Abstract
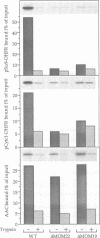
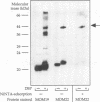
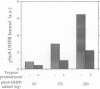
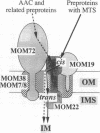
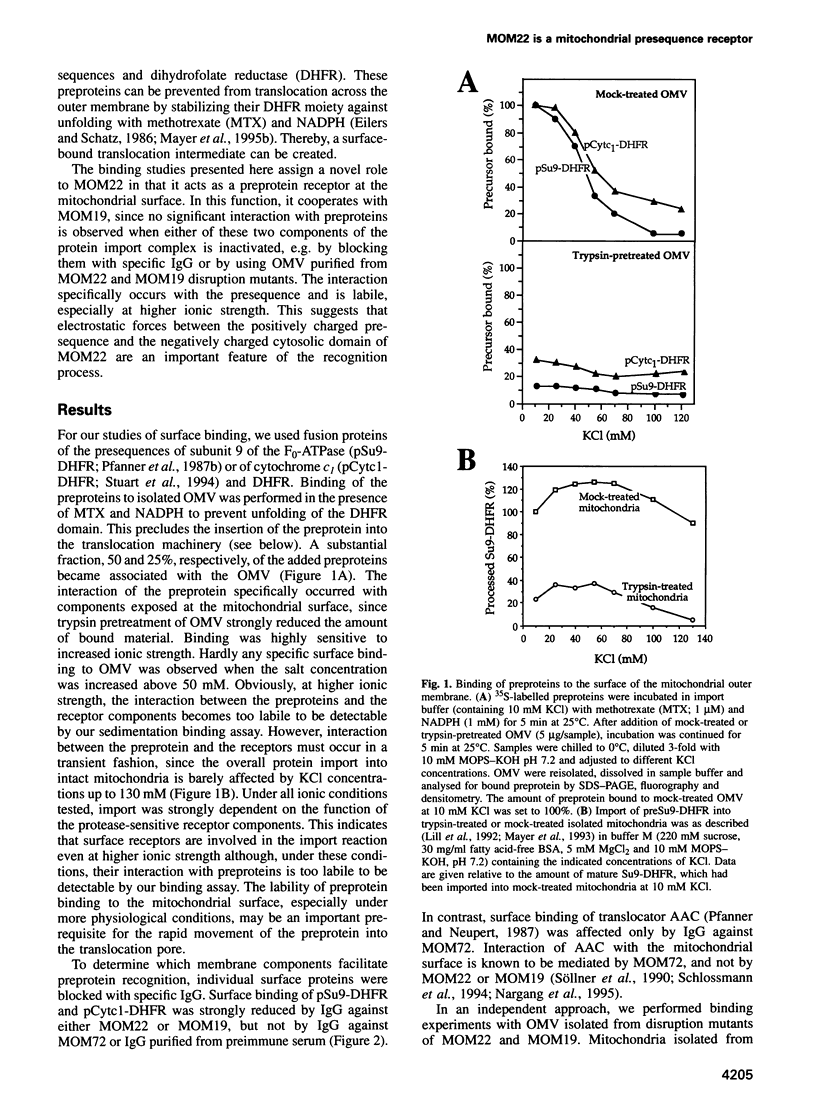
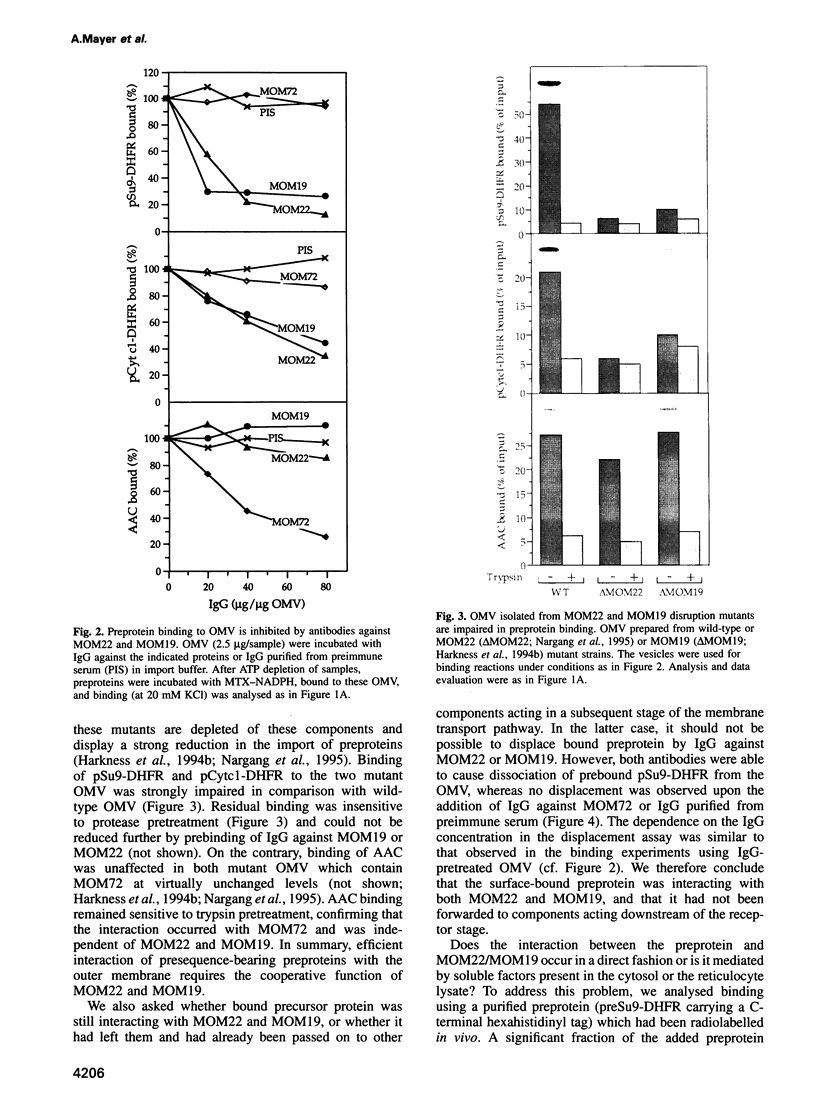
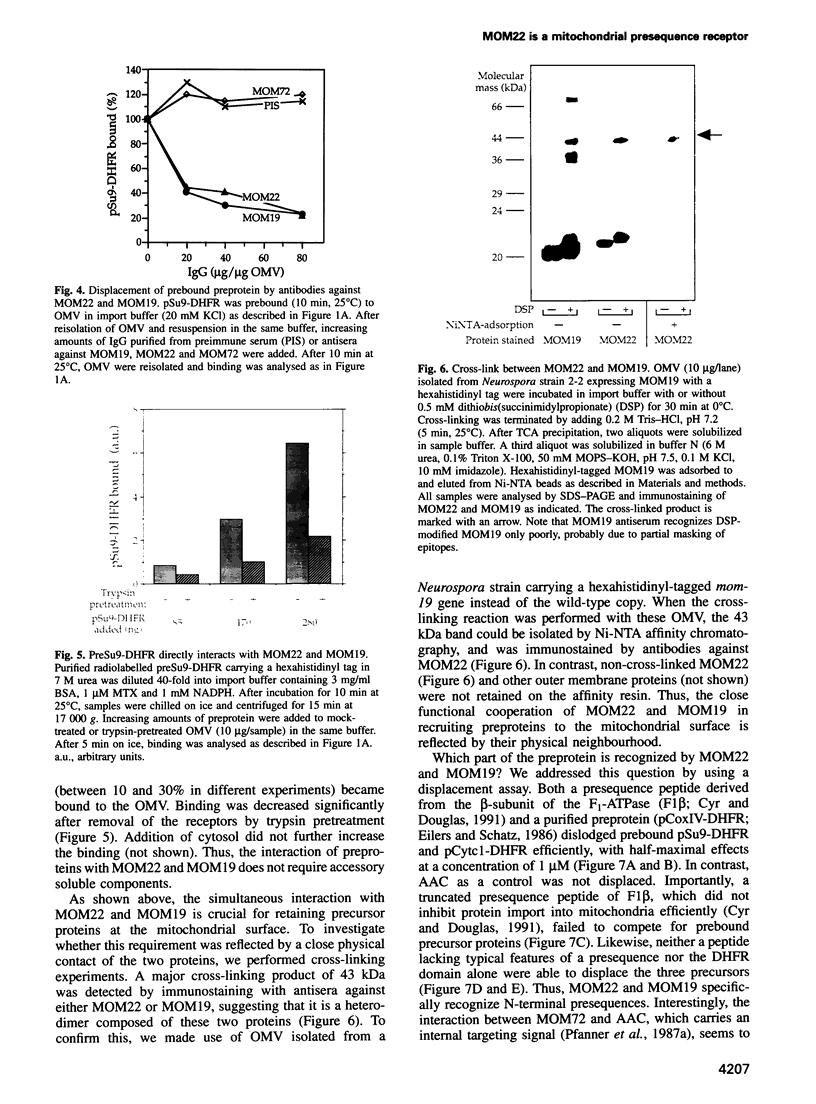
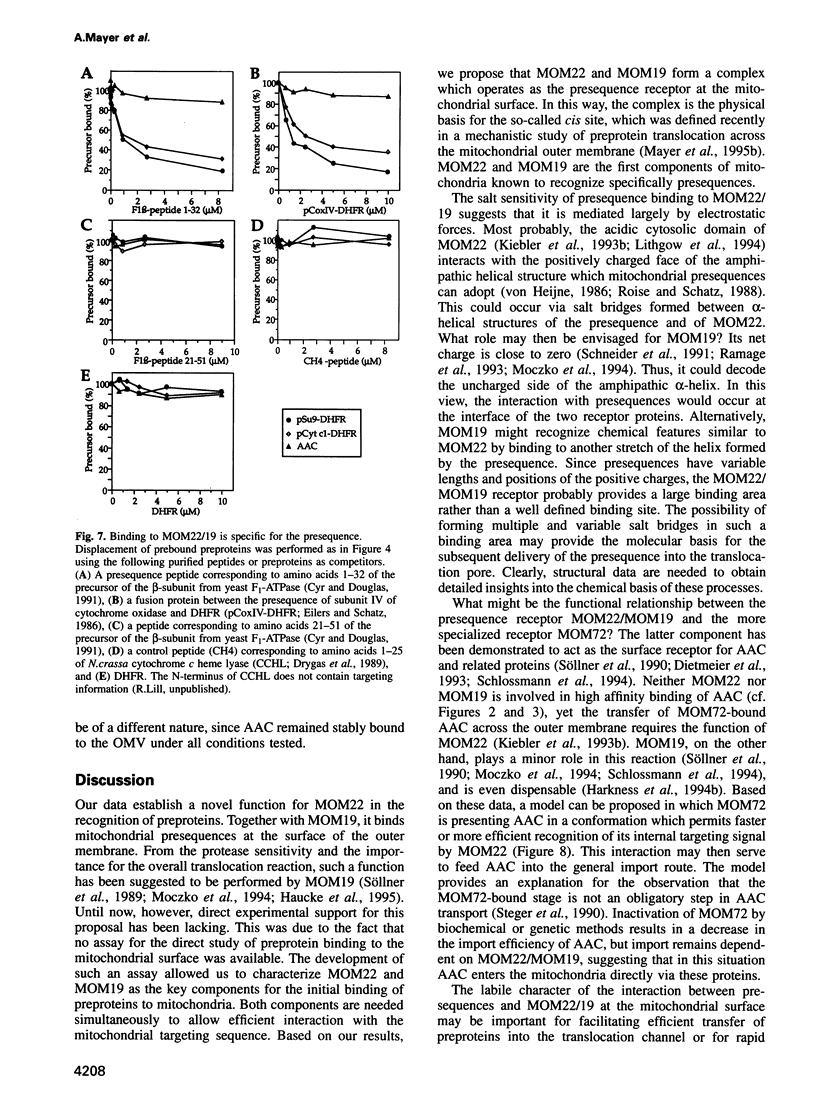
Recognition of targeting signals is a crucial step in protein sorting within the cell. So far, only a few components capable of deciphering targeting signals have been identified, and insights into the chemical nature of the interaction between the signals and their receptors are scarce. Using highly purified mitochondrial outer membrane vesicles, we demonstrate that MOM22 and MOM19, components of the protein import complex of the outer membrane, bind preproteins at the mitochondrial surface in a reversible fashion. Interaction specifically and directly occurs with the N-terminal presequence and is abolished after inactivation of either MOM22 or MOM19. Binding is salt sensitive, suggesting that recognition involves electrostatic forces between the positive charges of the presequence and the acidic cytosolic domain of MOM22. MOM19 and MOM22 can be cross-linked with high efficiency. We propose that the two proteins form a complex which functions as the presequence receptor at the mitochondrial surface and facilitates the movement of preproteins into the translocation pore.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arretz M., Schneider H., Guiard B., Brunner M., Neupert W. Characterization of the mitochondrial processing peptidase of Neurospora crassa. J Biol Chem. 1994 Feb 18;269(7):4959–4967. [PubMed] [Google Scholar]
- Austin B., Hall R. M., Tyler B. M. Optimized vectors and selection for transformation of Neurospora crassa and Aspergillus nidulans to bleomycin and phleomycin resistance. Gene. 1990 Sep 1;93(1):157–162. doi: 10.1016/0378-1119(90)90152-h. [DOI] [PubMed] [Google Scholar]
- Becker K., Guiard B., Rassow J., Söllner T., Pfanner N. Targeting of a chemically pure preprotein to mitochondria does not require the addition of a cytosolic signal recognition factor. J Biol Chem. 1992 Mar 15;267(8):5637–5643. [PubMed] [Google Scholar]
- Cyr D. M., Douglas M. G. Early events in the transport of proteins into mitochondria. Import competition by a mitochondrial presequence. J Biol Chem. 1991 Nov 15;266(32):21700–21708. [PubMed] [Google Scholar]
- Dietmeier K., Zara V., Palmisano A., Palmieri F., Voos W., Schlossmann J., Moczko M., Kispal G., Pfanner N. Targeting and translocation of the phosphate carrier/p32 to the inner membrane of yeast mitochondria. J Biol Chem. 1993 Dec 5;268(34):25958–25964. [PubMed] [Google Scholar]
- Drygas M. E., Lambowitz A. M., Nargang F. E. Cloning and analysis of the Neurospora crassa gene for cytochrome c heme lyase. J Biol Chem. 1989 Oct 25;264(30):17897–17906. [PubMed] [Google Scholar]
- Eilers M., Schatz G. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature. 1986 Jul 17;322(6076):228–232. doi: 10.1038/322228a0. [DOI] [PubMed] [Google Scholar]
- Gratzer S., Lithgow T., Bauer R. E., Lamping E., Paltauf F., Kohlwein S. D., Haucke V., Junne T., Schatz G., Horst M. Mas37p, a novel receptor subunit for protein import into mitochondria. J Cell Biol. 1995 Apr;129(1):25–34. doi: 10.1083/jcb.129.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachiya N., Komiya T., Alam R., Iwahashi J., Sakaguchi M., Omura T., Mihara K. MSF, a novel cytoplasmic chaperone which functions in precursor targeting to mitochondria. EMBO J. 1994 Nov 1;13(21):5146–5154. doi: 10.1002/j.1460-2075.1994.tb06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P., Bedwell D. M. Characterization of the mitochondrial binding and import properties of purified yeast F1-ATPase beta subunit precursor. Import requires external ATP. J Biol Chem. 1994 Mar 11;269(10):7192–7200. [PubMed] [Google Scholar]
- Harkness T. A., Metzenberg R. L., Schneider H., Lill R., Neupert W., Nargang F. E. Inactivation of the Neurospora crassa gene encoding the mitochondrial protein import receptor MOM19 by the technique of "sheltered RIP". Genetics. 1994 Jan;136(1):107–118. doi: 10.1093/genetics/136.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness T. A., Nargang F. E., van der Klei I., Neupert W., Lill R. A crucial role of the mitochondrial protein import receptor MOM19 for the biogenesis of mitochondria. J Cell Biol. 1994 Mar;124(5):637–648. doi: 10.1083/jcb.124.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haucke V., Lithgow T., Rospert S., Hahne K., Schatz G. The yeast mitochondrial protein import receptor Mas20p binds precursor proteins through electrostatic interaction with the positively charged presequence. J Biol Chem. 1995 Mar 10;270(10):5565–5570. doi: 10.1074/jbc.270.10.5565. [DOI] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Schatz G. The cleavable prepiece of an imported mitochondrial protein is sufficient to direct cytosolic dihydrofolate reductase into the mitochondrial matrix. FEBS Lett. 1984 Dec 10;178(2):306–310. doi: 10.1016/0014-5793(84)80622-5. [DOI] [PubMed] [Google Scholar]
- Kiebler M., Becker K., Pfanner N., Neupert W. Mitochondrial protein import: specific recognition and membrane translocation of preproteins. J Membr Biol. 1993 Sep;135(3):191–207. doi: 10.1007/BF00211091. [DOI] [PubMed] [Google Scholar]
- Kiebler M., Keil P., Schneider H., van der Klei I. J., Pfanner N., Neupert W. The mitochondrial receptor complex: a central role of MOM22 in mediating preprotein transfer from receptors to the general insertion pore. Cell. 1993 Aug 13;74(3):483–492. doi: 10.1016/0092-8674(93)80050-o. [DOI] [PubMed] [Google Scholar]
- Lill R., Dowhan W., Wickner W. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell. 1990 Jan 26;60(2):271–280. doi: 10.1016/0092-8674(90)90742-w. [DOI] [PubMed] [Google Scholar]
- Lill R., Stuart R. A., Drygas M. E., Nargang F. E., Neupert W. Import of cytochrome c heme lyase into mitochondria: a novel pathway into the intermembrane space. EMBO J. 1992 Feb;11(2):449–456. doi: 10.1002/j.1460-2075.1992.tb05074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithgow T., Junne T., Suda K., Gratzer S., Schatz G. The mitochondrial outer membrane protein Mas22p is essential for protein import and viability of yeast. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):11973–11977. doi: 10.1073/pnas.91.25.11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Lill R., Neupert W. Translocation and insertion of precursor proteins into isolated outer membranes of mitochondria. J Cell Biol. 1993 Jun;121(6):1233–1243. doi: 10.1083/jcb.121.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Neupert W., Lill R. Mitochondrial protein import: reversible binding of the presequence at the trans side of the outer membrane drives partial translocation and unfolding. Cell. 1995 Jan 13;80(1):127–137. doi: 10.1016/0092-8674(95)90457-3. [DOI] [PubMed] [Google Scholar]
- Moczko M., Ehmann B., Gärtner F., Hönlinger A., Schäfer E., Pfanner N. Deletion of the receptor MOM19 strongly impairs import of cleavable preproteins into Saccharomyces cerevisiae mitochondria. J Biol Chem. 1994 Mar 25;269(12):9045–9051. [PubMed] [Google Scholar]
- Moczko M., Gärtner F., Pfanner N. The protein import receptor MOM19 of yeast mitochondria. FEBS Lett. 1993 Jul 12;326(1-3):251–254. doi: 10.1016/0014-5793(93)81801-6. [DOI] [PubMed] [Google Scholar]
- Nakai M., Endo T. Identification of yeast MAS17 encoding the functional counterpart of the mitochondrial receptor complex protein MOM22 of Neurospora crassa. FEBS Lett. 1995 Jan 3;357(2):202–206. doi: 10.1016/0014-5793(94)01362-5. [DOI] [PubMed] [Google Scholar]
- Nargang F. E., Künkele K. P., Mayer A., Ritzel R. G., Neupert W., Lill R. 'Sheltered disruption' of Neurospora crassa MOM22, an essential component of the mitochondrial protein import complex. EMBO J. 1995 Mar 15;14(6):1099–1108. doi: 10.1002/j.1460-2075.1995.tb07093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson D. W., Köhler H., Neupert W. Import of cytochrome c into mitochondria. Cytochrome c heme lyase. Eur J Biochem. 1987 Apr 1;164(1):147–157. doi: 10.1111/j.1432-1033.1987.tb11006.x. [DOI] [PubMed] [Google Scholar]
- Pfaller R., Neupert W. High-affinity binding sites involved in the import of porin into mitochondria. EMBO J. 1987 Sep;6(9):2635–2642. doi: 10.1002/j.1460-2075.1987.tb02554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Hoeben P., Tropschug M., Neupert W. The carboxyl-terminal two-thirds of the ADP/ATP carrier polypeptide contains sufficient information to direct translocation into mitochondria. J Biol Chem. 1987 Nov 5;262(31):14851–14854. [PubMed] [Google Scholar]
- Pfanner N., Neupert W. Distinct steps in the import of ADP/ATP carrier into mitochondria. J Biol Chem. 1987 Jun 5;262(16):7528–7536. [PubMed] [Google Scholar]
- Pfanner N., Tropschug M., Neupert W. Mitochondrial protein import: nucleoside triphosphates are involved in conferring import-competence to precursors. Cell. 1987 Jun 19;49(6):815–823. doi: 10.1016/0092-8674(87)90619-2. [DOI] [PubMed] [Google Scholar]
- Ramage L., Junne T., Hahne K., Lithgow T., Schatz G. Functional cooperation of mitochondrial protein import receptors in yeast. EMBO J. 1993 Nov;12(11):4115–4123. doi: 10.1002/j.1460-2075.1993.tb06095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roise D., Schatz G. Mitochondrial presequences. J Biol Chem. 1988 Apr 5;263(10):4509–4511. [PubMed] [Google Scholar]
- Schlossmann J., Dietmeier K., Pfanner N., Neupert W. Specific recognition of mitochondrial preproteins by the cytosolic domain of the import receptor MOM72. J Biol Chem. 1994 Apr 22;269(16):11893–11901. [PubMed] [Google Scholar]
- Steger H. F., Söllner T., Kiebler M., Dietmeier K. A., Pfaller R., Trülzsch K. S., Tropschug M., Neupert W., Pfanner N. Import of ADP/ATP carrier into mitochondria: two receptors act in parallel. J Cell Biol. 1990 Dec;111(6 Pt 1):2353–2363. doi: 10.1083/jcb.111.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart R. A., Gruhler A., van der Klei I., Guiard B., Koll H., Neupert W. The requirement of matrix ATP for the import of precursor proteins into the mitochondrial matrix and intermembrane space. Eur J Biochem. 1994 Feb 15;220(1):9–18. doi: 10.1111/j.1432-1033.1994.tb18593.x. [DOI] [PubMed] [Google Scholar]
- Söllner T., Griffiths G., Pfaller R., Pfanner N., Neupert W. MOM19, an import receptor for mitochondrial precursor proteins. Cell. 1989 Dec 22;59(6):1061–1070. doi: 10.1016/0092-8674(89)90762-9. [DOI] [PubMed] [Google Scholar]
- Söllner T., Pfaller R., Griffiths G., Pfanner N., Neupert W. A mitochondrial import receptor for the ADP/ATP carrier. Cell. 1990 Jul 13;62(1):107–115. doi: 10.1016/0092-8674(90)90244-9. [DOI] [PubMed] [Google Scholar]
- Söllner T., Rassow J., Pfanner N. Analysis of mitochondrial protein import using translocation intermediates and specific antibodies. Methods Cell Biol. 1991;34:345–358. doi: 10.1016/s0091-679x(08)61689-1. [DOI] [PubMed] [Google Scholar]
- Söllner T., Rassow J., Wiedmann M., Schlossmann J., Keil P., Neupert W., Pfanner N. Mapping of the protein import machinery in the mitochondrial outer membrane by crosslinking of translocation intermediates. Nature. 1992 Jan 2;355(6355):84–87. doi: 10.1038/355084a0. [DOI] [PubMed] [Google Scholar]
- Walter P., Ibrahimi I., Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986 Jun;5(6):1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]