Abstract
K2 or Spice products are emerging drugs of abuse that contain synthetic cannabinoids (SCBs). Although assumed by many teens and first time drug users to be a “safe” and “legal” alternative to marijuana, many recent reports indicate that SCBs present in K2 produce toxicity not associated with the primary psychoactive component of marijuana, Δ9-tetrahydrocannabinol (Δ9-THC). This mini-review will summarize recent evidence that use of K2 products poses greater health risks relative to marijuana, and suggest that distinct pharmacological properties and metabolism of SCBs relative to Δ9-THC may contribute to the observed toxicity. Studies reviewed will indicate that in contrast to partial agonist properties of Δ9-THC typically observed in vitro, SCBs in K2 products act as full cannabinoid receptor type 1 (CB1R) and type 2 (CB2R) agonists in both cellular assays and animal studies. Furthermore, unlike Δ9-THC metabolism, several SCB metabolites retain high affinity for, and exhibit a range of intrinsic activities at, CB1 and CB2Rs. Finally, several reports indicate that although quasi-legal SCBs initially evaded detection and legal consequences, these presumed “advantages” have been limited by new legislation and development of product and human testing capabilities. Collectively, evidence reported in this mini-review suggests that K2 products are neither safe nor legal alternatives to marijuana. Instead, enhanced toxicity of K2 products relative to marijuana, perhaps resulting from the combined actions of a complex mixture of different SCBs present and their active metabolites that retain high affinity for CB1 and CB2Rs, highlights the inherent danger that may accompany use of these substances.
Keywords: CB1 Receptors, CB2 Receptors, Drug abuse, Drug metabolism, K2/Spice, Synthetic cannabis, Δ9-Tetrahydrocannabinol
Introduction
History and detection of synthetic cannabinoids (SCBs)
SCBs rapidly emerged as popular drugs of abuse when commercial preparations branded as “K2” in the United States or as “Spice” in Europe became readily available online and in “head shops” (Seely et al., 2011). Advertisements describe these products as “harmless incense blends”, often leading to the incorrect assumption by some users that these products are safe (Fattore and Fratta, 2011). Ready-to-use drug formulations generally contain around 3 g of plant material such as “wild dagga” (Leonotis leonurus) and “Indian warrior” (Pedicularis densiflora) (Zuba et al., 2011). The presence of the herbal substrate gives the consumer the impression that they are indeed smoking a natural product, but the purchased material has been purposefully laced with SCBs (EMCDDA, 2009, Fattore and Fratta, 2011, Seely et al., 2012b, Seely et al., 2011). To do this, the SCBs are first dissolved in a solvent such as acetone or ethanol. The plant material is then saturated and left to dry so that the solvent evaporates and leaves behind highly variable concentrations of these potentially toxic drugs (Fattore and Fratta, 2011, Vardakou et al., 2010).
Final commercial products, sold in attractive packaging and under appealing names like “Tropical Synergy” or “Yucatan Fire” (Griffiths et al., 2010), are priced at $30 to $40 each. In comparison to marijuana, SCB products are relatively low priced and are widely available and extremely tempting for young people who may want to try marijuana or other drugs, but are afraid of legal or social consequences (Fattore and Fratta, 2011, Vandrey et al., 2012). These factors, combined with the fact that SCBs are often not detected in standard drug screens, has spurred an epidemic of K2 use on college and high school campuses, where one in nine high school seniors admitted using K2 in 2011, making K2 the 2nd most prevalent illicit drug after marijuana (Johnston et al., 2011, Vandrey et al., 2012). Therefore, K2 use is a major public health concern, especially since clinical reports show K2 use can lead to acute CNS and cardiovascular toxicity (Gunderson et al., 2012).
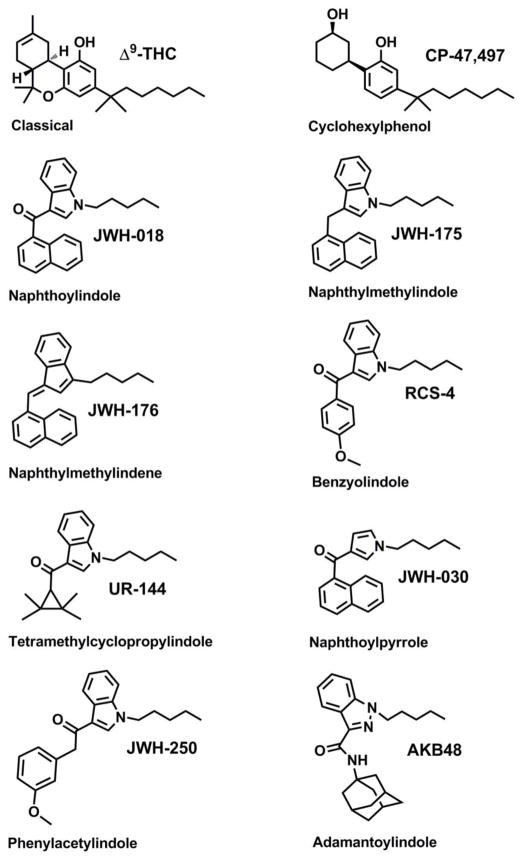
The public health dangers posed by these emerging drugs of abuse have prompted much regulation. For example, 14 European countries currently regulate many SCBs (Fattore and Fratta, 2011), and in the U.S. the most prevalent SCBs have been placed into the Schedule I class as defined by the Controlled Substances Act (Drug Enforcement Administration, 2011, SDAPA, 2012). However, such regulatory efforts are complicated by a lack of standardized tests that are capable of detecting the specific drugs present in commercial K2 preparations. Without increased analytical capacity, forensic testing laboratories are limited in their ability to evaluate emerging drugs of abuse (Moran, 2011). Analytical techniques like gas chromatography mass spectrometry (GC-MS) (Bretteville-Jensen et al., 2013, Emerson et al., 2013, Gottardo et al., 2012, Presley et al., 2013), liquid chromatography tandem mass spectrometry (LC-MS/MS) (Bretteville-Jensen et al., 2013, Presley et al., 2013, Teske et al., 2010, Wohlfarth et al., 2013) and time of flight mass spectrometry (TOF) (Bretteville-Jensen et al., 2013, Gottardo et al., 2012, Presley et al., 2013) are often required to identify and quantify SCBs present in K2 products. Recent reviews provide a comprehensive compilation of forensic data available for the historical identification of SCBs in these commercial preparations (Bretteville-Jensen et al., 2013, Presley et al., 2013). The chemical classification of the SCBs used to produce K2 is complex and includes classical cannabinoids, cyclohexylphenols, naphthoylindoles, naphthylmethylindoles, naphthylmethylindenes, benzoylindoles, phenylacetylindoles, adamantoylindoles, and tetramethylcyclopropylindoles (Bretteville-Jensen et al., 2013, Presley et al., 2013). Figure 1 depicts a specific example from each broad chemical classification, and illustrates important structural differences and similarities between groups.
Figure 1. Structures of representative SCBs commonly found in K2 products.
Synthetic cannabinoids are derived from diverse structural groups, including classical cannabinoids (Δ9-THC), cyclohexylphenols (CP-47,497), naphthoylindoles (JWH-018), naphthylmethylindoles (JWH-175), naphthylmethylindenes (JWH-176), benzoylindoles (RCS-4), phenylacetylindoles (JWH-250), adamantoylindoles (AKB48), and tetramethylcyclopropylindoles (UR-144).
Standardized testing of human specimens to confirm SCB use is another critical need for clinical, forensic, and public health laboratories tasked with identifying causative agents implicated in cases of impaired driving, and suspected drug abuse-related crimes and/or death. Additionally, standardization is necessary for drug testing associated with potential probation violations and drug abuse in the workplace and athletic programs. Although the science behind SCB testing is rapidly evolving, most analytical laboratories have yet to establish procedures to detect these compounds. Slight structural differences (Figure 1) and a virtual lack of appropriate metabolic reference standards makes human toxicological testing challenging. However, investigators are beginning to overcome these barriers. Several reference standards are commercially available and research laboratories are reporting new synthetic procedures (Emerson et al., 2013, Lovett et al., 2013). While many different analytical techniques have been investigated over the past three years, most human testing laboratories rely on LC-MS/MS techniques to assay parent SCBs in human blood (Hudson et al., 2010, Teske et al., 2010), and oxidized and conjugated metabolites in urine (Chimalakonda et al., 2011a, Chimalakonda et al., 2011b, Dresen et al., 2010, Dresen et al., 2011, ElSohly et al., 2011, Grigoryev et al., 2011a, Grigoryev et al., 2011b, Moller et al., 2011, Moran et al., 2011, Sobolevsky et al., 2010). LC-MS/MS applications for evaluating parent SCBs in oral fluid are also beginning to be developed and validated (Coulter et al., 2011, Kneisel et al., 2012).
Given the prevalence of SCB abuse in the US and around the world, a thorough understanding of the basic pharmacological and potential toxicological properties of SCBs found in K2 products is needed. In particular, such information will assist public health and regulatory initiatives aimed at understanding the distinct adverse effect profile of SCBs relative to marijuana. The material presented in this review will provide evidence that distinct pharmacological properties and metabolism of SCBs contribute to the toxicity of these drugs of abuse and perhaps provide a mechanistic explanation why SCBs are increasingly linked to clinical morbidity and mortality (Fattore and Fratta, 2011, Lapoint et al., 2011, Patton et al., 2013, Seely et al., 2012b, Seely et al., 2011).
Preclinical in vivo pharmacology: SCBs are high efficacy CB1R agonists relative to Δ9-THC
Cannabinoid tetrad
Administration of cannabinoid type 1 receptor (CB1R) agonists from multiple structural classes (see Figure 1) elicits a characteristic cluster of effects in laboratory animals. This cluster of four classical endpoints of hypothermia, analgesia, catalepsy, and locomotor suppression has been termed the cannabinoid tetrad (Compton et al., 1992, Little et al., 1988). The cannabinoid tetrad has been extremely useful in the characterization of the biological activity of cannabinoid ligands at CB1Rs. Ligands that fully activate cannabinoid receptors to produce maximal effects in a given system are referred to as full or high efficacy agonists (Neubig et al., 2003). In contrast, agonists that result in reduced maximal effects when compared to full agonists are designated as partial or low efficacy agonists. Interestingly, Δ9-THC tends to elicit tetrad effects of similar magnitude to higher efficacy cannabinoids such as WIN-55,212-2 and CP-55,940 (Fan et al., 1994). The administration of K2/Spice constituents JWH-018 and JWH-073 also produces characteristic tetrad effects in mice; however, both SCBs elicit greater decreases in absolute core temperatures when compared to Δ9-THC (Figure 2C) (Brents et al., 2012). Similar partial agonist activity of Δ9-THC with respect to hypothermia has been reported by others (Paronis et al., 2012). Tetrad measures other than hypothermia are unable to reliably distinguish intrinsic efficacy. In addition to efficacy, the potency of SCBs required to produce certain effects may also differ when compared to Δ9-THC. Potency is the amount of a drug (e.g., concentration or dose) required to produce a defined effect in a given system (Neubig et al., 2003). In our experience with the cannabinoid tetrad in mice, the rank order potency of the SCBs that we have examined is JWH-018 > JWH-073 > Δ9-THC. Finally, CB1R-mediated tetrad effects are also observed in mice exposed to smoke produced from combustion of an herbal incense product containing 5.4% JWH-018 (Wiebelhaus et al., 2012).
Figure 2. Comparison of the in vivo and in vitro efficacy for SCBs and Δ9-THC.

Panel A: CP-55,940, JWH-073 and the M1-metabolite of JWH-073 activate G-proteins with greater efficacy than Δ9-THC. Originally published in PLoS One, 6:e21917, 2011 (Brents et al., 2011). Copyright permission granted by PLoS One open access license. Panel B: CP-55,940, JWH-018 and the M1-metabolite of JWH-018 activate G-proteins more potently and efficaciously than Δ9-THC. Originally published in Biochemical Pharmacology, 83:952, 2012 (Brents et al., 2012). Copyright permission granted by Elsevier. Panel C: JWH-018 and the M1-metabolite of JWH-018 produce greater levels of hypothermia than Δ9-THC. Originally published in Biochemical Pharmacology, 83:952, 2012 (Brents et al., 2012). Copyright permission granted by Elsevier.
Discriminative stimulus effects
Cannabinoids elicit perceptual changes and other unobservable effects in man, and drug discrimination is useful as an animal model of these subjective effects. Centrally-active CB1R cannabinoid agonists reliably induce Δ9-THC-like effects in animals trained to discriminate Δ9-THC. For example, WIN-55,212-2 and 1-butyl-2-methyl-3-(1-naphthoyl)indole (Wiley et al., 1995), and R(−)-methanandamide (Jarbe et al., 2001) all substitute for Δ9-THC. Importantly, results from preclinical studies like these are predictive of the subjective effects of cannabis in humans (Balster and Prescott, 1992). To date, only a few studies have examined the discriminative stimulus effects of K2/Spice constituents. JWH-018 has been shown to substitute (occasion >80% selection of the drug-associated response option) for Δ9-THC and for methanandamide, and the interoceptive effects of JWH-018 are attenuated by prior administration of the CB1R antagonist rimonabant (Jarbe et al., 2011, Jarbe et al., 2010). Similarly, JWH-018 and JWH-073 fully substitute for the discriminative stimulus effects of Δ9-THC, and antagonist studies with rimonabant suggest that they do so via interactions with CB1Rs (Ginsburg et al., 2012).
Reinforcing effects
Assessment of the reinforcing effects of drugs is accomplished using drug self-administration, typically via the intravenous route. The reinforcing effects of cannabinoids have not been widely investigated in laboratory animals, perhaps because early studies on the reinforcing effects of cannabinoids failed to establish intravenous self-administration of Δ9-THC (Harris et al., 1974, Mansbach et al., 1994, Van Ree et al., 1978). However, these previous studies utilized high intravenous doses which, combined with poor water solubility, suggest that the Δ9-THC solutions used were, at best, in suspension, and not likely to be well absorbed (Justinova et al., 2003). More recently, the use of lower doses of Δ9-THC which clearly dissolve in solution, allowing the drug to rapidly penetrate the brain after intravenous administration, seems to readily maintain self-administration in squirrel monkeys (Justinova et al., 2003, Tanda et al., 2000). Additionally, self-administration of endogenous cannabinoids anandamide (Justinova et al., 2005) and 2-arachidonoylglycerol (Justinova et al., 2011) has also been demonstrated. To date, Δ9-THC has not been reported to maintain reliable self-administration behavior in rodents, although the higher efficacy cannabinoids WIN-55,212-2 and HU-210 are self-administered by mice and rats (Fattore et al., 2001, Martellotta et al., 1998, Navarro et al., 2001), suggesting that other high efficacy cannabinoids, such as those present in K2/Spice products, might also display reinforcing effects in self-administration procedures.
Tolerance, dependence and withdrawal
When a drug effect decreases in intensity as a function of repeated administration, “tolerance” to that particular drug effect is said to occur. Importantly, animals become tolerant to specific drug effects, not to drugs themselves. Specifically, among the cannabinoids, tolerance to antinociceptive effects, anticonvulsant activity, cataleptic effects, suppression of locomotor activity, hypothermia, hypotension, release of corticosteroids, static ataxia in the dog, and schedule-controlled responding in multiple species are readily observed (reviewed in (Adams and Martin, 1996)). Mechanisms for the development of tolerance to drug effects may be either pharmacokinetic or pharmacodynamic, or a combination of both. Among other drug classes, tolerance to drug effects produced by treatment with a low efficacy ligand can be at least partially surmounted by administration of a high efficacy agonist, while tolerance to effects induced by repeated treatment with a high efficacy compound will elicit profound cross-tolerance when low efficacy substances are tested. Thus, given chronic treatment with any given drug, cross-tolerance to the effects of a low efficacy agonist is greater than cross-tolerance to the effects of a high efficacy agonist. Consistent with this, Δ9-THC treatment decreases sensitivity to the discriminative stimulus effects of Δ9-THC in rhesus monkeys but resulted in less cross-tolerance to the Δ9-THC-like interoceptive effects of high efficacy cannabinoids CP-55,940, JWH-073, or JWH-018 (Hruba et al., 2012). However, chronic administration of Δ9-THC or WIN-55,212-2 results in similar levels of tolerance to drug-elicited hypoactivity, hypothermia, and antinociception (Sim-Selley and Martin, 2002). Interestingly, a greater degree of CB1R desensitization occurs in some brain regions of Δ9-THC-treated mice, despite the substantially lower CB1R efficacy. These data indicate that a better understanding of the relationship between tolerance to in vivo effects and regulation of expression and function of CB1Rs after chronic administration of cannabinoid agonists with varying efficacies is needed.
Discontinuation of chronic Δ9-THC administration usually does not induce spontaneous signs of withdrawal in laboratory animals (reviewed in (Maldonado, 2002)). However, withdrawal signs following discontinuation of repeated treatment with higher efficacy cannabinoids including CP-55,940 (Oliva et al., 2003), WIN-55,212-2 (Aceto et al., 2001), and perhaps JWH-018 (Zimmermann et al., 2009) and JWH-073 (Nacca et al., 2013) have been documented in laboratory species and in human K2/Spice users. Importantly, robust withdrawal effects are induced in laboratory animals repeatedly administered cannabinoid agonists and then challenged with the CB1R antagonist rimonabant (reviewed in (Gonzalez et al., 2005)). In rodents, cannabinoid antagonist-precipitated withdrawal is characterized by numerous observable signs, including wet dog shakes, head shakes, facial rubbing, front paw tremor, ataxia, hunched posture, body tremor, ptosis, piloerection, hypolocomotion, mastication, licking, rubbing, and scratching (reviewed by (Maldonado and Rodriguez de Fonseca, 2002)).
SCB Drug-Drug Interactions
K2 products rarely contain a single SCB, but instead are almost always laced with multiple SCBs in a single preparation (Seely et al., 2012b, Seely et al., 2011). As such, a potential exists for drug-drug interactions between multiple SCBs in a single product that may contribute to abuse-related and/or adverse effects associated with use of these drugs. Brents et al recently showed (Figure 3) showed that co-administration of JWH-018 and JWH-073 in mice produces additive, synergistic, or antagonistic interactions, depending upon the endpoint examined and/or the drug dose ratio employed (Brents et al., 2013). Specifically, synergistic interactions between SCBs were observed for Δ9-THC drug discrimination (Figure 3A), analgesia (Figure 3B) and displacement of radioligand from CB1 receptors (Figure 3C). The authors suggest that similar synergistic effects occurring between SCBs present in K2 products may increase their relative potency for both subjective and adverse effects and contribute to negative side effects commonly associated with use of these drugs.
Figure 3. In vivo and in vitro interactions between JWH-018 and JWH-073.
Co- administration of JWH-018 and JWH-073 results in synergistic interactions for Δ9-THC drug discrimination (Panel A), analgesia (Panel B) and displacement of radioligand from CB1 receptors (Panel C). Data presented in all panels were originally published in the Journal of Pharmacology and Experimental Therapeutics, 346:350, 2012 (Brents et al., 2013). Copyright permission granted by ASPET.
Metabolism: SCBs undergo extensive oxidation and conjugation with glucuronic acid
Due to the recent rise in SCB use, the metabolism of these agents is a rapidly growing area of interest. It has been shown that the sequence of the formation of metabolites is such that first, compounds are oxidized by a special group of enzymes called cytochromes P450s (CYPs), and then conjugated with a sugar moiety called glucuronic acid by a class of enzymes called UDP-glucuronosyltransferases (UGTs). This process is essential for the removal of these drugs from the body.
The metabolism of Δ9-THC is complex and has been the subject of much research (Huestis, 2005). In brief, Δ9-THC is initially metabolized via oxidation by the specific CYP subtypes CYP2C9 and CYP3A4 (Watanabe et al., 2007). In humans, hydroxylation of Δ9-THC by CPY2C9 forms a major active psychoactive metabolite, 11-hydroxy-Δ9-THC (Bornheim et al., 1992, Watanabe et al., 1995). Following initial hydroxylation, many hydroxyl groups undergo further oxidation to produce carboxylic groups at several positions along the alkyl side chain. Oxidation of the active 11-hydroxy-Δ9-THC leads to production of 11-nor-9-carboxy-Δ9-THC, an inactive metabolite. 11-nor-9-carboxy-Δ9-THC is then conjugated at the carboxyl position to form O-esterglucuronide, the major metabolite found in human urine (Yamamoto et al., 1987).
In contrast to the extensive knowledge concerning Δ9-THC pharmacokinetics, the earliest reports of SCB metabolism were published in the early 2000s and focused on the in vitro metabolism of the cannabinoid receptor ligands WIN-55,212-2 (Zhang et al., 2002), AM-630 (Zhang et al., 2004) and JWH-015 (Zhang et al., 2006). However, the emergence of JWH-018 as a popular recreational drug soon brought human in vivo metabolism to the forefront. In the first of these studies, gas- and liquid-chromatography mass spectrometry (GC-MS and LC-MS/MS) was utilized to identify metabolites in urine obtained from three individuals known to have consumed JWH-018 (Sobolevsky et al., 2010). Metabolites of JWH-018 were observed to be excreted almost entirely as glucuronide conjugates. This work was quickly followed by an in vitro metabolism study using human liver microsomes (Wintermeyer et al., 2010), and several studies analyzing urine from suspected users and/or rats dosed with JWH-018 (Grigoryev et al., 2011a, Moller et al., 2011), JWH-073 (Grigoryev et al., 2011b), and JWH-250 (Grigoryev et al., 2011a). All studies confirmed the formation of metabolites containing a single hydroxyl group as the primary urinary metabolites in humans that were subsequently glucuronidated for elimination.
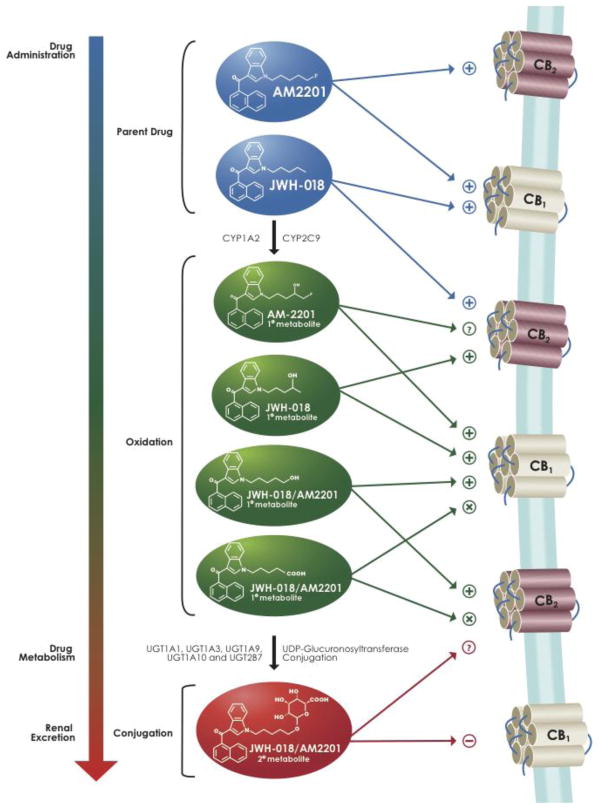
In 2011, reference standards for specific identification of the metabolites by LC-MS were developed allowing the first quantitative measurement of JWH-018 and JWH-073 metabolites in vivo and in vitro (Chimalakonda et al., 2011a, Chimalakonda et al., 2012, Moran et al., 2011). Four urine specimens were collected after individuals reportedly self-administered either JWH-018 or a mixture of JWH-018 and JWH-073. Analysis of human urine showed that all of the tested metabolites were excreted in high concentrations, and primarily as glucuronic acid conjugates (Chimalakonda et al., 2011a) (Figure 4). Since that time, additional laboratories have published similar findings from analysis of human urine (Beuck et al., 2011, de Jager et al., 2012, Dowling and Regan, 2011, ElSohly et al., 2011, Grigoryev et al., 2011a, Hutter et al., 2012, Sobolevsky et al., 2012).
Figure 4. Summary of known metabolism, excretion and downstream cellular signaling activity of JWH-018 and AM2201, two popular SCBs found in K2 products.
“+” indicates agonism, “−” indicates antagonism, “X” indicates no binding affinity, “?” indicates not known.
Oxidative Metabolism
The extensive evidence presented, documenting the formation of hydroxylated metabolites of these SCBs in humans, supports the involvement of CYPs in the biotransformation of these compounds (Chimalakonda et al., 2011a, Hutter et al., 2012, Moran et al., 2011, Sobolevsky et al., 2010, Wintermeyer et al., 2010, Zhang et al., 2002). In vitro metabolism studies, using recombinant CYPs, identified CYP2C9 and 1A2 as the primary hepatic P450 isoforms involved in the oxidation of JWH-018 and AM2201 (Chimalakonda et al., 2012) (Figure 4). Although typically associated with liver metabolism, CYPs are expressed throughout the body in a tissue specific manner. Given the high expression of CYP2C9 in the intestine (Paine et al., 2006), it is anticipated that this isoform will be involved in the intestinal metabolism of SCBs following oral administration. CYP1A2, which is abundant in the lung (Pavanello et al., 2012), could be especially important for metabolism of these compounds when smoked. Although the involvement of CYP2D6 in the metabolism of JWH-018 and AM2201 by the liver is minimal (Chimalakonda et al., 2012), this isoform is known to be expressed in the cerebral cortex, hippocampus, and cerebellum, regions known for high expression of CB1 receptors (Meyer et al., 2007). These observations suggest that CYP2D6 may play a role in regulating brain concentrations of these SCBs and their active metabolites.
Conjugative Metabolism
The presence of high concentrations of glucuronide metabolites of synthetic cannabinoids in human urine (Chimalakonda et al., 2011a, Moran et al., 2011, Sobolevsky et al., 2010, Wintermeyer et al., 2010), coupled with a virtual absence from serum samples (Dresen et al., 2011, Kacinko et al., 2011), indicates that glucuronic acid conjugation is required for the elimination of these drugs in urine. Studies using proteins generated in the laboratory determined that the major UGT isoforms involved in the metabolism of JWH-018 and JWH-073 in the liver were UGT1A1, UGT1A9, and UGT2B7 (Chimalakonda et al., 2011a). Significant activity was also seen with UGT1A10, which is only expressed outside the liver, as well as with isoforms known to be expressed in the lung (UGT1A7) and brain (UGT1A3 and UGT2B7) (Figure 4).
Comparison of these in vitro results with analysis of human urine samples suggests that human UGT1A3 and UGT2B7 are the predominant isoforms responsible for producing the major urinary metabolites of JWH-018 and JWH-073 (Chimalakonda et al., 2011b). It is important to highlight that although these isoforms are present in high levels in the liver, they are also expressed in the human brain. Therefore, the activity of these isoforms, such as CYP2D6 (described previously), may regulate the concentrations of active compounds available for CB1R binding and activation.
Cellular signaling: SCB metabolites retain affinity and activity at CB1 and cannabinoid type 2 (CB2Rs) receptors
SCBs in K2 products are high affinity CB1 and CB2R full agonists in vitro
Many studies investigating the pharmacological significance of SCB metabolism have been completed for two of the original SCBs detected in commercial K2 preparations (JWH-018 and JWH-073) (Seely et al., 2011). Similar to the naturally occurring cannabinoid Δ9-THC, both JWH-018 and JWH-073 bind with high affinity (in the low nanomolar range) to CB1 and CB2Rs (Aung et al., 2000, Brents et al., 2012, Brents et al., 2011, Chin et al., 1999, Rajasekaran et al., 2013). Several other SCBs more recently reported to be found in K2 products (see Figure 1) also have high affinity for CB1 and CB2Rs, including CP-47,497 (Huffman et al., 2008), JWH-175 (Huffman and Padgett, 2005), JWH-176 (Huffman and Padgett, 2005), UR-144 (Frost et al., 2010), JWH-030 (Griffin et al., 1998), JWH-250 (Huffman et al., 2005), AKB48 (Uchiyama et al., 2013) and AM-1248 (Jankovics et al., 2012). In addition to high affinity for CB1Rs, most SCBs present in K2 products also act as potent and fully efficacious CB1R agonists when evaluated by either in vitro (Figure 2A–B) or in vivo (Figure 2C) assays (Brents et al., 2012, Brents et al., 2011). Examples of SCBs exhibiting high CB1R affinity and full agonist activity include JWH-018 (Atwood et al., 2010, Brents et al., 2011), JWH-073 (Atwood et al., 2011, Brents et al., 2012), AM-1248 (Frost et al., 2010), CP-55-940 (Griffin et al., 1998), WIN-55,512-2 (Griffin et al., 1998) and HU-210 (Howlett et al., 1990).
The fact that SCBs possess both high CB1R affinity and are fully efficacious agonists may explain the marked abuse liability of these compounds in humans (Lindigkeit et al., 2009). Indeed, recent reports confirm that JWH-018 and JWH-073 exhibit higher in vitro efficacy at CB1Rs compared to Δ9-THC (Atwood et al., 2010, Brents et al., 2012, Brents et al., 2011, Lindigkeit et al., 2009) (Figure 2A–B). However, Δ9-THC usually produces effects in animals consistent with that of a full agonist (Fan et al., 1994) (see previous discussion), even though it curiously exhibits only partial agonist activity at CB1Rs when evaluated by in vitro assays (Griffin et al., 1998). Furthermore, discontinuation after prolonged marijuana exposure produces a withdrawal syndrome in humans (Allsop et al., 2011, Haney, 2005) and results in region-specific downregulation of central CB1Rs (Hirvonen et al., 2011). Therefore, it might be proposed that chronic use of the higher efficacy SCBs present in K2 products may accentuate chronic drug effects such as tolerance, dependence and withdrawal relative to Δ9-THC use. Consistent with this hypothesis, extended abuse of high doses of SCBs in “Spice Gold” has been shown to produce a relatively marked “withdrawal phenomena and dependence syndrome” in humans following abrupt discontinuation of use, characterized by drug craving, elevated blood pressure, nausea, tremor, profuse sweating and nightmares (Zimmermann et al., 2009).
Some SCBs metabolites retain affinity for and activity at CB1Rs in both in vitro and in vivo assays
Metabolism of Δ9-THC results in production of only a single major active metabolite (11-hydroxy-Δ9-THC) which exhibits reduced CB1R affinity (Kochanowski and Kala, 2005). However, recent reports show that several mono-hydroxylated metabolites of JWH-018, JWH-073 and AM2201 (three common SCBs found in K2 products) unexpectedly retain high nanomolar binding affinity for CB1Rs (Brents et al., 2012, Brents et al., 2011, Chimalakonda et al., 2012) (Figure 4). Unlike the mono-hydroxylated metabolites examined, a carboxylate metabolite of JWH-018, JWH-073 and AM2201 fails to bind to, or activate, CB1Rs.
In addition to retaining high CB1R affinity, the major mono-hydroxylated metabolites of JWH-018, JWH-073 and AM2201 appear to act as partial or full agonists at CB1Rs as measured by G-protein activation when compared to the full agonist CP-55,940 (Brents et al., 2012, Brents et al., 2011, Chimalakonda et al., 2012) (Figure 2A–B). Most importantly, mono-hydroxylated metabolites of JWH-018 and JWH-073 have also been shown to retain marked cannabimimetic effects in vivo, eliciting profound hypothermic (Figure 2C) and locomotor depressant effects in mice that were blocked by prior administration of the CB1R-preferring antagonist/inverse agonist AM251 (Brents et al., 2012). Collectively, these data indicate that several mono-hydroxylated metabolites of JWH-018, JWH-073 and AM2201 retain high CB1R affinity and activity and thus may participate in an additive and/or synergistic manner with other SCBs (Brents et al., 2013), resulting in enhanced toxicity. Other factors potentially contributing to negative side effects associated with use of SCBs include; (1) actions of drugs present in the K2 products other than SCBs, (2) a total lack of quality control leading to significant batch-to-batch differences in actual concentration of SCBs present in purchased products, and (3) increased vulnerability to some SCB adverse effects due to pre-existing conditions of drug users (Fattore and Fratta, 2011, Lapoint et al., 2011, Patton et al., 2013, Seely et al., 2012b, Seely et al., 2011).
Some oxidized products of SCBs retain CB1R affinity, but lack activity at CB1Rs
There is also evidence to suggest that some oxidized products of SCBs can act as antagonists at CB1Rs. While not commonly detected in human urine, the 7-hydroxyindole derivative of JWH-073 binds to CB1Rs with nanomolar affinity but does not activate G-proteins at pharmacologically relevant concentrations, up to 10 μM (Brents et al., 2012). Follow-up studies using Schild analysis confirmed that this oxidized derivative of JWH-073 competitively antagonizes G-protein activation in vitro, and the hypothermic effects elicited by JWH-018 were blunted in mice pretreated with the 7-hydroxyindole derivative of JWH-073. However, neither analgesic, cataleptic, nor locomotor effects of JWH-018 were altered by this metabolite. These combined observations, along with the fact that the hydroxyindole derivative of JWH-073 is not found in humans, indicates that this oxidized product of JWH-073 may not be formed in humans and may not readily penetrate the blood-brain barrier.
A major human glucuronidated metabolite of JWH-018 (5-hydroxypentyl-β-D-glucuronide) also retains significant affinity for CB1Rs and acts as a neutral antagonist in vitro (Seely et al., 2012a) (Figure 4). In marked contrast, in this same study a major THC glucuronide metabolite (11-nor-9-carboxy-Δ9-THC-β-D-glucuronide) was found to lack affinity for, or activity at, CB1Rs. These studies indicate that both hydroxylated and glucuronidated metabolites of JWH-073 and JWH-018 can retain significant affinity for CB1Rs, while lacking intrinsic activity. Therefore, metabolism of SCBs apparently can result in the formation of physiologically relevant CB1R antagonists.
Some SCBs metabolites retain affinity and activity for CB2Rs, the second major cannabinoid receptor subtype
Although the abuse potential of SCBs, such as JWH-018 and JWH-073, likely results from agonist action at CB1Rs, most SCBs present in K2 also have high affinity and significant activity at the second major cannabinoid receptor subtype, CB2Rs (Aung et al., 2000, Chin et al., 1999). Unlike CB1Rs, CB2Rs are most abundantly found on immune cells outside the CNS (Klein et al., 2003) and regulate a range of physiological processes from inflammation to bone formation (Patel et al., 2010). However, in preclinical models, activation of low numbers of CB2Rs in the CNS has also been shown to modulate the abuse-related properties of several drugs of abuse such as alcohol (Onaivi et al., 2008), nicotine (Gamaleddin et al., 2012) and cocaine (Xi et al., 2011). Similar to results detailed for metabolites of JWH-018 and JWH-073 acting at CB1Rs, recent reports show that several of the mono-hydroxylated metabolites of these SCBs also retain high affinity and exhibit significant activity at CB2Rs (Rajasekaran et al., 2013) (Figure 4). Interestingly, several studies have demonstrated that chronic activation of CB2Rs results in upregulation of 5-HT2A receptors in the prefrontal cortex of mice (Franklin and Carrasco, 2013, Franklin et al., 2012). Dysfunction in 5-HT2A receptor function has been associated with several mental disorders, including anxiety (Ghisleni et al., 2008) and psychosis (Morgan et al., 2013), and 5-HT2A receptors are the primary site of action for hallucinogenic drugs (Fantegrossi et al., 2008). Both anxiety and psychosis are common adverse effects of SCBs, not observed with Δ9-THC (Gunderson et al., 2012). Therefore, it is tempting to speculate that excessive 5-HT2A upregulation produced by SCBs and their metabolites, mediated via activation of CB2Rs, might contribute to anxiety and psychosis often observed following exposure to SCBs present in K2 products. In any case, these results suggest that future studies of the pharmacological and toxicological properties of synthetic cannabinoids present in K2 products should consider potential actions of these drugs at both CB1 and CB2Rs, as well as the functional consequences of such actions on expression and function of other, non-cannabinoid, receptor systems.
Conclusions
Although SCBs were initially quasi-legal and thought to be safe alternatives to marijuana by some users, it is clear that these emerging drugs of abuse are not safe and pose significant threats to public health. It is anticipated that morbidity and mortality rates will continue to increase, especially as laboratories incorporate new testing strategies into their standardized procedures. Recent toxicological findings clearly show that in contrast to partial agonist properties of Δ9-THC found in marijuana, SCBs present in K2 products (Figure 1) act as full CB1 and CB2R agonists in both cellular assays and animal studies (Figure 2). In addition, several SCB metabolites retain high affinity for, and exhibit a range of intrinsic activity at CB1 and CB2Rs when examined by in vitro and in vivo assays (Figures 2 and 4). Therefore, K2 products are not safe, legal alternate forms of marijuana. Instead, enhanced toxicity of K2 products relative to marijuana must be acknowledged, and might result from the combined actions of a complex mixture of not only different SCBs present in commercial preparations, but also due to the complex metabolic and cellular signaling pathways involved in SCB detoxification and excretion.
Acknowledgments
The authors would like to thank Ms. Amy L. Patton (Arkansas Department of Public Health) and Cayman Chemical (Ann Arbor, MI) for technical assistance in preparing the figures presented in this manuscript. This research was supported in part by the Association of Public Health Laboratories [Grant Innovations in Quality Public health Laboratory Practice] (JHM), the Centers for Disease Control [Contract 200-2007-21729] (JHM), an award from the University of Arkansas for Medical Sciences Translational Research Institute, which is funded by the National Center for Research Resources [1 UL 1RR029884, Curtis Lowery, PI, JHM and PLP, Co-I].
Footnotes
Conflict of Interest Statement:
The authors declare that they have no financial or personal conflicts of interest that influenced, or could be perceived to have influenced, this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aceto MD, Scates SM, Martin BB. Spontaneous and precipitated withdrawal with a synthetic cannabinoid, WIN 55212-2. Eur J Pharmacol. 2001;416:75–81. doi: 10.1016/s0014-2999(01)00873-1. [DOI] [PubMed] [Google Scholar]
- Adams IB, Martin BR. Cannabis: pharmacology and toxicology in animals and humans. Addiction. 1996;91:1585–614. [PubMed] [Google Scholar]
- Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend. 2011;119:123–9. doi: 10.1016/j.drugalcdep.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Huffman J, Straiker A, Mackie K. JWH018, a common constituent of ‘Spice’ herbal blends, is a potent and efficacious cannabinoid CB receptor agonist. Br J Pharmacol. 2010;160:585–93. doi: 10.1111/j.1476-5381.2009.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Lee D, Straiker A, Widlanski TS, Mackie K. CP47,497-C8 and JWH073, commonly found in ‘Spice’ herbal blends, are potent and efficacious CB(1) cannabinoid receptor agonists. Eur J Pharmacol. 2011;659:139–45. doi: 10.1016/j.ejphar.2011.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung MM, Griffin G, Huffman JW, Wu M, Keel C, Yang B, et al. Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB(1) and CB(2) receptor binding. Drug Alcohol Depend. 2000;60:133–40. doi: 10.1016/s0376-8716(99)00152-0. [DOI] [PubMed] [Google Scholar]
- Balster RL, Prescott WR. Delta 9-tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Rev. 1992;16:55–62. doi: 10.1016/s0149-7634(05)80051-x. [DOI] [PubMed] [Google Scholar]
- Beuck S, Moller I, Thomas A, Klose A, Schlorer N, Schanzer W, et al. Structure characterisation of urinary metabolites of the cannabimimetic JWH-018 using chemically synthesised reference material for the support of LC-MS/MS-based drug testing. Analytical and bioanalytical chemistry. 2011;401:493–505. doi: 10.1007/s00216-011-4931-5. [DOI] [PubMed] [Google Scholar]
- Bornheim LM, Lasker JM, Raucy JL. Human hepatic microsomal metabolism of delta 1-tetrahydrocannabinol. Drug Metab Dispos. 1992;20:241–6. [PubMed] [Google Scholar]
- Brents LK, Gallus-Zawada A, Radominska-Pandya A, Vasiljevik T, Prisinzano TE, Fantegrossi WE, et al. Mono-hydroxylated metabolites of the K2 synthetic cannabinoid JWH-073 retain high cannabinoid 1 receptor (CB1R) affinity and exhibit neutral antagonist to partial agonist activity. Biochem Pharmacol. 2012;83:952–61. doi: 10.1016/j.bcp.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brents LK, Reichard EE, Zimmerman SM, Moran JH, Fantegrossi WE, Prather PL. Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS One. 2011;6:c21917. doi: 10.1371/journal.pone.0021917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brents LK, Zimmerman SM, Saffell AR, Prather PL, Fantegrossi WE. Differential Drug-Drug Interactions of the Synthetic Cannabinoids JWH-018 and JWH-073: Implications for Drug Abuse Liability and Pain Therapy. J Pharmacol Exp Ther. 2013;346:350–61. doi: 10.1124/jpet.113.206003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretteville-Jensen AL, Tuv SS, Bilgrei OR, Fjeld B, Bachs L. Synthetic Cannabinoids and Cathinones: Prevalence and Markets. Forensic Science Review. 2013;25:7–26. [PubMed] [Google Scholar]
- Chimalakonda KC, Bratton SM, Le VH, Yiew KH, Dineva A, Moran CL, et al. Conjugation of Synthetic Cannabinoids JWH-018 and JWH-073, Metabolites by Human UDP-Glucuronosyltransferases. Drug Metab Dispos. 2011a;39:1967–76. doi: 10.1124/dmd.111.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimalakonda KC, Moran CL, Kennedy PD, Endres GW, Uzieblo A, Dobrowolski PJ, et al. Solid-phase extraction and quantitative measurement of omega and omega-1 metabolites of JWH-018 and JWH-073 in human urine. Anal Chem. 2011b;83:6381–8. doi: 10.1021/ac201377m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimalakonda KC, Seely KA, Bratton SM, Brents LK, Moran CL, Endres GW, et al. Cytochrome P450-mediated oxidative metabolism of abused synthetic cannabinoids found in K2/Spice: identification of novel cannabinoid receptor ligands. Drug Metab Dispos. 2012;40:2174–84. doi: 10.1124/dmd.112.047530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin CN, Murphy JW, Huffman JW, Kendall DA. The third transmembrane helix of the cannabinoid receptor plays a role in the selectivity of aminoalkylindoles for CB2, peripheral cannabinoid receptor. J Pharmacol Exp Ther. 1999;291:837–44. [PubMed] [Google Scholar]
- Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacological profile of a series of bicyclic cannabinoid analogs: classification as cannabimimetic agents. J Pharmacol Exp Ther. 1992;260:201–9. [PubMed] [Google Scholar]
- Coulter C, Garnier M, Moore C. Synthetic cannabinoids in oral fluid. J Anal Toxicol. 2011;35:424–30. doi: 10.1093/anatox/35.7.424. [DOI] [PubMed] [Google Scholar]
- de Jager AD, Warner JV, Henman M, Ferguson W, Hall A. LC-MS/MS method for the quantitation of metabolites of eight commonly-used synthetic cannabinoids in human urine - An Australian perspective. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2012 doi: 10.1016/j.jchromb.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Dowling G, Regan L. A method for CP 47, 497 a synthetic non-traditional cannabinoid in human urine using liquid chromatography tandem mass spectrometry. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2011;879:253–9. doi: 10.1016/j.jchromb.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Dresen S, Ferreiros N, Putz M, Westphal F, Zimmermann R, Auwarter V. Monitoring of herbal mixtures potentially containing synthetic cannabinoids as psychoactive compounds. J Mass Spectrom. 2010 doi: 10.1002/jms.1811. [DOI] [PubMed] [Google Scholar]
- Dresen S, Kneisel S, Weinmann W, Zimmermann R, Auwarter V. Development and validation of a liquid chromatography-tandem mass spectrometry method for the quantitation of synthetic cannabinoids of the aminoalkylindole type and methanandamide in serum and its application to forensic samples. J Mass Spectrom. 2011;46:163–71. doi: 10.1002/jms.1877. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration DoJ. Schedules of controlled substances: temporary placement of three synthetic cathinones in Schedule I. Final Order. Fed Regist. 2011;76:65371–5. [PubMed] [Google Scholar]
- ElSohly MA, Gul W, Elsohly KM, Murphy TP, Madgula VL, Khan SI. Liquid chromatography-tandem mass spectrometry analysis of urine specimens for K2 (JWH-018) metabolites. J Anal Toxicol. 2011;35:487–95. doi: 10.1093/anatox/35.7.487. [DOI] [PubMed] [Google Scholar]
- EMCDDA; Office for Official Publications of the European Communities, editor. European Monitoring Centre for Drugs and Drug Addiction EMCDDA 2009 Thematic paper. 2009. Understanding the ‘Spice’ phenomeneon; pp. 1–25. [Google Scholar]
- Emerson B, Durham B, Gidden J, Lay JO., Jr Gas chromatography-mass spectrometry of JWH-018 metabolites in urine samples with direct comparison to analytical standards. Forensic Sci Int. 2013;229:1–6. doi: 10.1016/j.forsciint.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Compton DR, Ward S, Melvin L, Martin BR. Development of cross-tolerance between delta 9-tetrahydrocannabinol, CP 55,940 and WIN 55,212. J Pharmacol Exp Ther. 1994;271:1383–90. [PubMed] [Google Scholar]
- Fantegrossi WE, Murnane KS, Reissig CJ. The behavioral pharmacology of hallucinogens. Biochem Pharmacol. 2008;75:17–33. doi: 10.1016/j.bcp.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Cossu G, Martellotta CM, Fratta W. Intravenous self-administration of the cannabinoid CB1 receptor agonist WIN 55,212-2 in rats. Psychopharmacology (Berl) 2001;156:410–6. doi: 10.1007/s002130100734. [DOI] [PubMed] [Google Scholar]
- Fattore L, Fratta W. Beyone THC: the new generation of cannabinoid designer drugs. Frontiers Behav Neurosci. 2011;5:1–12. doi: 10.3389/fnbeh.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin JM, Carrasco GA. G-Protein Receptor Kinase 5 Regulates the Cannabinoid Receptor 2-Induced Upregulation of Serotonin 2A Receptors. J Biol Chem. 2013 doi: 10.1074/jbc.M113.454843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin JM, Vasiljevik T, Prisinzano TE, Carrasco GA. Cannabinoid 2 receptor- and beta Arrestin 2-dependent upregulation of serotonin 2A receptors. Eur Neuropsychopharmacol. 2012 doi: 10.1016/j.euroneuro.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, et al. Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity. J Med Chem. 2010;53:295–315. doi: 10.1021/jm901214q. [DOI] [PubMed] [Google Scholar]
- Gamaleddin I, Zvonok A, Makriyannis A, Goldberg SR, Le Foll B. Effects of a selective cannabinoid CB2 agonist and antagonist on intravenous nicotine self administration and reinstatement of nicotine seeking. PLoS One. 2012;7:e29900. doi: 10.1371/journal.pone.0029900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisleni G, Kazlauckas V, Both FL, Pagnussat N, Mioranzza S, Rocha JB, et al. Diphenyl diselenide exerts anxiolytic-like effect in Wistar rats: putative roles of GABAA and 5HT receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1508–15. doi: 10.1016/j.pnpbp.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Ginsburg BC, Schulze DR, Hruba L, McMahon LR. JWH-018 and JWH-073: Delta(9)-tetrahydrocannabinol-like discriminative stimulus effects in monkeys. J Pharmacol Exp Ther. 2012;340:37–45. doi: 10.1124/jpet.111.187757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Cebeira M, Fernandez-Ruiz J. Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol Biochem Behav. 2005;81:300–18. doi: 10.1016/j.pbb.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Gottardo R, Chiarini A, Dal Pra I, Seri C, Rimondo C, Serpelloni G, et al. Direct screening of herbal blends for new synthetic cannabinoids by MALDI-TOF MS. J Mass Spectrom. 2012;47:141–6. doi: 10.1002/jms.2036. [DOI] [PubMed] [Google Scholar]
- Griffin G, Atkinson PJ, Showalter VM, Martin BR, Abood ME. Evaluation of cannabinoid receptor agonists and antagonists using the guanosine-5′-O-(3-[35S]thio)-triphosphate binding assay in rat cerebellar membranes. J Pharmacol Exp Ther. 1998;285:553–60. [PubMed] [Google Scholar]
- Griffiths P, Sedefov R, Gallegos A, Lopez D. How globalization and market innovation challenge how we think about and respond to drug use: ‘Spice’ a case study. Addiction. 2010;105:951–3. doi: 10.1111/j.1360-0443.2009.02874.x. [DOI] [PubMed] [Google Scholar]
- Grigoryev A, Melnik A, Savchuk S, Simonov A, Rozhanets V. Gas and liquid chromatography-mass spectrometry studies on the metabolism of the synthetic phenylacetylindole cannabimimetic JWH-250, the psychoactive component of smoking mixtures. J Chromatogr B Analyt Technol Biomed Life Sci. 2011a;879:2519–26. doi: 10.1016/j.jchromb.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Grigoryev A, Savchuk S, Melnik A, Moskaleva N, Dzhurko J, Ershov M, et al. Chromatography-mass spectrometry studies on the metabolism of synthetic cannabinoids JWH-018 and JWH-073, psychoactive components of smoking mixtures. J Chromatogr B Analyt Technol Biomed Life Sci. 2011b;879:1126–36. doi: 10.1016/j.jchromb.2011.03.034. [DOI] [PubMed] [Google Scholar]
- Gunderson EW, Haughey HM, Ait-Daoud N, Joshi AS, Hart CL. “Spice” and “K2” herbal highs: a case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. Am J Addict. 2012;21:320–6. doi: 10.1111/j.1521-0391.2012.00240.x. [DOI] [PubMed] [Google Scholar]
- Haney M. The marijuana withdrawal syndrome: diagnosis and treatment. Curr Psychiatr Rep. 2005;7:360–6. doi: 10.1007/s11920-005-0036-1. [DOI] [PubMed] [Google Scholar]
- Harris RT, Waters W, McLendon D. Evaluation of reinforcing capability of delta-9-tetrahydrocannabinol in rhesus monkeys. Psychopharmacologia. 1974;37:23–9. doi: 10.1007/BF00426679. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, et al. Reversible and regionally selective downregulation of brain cannabinoid CB(1) receptors in chronic daily cannabis smokers. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Champion TM, Wilken GH, Mechoulam R. Stereochemical effects of 11-OH-delta 8-tetrahydrocannabinol-dimethylheptyl to inhibit adenylate cyclase and bind to the cannabinoid receptor. Neuropharmacol. 1990;29:161–5. doi: 10.1016/0028-3908(90)90056-w. [DOI] [PubMed] [Google Scholar]
- Hruba L, Ginsburg BC, McMahon LR. Apparent inverse relationship between cannabinoid agonist efficacy and tolerance/cross-tolerance produced by Delta(9)-tetrahydrocannabinol treatment in rhesus monkeys. J Pharmacol Exp Ther. 2012;342:843–9. doi: 10.1124/jpet.112.196444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson S, Ramsey J, King L, Timbers S, Maynard S, Dargan PI, et al. Use of high-resolution accurate mass spectrometry to detect reported and previously unreported cannabinomimetics in “herbal high” products. J Anal Toxicol. 2010;34:252–60. doi: 10.1093/jat/34.5.252. [DOI] [PubMed] [Google Scholar]
- Huestis MA. Pharmacokinetics and metabolism of the plant cannabinoids, delta9-tetrahydrocannabinol, cannabidiol and cannabinol. Handb Exp Pharmacol. 2005:657–90. doi: 10.1007/3-540-26573-2_23. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Padgett LW. Recent developments in the medicinal chemistry of cannabimimetic indoles, pyrroles and indenes. Curr Med Chem. 2005;12:1395–411. doi: 10.2174/0929867054020864. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Szklennik PV, Almond A, Bushell K, Selley DE, He H, et al. 1-Pentyl-3-phenylacetylindoles, a new class of cannabimimetic indoles. Bioorg Med Chem Lett. 2005;15:4110–3. doi: 10.1016/j.bmcl.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Thompson AL, Wiley JL, Martin BR. Synthesis and pharmacology of 1-deoxy analogs of CP-47,497 and CP-55,940. Bioorg Med Chem. 2008;16:322–35. doi: 10.1016/j.bmc.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter M, Broecker S, Kneisel S, Auwarter V. Identification of the major urinary metabolites in man of seven synthetic cannabinoids of the aminoalkylindole type present as adulterants in ‘herbal mixtures’ using LC-MS/MS techniques. J Mass Spectrom. 2012;47:54–65. doi: 10.1002/jms.2026. [DOI] [PubMed] [Google Scholar]
- Jankovics P, Varadi A, Tolgyesi L, Lohner S, Nemeth-Palotas J, Balla J. Detection and identification of the new potential synthetic cannabinoids 1-pentyl-3-(2-iodobenzoyl)indole and 1-pentyl-3-(1-adamantoyl)indole in seized bulk powders in Hungary. Forensic Sci Int. 2012;214:27–32. doi: 10.1016/j.forsciint.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Deng H, Vadivel SK, Makriyannis A. Cannabinergic aminoalkylindoles, including AM678=JWH018 found in ‘Spice’, examined using drug (Delta(9)-tetrahydrocannabinol) discrimination for rats. Behav Pharmacol. 2011;22:498–507. doi: 10.1097/FBP.0b013e328349fbd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbe TU, Lamb RJ, Lin S, Makriyannis A. (R)-methanandamide and Delta 9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacology (Berl) 2001;156:369–80. doi: 10.1007/s002130100730. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Li C, Vadivel SK, Makriyannis A. Discriminative stimulus functions of methanandamide and delta(9)-THC in rats: tests with aminoalkylindoles (WIN55,212-2 and AM678) and ethanol. Psychopharmacology (Berl) 2010;208:87–98. doi: 10.1007/s00213-009-1708-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’malley PM, Bachman JG, Schulenberg JE. Marijuana use continues to rise among US teens, while alcohol use hits historic lows. University of Michigan News Service; Ann Arbor, MI: 2011. [Google Scholar]
- Justinova Z, Solinas M, Tanda G, Redhi GH, Goldberg SR. The endogenous cannabinoid anandamide and its synthetic analog R(+)-methanandamide are intravenously self-administered by squirrel monkeys. J Neurosci. 2005;25:5645–50. doi: 10.1523/JNEUROSCI.0951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology (Berl) 2003;169:135–40. doi: 10.1007/s00213-003-1484-0. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Yasar S, Redhi GH, Goldberg SR. The endogenous cannabinoid 2-arachidonoylglycerol is intravenously self-administered by squirrel monkeys. J Neurosci. 2011;31:7043–8. doi: 10.1523/JNEUROSCI.6058-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacinko SL, Xu A, Homan JW, McMullin MM, Warrington DM, Logan BK. Development and validation of a liquid chromatography-tandem mass spectrometry method for the identification and quantification of JWH-018, JWH-073, JWH-019, and JWH-250 in human whole blood. J Anal Toxicol. 2011;35:386–93. doi: 10.1093/anatox/35.7.386. [DOI] [PubMed] [Google Scholar]
- Klein T, Newton C, Larsen K, Lu L, Perkins I, Liang N, et al. The cannabinoid system and immune modulation. J Leukoc Biol. 2003;74:486–96. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- Kneisel S, Auwarter V, Kempf J. Analysis of 30 synthetic cannabinoids in oral fluid using liquid chromatography-electrospray ionization tandem mass spectrometry. Drug Test Anal. 2012 doi: 10.1002/dta.1429. [DOI] [PubMed] [Google Scholar]
- Kochanowski M, Kala M. Tetrahydrocannabinols in clinical and forensic toxicology. Przegl Lek. 2005;62:576–80. [PubMed] [Google Scholar]
- Lapoint J, James LP, Moran CL, Nelson LS, Hoffman RS, Moran JH. Severe toxicity following synthetic cannabinoid ingestion. Clin Toxicol. 2011;49:760–4. doi: 10.3109/15563650.2011.609822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindigkeit R, Boehme A, Eiserloh I, Luebbecke M, Wiggermann M, Ernst L, et al. Spice: a never ending story? Forensic Sci Int. 2009;191:58–63. doi: 10.1016/j.forsciint.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Little PJ, Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J Pharmacol Exp Ther. 1988;247:1046–51. [PubMed] [Google Scholar]
- Lovett DP, Yanes EG, Herbelin TW, Knoerzer TA, Levisky JA. Structure elucidation and identification of a common metabolite for naphthoylindole-based synthetic cannabinoids using LC-TOF and comparison to a synthetic reference standard. Forensic Sci Int. 2013;226:81–7. doi: 10.1016/j.forsciint.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Maldonado R. Study of cannabinoid dependence in animals. Pharmacol Ther. 2002;95:153–64. doi: 10.1016/s0163-7258(02)00254-1. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Rodriguez de Fonseca F. Cannabinoid addiction: behavioral models and neural correlates. J Neurosci. 2002;22:3326–31. doi: 10.1523/JNEUROSCI.22-09-03326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach RS, Nicholson KL, Martin BR, Balster RL. Failure of Delta(9)-tetrahydrocannabinol and CP 55,940 to maintain intravenous self-administration under a fixed-interval schedule in rhesus monkeys. Behav Pharmacol. 1994;5:219–25. doi: 10.1097/00008877-199404000-00014. [DOI] [PubMed] [Google Scholar]
- Martellotta MC, Cossu G, Fattore L, Gessa GL, Fratta W. Self-administration of the cannabinoid receptor agonist WIN 55,212-2 in drug-naive mice. Neuroscience. 1998;85:327–30. doi: 10.1016/s0306-4522(98)00052-9. [DOI] [PubMed] [Google Scholar]
- Meyer RP, Gehlhaus M, Knoth R, Volk B. Expression and function of cytochrome p450 in brain drug metabolism. Curr Drug Metab. 2007;8:297–306. doi: 10.2174/138920007780655478. [DOI] [PubMed] [Google Scholar]
- Moller I, Wintermeyer A, Bender K, Jubner M, Thomas A, Krug O, et al. Screening for the synthetic cannabinoid JWH-018 and its major metabolites in human doping controls. Drug Test Anal. 2011;3:609–20. doi: 10.1002/dta.158. [DOI] [PubMed] [Google Scholar]
- Moran CL, Le VH, Chimalakonda KC, Smedley AL, Lackey FD, Owen SN, et al. Quantitative measurement of JWH-018 and JWH-073 metabolites excreted in human urine. Anal Chem. 2011;83:4228–36. doi: 10.1021/ac2005636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JH. Smart resource allocation needed to study ‘legal highs’. Nat Med. 2011;17:1339. doi: 10.1038/nm1111-1339. [DOI] [PubMed] [Google Scholar]
- Morgan D, Kondabolu K, Kuipers A, Sakhuja R, Robertson KL, Rowland NE, et al. Molecular and behavioral pharmacology of two novel orally-active 5HT2 modulators: Potential utility as antipsychotic medications. Neuropharmacol. 2013 doi: 10.1016/j.neuropharm.2013.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacca N, Vatti D, Sullivan R, Sud P, Su M, Marraffa J. The Synthetic Cannabinoid Withdrawal Syndrome. J Addict Med. 2013 doi: 10.1097/ADM.0b013e31828e1881. [DOI] [PubMed] [Google Scholar]
- Navarro M, Carrera MR, Fratta W, Valverde O, Cossu G, Fattore L, et al. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J Neurosci. 2001;21:5344–50. doi: 10.1523/JNEUROSCI.21-14-05344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubig RR, Spedding M, Kenakin T, Christopoulos A, Drug C International Union of Pharmacology Committee on Receptor N. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on terms and symbols in quantitative pharmacology. Pharmacol Rev. 2003;55:597–606. doi: 10.1124/pr.55.4.4. [DOI] [PubMed] [Google Scholar]
- Oliva JM, Ortiz S, Palomo T, Manzanares J. Behavioural and gene transcription alterations induced by spontaneous cannabinoid withdrawal in mice. J Neurochem. 2003;85:94–104. doi: 10.1046/j.1471-4159.2003.01627.x. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, et al. Brain neuronal CB2 cannabinoid receptors in drug abuse and depression: from mice to human subjects. PLoS One. 2008;3:e1640. doi: 10.1371/journal.pone.0001640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DC. The human intestinal cytochrome P450 “pie”. Drug Metab Dispos. 2006;34:880–6. doi: 10.1124/dmd.105.008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paronis CA, Nikas SP, Shukla VG, Makriyannis A. Delta(9)-Tetrahydrocannabinol acts as a partial agonist/antagonist in mice. Behav Pharmacol. 2012;23:802–5. doi: 10.1097/FBP.0b013e32835a7c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KD, Davison JS, Pittman QJ, Sharkey KA. Cannabinoid CB(2) receptors in health and disease. Curr Med Chem. 2010;17:1393–410. doi: 10.2174/092986710790980041. [DOI] [PubMed] [Google Scholar]
- Patton AL, Chimalakonda KC, Moran CL, McCain KR, Radominska-Pandya A, James LP, et al. K2 Toxicity: Fatal Case of Psychiatric Complications Following AM2201 Exposure. J Forensic Sci. 2013 doi: 10.1111/1556-4029.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavanello S, Fedeli U, Mastrangelo G, Rota F, Overvad K, Raaschou-Nielsen O, et al. Role of CYP1A2 polymorphisms on lung cancer risk in a prospective study. Cancer Genet. 2012;205:278–84. doi: 10.1016/j.cancergen.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Presley BC, Janse-Carnum SA, Logan BK. Analysis of synthethc cannabinoids in botanical material: A review of analytical method and findings. Forensic Science Review. 2013;25:27–46. [PubMed] [Google Scholar]
- Rajasekaran M, Brents LK, Franks LN, Moran JH, Prather PL. Human metabolites of synthetic cannabinoids JWH-018 and JWH-073 bind with high affinity and act as potent agonists at cannabinoid type-2 receptors. Toxicol Appl Pharmacol. 2013;269:100–8. doi: 10.1016/j.taap.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SDAPA; Justic Do, editor. Synthetic Drug Abuse Prevention Act. 2012;XI [Google Scholar]
- Seely KA, Brents LK, Radominska-Pandya A, Endres GW, Keyes GS, Moran JH, et al. A Major Glucuronidated Metabolite of JWH-018 Is a Neutral Antagonist at CB1 Receptors. Chem Res Toxicol. 2012a;25:825–7. doi: 10.1021/tx3000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely KA, Lapoint J, Moran JH, Fattore L. Spice drugs are more than harmless herbal blends: A review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry. 2012b doi: 10.1016/j.pnpbp.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely KA, Prather PL, James LP, Moran JH. Marijuana-based drugs: innovative therapeutics or designer drugs of abuse? Mol Interv. 2011;11:36–51. doi: 10.1124/mi.11.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, Martin BR. Effect of chronic administration of R-(+)-[2,3-Dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)methanone mesylate (WIN55,212-2) or delta(9)-tetrahydrocannabinol on cannabinoid receptor adaptation in mice. J Pharmacol Exp Ther. 2002;303:36–44. doi: 10.1124/jpet.102.035618. [DOI] [PubMed] [Google Scholar]
- Sobolevsky T, Prasolov I, Rodchenkov G. Detection of JWH-018 metabolites in smoking mixture post-administration urine. Forensic Sci Int. 2010;200:141–7. doi: 10.1016/j.forsciint.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Sobolevsky T, Prasolov I, Rodchenkov G. Detection of urinary metabolites of AM-2201 and UR-144, two novel synthetic cannabinoids. Drug Test Anal. 2012 doi: 10.1002/dta.1418. [DOI] [PubMed] [Google Scholar]
- Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3:1073–4. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- Teske J, Weller JP, Fieguth A, Rothamel T, Schulz Y, Troger HD. Sensitive and rapid quantification of the cannabinoid receptor agonist naphthalen-1-yl-(1-pentylindol-3-yl)methanone (JWH-018) in human serum by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:2659–63. doi: 10.1016/j.jchromb.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Uchiyama N, Kawamura M, Kikura-Hanajiri R, Goda Y. URB-754: a new class of designer drug and 12 synthetic cannabinoids detected in illegal products. Forensic Sci Int. 2013;227:21–32. doi: 10.1016/j.forsciint.2012.08.047. [DOI] [PubMed] [Google Scholar]
- Van Ree JM, Slangen JL, de Wied D. Intravenous self-administration of drugs in rats. TJournal of Pharmacology and Experimental Therapeutics. 1978;204:547–57. [PubMed] [Google Scholar]
- Vandrey R, Dunn KE, Fry JA, Girling ER. A survey study to characterize use of Spice products (synthetic cannabinoids) Drug Alcohol Depend. 2012;120:238–41. doi: 10.1016/j.drugalcdep.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardakou I, Pistos C, Spiliopoulou C. Spice drugs as a new trend: mode of action, identification and legislation. Toxicol Lett. 2010;197:157–62. doi: 10.1016/j.toxlet.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Matsunaga T, Yamamoto I, Funae Y, Yoshimura H. Involvement of CYP2C in the metabolism of cannabinoids by human hepatic microsomes from an old woman. Biol Pharm Bull. 1995;18:1138–41. doi: 10.1248/bpb.18.1138. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Yamaori S, Funahashi T, Kimura T, Yamamoto I. Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes. Life Sci. 2007;80:1415–9. doi: 10.1016/j.lfs.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Wiebelhaus JM, Poklis JL, Poklis A, Vann RE, Lichtman AH, Wise LE. Inhalation exposure to smoke from synthetic “marijuana” produces potent cannabimimetic effects in mice. Drug Alcohol Depend. 2012;126:316–23. doi: 10.1016/j.drugalcdep.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Huffman JW, Balster RL, Martin BR. Pharmacological specificity of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rhesus monkeys. Drug Alcohol Depend. 1995;40:81–6. doi: 10.1016/0376-8716(95)01193-5. [DOI] [PubMed] [Google Scholar]
- Wintermeyer A, Moller I, Thevis M, Jubner M, Beike J, Rothschild MA, et al. In vitro phase I metabolism of the synthetic cannabimimetic JWH-018. Anal Bioanal Chem. 2010 doi: 10.1007/s00216-010-4171-0. [DOI] [PubMed] [Google Scholar]
- Wohlfarth A, Scheidweiler KB, Chen X, Liu HF, Huestis MA. Qualitative confirmation of 9 synthetic cannabinoids and 20 metabolites in human urine using LC-MS/MS and library search. Anal Chem. 2013;85:3730–8. doi: 10.1021/ac3037365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, et al. Brain cannabinoid CB receptors modulate cocaine’s actions in mice. Nat Neurosci. 2011;14:1160–6. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto I, Watanabe K, Kuzuoka K, Narimatsu S, Yoshimura H. The pharmacological activity of cannabinol and its major metabolite, 11-hydroxycannabinol. Chem Pharm Bull (Tokyo) 1987;35:2144–7. doi: 10.1248/cpb.35.2144. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Ma P, Cole RB, Wang G. Identification of in vitro metabolites of JWH-015, an aminoalkylindole agonist for the peripheral cannabinoid receptor (CB2) by HPLC-MS/MS. Analytical and bioanalytical chemistry. 2006;386:1345–55. doi: 10.1007/s00216-006-0717-6. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Ma P, Iszard M, Cole RB, Wang W, Wang G. In vitro metabolism of R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo [1,2,3-de]1,4-benzoxazinyl]-(1-naphthalenyl) methanone mesylate, a cannabinoid receptor agonist. Drug Metab Dispos. 2002;30:1077–86. doi: 10.1124/dmd.30.10.1077. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Ma P, Wang W, Cole RB, Wang G. Characterization of rat liver microsomal metabolites of AM-630, a potent cannabinoid receptor antagonist, by high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Journal of mass spectrometry: JMS. 2004;39:672–81. doi: 10.1002/jms.640. [DOI] [PubMed] [Google Scholar]
- Zimmermann US, Winkelmann PR, Pilhatsch M, Nees JA, Spanagel R, Schulz K. Withdrawal phenomena and dependence syndrome after the consumption of “spice gold”. Dtsch Arztebl Int. 2009;106:464–7. doi: 10.3238/arztebl.2009.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuba D, Byrska B, Maciow M. Comparison of “herbal highs” composition. Anal Bioanal Chem. 2011;400:119–26. doi: 10.1007/s00216-011-4743-7. [DOI] [PubMed] [Google Scholar]