Abstract
Previous studies showed that SOX9 plays a critical role in pancreatic ductal development. The aim of this study was to evaluate SOX9 as a marker for pancreatic ductal lineage. SOX9 expression was evaluated by immunohistochemistry in 146 benign pancreas (BP), 136 pancreatic ductal adenocarcinomas (PDAC), 47 pancreatic intraepithelial neoplasia (PanIN), 21 intraductal papillary mucinous neoplasms (IPMN), 14 mucinous cystic neoplasms (MCN), 10 serous cystadenomas (SCA), 39 pancreatic neuroendocrine tumors (PET), 9 acinar cell carcinomas (ACC) and 23 solid pseudopapillary neoplasms (SPN). Nuclear expression of SOX9 was detected in the centroacinar cells and ductal cells, but not in acinar or endocrine cells in 100% BP. Focal or diffuse SOX9 expression was detected in 100% PanIN, 100% IPMN, 100% MCN, 100% SCA, 89.0% PDAC, 2.6% PETs, 11.1% ACC and 0% SPN. SOX9 expression was lower in PanIN2 and PanIN3 than PanIN1 lesions (P<0.01). Compared to BP, IPMN had lower SOX9 expression (P<0.05). No correlation between SOX9 expression and other clinicopathologic parameters was identified. Our study showed that SOX9 is expressed in centroacinar and ductal epithelial cells of BP and is a useful marker for pancreatic ductal lineage of pancreatic neoplasms.
Keywords: SOX9, centroacinar cells, pancreatic ductal adenocarcinoma, PanIN, IPMN, MCN, SCA, ACC, PET, SPN
INTRODUCTION
The transcription factor SOX9 [SRY (Sex determining region Y)-box9] belongs to a family of transcription factors that are characterized by a highly conserved high mobility group (HMG) DNA binding domain, which was initially identified in SRY gene (1). Previous studies from mice and human have demonstrated that SOX9 play a critical role in the development and differentiation of multiple tissues during embryogenesis, including the differentiation of Sertoli cells of the testis (2), the development of prostate, chondrocyte differentiation (3–5), glial and neural crest differentiation (6–8), the formation of hair stem cells in the skin (9) and Paneth cells in the intestine (10–14). Recent studies have also showed that SOX9 plays a key role in the development of pancreas (15–17). SOX9, a downstream target of Notch, is expressed from the early stage of pancreatic development and is required for the maintenance of the pancreatic progenitor pool and for establishment of the pancreatic endocrine and exocrine cell fates (15, 17). A dose-dependent expression of Notch has been suggested in this process. Intermediate levels of Notch are required to activate SOX9. SOX9 in turn leads to activation of neurogenin 3 (Ngn3), a gene necessary and sufficient for establishment of an endocrine cell fate in the developing pancreas (18–20). Once activated, Ngn3 subsequently down-regulates SOX9 expression in the endocrine cell compartment (20). At high-levels of Notch signaling, the Ngn3 repressor, Hes1 (hairy and enhancer of split-1) is induced. The progenitor cells consequently retain SOX9 expression and undergo conversion to a ductal fate (20). A recent study using a zebrafish model system has shown that the formation of pancreatic and biliary ductal system is severely impaired inSOX9 mutants while the endocrine and acinar compartments of the pancreas appear unaffected (21). These data suggest that SOX9 is a key regulator for the development of pancreatic ductal system.
The pancreatic ductal system is composed of the centroacinar cells, the intercalated ducts, intralobular, interlobular and main pancreatic ducts. Located in the middle of the acini, the centroacinar cells are small, inconspicuous, flat to cuboidal cells with minimal pale or lightly eosinophilic cytoplasm and central oval nuclei. These cells constitute the beginning of the ductal system and convey the secretions from acinar cells to the intercalated ducts, which in turn fuse to form the intralobular and subsequently the interlobular pancreatic ducts. A spectrum of non-invasive lesions with mucinous histology of the pancreatic ductal system, including pancreatic intraepithelial neoplasia (PanIN), mucinous cystic neoplasms (MCN) and intraductal papillary mucinous neoplasms (IPMN) have been determined to be precursors for pancreatic ductal adenocarcinoma (PDAC) (22). However, the cell of origin or lineage for pancreatic ductal carcinogenesis has been a topic of debate in the literature (23–28). A recent study by Kopp et al. showed that SOX9 accelerates the formation of precursor lesions of PDAC when co-expressed with oncogenic Kras. By lineage tracing, their study also suggests that PanIN lesions and subsequently pancreatic ductal adenocarcinoma arise from ductal metaplasia of the pancreatic acinar cells, a phenomenon known as acinar-to-ductal metaplasia (29). SOX9 has been shown to be expressed in PDAC and IPMNs, however, the expression pattern of SOX9 in normal pancreas, PanIN lesions, MCN and other types of pancreatic neoplasms has not been examined in detail. We therefore examined the expression of SOX9 in the various compartments of benign pancreatic tissue (BP) and different types of precursor lesions for PDAC, including PanIN, MCN and IPMN. In addition, we also compared the SOX9 expression in different types of pancreatic neoplasms and correlated SOX9 expression with clinicopathologic parameters. Our study showed that SOX9 is expressed in centroacinar cells, pancreatic ductal cells, PDAC and its precursor lesions, but rarely in other pancreatic neoplasms.
MATERIALS AND METHODS
Case selection
We retrospectively reviewed medical records and tissue specimens of 146 benign pancreatic (BP) tissue (109 normal pancreas and 37 chronic pancreatitis) and 136 paired PDAC, 47 PanIN lesions of different histologic grades, 21 patients with IPMN, 14 patients with MCN, 10 patients with serous cystadenoma (SCA), 39 patients with low-grade pancreatic neuroendocrine tumor (PET), 9 patients with acinar cell carcinoma (ACC) and 23 patients with solid pseudopapillary neoplasm (SPN) who underwent pancreatectomy at the University of Texas MD Anderson Cancer Center between 1990 and 2011. Clinicopathologic parameters such as age, gender, tumor differentiation, tumor stage, and survival data were obtained from medical records. This study was approved by the Institutional Review Board of the University of Texas MD Anderson Cancer Center.
Tissue Microarray construction
To construct the tissue microarrays for PDAC, SPN, ACC, PET and BP cases used in this study, formalin-fixed, paraffin-embedded archival tissue blocks and their matching hematoxylin and eosin-stained (H & E) slides were retrieved, reviewed and screened for representative tumor regions by a pathologist. For each patient, two cores of tumor were sampled from representative areas using a 1.0-mm punch. The tissue microarray was constructed with a tissue microarrayer (Beecher Instruments, Sun Prairie, WI) as described previously (30).
Immunohistochemical analysis for SOX9 and Double immunohistochemical stain for CK19/SOX9
Immunohistochemical staining for SOX9 was performed either on 4-μm unstained sections from the tissue microarray blocks for PDACs and their paired benign pancreas tissue, ACCs, PETs, SPTs or on 4-μm whole sections from the formalin-fixed paraffin-embedded archival tissue blocks for IPMNs, MCNs, SCAs, and PanIN lesions using a polyclonal antibody against SOX9 (Millipore, Billerica, MA). Following deparaffinization, antigen retrieval was performed on thetissue sections at 100 °C in a steamer containing 10 mmol citrate buffer (pH, 6.0) for 5 min. The sections were then washed and immersed in anti-SOX9antibody (1:2000 dilution) at 35°C for 15 min. Subsequently, thesections were immersed in 3.0% hydrogen peroxidase at 35 °C for 5 min to block the endogenous peroxidase activity. A primary enhancer solution was then applied to the slides and incubated at 35°C for 8 minutes. The sections were then incubated with secondary Poly-HRP anti-mouse/anti-rabbitimm unoglobulin at 35°C for 8 min. Diaminobenzidine (DAB) was used as a chromogen and DAB enhancer was applied, and hematoxylin was used for counterstaining.
Double immunohistochemical stain for CK19 (RCK108, Dako, Carpinteria, CA) and SOX9 was performed on 4-μm unstained sections from the formalin-fixed paraffin-embedded archival tissue blocks. The sections were stained for SOX9 first using the conditions described above. The sections were then washed and immersed in anti-CK19 antibody (1:50 dilution) at 35°C for 15 min. A post primary enhancer was applied after to the slides after two washes and incubated at 35°C for 20 minutes. The sections were then washed and 3-amino-9-ethylcarbazole (AEC) was applied at 35°C for 15 min. Hematoxylin was used for counterstaining.
Measurement of SOX9 expression
The immunohistochemically stained slides of tissue microarrays and the formalin-fixed paraffin-embedded archival tissue were examined using standard light microscopy (Olympus BX41). The staining results were independently scored semiquantitatively by two pathologists (S.S. and H.W.), who were blinded to the clinicopathologic data. Only moderate to strong nuclear staining for SOX9 was considered as positive. The percentage of positive cells was estimated as a ratio between the number of the positively stained nuclei and the total number of tumor cell nuclei. The average percentage of SOX9 positive nuclei from the two pathologists were recorded and used for statistical analyses in all cases. Nuclear expression of SOX9 was categorized as negative (no staining or staining in less than 5% of the tumor cells), focal staining (5–30% nuclear staining in tumor cells) or diffuse staining (>30% nuclear staining in tumor cells).
Statistical analysis
Independent t test was used to compare the mean percentages of positive cells between the different histologic grades of PanINs, IPMNs or MCNs. The immunohistochemical staining results were correlated with the histology type of the pancreatic neoplasms by Fisher’s exact tests or Chi-square test. Statistical analysis was performed using Statistical Package for Social Sciences software (for Windows 12.0, SPSS Inc., Armonk, New York). We used a two-sided significance level of 0.05 for all statistical analyses.
RESULTS
SOX9 expression in benign pancreatic tissue
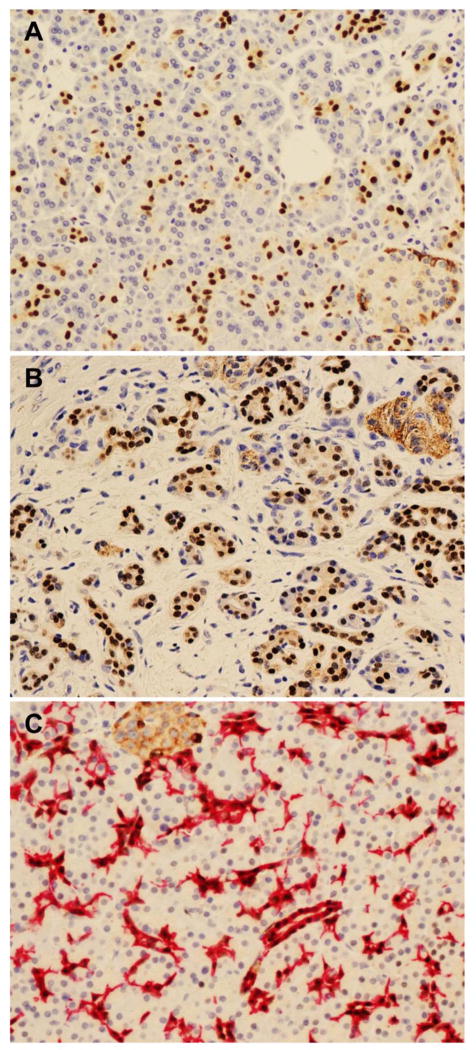
Diffuse and uniform nuclear expression of SOX9 was detected in the centroacinar cells and the epithelial cells of the pancreatic ductal system in all 109 (100%) normal pancreas samples (Figure 1A). Diffuse and uniform nuclear SOX9 expression was also detected in the ductal cells in all 37 (100%) chronic pancreatitis tissue samples (Figure 1B). There was no difference in SOX9 expression in the ductal cells between normal pancreas and chronic pancreatitis (Table 1). To confirm the expression of SOX9 in centroacinar cells and pancreatic ductal cells, double staining for SOX9 and cytokeratin 19, which is a known marker for the pancreatic ductal system including the intercalated ducts and centroacinar cells (31), were performed. The centroacinar and ductal epithelial cells that were positive for cytokeratin 19 co-expressed SOX9 (Figure 1C). No nuclear expression of SOX9 was detected in either pancreatic acinar cells or the pancreatic islet cells. The pancreatic islet cells demonstrated weak cytoplasmic staining for SOX9, which may be due to non-specific cross-reaction (Figure 1A).
Figure 1.
Nuclear SOX9 expression is present in centroacinar cells and pancreatic ductal cells, but not in acinar or islet cells in normal pancreatic tissue (A) and chronic pancreatitis (B). Nuclear expression of SOX9 (brown) is observed in all cells expressing CK19 (red) by double immunohistochemical stain (C). Original magnification, 200x for A–C.
Table 1.
Expression of SOX9 in benign pancreatic tissue, pancreatic intraepithelial neoplasia and cystic neoplasms of the pancreas
| Type of tissue | Number of cases | Negative (%) | Focal staining (%) | Diffuse staining (%) | P valueb |
|---|---|---|---|---|---|
| Benign pancreatic tissue | 146 | ||||
| Normal | 109 | 0 (0) | 0 (0) | 109 (100) | |
| Chronic pancreatitis | 37 | 0 (0) | 0 (0) | 37 (100) | 1.00 |
| Pancreatic intraepithelial neoplasia | |||||
| Grade 1 | 18 | 0 (0) | 0 (0) | 18 (100) | 1.00 |
| Grade 2 | 21 | 0 (0) | 6 (28.6) | 15 (71.4) | <0.01 |
| Grade 3 | 8 | 0 (0) | 2 (25.0) | 6 (75.0) | 0.004 |
| Intraductal Papillary Mucinous Neoplasm | 21 | ||||
| Low Grade Dysplasia | 13a | 0 (0) | 3 (23.1) | 10 (76.9) | 0.001 |
| High Grade Dysplasia | 11 | 0 (0) | 2 (18.2) | 11 (81.8) | 0.01 |
| Mucinous cystic neoplasm | 14 | ||||
| Low Grade Dysplasia | 14a | 0 (0) | 0 (0) | 14 (100) | 1.00 |
| High Grade Dysplasia | 3 | 0 (0) | 0 (0) | 3 (100) | 1.00 |
| Serous cystadenoma | 10 | 0 (0) | 0 (0) | 10 (100) | 1.00 |
Including three patients with both low-grade and high-grade dysplasia;
P values compared to normal pancreas
SOX9 expression in PanIN, IPMN and MCN
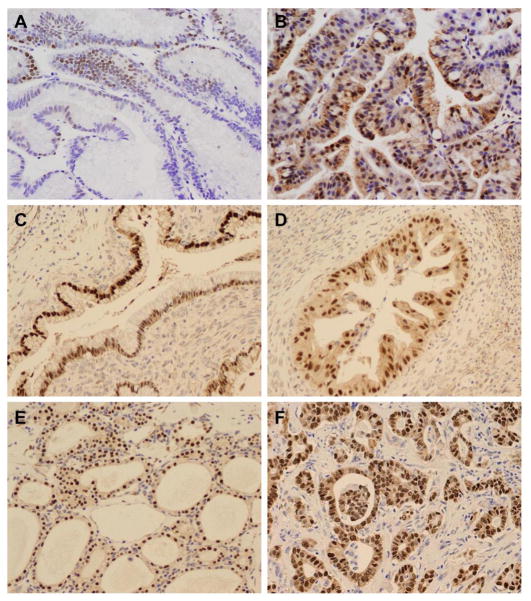
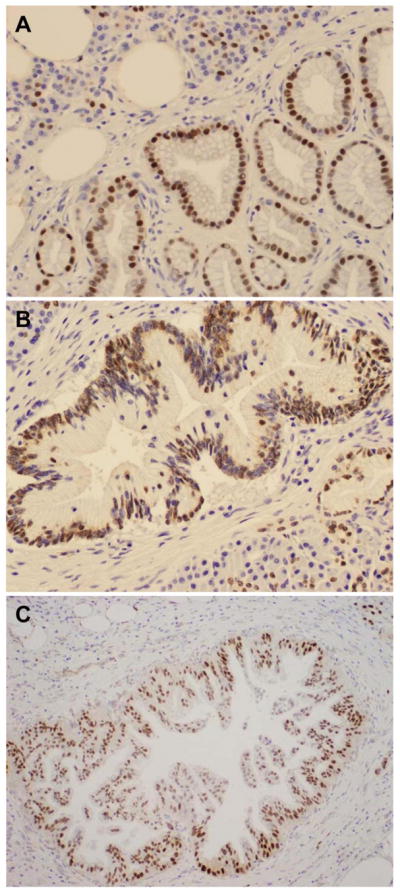
The immunohistochemical staining results for SOX9 in PanIN lesions, IPMN and MCN are summarized in Table 1 and Table 2. Although nuclear expression of SOX9 was noted in all grade 1 (18/18), grade 2 (21/21), and grade 3 (8/8) PanIN lesions (Figure 2A–2C), SOX9 staining was focal in 0 (0%) of PanIN1, 6 (28.6%) of PanIN2 and 2 (25%) of PanIN3 lesions. The mean percentages of SOX9 positive cells were 78.9 ± 11.3, 54.5 ± 23.9, and 53.4 ± 25.2 for PanIN1, PanIN2 and PanIN3 respectively (Table 2). PanIN1 had higher SOX9 expression than PanIN2 (P<0.001) and PanIN3 (P=0.002). However, there was no difference in the percentage of SOX9 positive cells between PanIN2 and PanIN3 (P=0.94). All 21 (100%) of IPMN and 14 (100%) of MCN were also positive for SOX9. The nuclear staining for SOX9 was focal and heterogenous in 23.1% (3/13) of IPMN with low-grade dysplasia and 18.2% (2/11) of IPMN with high-grade dysplasia. Compared to normal pancreatic ductal cells, the expression of SOX9 in IPMN was decreased (p<0.05). In the three IPMN cases with both low-grade and high-grade dysplasia, the percentages of SOX9 positive cells were 25%, 50% and 60% respectively in the low-grade areas and 60%, 70% and 25% respectively in the high-grade areas. In contrast, all 14 MCN with low-grade and/or high-grade dysplasia showed diffuse nuclear staining for SOX9 (Figure 3C & 3D). There were no correlation between the percentage of SOX9 positive cells or the frequencies of SOX9 expression and the degree of dysplasia in IPMNs and MCNs. In addition, strong nuclear staining for SOX9 was also detected in the tumor cells in all 10 (100%) cases of SCA (Figure 3E and Table 1).
Table 2.
Percentage of positive tumor cells in pancreatic ductal adenocarcinoma and its precursor lesions
| Types of tissue | Number of cases | Percentage of positive cells (%, Mean ± SD) | P value |
|---|---|---|---|
| Pancreatic intraepithelial neoplasia | |||
| Grade 1 | 18 | 78.9 ± 11.3 | |
| Grade 2 | 21 | 54.5 ± 23.9 | <0.01 |
| Grade 3 | 8 | 53.4 ± 25.2 | 0.002 |
| Intraductal papillary mucinous neoplasm | 21 | ||
| Low Grade Dysplasia | 13* | 50.8 ± 18.1 | |
| High Grade Dysplasia | 11 | 58.2 ± 19.0 | 0.34 |
| Mucinous cystic neoplasm | 14 | ||
| Low Grade Dysplasia | 14* | 78.6 ± 12.2 | |
| High Grade Dysplasia | 3 | 70.0 ± 20.0 | 0.33 |
| Pancreatic ductal adenocarcinoma | 136 | 68.0 ± 31.6 | NA |
Including three patients with both low-grade and high-grade dysplasia
Figure 2.
Representative micrographs showing nuclear SOX9 expression in pancreatic intraepithelial neoplasia (PanIN) 1 (A), PanIN2 (B) and PanIN3 lesions (C). Original magnification, 200x for A–B and 100x for C.
Figure 3.
Representative micrographs showing nuclear SOX9 expression in intraductal papillary mucinous neoplasm (IPMN) with low grade dysplasia (A), IPMN with high grade dysplasia (B), mucinous cystic neoplasm (MCN) with low grade dysplasia (C), MCN with high grade dysplasia (D), serous cystadenoma (E) and pancreatic ductal adenocarcinoma (F). Original magnification, 200x for A–F.
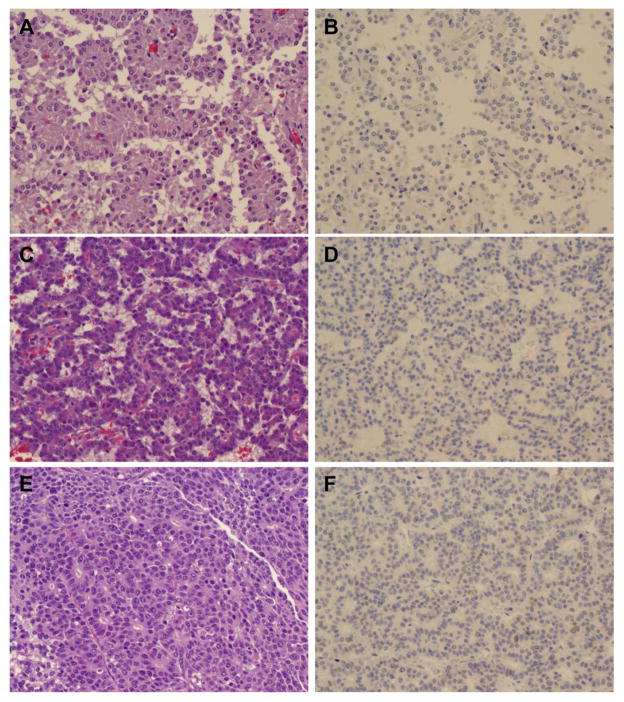
SOX9 expression in PDAC, ACC, PET and SPN
The results of the immunohistochemical staining for SOX9 in pancreatic tumors, including PDAC, ACC, PET and SPN, are summarized in Table 3. Diffuse and strong nuclear SOX9 expression was observed in 83.1% (113/136) of the PDAC cases (Figure 3F). In contrast, nuclear SOX9 expression was detected only in 2.6% (1/39) PETs, 11.1% (1/9) ACC, and 0% (0/23) SPTs examined (P<0.05, Figure 4A–4F). Compared to the paired benign pancreatic tissue samples, PDAC has decreased nuclear expression of SOX9 (P=0.0001). Our data suggested that SOX9 may be used as a marker for the ductal linage of pancreatic neoplasms. However, no correlation between SOX9 expression and the degree of tumor differentiation or grades, lymph node metastasis, other clinicopathological factors or survival was observed in the patients with any types of the above-mentioned pancreatic tumors (P>0.05, data not shown).
Table 3.
Expression of SOX9 in different types of pancreatic neoplasms
| Types of tumor | Number of cases | Negative (%) | Focal staining (%) | Diffuse staining (%) | P values |
|---|---|---|---|---|---|
| Pancreatic ductal adenocarcinoma | 136 | 15 (11.0) | 8 (5.9) | 113 (83.1) | NA |
| Pancreatic endocrine neoplasm | 39 | 38 (97.4) | 0 (0) | 1 (2.6) | <0.001 |
| Acinar cell carcinoma | 9 | 8 (88.9) | 0 (0) | 1 (11.1) | <0.001 |
| Solid pseudopapillary neoplasm | 23 | 23 (100.0) | 0 (0) | 0 (0.0) | <0.001 |
Figure 4.
Representative micrographs showing the hematoxylin & eosin (H & E) staining and SOX9 expression in solid pseudopapillary neoplasm (A & B), pancreatic neuroendocrine tumor (C & D) and acinar cell carcinoma (E and F). No nuclear staining was detected in solid pseudopapillary neoplasm (B), pancreatic neuroendocrine tumor (D) and acinar cell carcinoma (F). Original magnification, 200x for A–F.
DISCUSSION
Several animal studies have demonstrated the key role of SOX9 in pancreatic development and pancreatic ductal carcinogenesis. In this study, we examined the expression of SOX9 in a large cohort of benign pancreatic tissue, all types of precursor lesions of PDAC, and different types of pancreatic neoplasms, including PDAC, ACC, PET, SPN, and SCA. Our data showed that nuclear expression of SOX9 is present in centroacinar cells and ductal epithelial cells in benign pancreatic tissue and all types of pancreatic lesions/neoplasms of ductal origin, but rare in other pancreatic neoplasms. Although SOX9 expression in benign pancreatic tissue is identical to that of cytokeratin 19, recent studies showed that cytokeratin 19 is expressed in 86% of ACCs and 70% of PETs (32, 33). In addition, SOX9 is a nuclear marker. Thus SOX9 is a useful marker for the epithelial cells of pancreatic ductal system, including centroacinar cells, and for the ductal linage of pancreatic neoplasms.
In this study, we examine the expression of SOX9 in 109 normal pancreas samples and 37 chronic pancreatitis samples and we observed uniform strong nuclear expression of SOX9 in the centroacinar cells, normal pancreatic ductal cells and the proliferating ductules of chronic pancreatitis in all cases. No nuclear expression of SOX9 was observed in acinar cells and the islet cells of the pancreas. Our findings are consistent with the previous studies which showed that the central acinar cell and ductal cell specific nuclear expression of SOX9 (17, 29). These results support the critical role of SOX9 in the development of pancreatic ductal system.
In a recent study, Tanaka et al. showed that the percentage of cells expressing SOX9 was lower in invasive PDAC than normal pancreatic ducts and that the decrease of nuclear SOX9 expression correlated with the progression of IPMN (34). In this study, we observed significantly lower nuclear expression of SOX9 in PanIN2 and PanIN3 lesions than PanIN1 lesions (P<0.01). Consistent with their results, we also observed decreased nuclear expression of SOX9 in IPMNs with either low-grade or high-grade dyaplasia compared normal pancreatic ducts. However, we did not observe any significant difference in the nuclear SOX9 expression between IPMN with low-grade dysplasia and IPMN with high-grade dysplasia. In addition, there were no significant difference in the nuclear expression of SOX9 between PanIN2 and PanIN3 lesions or between the MCNs with low-grade and those with high-grade dysplasia. Furthermore, diffuse and strong nuclear SOX9 expression was observed in 83.1% of 136 invasive PDAC samples examined in this study. Therefore, our findings did not support their observations that there is a progressive loss of nuclear SOX9 expression with the progression of IPMN (34). The difference between ours and their study could be attributed in part to the difference in the antibodies used in these two studies. On the other hand, our findings are consistent with the previous study by Kopp et al. which reported SOX9 expression in 82% of MCNs and 95% IPMNs (29). Our observation that nuclear expression of SOX9 in PDAC samples and all precursor lesions of PDAC suggest that SOX9 is a sensitive marker of pancreatic ductal lineage. Since SOX9 is expressed in all cells of the ductal lineage, it cannot be utilized to distinguish between chronic pancreatitis and PDAC.
The expression of SOX9 in PET, SPT, ACC, and SCA has not been reported. In this study, we observe strong nuclear SOX9 expression in 100% of SCAs examined, which was consistent with the ductal origin of this tumor. The cell origin of pancreatic SPN is not clear. None of the 23 SPNs examined in this study were positive for SOX9 expression. Our results would argue against the ductal origin of this tumor. Interestingly, we observed nuclear expression of SOX9 in one of 9 ACCs examined. Careful histologic review and immunohistochemical study did not show unequivocal ductal differentiation in this case. We also observed diffuse nuclear expression of SOX9 in one PET case (2.6%). Although the number of cases for PET and ACC examined in this study is relatively small, our study demonstrated that nuclear SOX9 expression may be used asa marker for ductal lineage, especially on challenging core needle biopsies of pancreatic tumors to differentiate PDAC from acinar cell carcinoma, neuroendocrine tumor and solid pseudopapillary neoplasms of the pancreas.
In summary, our study showed that SOX9 is expressed in the centroacinar and pancreatic ductal cells in benign pancreatic tissue, PDAC and all known types of its precursor lesions, and SCA, but rare in PET, SPT, and ACC. Our study demonstrates that nuclear expression of SOX9 is a useful marker for the pancreatic ductal lineage of pancreatic neoplasms, which may be used in the differential diagnosis of pancreatic tumors.
Acknowledgments
Supported by the National Institutes of Health grant (1R21CA149544-01A1); G. S. Hogan Gastrointestinal Cancer Research Fund, and the Khalifa Bin Zayed Al Nahyan Foundation at The University of Texas M.D. Anderson Cancer Center
Footnotes
The authors have no conflicts of interest or funding to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gordon CT, Tan TY, Benko S, Fitzpatrick D, Lyonnet S, Farlie PG. Long-range regulation at the SOX9 locus in development and disease. J Med Genet. 2009;46:649–656. doi: 10.1136/jmg.2009.068361. [DOI] [PubMed] [Google Scholar]
- 2.Chaboissier MC, Kobayashi A, Vidal VI, Lutzkendorf S, van de Kant HJ, Wegner M, de Rooij DG, Behringer RR, Schedl A. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development. 2004;131:1891–1901. doi: 10.1242/dev.01087. [DOI] [PubMed] [Google Scholar]
- 3.Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Sox9 is required for cartilage formation. Nat Genet. 1999;22:85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- 4.Bi W, Huang W, Whitworth DJ, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc Natl Acad Sci U S A. 2001;98:6698–6703. doi: 10.1073/pnas.111092198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stolt CC, Lommes P, Sock E, Chaboissier MC, Schedl A, Wegner M. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 2003;17:1677–1689. doi: 10.1101/gad.259003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung M, Briscoe J. Neural crest development is regulated by the transcription factor Sox9. Development. 2003;130:5681–5693. doi: 10.1242/dev.00808. [DOI] [PubMed] [Google Scholar]
- 8.McCauley DW, Bronner-Fraser M. Importance of SoxE in neural crest development and the evolution of the pharynx. Nature. 2006;441:750–752. doi: 10.1038/nature04691. [DOI] [PubMed] [Google Scholar]
- 9.Vidal VP, Chaboissier MC, Lutzkendorf S, Cotsarelis G, Mill P, Hui CC, Ortonne N, Ortonne JP, Schedl A. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol. 2005;15:1340–1351. doi: 10.1016/j.cub.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 10.Barrionuevo FJ, Burgos M, Scherer G, Jimenez R. Genes promoting and disturbing testis development. Histol Histopathol. 2012;27:1361–1383. doi: 10.14670/HH-27.1361. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura R, Hata K, Matsubara T, Wakabayashi M, Yoneda T. Regulation of bone and cartilage development by network between BMP signalling and transcription factors. J Biochem. 2012;151:247–254. doi: 10.1093/jb/mvs004. [DOI] [PubMed] [Google Scholar]
- 12.Stuhlmiller TJ, Garcia-Castro MI. Current perspectives of the signaling pathways directing neural crest induction. Cell Mol Life Sci. 2012;69:3715–3737. doi: 10.1007/s00018-012-0991-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wuelling M, Vortkamp A. Chondrocyte proliferation and differentiation. Endocr Dev. 2011;21:1–11. doi: 10.1159/000328081. [DOI] [PubMed] [Google Scholar]
- 14.Blache P, van de Wetering M, Duluc I, Domon C, Berta P, Freund JN, Clevers H, Jay P. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J Cell Biol. 2004;166:37–47. doi: 10.1083/jcb.200311021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lynn FC, Smith SB, Wilson ME, Yang KY, Nekrep N, German MS. Sox9 coordinates a transcriptional network in pancreatic progenitor cells. Proc Natl Acad Sci U S A. 2007;104:10500–10505. doi: 10.1073/pnas.0704054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piper K, Ball SG, Keeling JW, Mansoor S, Wilson DI, Hanley NA. Novel SOX9 expression during human pancreas development correlates to abnormalities in Campomelic dysplasia. Mech Dev. 2002;116:223–226. doi: 10.1016/s0925-4773(02)00145-4. [DOI] [PubMed] [Google Scholar]
- 17.Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci U S A. 2007;104:1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 20.Shih HP, Kopp JL, Sandhu M, Dubois CL, Seymour PA, Grapin-Botton A, Sander M. A Notch-dependent molecular circuitry initiates pancreatic endocrine and ductal cell differentiation. Development. 2012;139:2488–2499. doi: 10.1242/dev.078634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delous M, Yin C, Shin D, Ninov N, Debrito Carten J, Pan L, Ma TP, Farber SA, Moens CB, Stainier DY. Sox9b is a key regulator of pancreaticobiliary ductal system development. PLoS Genet. 2012;8:e1002754. doi: 10.1371/journal.pgen.1002754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 23.Maitra A, Leach SD. Disputed paternity: the uncertain ancestry of pancreatic ductal neoplasia. Cancer Cell. 2012;22:701–703. doi: 10.1016/j.ccr.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, Redston MS, DePinho RA. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 26.De La OJ, Emerson LL, Goodman JL, Froebe SC, Illum BE, Curtis AB, Murtaugh LC. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc Natl Acad Sci U S A. 2008;105:18907–18912. doi: 10.1073/pnas.0810111105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Habbe N, Shi G, Meguid RA, Fendrich V, Esni F, Chen H, Feldmann G, Stoffers DA, Konieczny SF, Leach SD, Maitra A. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci U S A. 2008;105:18913–18918. doi: 10.1073/pnas.0810097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kopp JL, von Figura G, Mayes E, Liu FF, Dubois CL, Morris JPt, Pan FC, Akiyama H, Wright CV, Jensen K, Hebrok M, Sander M. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:737–750. doi: 10.1016/j.ccr.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Zhang W, Fuller GN. Tissue microarrays: applications in neuropathology research, diagnosis, and education. Brain Pathol. 2002;12:95–107. doi: 10.1111/j.1750-3639.2002.tb00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouwens L. Cytokeratins and cell differentiation in the pancreas. J Pathol. 1998;184:234–239. doi: 10.1002/(SICI)1096-9896(199803)184:3<234::AID-PATH28>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 32.La Rosa S, Adsay V, Albarello L, Asioli S, Casnedi S, Franzi F, Marando A, Notohara K, Sessa F, Vanoli A, Zhang L, Capella C. Clinicopathologic study of 62 acinar cell carcinomas of the pancreas: insights into the morphology and immunophenotype and search for prognostic markers. The American journal of surgical pathology. 2012;36:1782–1795. doi: 10.1097/PAS.0b013e318263209d. [DOI] [PubMed] [Google Scholar]
- 33.Han X, Zhao J, Ji Y, Xu X, Lou W. Expression of CK19 and KIT in resectable pancreatic neuroendocrine tumors. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2013 doi: 10.1007/s13277-013-0850-8. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka T, Kuroki T, Adachi T, Ono S, Hirabaru M, Soyama A, Kitasato A, Takatsuki M, Hayashi T, Eguchi S. Evaluation of SOX9 expression in pancreatic ductal adenocarcinoma and intraductal papillary mucinous neoplasm. Pancreas. 2013;42:488–493. doi: 10.1097/MPA.0b013e318269d281. [DOI] [PubMed] [Google Scholar]