclusion of multiple other risk factors for SCD (Figure 1A, HR per 10 cm = 0.84 without risk factors vs. 0.86 with risk factors). There was no significant heterogeneity between studies in any of the analyses (see Figure 1A and 1B for statistics). When tested formally, the interaction term for height and sex was not statistically significant in either cohort at baseline or with inclusion of risk factors (pooled p interaction = 0.16). These results were also consistent with findings from total SCD (Supplemental Table 1).
The most common associated risk factor with SCD is CHD, which has been inversely associated with height in previous studies(19–21). We found no significant interaction of height with prevalent CHD in pooled analyses. We examined incident non-fatal CHD as a time-varying cofactor in models for effect attenuation, and found that inclusion of incident non-fatal CHD had minimal effect on the ability of height to predict risk of SCD (HRWith 0.84 (95%CI 0.72, 0.98, p = 0.03; HRWithout 0.85 (95%CI 0.73, 1.00, p = 0.05). This effect was consistent whether prevalent CHD was modeled as a separate variable or included as incident non-fatal CHD present at time 0 (Supplemental Table 2).
We observed no significant interaction between African American race and height in ARIC (p = 0.55) or CHS (p = 0.89), or in the combined analysis (p = 0.44).
DISCUSSION
In this combined analysis of 21,189 participants with 426 cases of SCD, height was inversely associated with the risk of SCD, an association that was statistically significant in men. The association was not significantly attenuated with inclusion of other potential mediators, including left ventricular mass by ECG.
Sudden cardiac death is increasingly recognized as a public health concern, and remains a challenge from the research perspective. Aside from the heterogenous nature of the condition, which can occur through a variety of related and unrelated disease mechanisms, event confirmation is difficult, typically requiring formal adjudication by trained physicians, although previous studies using administrative coding have hinted at such a relationship.(22) In our two cohorts of different age groups, the event rate was 0.1 – 0.3% per person-year (similar to previous results among individuals older than 35 years(23)), further emphasizing the challenge of studying this outcome. To our knowledge, this is the largest study of height and adjudicated SCD currently available.
Short stature is a well-described risk factor for CHD(19–21), and has been associated with an increased risk of congestive heart failure(24), stroke(25), and all-cause mortality(22). Vascular disease, and CHD in particular, is an important mechanistic link between height and risk of SCD. Coronary heart disease is a well-described risk factor for SCD, being responsible for an estimated 75% of all events(23, 26, 27), and lower CHD risk among taller individuals is one possible explanation for our findings. That we did not detect substantial attenuation of the association of height and SCD with inclusion of baseline CHD or incident non-fatal CHD as a covariate, nor a significant interaction of prevalent CHD with height, suggests that height may relate to lower SCD risk through other pathways as well.
Among our findings of subgroup analysis was the suggestion that men appeared to have a greater associations of height with lower SCD risk. Although we had no clear explanation for this finding, compared with women, men are at an increased risk of SCD. In a study of five primary prevention studies of implantable cardioverter-defibrillators (ICDs), women were found to have the same mortality as men, yet experienced fewer appropriate ICD interventions(28). This finding has been noted in other studies, in which women had less benefit of ICD implantation for primary or secondary prevention,(29) and less inducible ventricular tachycardia after myocardial infarction(30). These observations suggest that men may be particularly susceptible to ventricular arrhythmias (which benefit from ICD placement) as a cause of SCD and thus height may have particular importance for cases of SCD related to arrhythmias. However, no studies have directly examined the risk of ventricular arrhythmias per se and height to our knowledge.
In many ways, height is an intriguing risk factor. It is more easily objectively measured than other risk factors, including blood pressure, and the measures do not fluctuate over time, as with ECG intervals. It also reflects two factors in chronic disease that are difficult to quantify: environment and genetics. A number of genome-wide association studies have identified genes associated with height(31–38). The largest of these to date, the GIANT study, was performed in 183,727 people of European ancestry and identified 180 loci associated with height(38), which explain 0.3%(32) to 20%(38) of the heritable variation in height. Although no study has directly cross-referenced an association between these SNPs and the risk of SCD, in a genome-wide association study of the Oregon Sudden Unexpected Death Study (Oregon-SUDS), a minor allele of GPC5 (Glypican 5) was associated with a lower risk of SCD(39) and interestingly, this same gene was found to be associated with height in the GIANT study(38). While this finding may be spurious, it suggests that there could be genetic mechanisms for the protective effect of height on SCD.
In addition to the typical limitations discussed above relevant to study of SCD—event adjudication and limited numbers for power—our study was also limited in the combination of two somewhat different populations in terms of age and race. ARIC is a cohort of middle-aged individuals, with a larger percentage of African Americans than CHS, which is predominantly older. Middle-age height likely has different implications in terms of health overall than height in older age, which might imply the absence of conditions such as osteoporosis. Although we observed no significant heterogeneity, and there is not a well-described association between osteoporosis and sudden cardiac death, this difference could potentially limit meta-analysis of these two populations. We also had small numbers of individuals in particular sex-race categories, limiting our ability to study the observed interactions with precision.
In conclusion, in this combined meta-analysis of two large prospective cohorts, taller height was associated with lower risk of SCD. Subgroup analyses suggested that this association was most prominent in men, a group associated with an increased risk of SCD. More research is necessary to understand the mechanisms by which height relates to SCD and whether these can be harnessed for clinical benefit.
Supplementary Material
Acknowledgments
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/PI.htm.
List of Abbreviations (in order of appearance)
- SCD
Sudden cardiac death
- CHS
Cardiovascular Health Study cohort
- ARIC
Atherosclerosis Risk in Communities study cohort
- LV
Left ventricular
- CHD
Coronary heart disease
- HF
Heart failure
- LVH
Left ventricular hypertrophy
- ECG
Electrocardiogram
- HR
Hazard ratio
- CI
Confidence interval
- ICD
Implantable cardioverter-defibrillator
- GPC5
Glypican 5
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010 Feb 23;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. Epub 2009/12/19.eng. [DOI] [PubMed] [Google Scholar]
- 2.Chugh SS, Reinier K, Teodorescu C, Evanado A, Kehr E, Al Samara M, et al. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis. 2008 Nov-Dec;51(3):213–28. doi: 10.1016/j.pcad.2008.06.003. Epub 2008/11/26.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998 Nov;32(5):1454–9. doi: 10.1016/s0735-1097(98)00407-0. Epub 1998/11/11.eng. [DOI] [PubMed] [Google Scholar]
- 4.Verma A, Meris A, Skali H, Ghali JK, Arnold JM, Bourgoun M, et al. Prognostic implications of left ventricular mass and geometry following myocardial infarction: the VALIANT (VALsartan In Acute myocardial iNfarcTion) Echocardiographic Study. JACC Cardiovasc Imaging. 2008 Sep;1(5):582–91. doi: 10.1016/j.jcmg.2008.05.012. Epub 2009/04/10.eng. [DOI] [PubMed] [Google Scholar]
- 5.Tracy RE, Sander GE. Histologically measured cardiomyocyte hypertrophy correlates with body height as strongly as with body mass index. Cardiology research and practice. 2011;2011:658958. doi: 10.4061/2011/658958. Epub 2011/07/09.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gidding SS. Controversies in the assessment of left ventricular mass. Hypertension. 2010 Jul;56(1):26–8. doi: 10.1161/HYPERTENSIONAHA.110.153346. Epub 2010/05/12.eng. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg MA, Patton KK, Sotoodehnia N, Karas MG, Kizer JR, Zimetbaum PJ, et al. The impact of height on the risk of atrial fibrillation: the Cardiovascular Health Study. Eur Heart J. 2012 Sep 12; doi: 10.1093/eurheartj/ehs301. Epub 2012/09/15. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004 Sep 15;44(6):1268–75. doi: 10.1016/j.jacc.2004.06.029. Epub 2004/09/15.eng. [DOI] [PubMed] [Google Scholar]
- 9.Parker DR, Lapane KL, Lasater TM, Carleton RA. Short stature and cardiovascular disease among men and women from two southeastern New England communities. Int J Epidemiol. 1998 Dec;27(6):970–5. doi: 10.1093/ije/27.6.970. Epub 1999/02/19.eng. [DOI] [PubMed] [Google Scholar]
- 10.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. American journal of epidemiology. 1989 Apr;129(4):687–702. Epub 1989/04/01.eng. [PubMed] [Google Scholar]
- 11.Eriksson H, Caidahl K, Larsson B, Ohlson LO, Welin L, Wilhelmsen L, et al. Cardiac and pulmonary causes of dyspnoea--validation of a scoring test for clinical-epidemiological use: the Study of Men Born in 1913. European heart journal. 1987 Sep;8(9):1007–14. doi: 10.1093/oxfordjournals.eurheartj.a062365. Epub 1987/09/01.eng. [DOI] [PubMed] [Google Scholar]
- 12.Rautaharju PM, MacInnis PJ, Warren JW, Wolf HK, Rykers PM, Calhoun HP. Methodology of ECG interpretation in the Dalhousie program; NOVACODE ECG classification procedures for clinical trials and population health surveys. Methods of information in medicine. 1990 Sep;29(4):362–74. Epub 1990/09/01.eng. [PubMed] [Google Scholar]
- 13.Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P. Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation. 1987 Mar;75(3):565–72. doi: 10.1161/01.cir.75.3.565. Epub 1987/03/01.eng. [DOI] [PubMed] [Google Scholar]
- 14.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991 Feb;1(3):263–76. doi: 10.1016/1047-2797(91)90005-w. Epub 1991/02/01.eng. [DOI] [PubMed] [Google Scholar]
- 15.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995 Jul;5(4):270–7. doi: 10.1016/1047-2797(94)00092-8. Epub 1995/07/01.eng. [DOI] [PubMed] [Google Scholar]
- 16.Hiscock R, Bauld L, Amos A, Fidler JA, Munafo M. Socioeconomic status and smoking: a review. Annals of the New York Academy of Sciences. 2012 Feb;1248:107–23. doi: 10.1111/j.1749-6632.2011.06202.x. Epub 2011/11/19.eng. [DOI] [PubMed] [Google Scholar]
- 17.Furberg CD, Manolio TA, Psaty BM, Bild DE, Borhani NO, Newman A, et al. Major electrocardiographic abnormalities in persons aged 65 years and older (the Cardiovascular Health Study). Cardiovascular Health Study Collaborative Research Group. Am J Cardiol. 1992 May 15;69(16):1329–35. doi: 10.1016/0002-9149(92)91231-r. Epub 1992/05/15.eng. [DOI] [PubMed] [Google Scholar]
- 18.Team RC. R: A language and environment for statistical computing. 2013 Available from: http://www.R-project.org/
- 19.Gray L, Davey Smith G, McConnachie A, Watt GC, Hart CL, Upton MN, et al. Parental height in relation to offspring coronary heart disease: examining transgenerational influences on health using the west of Scotland Midspan Family Study. Int J Epidemiol. 2012 Oct 19; doi: 10.1093/ije/dys149. Epub 2012/10/23. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paajanen TA, Oksala NK, Kuukasjarvi P, Karhunen PJ. Short stature is associated with coronary heart disease: a systematic review of the literature and a meta-analysis. Eur Heart J. 2010 Jul;31(14):1802–9. doi: 10.1093/eurheartj/ehq155. Epub 2010/06/10.eng. [DOI] [PubMed] [Google Scholar]
- 21.Kannam JP, Levy D, Larson M, Wilson PW. Short stature and risk for mortality and cardiovascular disease events. The Framingham Heart Study. Circulation. 1994 Nov;90(5):2241–7. doi: 10.1161/01.cir.90.5.2241. Epub 1994/11/01.eng. [DOI] [PubMed] [Google Scholar]
- 22.Adult height and the risk of cause-specific death and vascular morbidity in 1 million people: individual participant meta-analysis. Int J Epidemiol. 2012 Oct;41(5):1419–33. doi: 10.1093/ije/dys086. Epub 2012/07/25. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myerburg RJ, Castellanos A. Emerging paradigms of the epidemiology and demographics of sudden cardiac arrest. Heart rhythm : the official journal of the Heart Rhythm Society. 2006 Feb;3(2):235–9. doi: 10.1016/j.hrthm.2005.09.023. Epub 2006/01/31.eng. [DOI] [PubMed] [Google Scholar]
- 24.Akinkuolie AO, Aleardi M, Ashaye AO, Gaziano JM, Djousse L. Height and risk of heart failure in the Physicians' Health Study. Am J Cardiol. 2012 Apr 1;109(7):994–7. doi: 10.1016/j.amjcard.2011.11.032. Epub 2012/01/10.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honjo K, Iso H, Inoue M, Tsugane S. Adult height and the risk of cardiovascular disease among middle aged men and women in Japan. Eur J Epidemiol. 2011 Jan;26(1):13–21. doi: 10.1007/s10654-010-9515-8. Epub 2010/10/19.eng. [DOI] [PubMed] [Google Scholar]
- 26.de Vreede-Swagemakers JJ, Gorgels AP, Dubois-Arbouw WI, van Ree JW, Daemen MJ, Houben LG, et al. Out-of-hospital cardiac arrest in the 1990's: a population-based study in the Maastricht area on incidence, characteristics and survival. J Am Coll Cardiol. 1997 Nov 15;30(6):1500–5. doi: 10.1016/s0735-1097(97)00355-0. Epub 1997/11/15.eng. [DOI] [PubMed] [Google Scholar]
- 27.Deo R, Albert CM. Epidemiology and genetics of sudden cardiac death. Circulation. 2012 Jan 31;125(4):620–37. doi: 10.1161/CIRCULATIONAHA.111.023838. Epub 2012/02/02.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santangeli P, Pelargonio G, Dello Russo A, Casella M, Bisceglia C, Bartoletti S, et al. Gender differences in clinical outcome and primary prevention defibrillator benefit in patients with severe left ventricular dysfunction: a systematic review and meta-analysis. Heart rhythm : the official journal of the Heart Rhythm Society. 2010 Jul;7(7):876–82. doi: 10.1016/j.hrthm.2010.03.042. Epub 2010/04/13.eng. [DOI] [PubMed] [Google Scholar]
- 29.Curtis LH, Al-Khatib SM, Shea AM, Hammill BG, Hernandez AF, Schulman KA. Sex differences in the use of implantable cardioverter-defibrillators for primary and secondary prevention of sudden cardiac death. JAMA. 2007 Oct 3;298(13):1517–24. doi: 10.1001/jama.298.13.1517. Epub 2007/10/04.eng. [DOI] [PubMed] [Google Scholar]
- 30.Russo AM, Stamato NJ, Lehmann MH, Hafley GE, Lee KL, Pieper K, et al. Influence of gender on arrhythmia characteristics and outcome in the Multicenter UnSustained Tachycardia Trial. J Cardiovasc Electrophysiol. 2004 Sep;15(9):993–8. doi: 10.1046/j.1540-8167.2004.04050.x. Epub 2004/09/15.eng. [DOI] [PubMed] [Google Scholar]
- 31.Weedon MN, Lettre G, Freathy RM, Lindgren CM, Voight BF, Perry JR, et al. A common variant of HMGA2 is associated with adult and childhood height in the general population. Nat Genet. 2007 Oct;39(10):1245–50. doi: 10.1038/ng2121. Epub 2007/09/04.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanna S, Jackson AU, Nagaraja R, Willer CJ, Chen WM, Bonnycastle LL, et al. Common variants in the GDF5-UQCC region are associated with variation in human height. Nat Genet. 2008 Feb;40(2):198–203. doi: 10.1038/ng.74. Epub 2008/01/15.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lettre G, Jackson AU, Gieger C, Schumacher FR, Berndt SI, Sanna S, et al. Identification of ten loci associated with height highlights new biological pathways in human growth. Nat Genet. 2008 May;40(5):584–91. doi: 10.1038/ng.125. Epub 2008/04/09.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weedon MN, Lango H, Lindgren CM, Wallace C, Evans DM, Mangino M, et al. Genome-wide association analysis identifies 20 loci that influence adult height. Nat Genet. 2008 May;40(5):575–83. doi: 10.1038/ng.121. Epub 2008/04/09.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johansson A, Marroni F, Hayward C, Franklin CS, Kirichenko AV, Jonasson I, et al. Common variants in the JAZF1 gene associated with height identified by linkage and genome-wide association analysis. Hum Mol Genet. 2009 Jan 15;18(2):373–80. doi: 10.1093/hmg/ddn350. Epub 2008/10/28.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lei SF, Tan LJ, Liu XG, Wang L, Yan H, Guo YF, et al. Genome-wide association study identifies two novel loci containing FLNB and SBF2 genes underlying stature variation. Hum Mol Genet. 2009 May 1;18(9):1661–9. doi: 10.1093/hmg/ddn405. Epub 2008/11/29.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soranzo N, Rivadeneira F, Chinappen-Horsley U, Malkina I, Richards JB, Hammond N, et al. Meta-analysis of genome-wide scans for human adult stature identifies novel Loci and associations with measures of skeletal frame size. PLoS Genet. 2009 Apr;5(4):e1000445. doi: 10.1371/journal.pgen.1000445. Epub 2009/04/04.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lango Allen H, Estrada K, Lettre G, Berndt SI, Weedon MN, Rivadeneira F, et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010 Oct 14;467(7317):832–8. doi: 10.1038/nature09410. Epub 2010/10/01.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arking DE, Reinier K, Post W, Jui J, Hilton G, O'Connor A, et al. Genome-wide association study identifies GPC5 as a novel genetic locus protective against sudden cardiac arrest. PloS one. 2010;5(3):e9879. doi: 10.1371/journal.pone.0009879. Epub 2010/04/03.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.