Abstract
BACKGROUND
Quantitation of circulating tumor cells (CTCs) has utility in managing breast, colon and prostate carcinomas.
OBJECTIVE
Determine whether a commercially available CTC assay provides prognostic information in MCC and/or insight into treatment responses.
METHODS
We analyzed CTCs in 52 specimens from 34 MCC patients.
RESULTS
The presence of CTCs correlated with extent of disease at blood draw (p=0.004). Among 15 patients with regional nodal disease, CTC-negative patients had 80% disease-specific survival at 2 years after the test, versus 29% for CTC-positive patients (p=0.015). Among the entire cohort, those without CTCs had 72% MCC-specific survival while CTC-positive patients had 25% survival (n=34, median follow-up 19 months, p=0.0003). 57% of MCC patients had a cytokeratin “dot” visible in ≥20% of CTCs, a feature that was absent among CTCs from other carcinomas (zero of 13 cases).
LIMITATIONS
CTC assay was performed at variable times after diagnosis and heterogeneity in extent of disease affects interpretability of the data.
CONCLUSION
CTC detection in MCC is feasible and appears to add prognostic information, particularly in patients with regional nodal disease. It may also assist clinical management in certain situations, including differentiating metastatic MCC cells from those of other carcinomas.
Introduction
Merkel cell carcinoma (MCC) is an aggressive neuroendocrine skin cancer with a five-year disease-associated mortality of 30–40%1,2. Its reported incidence has tripled in the past 20 years to 1,600 cases/year in the US3. MCC commonly arises on sun-exposed skin of Caucasians who are older than 50. Etiologic factors include ultraviolet exposure, advanced age, immune suppression4 and the associated Merkel cell polyomavirus (MCPyV)5.
Although the levels of antibodies to the MCPyV T antigen oncoprotein in the serum can be used to track disease status6, only about 50% of MCC patients produce such antibodies. Sentinel lymph node biopsy is the gold standard for detecting early occult metastases at diagnosis. Radiological imaging modalities (CT or PET-CT scans) are the major tools to determine extent of metastatic disease and response to therapy in sites not easily accessible by physical examination. These imaging studies are costly, expose the patient to clinically significant radiation and are prone to false positive and false negative results7. There is a need for less invasive and less costly biomarkers for prognosis and disease status monitoring.
Circulating tumor cells (CTCs) have been studied extensively in several cancers including prostate8, colon9, breast10, ovarian11, pancreatic12 and neuroendocrine tumors such as small cell lung carcinoma13. CTCs have been proven to be useful prognostic markers in several carcinomas in which they correlate to disease progression and predict relapse14,15. In MCC, the presence of CTCs has been previously reported in 4 patients, typically as a single case 16,17,18. None of these 3 reports focused on assessing the utility of CTCs. In this study we have analyzed 52 samples from 34 patients and correlated CTCs with outcomes to evaluate the clinical utility of CTCs in MCC.
Methods
Human subjects and clinical samples
This study was approved by the Fred Hutchinson Cancer Research Center IRB (Protocol #6585) and performed in accordance with Helsinki principles. All patients gave informed consent. Patients’ blood samples (7.5 ml) were collected in CellSave Vacutainers™ containing EDTA and a cell stabilizing reagent (Veridex LLC™, Warren, NJ, USA).
CTC quantitation
Blood samples collected from MCC patients were maintained at room temperature and processed within 72h after collection. The CellSearch™ system (Veridex LLC™, Warren, NJ, USA) was used for isolation and counting of CTCs. The CellSearch Epithelial Cell Kit™ contains EpCAM(epithelial cell adhesion molecule)-specific antibodies conjugated to ferromagnetic particles to enrich epithelial cells. Isolated cells were fluorescently labeled with the nucleic acid dye 4′, 6-diamidino-2-phenylindole (DAPI) and monoclonal antibodies specific for leukocytes (CD45-allophycocyanin) and epithelial cells (cytokeratin 8, 18,19-phycoerythrin). To be defined as a CTC, an object must be round or oval, have a nucleus (DAPI-positive) contained within an epithelial cell (cytokeratin 8, 18, 19-positive), and lack expression of CD45. Identification and enumeration of putative CTCs were performed by the CellTracks Analyzer II™ and then subsequently verified by a trained operator 19. Samples containing one or more CTCs per 7.5 ml blood were considered CTC-positive, whereas samples containing no CTCs were considered negative.
Cytokeratin Staining Pattern Evaluation
CTC results (earliest positive draw) were analyzed in the 14 MCC patients with a positive result, and in 13 consecutive patients with positive CTCs in three other cancers (4 breast, 4 colon, 5 prostate). One expert technician, blinded as to diagnosis, counted and sorted CTCs into 2 categories according to their cytokeratin (CK) staining pattern: “typical dot” CK staining or “other” CK-staining pattern. In 2 patients whose CTC count exceeded 80 per 7.5 ml, 27 cells were analyzed in each case for staining pattern.
Statistical Analyses
Study analysis was performed using GraphPad Prism 5™ (GraphPad Software™) with values of p<0.05 considered significant. Differences in baseline characteristics between positive and negative CTC were analyzed with chi-squared and t tests. Association between presence of CTCs and extent of disease was assessed with Fisher’s test for trend. A one-way ANOVA was used to analyze the relationship between CTC count and extent of disease. Overall survival was defined as the time between blood draw and either time of death or last follow-up. Median overall survival rates were calculated using Kaplan-Meier analysis, and differences between curves were analyzed by log-rank test. Differences between MCC and other cancers were analyzed by Fisher’s exact test and t-test.
Results
Patients
Between June 2010 and March 2011, 34 patients seen in a multidisciplinary MCC clinic were recruited into the study and followed through the end of the study period (September 2012) with respect to outcome. Patients with no evidence of disease, localized, nodal or distant disease were all represented, as were both newly diagnosed and follow-up patients. This cohort thus represents a cross-section of patients typical for a tertiary care center. Clinical characteristics of the patients are shown in Table I. Median age at diagnosis was 68.5 years (range 41 to 90 years); 26 patients were men. Tumor site distribution was as follows: head or neck (12 patients), trunk (3), upper limb (5), lower limb (9), nodal disease with unknown primary (5). AJCC stage of MCC at diagnosis and stage at time of initial CTC assay are shown in Table 1. Median time from diagnosis to initial CTC count was 224 days.
Table I.
Clinical characteristics of MCC patients who underwent a CTC study
| Number of patients | % | |
|---|---|---|
| All patients | 34 | |
| Gender | ||
| Male | 26 | 76% |
| Female | 8 | 24% |
| Median age at diagnosis: 68.5 years (range: 41–90) | ||
| Stage at diagnosis (AJCC 7th edition) | ||
| Stage I (≤2 cm primary) | 14 | 41% |
| Stage II (>2 cm primary) | 5 | 15% |
| Stage III (nodal) | 15 | 44% |
| Stage IV (distant) | 0 | 0% |
| Median primary tumor diameter: 1.6 cm (range: 0.3–6) | ||
| Pathologic nodal evaluation at diagnosis (n=19) | ||
| negative | 12 | 63% |
| positive | 7 | 37% |
| Median time from diagnosis to 1st CTC assay: 224 days (range: 11–3719) | ||
| Extent of disease at 1st CTC sample | ||
| No evidence of disease | 7 | 20% |
| Local disease | 6 | 18% |
| Nodal disease | 15 | 44% |
| Distant disease | 6 | 18% |
52 blood samples from 34 patients were analyzed. CTCs were detected in 21/52 blood samples (40%). Median number of CTCs detected was 2 CTCs/7.5 ml (range: 1 to 711). 14 of 34 (41%) patients had CTCs detected in one or more blood draw.
There was no correlation between presence of CTCs at time of 1st blood draw and clinical characteristics of the tumor at initial diagnosis: stage, primary tumor size, sentinel lymph node status or lymphovascular invasion (data not shown). In contrast, CTC detection was strongly associated with extent of disease at the time of the assay. Specifically, correlation between CTC positivity at time of 1st CTC assay and extent of disease was as follows: 0 of 7 positive among patients with no clinical/radiographic evidence of disease, 1 of 6 positive among those with microscopic or local disease, 7 of 15 positive among those with regional disease, and 4 of 6 positive in patients with distant disease (p=0.004, Fisher’s test for trend). Among 6 of 15 patients with regional disease on whom we were able to ascertain the number of involved nodes, there was no significant correlation between number of involved nodes and presence of CTCs.
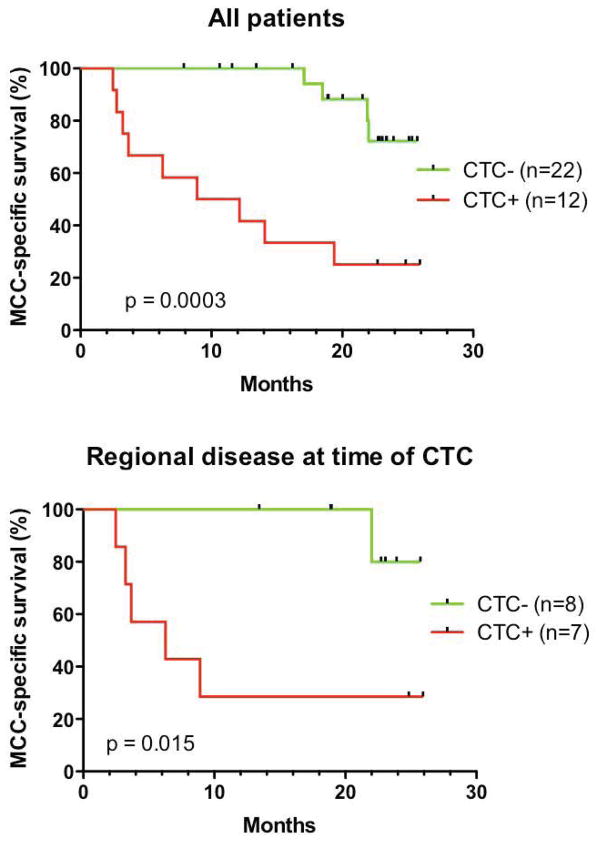
Median time between initial CTC draw and last follow-up was 19 months. Presence of CTCs was strongly correlated with subsequent disease progression (p=0.009, Fisher’s exact test) (Fig. 1). Thirteen patients had died and 21 were alive at the completion of the study. All deaths in this cohort were due to MCC, therefore overall survival and MCC-specific survival were identical in this study. Median overall survival time among all 34 patients had not been reached at 24 months of follow-up. Median survival time for patients with initial positive CTC was 10.5 months, while it had not yet been reached at 25.6 months for patients with negative CTC. As shown in Fig. 2 panel A, a statistically significant difference in overall survival was found between CTC-positive and CTC-negative groups of patients (p=0.0003). When grouped by extent of disease at time of CTC assay, the correlation between CTC status and survival remained significant for patients with regional nodal disease (Fig. 2 panel B).
Fig. 1.
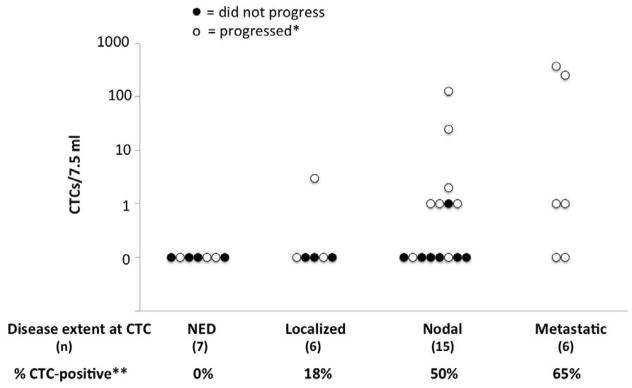
Correlation of CTCs with extent of disease and progression. The percentage of patients in each category of disease burden at time of CTC assay is indicated. Fisher’s test for trend was significant for increased CTC positivity with advancing disease (**p=0.004). NED = no evidence of disease; Localized = primary tumor still in place or cutaneous recurrence at the primary site; Nodal = disease presenting or recurring in a lymph node; Metastatic = disease at a distant site. Each case is represented as an open or closed circle to indicate whether that patient did or did not progress (respectively) during follow-up. The presence of CTC was strongly correlated with subsequent disease progression (*median follow-up=582 days, p=0.009).
Fig. 2.
CTC status is associated with outcome in MCC patients. Among 34 patients, blood was collected at varying time points (11 to 3719 days) after initial MCC diagnosis. Among all patients, CTC status was strongly correlated with survival (panel A, p=0.0003). While the number of patients was small in other groups, CTC status was significantly associated with survival in patients who had regional nodal disease at the time of CTC assay (panel B, p=0.015).
CK staining pattern in CTCs
Images of 396 CTCs were evaluated for CK staining pattern (range: 1 to 128 per patient). Among 14 MCC patients, 8 had “typical-dot” CTCs (57%; Fig. 3). Among 13 patients with other cancers, only 4 had “typical-dot” CTCs (23%, NS). The percentage of “typical-dot” cells among all CTCs was significantly higher in MCC patients (median=35%) as opposed to other cancers (median=0%) (p=0.0024). In three patients with previously diagnosed MCC, the “typical-dot” pattern of the CTC was felt to indicate that a new, highly suspicious visceral or osseous lesion was likely to be MCC and hence management proceeded without biopsy of the new lesion.
Fig 3.
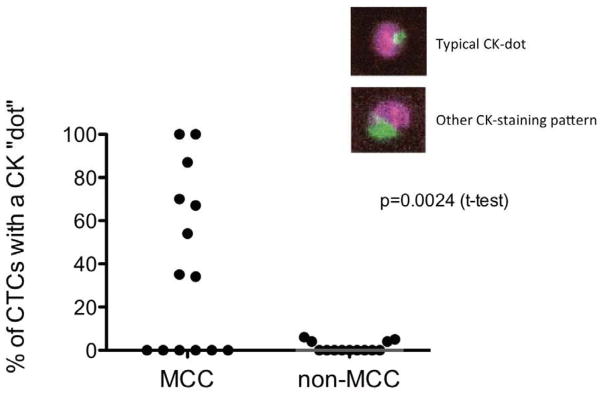
“Dot-like” cytokeratin staining in CTCs from Merkel cell carcinoma. Left: 8 of 14 MCCs showed the frequent presence of dot-like CK in CTCs. Right: Of 13 non-MCC carcinomas (breast, prostate, colon), none contained a significant fraction of CTCs with dot-like CK.
Longitudinal studies
Eleven patients had blood samples drawn for CTC analyses at more than one time point (median draws per patient: 2.7, range: 2 to 7). In 7 patients, CTC levels correlated with tumor burden and accurately reflected responses to treatment. In 2 patients, CTC results could not be linked to a clearly defined disease burden (CTC counts were done during therapy and/or disease status assessment was not recorded at the time of blood draw). Finally, in 2 patients CTC counts were consistently negative, regardless of measurable disease. In managing our patients, in 6 cases the CTC test proved beneficial in clinical management in the following ways: 3 patients avoided a biopsy of a highly suspicious lesion in a difficult-to-biopsy location (as described above), 1 patient showed early and prolonged disease remission, and 2 patients showed insufficient response to treatment, suggesting a need for particularly close follow-up.
Case vignette (Fig. 4)
Fig. 4.
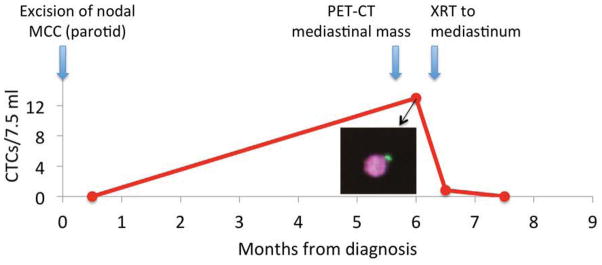
MCC case vignette illustrating the clinical utility of CTCs (See text for details). Typical “dot-like” cytokeratin (inset) in a CTC indicates that a mediastinal mass is MCC, sparing the patient a visceral biopsy.
A 69-year-old man with no known immune suppression was diagnosed with a stage IIIB MCC in the left parotid lymph node basin with no known primary. A blood draw 2 weeks after the diagnostic parotidectomy showed no CTCs. The patient was treated with definitive fractionated radiation (60 Gy) to the left parotid and left neck. 3 months after the end of the treatment, a routine PET-CT showed a 4.5 cm mass in the posterior mediastinum. A blood draw 10 days later showed 13 CTCs/7.5ml. Importantly, several CTCs had a characteristic “dot-like” cytokeratin staining pattern. A single dose of ablative radiotherapy (6 Gy) was given to the mediastinal mass. CTC counts rapidly decreased to 1 CTC/7.5 ml by two weeks after radiation, and were negative 3 weeks later. In this case, the CTC results spared the patient an invasive biopsy of the mediastinal mass and provided reassuring data of therapeutic efficacy of palliative radiation.
Discussion
MCC is an aggressive cancer with a high tendency to develop metastases and a mortality rate that is three times higher than melanoma. The use of imaging remains controversial in this cancer because of its cost and lack of specificity and sensitivity7,20. There is a need to identify blood-based biomarkers to help track the disease and predict prognosis and response to treatment. CTCs are thought to be important mediators of tumor dissemination and metastatic disease progression21. We used a commercially available test that is FDA-approved for other cancers to identify CTCs in MCC. To our knowledge, this study is the first formal evaluation of CTCs in MCC, and the first exploration of the clinical utility of CTCs in the management of MCC patients. Among 52 samples from 34 patients, CTCs were found to reflect burden of disease and their presence showed a significant association with survival. In individual patients, serial CTC counts helped assess response to treatment and finding the unique CK ‘dot-like’ staining pattern in CTCs was used to help guide clinical management in several individuals with MCC.
CTCs have been shown to aid in the therapeutic management of patients in a large number of different carcinoma types. In multivariate analyses carried out on breast, prostate and colorectal cancers, CTCs at baseline were an independent predictor of progression-free survival and overall survival10,22,23. Moreover, CTCs can act as a surrogate marker to determine response to treatment, and CTC changes during therapy can predict survival benefit from the treatment18,24,25. In MCC, publications have reported the presence of CTCs on peripheral blood smears in three patients with metastatic MCC to the bone marrow16,17. Furthermore, the presence of Merkel cell polyomavirus DNA in the peripheral blood of MCC patients (presumably indirectly reflecting the presence of CTCs) has been shown to predict poor survival26. In addition, CTC analysis using the CellSearch platform has been used to assess the response to treatment in one MCC patient27.
Our data show that CTC detection is feasible in MCC. This test did not reliably detect the presence of disease as 15 of 27 cases with disease at the time of blood draw had negative CTC results. However, CTCs were not detected in any patient without evidence of disease at the time of blood draw (n=7). More importantly, the presence of CTCs correlated with subsequent disease progression, and overall survival was significantly shorter in CTC-positive as compared to CTC-negative patients. Our study also demonstrates that in a majority of patients with serial blood draws, CTC counts correlated with tumor burden and response to treatment. Specifically, 2 patients had consistently negative CTCs despite measurable disease. In contrast, all 7 patients who had at least one positive CTC result had changes in counts that reflected tumor burden and response to treatment. Furthermore, in several cases, changes in CTC values occurred earlier than evaluable responses in imaging studies. Therefore, while this assay may fail to detect patients with measurable disease, for patients with a positive CTC result, serial monitoring of CTC counts may be appropriate to better inform clinicians of changes in extent of disease and therapeutic efficacy.
Our study also reveals that MCC CTCs often have a very specific cytokeratin staining pattern that is analogous to the classic MCC pattern seen on immunohistochemical staining of tissue. This finding could prove useful in differentiating CTCs derived from a MCC as compared to CTCs from other carcinomas. This would be particularly useful when imaging studies reveal a metastasis of uncertain origin. Although not a substitute for imaging nor pathological evaluation, a CTC study may provide earlier information on response to treatment and better discriminate between active disease and surgery- or radiation-associated inflammation.
There are several limitations to this study, including the fact that it is not a comprehensive longitudinal analysis. Its cross-sectional nature, with new and follow-up patients presenting with varying disease burdens, limits the interpretability of the data. Our patient population may not be representative of the general population of MCC patients because these patients all sought care at a tertiary center. Furthermore, because of the small number of subjects, the prognostic significance of CTCs can not be determined in a multivariate analysis. Specifically, these preliminary data are confounded by the link between extent of disease at blood draw and CTC count. In addition, the median 19-month follow-up period is sufficient to capture most recurrences but may not capture the majority of deaths from MCC. Indeed, patients with negative CTCs may have metastatic disease with a lower tumor burden than patients with positive CTCs, and therefore tend to recur and die later. These results need to be validated on a larger cohort in a prospective longitudinal study that includes a baseline CTC analysis at diagnosis.
This study demonstrates that the CTC assay can provide insight into prognosis, therapeutic efficacy and help improve follow-up care in MCC patients, particularly those with nodal disease at the time of evaluation. Further studies will be needed to determine the patient setting in which this assay will be most informative. As is the case for other cancers, the ability to detect CTCs may have an impact on the prognosis and treatment of patients with MCC by providing insight into the risk of the development of future metastases and disease progression, and a peripheral marker for treatment susceptibility and cancer surveillance.
Acknowledgments
Supported by American Cancer Society RSG-08-115-01-CCE, NIH K24-CA139052, NIH R01-CA162522-01, the David & Rosalind Bloom Endowment for MCC Research, the Michael Piepkorn Endowment Fund and the UW MCC Patient Gift Fund.
Abbreviations used
- MCC
Merkel cell carcinoma
- CTCs
circulating tumor cells
- MCPyV
Merkel cell polyomavirus
- CK
cytokeratin
- NED
no evidence of disease
- CT
computed tomography
- PET
positron emission tomography
- MRI
magnetic resonance imaging
- XRT
radiation therapy
- AJCC
American Joint Committee on Cancer
Footnotes
Authors’ disclosures of potential conflicts of interest: DS receives salary support and research funding from RareCyte Corporation, a start-up company that is developing a competing technology for CTC detection.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lemos BD, Storer BE, Iyer JG, Phillips JL, Bichakjian CK, Fang LC, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: Analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol. 2010;63:751–761. doi: 10.1016/j.jaad.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santamaria-Barria JA, Boland GM, Yeap BY, Nardi V, Dias-Santagata D, Cusack JC., Jr Merkel Cell Carcinoma: 30-Year Experience from a Single Institution. Ann Surg Oncol. doi: 10.1245/s10434-012-2779-3. Published online December 1, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Albores-Saavedra J, Batich K, Chable-Montero F, Sagy N, Schwartz AM, Henson DE. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2010;37:20–7. doi: 10.1111/j.1600-0560.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- 4.Heath M, Jaimes N, Lemos B, Mostaghimi A, Wang LC, Penas PF, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375–381. doi: 10.1016/j.jaad.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulson KG, Carter JJ, Johnson LG, Cahill KW, Iyer JG, Schrama D, et al. Antibodies to merkel cell polyomavirus T antigen oncoproteins reflect tumor burden in merkel cell carcinoma patients. Cancer Res. 2010;70:8388–8397. doi: 10.1158/0008-5472.CAN-10-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawryluk EB, O’Regan KN, Sheehy N, Guo Y, Dorosario A, Sakellis CG, et al. Positron emission tomography/computed tomography imaging in Merkel cell carcinoma: A study of 270 scans in 97 patients at the Dana-Farber/Brigham and Women’s Cancer Center. J Am Acad Dermatol. doi: 10.1016/j.jaad.2012.08.042. Published online November 2, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Olmos D, Arkenau HT, Ang JE, Ledaki I, Attard G, Carden CP, et al. Circulating tumour cell (CTC) counts as intermediate end points in castration-resistant prostate cancer (CRPC): a single-centre experience. Ann Oncol. 2009;20:27–33. doi: 10.1093/annonc/mdn544. [DOI] [PubMed] [Google Scholar]
- 9.Negin BP, Cohen SJ. Circulating tumor cells in colorectal cancer: past, present, and future challenges. Curr Treat Options Oncol. 2010;11:1–13. doi: 10.1007/s11864-010-0115-3. [DOI] [PubMed] [Google Scholar]
- 10.Liu MC, Shields PG, Warren RD, Cohen P, Wilkinson M, Ottaviano YL, et al. Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer. J Clin Oncol. 2009;27:5153–5159. doi: 10.1200/JCO.2008.20.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poveda A, Kaye SB, McCormack R, Wang S, Parekh T, Ricci D, et al. Circulating tumor cells predict progression free survival and overall survival in patients with relapsed/recurrent advanced ovarian cancer. Gynecol Oncol. 2011;122:567–572. doi: 10.1016/j.ygyno.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 12.Kurihara T, Itoi T, Sofuni A, Itokawa F, Tsuchiya T, Tsuji S, et al. Detection of circulating tumor cells in patients with pancreatic cancer: a preliminary result. J Hepatobiliary Pancreat Surg. 2008;15:189–195. doi: 10.1007/s00534-007-1250-5. [DOI] [PubMed] [Google Scholar]
- 13.Khan MS, Tsigani T, Rashid M, Rabouhans JS, Yu D, Luong TV, et al. Circulating tumor cells and EpCAM expression in neuroendocrine tumors. Clin Cancer Res. 2011;17:337–345. doi: 10.1158/1078-0432.CCR-10-1776. [DOI] [PubMed] [Google Scholar]
- 14.Qiu MZ, Li ZH, Zhou ZW, Li YH, Wang ZQ, Wang FH, et al. Detection of carcinoembryonic antigen messenger RNA in blood using quantitative real-time reverse transcriptase-polymerase chain reaction to predict recurrence of gastric adenocarcinoma. J Transl Med. 2010;8:107. doi: 10.1186/1479-5876-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pachmann K, Camara O, Kohlhase A, Rabenstein C, Kroll T, Runnebaum IB, et al. Assessing the efficacy of targeted therapy using circulating epithelial tumor cells (CETC): the example of SERM therapy monitoring as a unique tool to individualize therapy. J Cancer Res Clin Oncol. 2011;137:821–828. doi: 10.1007/s00432-010-0942-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tam CS, Turner P, McLean C, Whitehead S, Cole-Sinclair M. ‘Leukaemic’ presentation of metastatic Merkel cell carcinoma. Br J Haematol. 2005;129:446. doi: 10.1111/j.1365-2141.2005.05475.x. [DOI] [PubMed] [Google Scholar]
- 17.Nemoto I, Sato-Matsumura KC, Fujita Y, Natsuga K, Ujiie H, Tomita Y, et al. Leukaemic dissemination of Merkel cell carcinoma in a patient with systemic lupus erythematosus. Clin Exp Dermatol. 2008;33:270–272. doi: 10.1111/j.1365-2230.2007.02618.x. [DOI] [PubMed] [Google Scholar]
- 18.Koyanagi K, O’Day SJ, Boasberg P, Atkins MB, Wang HJ, Gonzalez R, et al. Serial monitoring of circulating tumor cells predicts outcome of induction biochemotherapy plus maintenance biotherapy for metastatic melanoma. Clin Cancer Res. 2010;16:2402–2408. doi: 10.1158/1078-0432.CCR-10-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 20.Gupta SG, Wang LC, Penas PF, Gellenthin M, Lee SJ, Nghiem P. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: The Dana-Farber experience and meta-analysis of the literature. Arch Dermatol. 2006;142:685–690. doi: 10.1001/archderm.142.6.685. [DOI] [PubMed] [Google Scholar]
- 21.Alix-Panabieres C, Riethdorf S, Pantel K. Circulating tumor cells and bone marrow micrometastasis. Clin Cancer Res. 2008;14:5013–5021. doi: 10.1158/1078-0432.CCR-07-5125. [DOI] [PubMed] [Google Scholar]
- 22.Mego M, De Giorgi U, Hsu L, Ueno NT, Valero V, Jackson S, et al. Circulating tumor cells in metastatic inflammatory breast cancer. Ann Oncol. 2009;20:1824–1828. doi: 10.1093/annonc/mdp207. [DOI] [PubMed] [Google Scholar]
- 23.Goodman OB, Jr, Fink LM, Symanowski JT, Wong B, Grobaski B, Pomerantz D, et al. Circulating tumor cells in patients with castration-resistant prostate cancer baseline values and correlation with prognostic factors. Cancer Epidemiol Biomarkers Prev. 2009;18:1904–1913. doi: 10.1158/1055-9965.EPI-08-1173. [DOI] [PubMed] [Google Scholar]
- 24.Nole F, Munzone E, Zorzino L, Minchella I, Salvatici M, Botteri E, et al. Variation of circulating tumor cell levels during treatment of metastatic breast cancer: prognostic and therapeutic implications. Annals of Oncol. 2008;19:891–897. doi: 10.1093/annonc/mdm558. [DOI] [PubMed] [Google Scholar]
- 25.Hiraiwa K, Takeuchi H, Hasegawa H, Saikawa Y, Suda K, Ando T, et al. Clinical significance of circulating tumor cells in blood from patients with gastrointestinal cancers. Ann Surg Oncol. 2008;15:3092–3100. doi: 10.1245/s10434-008-0122-9. [DOI] [PubMed] [Google Scholar]
- 26.Laude HC, Jonchere B, Maubec E, Carlotti A, Marinho E, Couturaud B, et al. Distinct merkel cell polyomavirus molecular features in tumour and non tumour specimens from patients with merkel cell carcinoma. PLoS Pathog. doi: 10.1371/journal.ppat.1001076. Published online August 26, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yap TA, Olmos D, Brunetto AT, Tunariu N, Barriuso J, Riisnaes R, et al. Phase I trial of a selective c-MET inhibitor ARQ 197 incorporating proof of mechanism pharmacodynamic studies. J Clin Oncol. 2011;29:1271–1279. doi: 10.1200/JCO.2010.31.0367. [DOI] [PubMed] [Google Scholar]