Abstract
Ancient human remains of paleopathological interest typically contain highly degraded DNA in which pathogenic taxa are often minority components, making sequence-based metagenomic characterization costly. Microarrays may hold a potential solution to these challenges, offering a rapid, affordable, and highly informative snapshot of microbial diversity in complex samples without the lengthy analysis and/or high cost associated with high-throughput sequencing. Their versatility is well established for modern clinical specimens, but they have yet to be applied to ancient remains. Here we report bacterial profiles of archaeological and historical human remains using the Lawrence Livermore Microbial Detection Array (LLMDA). The array successfully identified previously-verified bacterial human pathogens, including Vibrio cholerae (cholera) in a 19th century intestinal specimen and Yersinia pestis (“Black Death” plague) in a medieval tooth, which represented only minute fractions (0.03% and 0.08% alignable high-throughput shotgun sequencing reads) of their respective DNA content. This demonstrates that the LLMDA can identify primary and/or co-infecting bacterial pathogens in ancient samples, thereby serving as a rapid and inexpensive paleopathological screening tool to study health across both space and time.
Research into the origins of infectious diseases and population health through time faces many challenges, such as biased archival records and ambiguous paleopathological skeletal indicators of actual pathogen infection levels1. Despite its inherent fragility, ancient DNA (aDNA) remains a highly informative paleopathological study target, having been recovered and characterized from a variety of contexts, age depths and specimen types2. Recently, high-throughput sequencing (HTS), often coupled with targeted enrichment (TE), has allowed for the recovery of large genomic targets from archaeological specimens, including full pathogen genomes3,4,5. However, TE-HTS is only useful when the primary pathogen(s) are known or suspected to be present, and necessarily ignores non-targeted taxa and genomic loci. This is problematic because the primary pathogenic agent in an ancient paleopathological specimen can be elusive, and furthermore the entire microbiome likely played a significant role in past human health, as it does today6. Therefore establishing detailed levels of commensal and co-infecting pathogens is essential for accurately reconstructing past epidemics, population health, and disease susceptibility. As such, for paleopathologists wishing to examine changes in microbial co-infection levels across space and time, more comprehensive metagenomic characterization is necessary. One way to achieve this is by sequencing amplicons of conserved loci (such as 16S rRNA) that can to a degree measure the metagenomic content of a sample. However, by design, amplicon datasets ignore potential taxonomically-informative diversity in more variable genomic regions, and for that matter can be biased by polymerase or disparate target abundances7,8. Metagenomic “shotgun” HTS on the other hand is arguably the most comprehensive and least biased method currently available for total microbial characterization for modern and aDNA specimens9,10, but very deep sequencing is often required to identify pathogens confidently. While certainly powerful, both of these metagenomic approaches can be labour- and time-intensive, thereby representing significant barriers for groups that would like to thoroughly profile or screen the microbial content of large or difficult paleopathological sample sets.
One potential technological solution to this issue is the microarray, which over the past two decades has been used for the large-scale study of gene expression and genic content of simple and complex samples11. Microarrays are glass slides densely spotted with clusters of single-stranded synthetic oligonucleotides that are allowed to hybridize with fluorophore-labeled DNA from a sample, and the resulting fluorescence signals are interpreted to determine sequence composition and/or taxonomic content. Recently, microarrays designed specifically for characterizing the microbial content of complex samples have been successfully used (e.g.12,13,14,15,16,17), particularly in cases where traditional clinical methods are inconclusive, time-consuming, and/or expensive17. Microarrays can contain up to millions of unique oligonucleotides and their use and analysis involve low processing time and cost14. Therefore, they potentially provide a more practical alternative to metagenomic HTS for characterizing the microbial content of paleopathological specimens. However, microarray detection techniques have not yet been applied to aDNA extracts, which due to short fragment length and base damage may present challenges.
To assess the potential value of microarrays for pathogen detection in ancient samples, we compared microbial profiles of two archaeological human specimens generated with a recently-developed pathogen detection microarray to profiles generated with standard metagenomic HTS analysis. For microarray analysis, we used the Lawrence Livermore Microbial Detection Array (LLMDA) designed by the Lawrence Livermore National Laboratory12, one of several array platforms developed in the last decade to identify pathogens in experimental mixtures and clinical samples14. The LLMDA v5 12-plex 135K array contains probes designed from all published vertebrate-infecting pathogen genomes. LLMDA probes target conserved regions amongst all known species/strains of a family (or equivalent unit), but due to the high number and overall diversity of probes, unique combinations of matching probes across an individual genome sequence allow for species or strain identification. Florescence data are analysed using a likelihood maximization algorithm to identify the combination of species that best explains the resulting signal. To achieve this, each signal set is compared against a current database of full microbial genomes and analysed for the expected vs. detected combined probe fluorescence signal, resulting in a species list ranked by likelihood of presence. If desired, these results can then be parsed to calculate overall likelihoods of higher taxa presence by summing the likelihoods of relevant species-level hits (see Supplementary Information for full description). The specimens we analysed here were a preserved medical intestine sample from an 1849AD cholera victim (specimen 3090.13)3 and a tooth from a 1348AD Black Death plague victim (specimen 8291)4. Both were previously confirmed with TE-HTS to contain their relevant pathogens, though they constitute very low levels in shotgun HTS datasets (3090.13: 0.03% alignable with bowtie18 to Vibrio cholerae, the etiological agent of cholera; 8291: 0.08% alignable to Yersinia pestis, the etiological agent of the plague or Black Death). Both of these pathogens' families (Vibrionaceae and Enterobacteriaceae) have probes on the LLMDA, and therefore species in these families should be detectable. We specifically assessed (1) whether LLMDA would detect the previously determined pathogens in our ancient samples, (2) which additional bacterial taxa were detectable by both LLMDA and HTS, and (3) which bacterial taxa were detected by either LLMDA or HTS alone.
Results
We have restricted our taxonomic comparisons to bacteria, since the sequencing libraries were built from DNA only and thus not appropriate for a complete viral survey. While both HTS and LLMDA analyses are capable of species-level identification (as LLMDA analysis calculates the likelihood of presence for individual species/strain genomes), for the purposes of this paper we have focused on family-level identification for ease of comparison. Note that the v5 12x135K LLMDA probes were derived from all complete vertebrate-infecting pathogen genome sequences available at the time of design (December 2011); however, as the hybridization patterns were interpreted using an updated database (April 2012), probes may match new genomes from other taxa (even non-vertebrate infecting species) and therefore potential taxonomic calls are not limited to those used for probe design. For the metagenomic HTS data, taxonomic assignments were identified by BLAST (blastn-megablast)19 and MEGAN420 analysis against the National Center for Biotechnology Information (NCBI) RefSeq genome database21 (October 2012). A schematic comparison is provided in Fig. 1 and results for both methods are given in Table 1 and Tables S1 and S2.
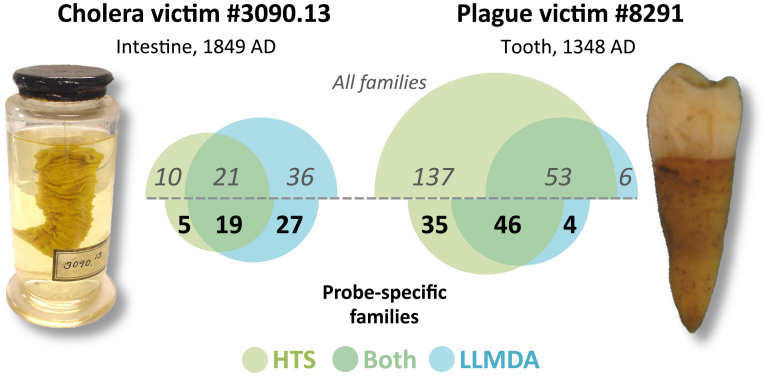
Figure 1. Number of bacterial families detected by HTS and/or LLMDA.
Number of bacterial families (or less-specific higher taxonomic level) detected by HTS sequencing (green circles) and LLMDA analyses (blue circles). Families detected by both methods are indicated where the circles overlap. Values above the midline include all detected families, whereas values below the midline include only those families used for LLMDA probe design. Image of cholera victim specimen 3090.13 was taken by AMD; image of plague victim specimen 8291 was taken by HNP.
Table 1. Summary of LLMDA and HTS results. Only taxa with probes designed on the LLMDA array are shown (see Table S1 for full results). Only taxa with at least 5 reads are called with HTS-MEGAN4 analysis. Reads = number of HTS reads assigned to that taxonomic level (- = not found in HTS dataset). LO score = LLMDA log odds score (- = not called with LLMDA). Phyla abbreviations = Act, Actinobacteria; Bac, Bacteroidetes; Chla, Chlamydiae; Chlo, Chlorobi; Chl, Chloroflexi; Fib, Fibrobacteres; Fir, Firmicutes; Fus, Fusobacteria; Pro, Proteobacteria; Spi, Spirochaetes; Syn, Synergistetes; Ten, Tenericutes; The, Thermotogae; Ver, Verrucomicrobia.
| Cholera victim specimen 3090.13 | Plague victim specimen 8291 | ||||||
|---|---|---|---|---|---|---|---|
| Phylum | Family | Reads | LO score | Phylum | Family | Reads | LO score |
| Pro | Vibrionaceae | 10,600 | 4,470.7 | Pro | Enterobacteriaceae | 15,062 | 1,640.8 |
| Pro | Aeromonadaceae | 1,877 | 480.0 | Pro | Alcaligenaceae | 11,976 | 880.0 |
| Pro | Enterobacteriaceae | 1,072 | 4,944.3 | Pro | Bradyrhizobiaceae | 8,189 | 174.4 |
| Fir | Erysipelotrichaceae | 1,039 | 561.7 | Pro | Burkholderiaceae | 7,298 | 10,155.0 |
| Fir | Clostridiaceae | 989 | 2,023.6 | Fir | Clostridiaceae | 5,188 | 1,861.8 |
| Fir | Streptococcaceae | 387 | 486.6 | Act | Pseudonocardiaceae | 4,876 | 474.1 |
| Pro | Comamonadaceae | 233 | 496.6 | Pro | Comamonadaceae | 3,704 | 466.2 |
| Fir | Peptostreptococcaceae | 216 | - | Pro | Pseudomonadaceae | 2,778 | 3,461.5 |
| Pro | Pseudomonadaceae | 178 | 4,313.1 | Pro | Xanthomonadaceae | 2,720 | 197.3 |
| Pro | Moraxellaceae | 122 | 105.2 | Act | Streptomycetaceae | 2,135 | 506.1 |
| Pro | Xanthomonadaceae | 93 | 228.0 | Pro | Methylobacteriaceae | 1,195 | 118.3 |
| Pro | Burkholderiaceae | 22 | 11,233.8 | Pro | Oxalobacteraceae | 1,045 | 119.6 |
| Fir | Veillonellaceae | 22 | 130.2 | Pro | Neisseriaceae | 903 | 232.0 |
| Act | Corynebacteriaceae | 19 | 309.5 | Pro | Sphingomonadaceae | 747 | - |
| Fir | Staphylococcaceae | 14 | 273.6 | Act | Mycobacteriaceae | 642 | 1,368.6 |
| Pro | Pasteurellaceae | 11 | - | Pro | Caulobacteraceae | 606 | 106.0 |
| Act | Micrococcaceae | 8 | 358.5 | Pro | Acetobacteraceae | 492 | 222.6 |
| Pro | Neisseriaceae | 8 | - | Fir | Peptostreptococcaceae | 324 | - |
| Fir | Enterococcaceae | 6 | 204.0 | Act | Nocardiaceae | 310 | 282.7 |
| Bac | Flavobacteriaceae | 6 | - | Pro | Brucellaceae | 274 | - |
| Fir | Bacillaceae | 5 | 3,077.2 | Pro | Halomonadaceae | 204 | - |
| Act | Streptomycetaceae | 5 | 523.1 | Pro | Aeromonadaceae | 167 | - |
| Act | Coriobacteriaceae | 5 | 123.6 | Pro | Desulfovibrionaceae | 158 | 218.4 |
| Fus | Fusobacteriaceae | 5 | - | Fir | Lachnospiraceae | 131 | 707.8 |
| Fir | Paenibacillaceae | - | 1,100.2 | Fir | Eubacteriaceae | 122 | 74.3 |
| Fir | Lachnospiraceae | - | 1,016.1 | Act | Micrococcaceae | 111 | 349.9 |
| Act | Propionibacteriaceae | - | 947.8 | Fus | Fusobacteriaceae | 99 | - |
| Pro | Alcaligenaceae | - | 745.0 | Fir | Peptococcaceae | 97 | 116.1 |
| Fir | Lactobacillaceae | - | 677.9 | Act | Propionibacteriaceae | 95 | 950.6 |
| Pro | Desulfovibrionaceae | - | 390.9 | Act | Cellulomonadaceae | 92 | - |
| Act | Actinomycetaceae | - | 231.2 | Pro | Sutterellaceae | 85 | - |
| Act | Bifidobacteriaceae | - | 225.6 | Act | Gordoniaceae | 84 | - |
| Act | Micrococcineae | - | 213.2 | Pro | Piscirickettsiaceae | 82 | - |
| Fir | Carnobacteriaceae | - | 207.6 | Fir | Streptococcaceae | 79 | 104.2 |
| Act | Mycobacteriaceae | - | 185.0 | Act | Coriobacteriaceae | 77 | 112.0 |
| Fir | Listeriaceae | - | 164.0 | Act | Actinomycetaceae | 74 | 216.8 |
| Fir | Planococcaceae | - | 157.1 | Pro | Cardiobacteriaceae | 70 | - |
| Fir | Aerococcaceae | - | 135.2 | Fir | Lactobacillaceae | 66 | 378.8 |
| Pro | Deferribacteraceae | - | 128.3 | Fir | Veillonellaceae | 65 | 228.7 |
| Fir | Peptococcaceae | - | 127.7 | Fir | Bacillaceae | 63 | 2,764.1 |
| Ver | Verrucomicrobiaceae | - | 127.4 | Pro | Moraxellaceae | 62 | 203.2 |
| Act | Jonesiaceae | - | 126.6 | Act | Corynebacteriaceae | 54 | 562.3 |
| Pro | Helicobacteraceae | - | 124.7 | Act | Intrasporangiaceae | 53 | - |
| Pro | Caulobacteraceae | - | 117.6 | Act | Bifidobacteriaceae | 52 | 748.5 |
| Chl | Herpetosiphonaceae | - | 112.8 | Spi | Spirochaetaceae | 52 | - |
| Act | Brevibacteriaceae | - | 112.7 | Pro | Erythrobacteraceae | 44 | - |
| Act | Dermabacteraceae | - | 111.3 | Fir | Staphylococcaceae | 43 | 176.7 |
| Fir | Leuconostocaceae | - | 111.0 | Syn | Synergistaceae | 40 | 108.5 |
| Pro | Campylobacteraceae | - | 107.9 | Fir | Ruminococcaceae | 30 | 100.2 |
| Fir | Eubacteriaceae | - | 95.6 | Pro | Pasteurellaceae | 30 | 80.1 |
| Fib | Fibrobacteraceae | - | 90.5 | Bac | Flavobacteriaceae | 25 | 108.9 |
| Pro | Vibrionaceae | 24 | - | ||||
| Act | Dermabacteraceae | 21 | 108.1 | ||||
| Act | Dermacoccaceae | 21 | - | ||||
| Act | Segniliparaceae | 21 | - | ||||
| Act | Tsukamurellaceae | 21 | - | ||||
| Spi | Leptospiraceae | 20 | - | ||||
| Bac | Rikenellaceae | 19 | - | ||||
| Fir | Erysipelotrichaceae | 17 | 322.5 | ||||
| Bac | Bacteroidaceae | 17 | - | ||||
| Pro | Campylobacteraceae | 17 | - | ||||
| Pro | Succinivibrionaceae | 17 | - | ||||
| The | Thermotogaceae | 16 | - | ||||
| Pro | Desulfomicrobiaceae | 15 | - | ||||
| Bac | Prevotellaceae | 14 | - | ||||
| Pro | Helicobacteraceae | 11 | 120.6 | ||||
| Pro | Bartonellaceae | 11 | - | ||||
| Ten | Mycoplasmataceae | 10 | - | ||||
| Fir | Leuconostocaceae | 9 | 191.4 | ||||
| Fir | Enterococcaceae | 9 | 177.6 | ||||
| Fir | Listeriaceae | 9 | 164.6 | ||||
| Bac | Porphyromonadaceae | 9 | - | ||||
| Pro | Legionellaceae | 9 | - | ||||
| Fir | Aerococcaceae | 8 | 257.5 | ||||
| Pro | Anaplasmataceae | 7 | - | ||||
| Pro | Coxiellaceae | 7 | - | ||||
| Fir | Carnobacteriaceae | 6 | 184.4 | ||||
| Pro | Bdellovibrionaceae | 6 | 85.0 | ||||
| Chla | Parachlamydiaceae | 6 | - | ||||
| Pro | Francisellaceae | 6 | - | ||||
| Pro | Rickettsiaceae | 5 | - | ||||
| Act | Jonesiaceae | - | 108.3 | ||||
| Chlo | Herpetosiphonaceae | - | 101.2 | ||||
| Act | Brevibacteriaceae | - | 101.1 | ||||
| Fib | Fibrobacteraceae | - | 79.3 | ||||
Taxa detected by both LLMDA & HTS
For cholera victim 3090.13, twenty-one bacterial families were detected by both LLMDA and the 118 million BLASTed HTS reads from the sample (Fig. 1), representing 36.8% and 40.4% of the families called by each respective method. For plague victim 8291, fifty-three families were detected by both approaches, representing 89.8% and 27.9% of the families called by LLMDA and 83 million HTS reads, respectively. When we considered only the families with specific probes designed for them on the LLMDA array, we detected 19 families in the cholera victim 3090.13 by both LLMDA and HTS, representing 41.3% and 79.2% of the families called by each respective method and 46 families for the plague victim 8291, representing 92.0% and 56.8% of the families with probes on the array (LLMDA and HTS).
The taxa detected by both methods included many groups with relatively high read counts in the HTS data (e.g. Aeromonadaceae and Enterobacteriaceae for 3090.13; Burkholderiaceae, Comamonadaceae, and Pseudomonadaceae for 8291). In addition, both methods detected the previously confirmed significant pathogens to the species level within the calls for their respective families (see Fig. S1 and Table S3 for the LLMDA probe data supporting these pathogen calls). For 3090.13, 10,379 (0.009% of BLAST reads) were V. cholerae, and LLMDA called the family Vibrionaceae with V. cholerae chromosomal sequences at a high log odds value (4,470.7). The detection of only V. cholerae chromosomal sequences amongst Vibrionaceae is not unexpected, given that Vibrionaceae species are aquatic and only opportunistically infect humans22. For 8291, 1,272 (0.001% of BLAST reads) were Y. pestis, and LLMDA called the family Enterobacteriaceae including Y. pestis pPCP1 plasmid sequences (among multiple other species) at a high odds value (1,640.8). Y. pestis pPCP1 was detected amongst a variety of other Enterobacteriaceae, which is a large family containing many normal microbiomic species such as E. coli23. The detection of pPCP1 is not surprising, given that the plasmid is both highly specific to Y. pestis and is found at high copy number within the bacterium24, which may have facilitated detection.
Taxa detected by only one method
BLAST analyses of HTS reads identified many bacterial families that were not detected by LLMDA analyses (for cholera victim 3090.13, n = 10, 32.3% of all HTS; for plague victim 8291, n = 137, 72.1% of all HTS), such as Neisseriaceae and Shewanellaceae in sample 3090.13, Cellulomonadaceae and Rhizobiaceae in sample 8291, and Fusobacteriaceae and Peptostreptococcaceae in both samples. Likewise, LLMDA analysis identified many families that HTS did not (for 3090.13, n = 36, 63.1% of all LLMDA; for 8291, n = 6, 10.2% of all LLMDA). However, when excluding taxonomic groups without specific probes represented on the array. However, when excluding taxonomic groups without specific probes represented on the array, only 5 families were detected by HTS alone (20.8% of all HTS) for sample 3090.13 and 35 (43.2%) for 8291, while for LLMDA alone, 27 (58.7% of all LLMDA) were detected for 3090.13 and 4 (8.0%) for 8291.
Discussion
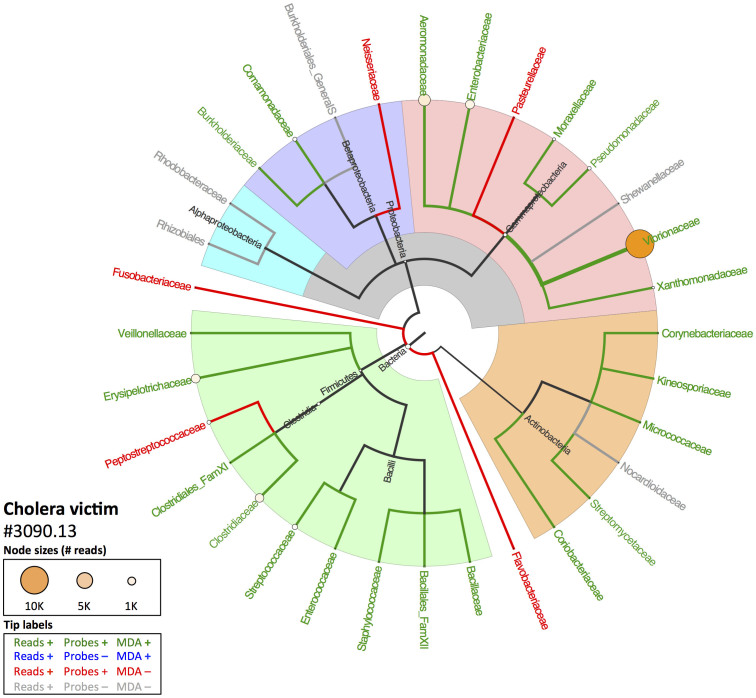
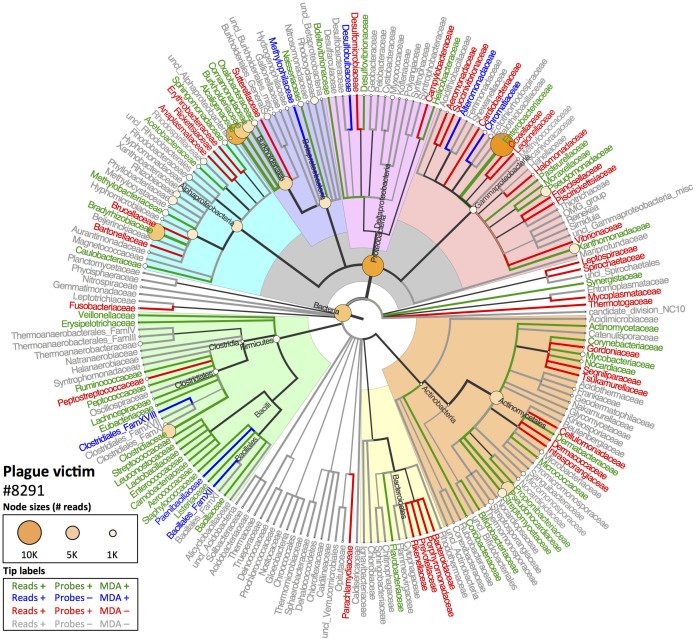
Figure 2 (cholera victim 3090.13) and Figure 3 (plague victim 8291) display the MEGAN4 output of the NCBI taxonomy for all family-level taxa identified with BLAST analysis of the HTS data and whether they were also detected with LLMDA. Overall, the LLMDA profiles reflect the major HTS-identified components well. Not only were the previously-identified pathogen bacterial species/families detected via both methods, but a number of major environmental, microbiomic and pathogenic taxa were identified to at least the order level (e.g., Actinomycetales, Bacilliales, Clostridiales, or Rhizobiales). While promising, a number of disparities between the profiles generated by each method encourage further investigation into their origin, discussed below.
Figure 2. Comparison of HTS and LLMDA results for cholera victim 3090.13.
Cladogram based on NCBI Genbank taxonomy indicating results of the BLASTN/MEGAN4 HTS analysis at the family level and above compared to LLMDA results. At the leaves, circle size reflects the relative number of reads assigned to those taxa (internal node sizes only indicated if >10 reads). Colors of taxon names indicate whether that taxon had (1) reads present in the HTS data, (2) probes designed for that family on the LLMDA, and (3) LLMDA call for that taxon. Bacterial phyla and major clades are highlighted.
Figure 3. Comparison of HTS and LLMDA results for plague victim 8291.
See caption for Figure 2.
When comparing metagenomic profiles generated by each method, it is important to be aware of the fundamental differences in their taxonomic identification strategies. For the analysis of BLAST output from HTS data, default parameters in MEGAN4 require five sequence reads to assign a taxon as being present; furthermore, the reads do not have to be assigned to the same species for family-level calls20. MEGAN4 also gives equal weight to read mappings that are concentrated in narrow regions of a target genome, which are inherently less specific as indicators of the target's presence. A common possible scenario leading to false positive taxon assignments could occur in both HTS and microarray analysis, when reads or probes map to ribosomal RNA or housekeeping genes that are relatively conserved between related taxa. Microarray probes can be designed to avoid these conserved regions, but in general sequence reads mapping to such regions are not filtered out in metagenomic analysis. Therefore, BLAST/MEGAN4 analysis of HTS data emphasizes sensitivity at the expense of specificity. The CLiMax algorithm used for LLMDA analysis requires that a family satisfy more stringent criteria to be considered present. The initial CLiMax analysis is performed at the target genome level rather than the family level; for a target to be called present, a minimum of 4 probes or 20% of the probes matching a specific genome (whichever is greater) must have intensities above an array-specific significance threshold. In addition, targets for which the high intensity probes are concentrated in narrow genomic regions are filtered out as potential false positives (see Supplementary Information for description of methods). When this filtering is removed, or if the minimum probe criteria are relaxed, CLiMax predicts the presence of several previously undetected families (data not shown). However, our previous experiments in which the LLMDA was hybridized to samples of known microbial content indicate that stringent filtering is necessary to avoid false positives12. Therefore, the CLiMax analysis is much more conservative in its predictions than BLAST/MEGAN4 analysis, emphasizing specificity over sensitivity, and possibly explaining some of the apparent undetected taxa in the LLMDA data.
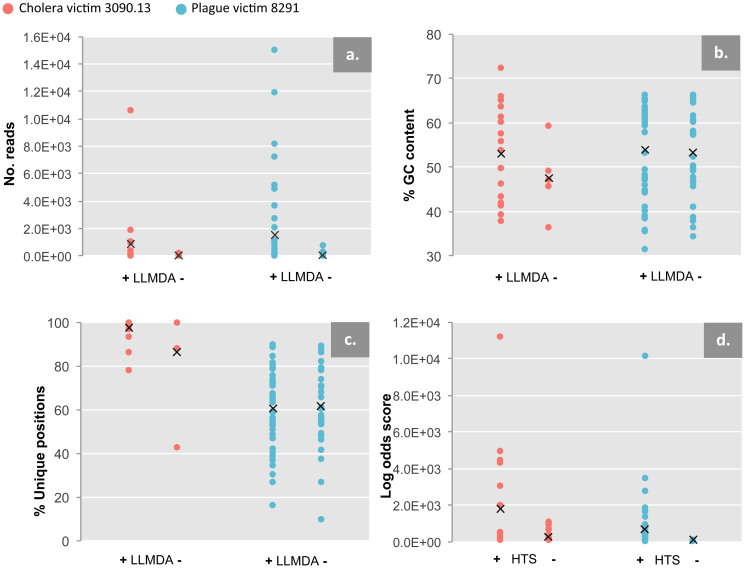
Several taxa detected with HTS were not detected with LLMDA. Many of these are unsurprising, as no probes designed from their genomes were present on the array. However, for those taxa with probes on the array, one possibility is that the LLMDA is simply not as sensitive as HTS at these sequencing depths: in plague victim 8291, taxa not detected with LLMDA had significantly fewer HTS reads than those that were (one-tailed, unequal variance Student's t-test, p = 0.002; Fig. 4a), though these variables are not significantly related for the cholera victim sample 3090.13 (p = 0.076). Furthermore, several taxa with relatively high read counts and with probes designed on the array were surprisingly not called (e.g., Sphingomonadaceae in sample 8291; Peptostreptococcaceae in both samples). That said, in the majority of cases where a family with probes designed on the array was declared present by BLAST/MEGAN4, but not called with LLMDA, a closely-related taxon was called (e.g., in both samples, Clostridiaceae was called although its close relative Peptostreptococcaceae was not).
Figure 4. HTS vs. LLMDA comparisons.
HTS readcounts, GC content, unique genomic positions sequenced, and maximum log odds scores for both specimens plotted against whether they were detected (+) or not detected (-) with LLMDA (a–c) or HTS (d). For HTS read counts, all HTS-identified families are analyzed (a); GC content and unique genomic positions are analyzed only for families that were used for LLMDA probe design (b,c); log odds scores are only analyzed for families detected with LLMDA.
To better understand the data used by MEGAN4 to call the family Peptostreptococcaceae as present, we examined the ribosomal RNA gene, and other feature annotations for the mapped read positions in Clostridium difficile strain 630 (RefSeq accession NC_009089.1), one of the fully sequenced genomes in this family. Notably, 915 of 1328 (69%) reads mapping to this genome from cholera victim 3090.13 and 146 of 319 (46%) from plague victim 8291 were within rRNA genes. Since rRNA genes only cover 1.1% of the C. difficile 630 genome, these read counts are much higher than would be expected by chance alone. Consequently, we suspect that a large part of the data used by MEGAN4 to call this family as present is based on reads that map to highly conserved genes, and could also support the presence of a related taxon. Although a detailed analysis of MEGAN4 performance is beyond the scope of this study, our preliminary results suggest that its relative non-specificity could underlie some of the discrepancies between HTS and microarray identifications.
We also considered the possibility that relatively low GC content of the targets could compromise hybridization-based LLMDA detection. Average log (fluorescence) intensity of probes for a given taxon is strongly correlated with the average GC% of that probe set (r = 0.56, p = 0.0028, R2 = 0.368 for cholera victim 3090.13; r = 0.65, p = 2.5 × 10−13, R2 = 0.653 for plague victim 8291; Fig. S3), but LLMDA detected taxa across the range of average log intensities. Furthermore, for taxa used for probe design, there was no significant difference in GC content between LLMDA-positive and LLMDA-negative HTS reads (two-tailed, unequal variance Student's t-test, p = 0.252 for 3090.13, p = 0.779 for 8291; Fig. 4b). This indicates that GC content alone cannot explain a taxon's presence or absence from the LLMDA calls. We also considered the possibility that confident LLMDA identification may be compromised if regional preservation or amplification biases reduce the evenness of genomic representation amongst the reads. However, for taxa with probes on the array, there was no significant difference between the proportions of unique genomic bases covered by HTS reads for LLMDA-positive and LLMDA-negative taxa (two-tailed, unequal variance Student's t-test, p = 0.365 for 3090.13, p = 0.843 for 8291; Fig. 4c).
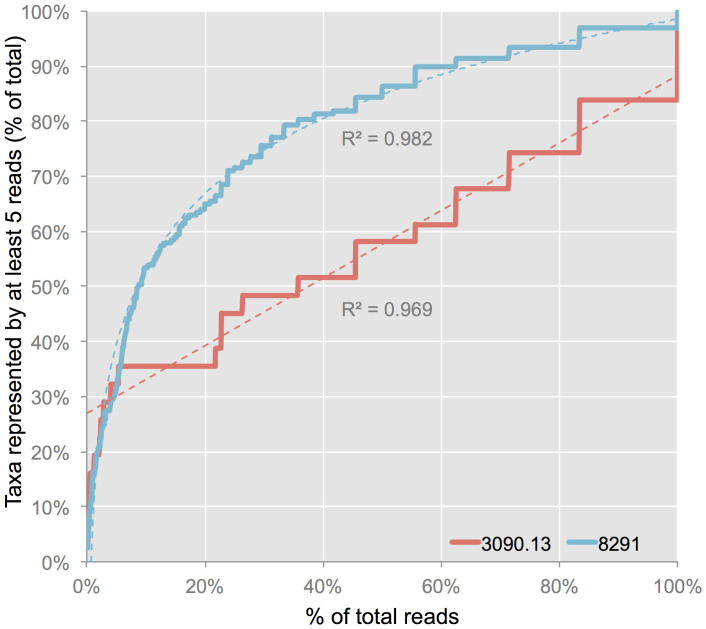
Several taxa were detected only with LLMDA. This may suggest that the LLMDA is more sensitive than HTS to certain taxa, as a rarefaction analysis of the HTS data suggests that in neither sample have all the HTS-detectable families likely been observed at these sequencing depths (Fig. 5). Cholera victim 3090.13 in particular shows a near-linear rarefaction curve, potentially explaining why it has so many more LLMDA-only calls than does plague victim 8291. However, taxa detected by both HTS and LLMDA still have significantly higher LLMDA log odds scores than taxa detected by LLMDA alone (one-tailed, unequal variance Student's t-test, p = 0.015 for 3090.13, p = 0.007 for 8291; Fig. 4d). This difference likely reflects the fact that LLMDA calls with smaller log odds scores are supported by fewer detected probes, and are thus inherently less reliable. However, the relationship between log odds scores and HTS observations is imperfect, as several taxa with relatively high read counts have maximum log odds score values within the range of LLMDA-only calls (e.g., Caulobacteraceae for sample 8291 and Moraxellaceae for sample 3090.13). Again as noted above, there is no significant difference between the proportion of unique genomic bases covered by HTS reads for LLMDA-positive and LLMDA-negative taxa (Fig. 4c).
Figure 5. HTS rarefaction analysis.
Rarefaction curves showing the number of bacterial families represented by at least 5 reads as a percent of the total observed families per sample with increasing read depth (0.1% increments). Dashed lines represent lines of best-fit; cholera victim specimen 3090.13 is a linear curve (R2 = 0.96936), plague victim specimen 8291 is a logarithmic curve (R2 = 0.98217).
We have demonstrated that the LLMDA provides similar bacterial family-level metagenomic profiles of archaeological and archival specimens as HTS, especially for the most abundant taxa, and successfully detected the previously-verified infecting pathogen species in both specimens. Furthermore, as demonstrated with cholera victim 3090.13, it is potentially capable of detecting bacterial taxa that are insufficiently or unable to be detected even with very large HTS datasets, due to the very deep sequencing depths required to observe low abundance HTS taxa, likely common for many co-infecting pathogens in complex aDNA extracts. This is encouraging, since LLMDA analysis is at least one order of magnitude less expensive and labor-intensive than metagenomic HTS. As such, the technique could be productively applied in a number of research settings, depending on the specific question and the nature of the specimens. For instance, dozens of samples could be rapidly assessed for the most abundant pathogen constituents. Use of the LLMDA may also integrate well into TE-HTS studies not only by narrowing the range of targets for hybridization capture, but also by generating enriched libraries via elution from the microarray itself, which can be later sequenced. However the profiles generated by the LLMDA and HTS are not identical, and criteria for confident taxon identification with both platforms remains imperfect. We have shown that no simple variable completely explains the signal disparities, and it is likely that a combination of analysis techniques, sequence factors such as GC content, and probe design drive the disagreements between the LLMDA and HTS. Further methodological evaluation may be able to refine these disparities. We expect that microarrays will progress in the near future to become an excellent screening tool for archaeological samples where microbial profiles can be swiftly, cheaply, and accurately reconstructed thereby aiding the elucidation of population health through deep time.
Methods
Libraries from these specimens were both shotgun HTS sequenced (divided across one HiSeq 1000 lane: 141,039,627 reads for cholera victim 3090.13, 122,830,910 reads for plague victim 8291) and utilized for LLMDA analysis. HTS datasets were compared to the NCBI RefSeq database21 using BLAST 2.2.26+19 and the resulting BLAST reports were parsed using MEGAN4 v.4.70.4 with the default settings20. Taxonomic trees were illustrated manually using FigTree (v.1.4.0; http://tree.bio.ed.ac.uk/software/figtree) based on MEGAN4 results. Indexed libraries were sent to Lawrence Livermore National Laboratory (LLNL) for blind analysis using the 12-plex 135K Roche NimbleGen version of the LLMDA v5 array, which is designed to target 3521 vertebrate-infecting species from 215 families (including bacteria, archaea, viruses, protozoa and fungi). A brief summary of the LLMDA workflow is as follows: libraries are linearly amplified via random hexamers (Cy-3 labeled) to add the necessary fluorescent signal, hybridized to the LLMDA array for 65 h, washed, scanned, and analysed. Unlike other aDNA experiments utilizing in-solution or array hybridization, “blocking oligonucleotides” were not used, as this is not a standard component of the LLMDA procedure. Arrays were analysed using the CLiMax algorithm12 with probe intensity threshold set to the 95th percentile of negative controls. See Supplementary Information for all further details.
Author Contributions
H.N.P., M.B., C.J. conceived of the research. A.M.D., C.J., J.T., J.M.E. designed and performed experiments. A.M.D., C.J., S.G., T.M.P., J.M.E., J.T., J.A., K.M. and H.N.P. analysed data. A.N.D. and S.N.D. provided samples. All authors contributed to the manuscript preparation.
Additional information
Accession Codes: Sequencing data has been deposited to the National Center for Biotechnology Information under BioProjects PRJNA239173 (sample 3090.13) and PRJNA239147 (sample 8291).
Supplementary Material
Table S1
Table S2
Table S3
Supplementary Information
Acknowledgments
We would like to thank David J.D. Earn, David Fisman, Joseph Tien, G. Brian Golding, Nicholas Waglechner, Debi Poinar, Melanie Kuch, D. Ann Herring, Kirsten Bos, Johannes Krause, and the members of the McMaster Ancient DNA Centre for their ongoing insights and contributions to this work in the field of paleopathological research. We thank Rebecca Redfern and Jelena Bekvalac at the Museum of London Centre for Human Bioarchaeology for providing access to the 8291 specimen. AMD was supported by an Ontario Graduate Scholarship. HNP was supported by a CRC (Canada Research Chair) and NSERC grants. TMP was funded by the Government of Canada through Genome Canada and the Ontario Genomics Institute through the Biomonitoring 2.0 project (OGI-050).
References
- Ortner D. J. Human skeletal paleopathology. Int. J. Paleopathol. 1, 4–11, 10.1016/j.ijpp.2011.01.002 (2011). [DOI] [PubMed] [Google Scholar]
- Rizzi E., Lari M., Gigli E., De Bellis G. & Caramelli D. Ancient DNA studies: new perspectives on old samples. Genet. Sel. Evol. 44, 21, 10.1186/1297-9686-44-21 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devault A. M. et al. Second-Pandemic Strain of Vibrio cholerae from the Philadelphia Cholera Outbreak of 1849. N. Engl. J. Med. 370, 334–340, 10.1056/NEJMoa1308663 (2014). [DOI] [PubMed] [Google Scholar]
- Bos K. I. et al. A draft genome of Yersinia pestis from victims of the Black Death. Nature 478, 506–510, 10.1038/nature10549 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuenemann V. J. et al. Genome-wide comparison of Medieval and modern Mycobacterium leprae. Science 341, 179–183, 10.1126/science.1238286 (2013). [DOI] [PubMed] [Google Scholar]
- Brogden K. A., Guthmiller J. M. & Taylor C. E. Human polymicrobial infections. Lancet 365, 253–255, 10.1016/s0140-6736(05)70155-0 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J. M., Portillo M. C., Belda-Ferre P. & Mira A. Amplification by PCR artificially reduces the proportion of the rare biosphere in microbial communities. PLoS ONE 7, e29973, 10.1371/journal.pone.0029973 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabney J. & Meyer M. Length and GC-biases during sequencing library amplification: A comparison of various polymerase-buffer systems with ancient and modern DNA sequencing libraries. Biotechniques 52, 87–+, 10.2144/000113809 (2012). [DOI] [PubMed] [Google Scholar]
- Khairat R. et al. First insights into the metagenome of Egyptian mummies using next-generation sequencing. J. Appl. Genetics 54, 309–325, 10.1007/s13353-013-0145-1 (2013). [DOI] [PubMed] [Google Scholar]
- Keller A. et al. New insights into the Tyrolean Iceman's origin and phenotype as inferred by whole-genome sequencing. Nat. Commun. 3, 698, 10.1038/ncomms1701 (2012). [DOI] [PubMed] [Google Scholar]
- Hoheisel J. D. Microarray technology: beyond transcript profiling and genotype analysis. Nat. Rev. Genet. 7, 200–210, 10.1038/nrg1809 (2006). [DOI] [PubMed] [Google Scholar]
- Gardner S., Jaing C., McLoughlin K. & Slezak T. A microbial detection array (MDA) for viral and bacterial detection. BMC Genomics 11, 668, 10.1186/1471-2164-11-668 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandsson L., Rosenstierne M. W., McLoughlin K., Jaing C. & Fomsgaard A. The Microbial Detection Array combined with random Phi29-amplification used as a diagnostic tool for virus detection in clinical samples. PLoS ONE 6, e22631, 10.1371/journal.pone.0022631 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin K. S. Microarrays for pathogen detection and analysis. Brief. Funct. Genom. 10, 342–353, 10.1093/bfgp/elr027 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. et al. Microarray-based detection and genotyping of viral pathogens. Proc. Natl. Acad. Sci. U. S. A. 99, 15687–15692, 10.1073/pnas.242579699 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios G. et al. Panmicrobial oligonucleotide array for diagnosis of infectious diseases. Emerg. Infect. Dis 13, 73–81, 10.3201/eid1301.060837 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoria J. G. et al. Viral nucleic acids in live-attenuated vaccines: Detection of minority variants and an adventitious virus. J. Virol. 84, 6033–6040, 10.1128/jvi.02690-09 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B., Trapnell C., Pop M. & Salzberg S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25, 10.1186/gb-2009-10-3-r25 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W. & Lipman D. J. Basic Local Alignment Search Tool. J. Mol. Biol. 215, 403–410, 10.1006/jmbi.1990.9999 (1990). [DOI] [PubMed] [Google Scholar]
- Huson D. H., Mitra S., Ruscheweyh H.-J., Weber N. & Schuster S. C. Integrative analysis of environmental sequences using MEGAN4. Genome Res. 21, 1552–1560, 10.1101/gr.120618.111 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt K. D., Tatusova T., Brown G. R. & Maglott D. R. NCBI Reference Sequences (RefSeq): current status, new features and genome annotation policy. Nucleic Acids Res. 40, D130–D135, 10.1093/nar/gkr1079 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reen F. J., Almagro-Moreno S., Ussery D. & Boyd E. F. The genomic code: inferring Vibrionaceae niche specialization. Nat. Rev. Microbiol. 4, 697–704, 10.1038/nrmicro1476 (2006). [DOI] [PubMed] [Google Scholar]
- Brenner D. J. & Farmer J. J. III in Bergey's Manual of Systematic Bacteriology - Volume 2: The Proteobacteria, Part B: The Gammaproteobacteria (eds Don J. Brenner., Noel R. Krieg. & James R. Staley) 587–850 (Springer, 2005).
- Parkhill J. et al. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413, 523–527, 10.1038/35097083 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Supplementary Information