Abstract
Purpose
To develop a bilateral coil and optimized fat suppressed T1-weighted sequence for 7T breast MRI.
Materials and Methods
A dual-solenoid coil and 3D T1w gradient echo sequence with B1+ insensitive fat suppression (FS) were developed for 7T. T1w FS image quality was characterized through image uniformity and fat/water contrast measurements in 11 subjects. Signal-to-noise ratio (SNR) and flip angle maps were acquired to assess the coil performance. Bilateral contrast-enhanced and unilateral high resolution (0.6 mm isotropic, 6.5 min acquisition time) imaging highlighted the 7 T SNR advantage.
Results
Reliable and effective FS and high image quality was observed in all subjects at 7T, indicating that the custom coil and pulse sequence were insensitive to high-field obstacles such as variable tissue loading. 7T and 3T T1w FS image uniformity was similar (P=0.24), indicating adequate 7T B1+ uniformity. High 7T SNR and fat/water contrast enabled 0.6 mm isotropic imaging and visualization of a high level of fibroglandular tissue detail.
Conclusion
7T T1w FS bilateral breast imaging is feasible with a custom RF coil and pulse sequence. Similar image uniformity was achieved at 7T and 3T, despite different RF field behavior and variable coil-tissue interaction due to anatomic differences that might be expected to alter magnetic field patterns.
Keywords: bilateral breast magnetic resonance imaging, high-field MRI, fat suppression, RF coil, breast cancer
INTRODUCTION
Breast cancer is the most common newly diagnosed cancer and the second leading cause of cancer-related mortality in women in the United States (1). MRI has emerged as an important clinical tool for the detection of breast cancer and for local staging. Clinical breast MRI performed at 1.5 T and 3 T has been shown to be more sensitive to the detection of breast tumors than conventional modalities such as ultrasound or digital mammography (2), and MRI is particularly advantageous for imaging dense breast parenchyma. High-field MRI (≥ 7 T) can provide improvements in signal-to-noise ratio (SNR), fibroglandular/fat contrast, and spatial/temporal resolution due to its inherent sensitivity advantage over lower field strengths, which may further improve the modality’s sensitivity and pave the way for novel contrast mechanisms to improve specificity (3-4). However, increased magnetic field strength results in longer T1 relaxation time, shorter T2* decay time, greater radiofrequency (RF) specific absorption rate (SAR), and reduced transmit field (B1+) homogeneity all of which can limit 7 T SNR gain in practice. Preliminary work indicates that these deterrents can be overcome, and studies have demonstrated greater than two-fold improvements in in vivo breast SNR at 7 T over 3 T (5-7). Further, given that the anatomic dimensions of the breast are similar to the RF wavelength in fibroglandular tissue and less than the wavelength in adipose tissue at 7 T, classic high-field RF hindrances such as poor B1+ penetration and B1+ inhomogeneity are expected to be mild compared to high-field abdominal imaging (8), while prolonged T1 relaxation time at high-field may be an advantage in contrast-enhanced examinations (9-11).
Preliminary 7 T breast MRI studies have shown promising results (5-7,12-17), although coil limitations have restricted many studies to unilateral examinations with inadequate coverage in a small number of subjects. To date, no study has provided a quantitative assessment of image quality. Further study is therefore warranted to investigate 7 T bilateral breast imaging in a cohort of subjects which is particularly important due to anatomic and electrical property variability (18) which can influence 7 T imaging more strongly than at lower-field. Image uniformity is a pertinent concern in breast imaging (19), particularly at high-field where uniformity is strongly influenced by the coil and pulse sequence. Further, fat suppression (FS) is preferred, particularly in clinics in the United States, due to intermingled fat/fibroglandular tissue which can interfere with lesion assessment in the breast. In this work, we address these issues by implementing a bilateral 7 T breast coil and B1+ insensitive FS technique. These advancements are quantitatively assessed through image uniformity and fat/water image contrast measurements in 11 subjects.
METHODS
This prospective study was approved by our local IRB and 11 subjects (age = 30 +/− 3 years, age range = 23 to 46 years) were scanned after written informed consent was obtained. 7 T data was acquired on a whole body scanner (MAGNETOM 7 T, Siemens Medical Solutions, Erlangen, Germany) with maximum gradient amplitude of 40 mT·m−1 and maximum slew rate of 150 mT·m−1·s−1, and a custom bilateral transmit-receive coil described in the following section. 3 T images were acquired on a TIM Trio scanner (Siemens Medical Solutions) with the same gradient specifications as those listed for the 7 T system. At 3 T, the body coil provided RF excitation and a bilateral seven-channel coil (single breast aperture = 2674 cm3, Invivo Corp., Gainsville, Fl.) was used for RF reception. 7 T contrast-enhanced imaging was performed in one subject with Gadolinium-DTPA (0.1 mmol/kg Magnevist, Bayer HealthCare Pharmaceuticals Inc., Wayne, NJ), which was hand injected at a rate of 2 mL/s.
7 T Bilateral Breast Coil
7 T whole body scanners do not routinely include a transmit body coil due to the technical difficulties in constructing a large resonant structure at high field. Accordingly, a custom local transmit/receive RF coil was constructed for 7 T bilateral breast imaging (Figure 1). We implemented a dual solenoid coil because of its high magnetic field uniformity, high efficiency, and convenient geometry. The geometry of each solenoid was two-turn, 15.5 cm diameter, and 7 cm height. The coil was housed in a repurposed commercially available former (1590 cm3 aperture for each breast, bilateral four channel breast coil, Siemens Healthcare) which is identical to that utilized in clinical reports (20-21). The solenoids were made of 1.3 cm wide copper sheets and tuned using ~8.2 pF capacitors distributed at 16 locations. In this configuration, the quality factor (Q) of an isolated solenoid (the contralateral solenoid was open-circuited during this measurement) was 85 at 222.8 MHz when unloaded and 13 at 220.0 MHz when loaded with a cylindrical pork phantom (0.9 kg, 11.5 cm diameter, and 15 cm height) (Q-ratio = 6.5). The low unloaded Q indicated substantial radiation loss. Moreover, resonance frequency splitting due to coupling between the solenoids was intolerable (22.5 MHz). These problems were alleviated by surrounding the solenoids with passive (“floating ground”) square-tubular conductive RF shields (17 cm length and 9 cm height). The two shields (left and right) were split into shorter square-tubular sections approximately 3 cm in height through two divisions in the coronal plane and further split at one point in the transverse plane. Gaps between these shield segments were connected with 470 pF capacitors to block gradient-induced currents while allowing RF currents. The Q-ratio improved to 12.4 with the shields in place, indicating reduced radiation loss (unloaded Q of the solenoid was 235 at 299.2 MHz and the loaded Q was 19 at 297.2 MHz). No resonance frequency splitting was observed in either the unloaded or loaded case, and the transmission coefficient (S21) between the two solenoids was −36 dB when loaded with the pork phantoms. Both solenoids were well matched; reflection coefficients S11 ≈ S22 ≈ −16 dB.
Figure 1.
Photographs of the coil (a), assembled coil and shield (b), shield (c), and full assembly (d).
Compared to a single solenoid that encompasses both breasts, the dual-solenoid array provided improved efficiency and enabled parallel imaging in the right/left direction. RF pulses were delivered to the solenoids through a Wilkinson power divider, whose outputs each presented one-half of the input power to two transmit/receive switches built in-house (insertion loss < 0.6 dB, isolation between the transmit and preamplifier ports > 26 dB). The transmit/receive switches were subsequently connected to the drive points of each solenoid and to low input impedance preamplifiers (model E8, Siemens Medical Solutions).
7 T Power Limit
The local 7 T transmit coil required custom RF power limits to restrict tissue heating caused by electric fields. To experimentally determine the safe operating limit, the relative temperature of pork phantoms was measured using proton resonance frequency shift method after a 600 s RF irradiation period. Due to the complex interaction between electric fields and tissue at 7 T, and breast anatomic variability, heating experiments were performed with two sets of cylindrical pork phantoms: each large phantom weighed 0.9 kg and was 11.5 cm in diameter and 15 cm in height, each small phantom weighed 0.4 kg and was 5.1 cm in diameter and 15 cm in height). In heating experiment 1, the large phantoms were centered in the each solenoid. In experiment 2, each small phantom was placed at the position of maximum heating found in experiment 1.
Before and after RF irradiation, a GRE acquisition was performed to measure the image phase for conversion into relative temperature maps (3D acquisition, voxel size = 1.7 × 1.7 × 5.0, 32 slices, 53 s acquisition time, TE = 10 ms, TR = 12 ms). Oil phantoms were placed adjacent to the pork phantoms to provide stable phase references. Gradients were disabled during the irradiation period to eliminate gradient-induced heating of the magnet and associated shifts in the baseline phase. In experiment 1, the time-averaged RF power measured at the power amplifier was 48.8 W / 10 s and the maximum local temperature increase during the 600 s irradiation period was 6.2 °C in the medial region in each solenoid. In experiment 2, the time-averaged RF power measured at the power amplifier was 45.1 W / 10 s and the maximum local temperature increase was 7.4 °C.
Starting from the 10 W/kg SAR limit (6 kJ/kg over the 600 s irradiation period) set by the International Electrotechnical Commission (International Standard IEC 60601-2-33 2010) and assuming a conservative value for the specific heat of tissue = 4 kJ/kg °C (22), we established a maximum heating limit of 1.5° C / 600 s. Given the maximum temperature increase in experiment 2 and assuming a linear relationship between RF irradiation and temperature change, the time-averaged power limit was set to 9.1 W / 10 s = 45.1 W · 1.5° C / (10 s · 7.4 °C). While complex tissue-electric field interactions and accentuated local heating are possible in vivo, these effects are expected to be roughly offset by perfusion. Further, given the complexity of computer-based SAR prediction models, variable subject anatomy, and the added complexity of aligning simulated results to those in situ, we prefer to rely upon experimental phantom heating measurements to determine power limits.
7 T and 3 T Coil Benchmarks
To compare the custom 7 T bilateral transmit/receive coil with the commercial 3 T body transmit and 3 T 7-channel bilateral receive coil, flip angle, SNR, and geometry factor maps were acquired in one subject and in water phantoms. The phantoms were doped with sugar and salt to realize the following electrical properties relative permittivity = 32 and conductivity = 0.24 S/m (measured using a dielectric probe at 297.2 MHz [model 85070E, Agilent Technologies, Santa Clara, CA]). These properties were chosen to emulate a blend of breast fibroglandular (18) and adipose (23) tissues whose properties are, respectively: relative permittivity = 49 and 5.5, and conductivity = 0.73 and 0.03 S/m. Receive signal for SNR analysis was acquired in three planes with a gradient echo pulse sequence with the following parameters: TE = 4.9 ms (3 T) and 5.1 ms (7 T), TR = 1000 ms, voxel size = 2.7 × 2.7 × 5 mm3, acquisition matrix = 128 × 128, flip angle = 10°, receiver bandwidth = 540 Hz/pixel (phantoms) and 1000 Hz/pixel (in vivo), and acquisition time = 2 min 7 s. The noise correlation matrix was acquired without RF excitation to allow construction of SNR maps using the optimal array combination method (24). The coil geometry factor (g) was derived from SNR data to assess parallel imaging performance (25). Flip angle maps were acquired using the method described in Ref. (26).
In Vivo 7 T B1+ Calibration
At 7 T, the transmit voltage necessary to generate a 180° flip angle with a 1 ms hard pulse was calibrated in each subject using a gradient echo based sequence with a series of preconditioning pulse amplitudes (27). Due to the spatially varying transmit field, the transmit voltage was calibrated in both the central fibroglandular tissue and peripheral fat. Separate fibroglandular and fat transmit calibration scans were necessary with the preconditioning pulse set to the appropriate frequency, because the frequency offset between the two species was of the same order as the pulse bandwidth. At 3 T, the transmit voltage was determined automatically by the vendor-provided algorithm. 3 T and 7 T flip angle maps were acquired in one subject using the method described in Ref. (28).
3D T1-weighted Fat Suppressed Imaging
A 3D T1-weighted gradient echo pulse sequence (volumetric interpolated breath-hold examination [VIBE] sequence on Siemens systems) was customized for breast MRI at 7 T. Effective fat suppression (FS) is essential for breast lesion detection. Because its adiabatic pulse reduces sensitivity to B1+ variation, we utilized spectrally selective adiabatic inversion recovery (SPAIR) FS. Two key modifications were made to convert the 3 T product pulse sequence to 7 T; the pulse duration was reduced from 23 ms to 10 ms to match the increase in fat-water spectral separation and the assumed T1 value of fat was increased to 540 ms based on preliminary phantom and in vivo measurements (5). To test the robustness of SPAIR FS to B1+ variation, images were acquired over a range of SPAIR pulse amplitudes. Other imaging parameters were similar to those used in 3 T clinical scans (Table 1).
Table 1.
3D T1w FS pulse sequence parameters.
| Sequence description | 3 T unilateral |
3 T bilateral |
7 T unilateral |
7 T unilateral high resolution |
7 T bilateral |
|---|---|---|---|---|---|
| Number of subjects | 10 | 1 | 10 | 1 | 2 |
| FS method | Saturation | Saturation | SPAIR | SPAIR | SPAIR |
| TE (ms) | 1.5 | 1.3 | 2.1 | 2.8 | 1.9 |
| TR (ms) | 3.8 | 3.8 | 4.5 | 5.4 | 4.2 |
| Nominal flip angle (degrees)* | 12 | 12 | 5-10 | 5 | 10 |
| Bandwidth (Hz/pixel) | 543 | 540 | 545 | 540 | 540 |
| FS lines per shot | 40 | 60 | 60 | 100 | 60 - 100 |
| Number of slices | 144 | 144 | 144 | 208 | 120 - 144 |
| Slice orientation | Sagittal | Transverse | Sagittal | Sagittal | Transverse |
| Field-of-view (mm) | 190 | 310 | 190 | 190 | 310 |
| Acquisition matrix | 1762 | 2882 | 1742 | 3202 | 2242 - 2882 |
| True voxel size (mm2) | 1.1 × 1.1 | 1.1 × 1.1 | 1.1 × 1.1 | 0.6 × 0.6 | 1.1 × 1.1 - 1.4 × 1.4 |
| Slice thickness (mm) | 1.6 | 1.0 | 1.6 | 0.6 | 1.0 - 1.4 |
| Parallel imaging acceleration | None | 2 | None | None | 2 |
| Acquisition time (s) | 71 | 109 | 119 | 390 | 108 - 163 |
7T flip angles were calibrated in the peripheral fat tissue to ensure efficacy of the SPAIR module. The flip angle in the center of the breast is expected to be approximately 25% greater (see flip angle maps in Figure 3).
Whereas SPAIR FS is applicable to 7 T breast imaging due to reduced B1+ uniformity, the technique requires fine tuning of the inversion recovery time, prolonged scan times, and may provide less benefit for 3 T breast imaging due to the high level of B1+ uniformity at this field strength. Correspondingly, 3 T T1w images in this article were acquired using the product VIBE sequence with saturation-based FS (quick fat suppression on Siemens systems) (see Table 1). Further 3 T FS optimization may be warranted but is beyond the scope of this work.
Image Assessment
Signal uniformity and FS efficacy are important metrics to assess breast image quality. These measurements were made in bilateral and unilateral 3D T1w FS images without Gadolinium to eliminate associated uptake variability (sequence parameters in Table 1). Further, to investigate the potential of trading the substantial 7 T SNR gain for increased spatial resolution, 7 T unilateral images were acquired with 0.6 mm isotropic resolution.
Image uniformity and FS efficacy were measured in a single slice near the center of the breast. Effort was made to select a slice that contained a comparable number of water and fat voxels (peripheral slices or those dominated by fat voxels were not selected) and to select a similar slice in each subject at both field strengths. Water and fat signal was defined as the mean amplitude in 3D T1w images within respective region of interests (ROIs) ( and ). Water and fat ROIs are described in the following paragraph. FS efficacy, or image contrast, was calculated according to , where c = 1 indicates ideal FS and c = 0 indicates that fat and water are not distinguishable. Image uniformity was defined as 1 minus the standard deviation divided by the mean of the water pixel intensities: . The two-tailed paired student’s t-test was used to determine the statistic significance between the mean contrast and uniformity at 7 T and 3 T. P-values less than 0.01 were considered significant.
Due to anatomic variation, subject-specific water and fat ROIs were generated using the three-point Dixon chemical species separation method which produces water, fat, and frequency maps (29-30). For this purpose, an additional series of 2D gradient echo images were acquired in the VIBE slice selected for quantitative image assessment (single slice, TR = 50 ms, TE = 2.7, 2.9, 3.1, 3.3 ms [7 T], TE = 3.5, 4.0, 4.5, 5.0 ms [3 T], flip angle = 20°, resolution = 0.7 × 0.7 mm2 [unilateral sagittal acquisitions], resolution = 1.2 × 1.2 mm2 [bilateral transverse acquisitions], and slice thickness = 3 mm). From the water and fat maps, pixels with greater than 85% water or fat content were included in respective ROIs. The ROIs were manually cropped to exclude tissue posterior to the boundary between the breast and pectoralis muscle. The ROIs were subsequently eroded by three pixels (using the imerode function in Matlab software [version 2009b, The MathWorks, Natick, MA]) to reduce the likelihood of misregistration between Dixon and VIBE acquisitions and interpolated to match the spatial resolution of the VIBE images.
RESULTS
Coil Performance
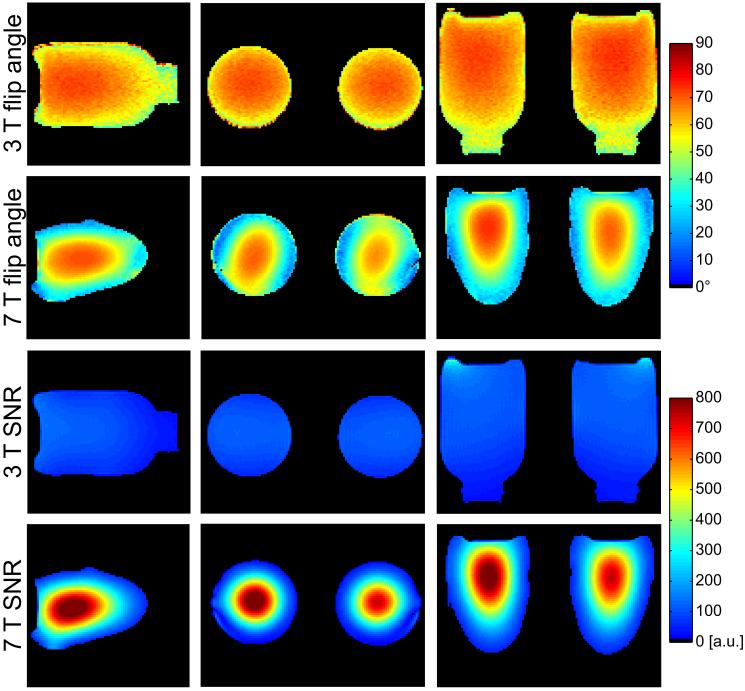
The transmit voltage required to generate a 180° flip angle in the center of the phantom with a 1 ms hard pulse was 166 v at 7 T and 315 v at 3 T, indicating that the local 7 T transmit coil was more efficient than the 3 T body coil. 7 T phantom flip angle maps illustrate constructive interference in the center of the phantoms characteristic of high-field RF behavior (Figure 2). 7 T phantom SNR maps demonstrate a 7-fold increase in the center of the phantom and similar SNR in the periphery compared to 3 T. Note that 7 T SNR is biased by underlying B1+ variation, where reduced SNR in the periphery is accentuated by a low flip angle. Due to the high level of isolation between the 7 T solenoids, the maximum g-factor was approximately 1 (no coil-related SNR penalty) for a parallel imaging acceleration rate of 2 in the left-right direction. Acceleration at rates greater than 2 or in other directions is not permitted by the 2-channel coil.
Figure 2.
Three-plane phantom flip angle maps at 3 T (first row) and 7 T (second row) illustrate accentuated RF interference pattern in the center of the phantoms which is characteristic of high-field MRI. SNR maps demonstrate a substantial 7 T advantage over 3 T (third and fourth rows).
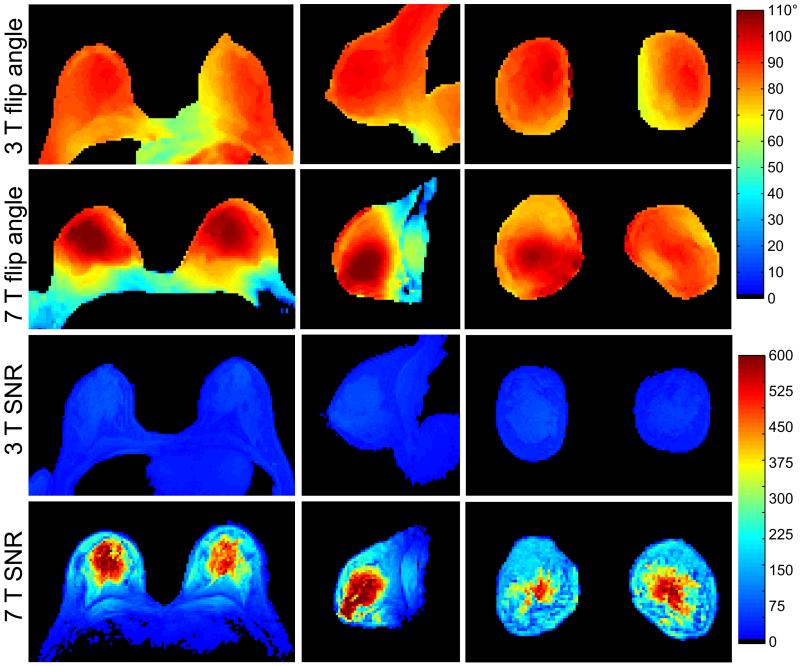
In vivo flip angle maps indicate non-uniformity of less than 25% at 7 T (Figure 3). The high level of symmetry between flip angle in the left and right breasts suggests the feasibility of bilateral 7 T imaging. 3 T flip angle non-uniformity was roughly 10% in the same subject. In 11 subjects, the 7 T transmit voltage required for a bilateral 180° excitation with a 1 ms hard pulse was 126 ± 16 V in the central fibroglandular tissue and 150 ± 22 V in the peripheral fat tissue. In the same 11 subjects, the transmit voltage required to generate the same excitation with the 3 T body coil was 508 ± 25 V (no distinction was made between that required in the central and peripheral tissue). 7 T in vivo SNR was more than 6-times greater than 3 T SNR in the central fibroglandular tissue and approximately 3-times greater in the peripheral adipose tissue. Greater field strength, reduced coil aperture, and increased coil efficiency attributed to the 7 T SNR advantage.
Figure 3.
In vivo bilateral flip angle and SNR maps at 3 T and 7 T. The flip angles at 3 T and 7 T were, respectively, 85.8 ± 6.5° (uniformity = 0.92) and 84.3 ± 20.3° (uniformity = 0.76) in the axial map in a ROI including both breasts to the pectoralis muscle boundary. Similar flip angles and uniformity can be observed in the sagittal and coronal maps. 7 T SNR was more than 6 times greater 3 T SNR in the central fibroglandular tissue and approximately 3 times greater in the peripheral adipose tissue.
3D T1-weighted Fat Suppressed Imaging
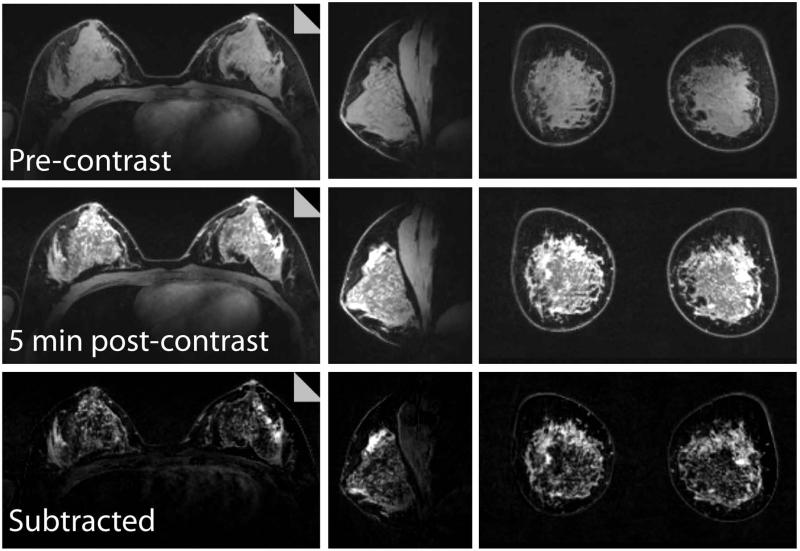
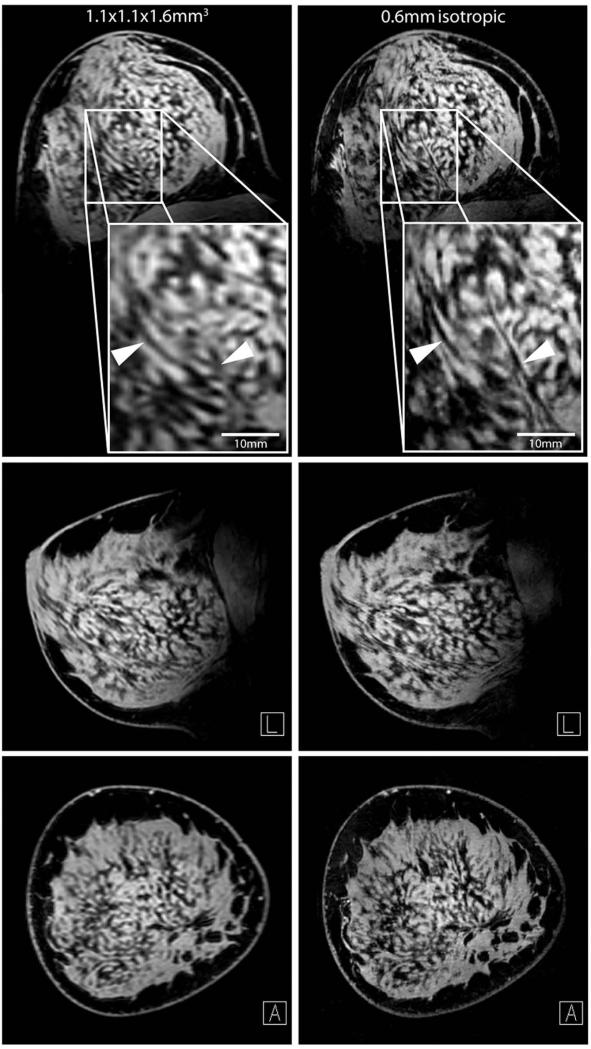
Three-plane views of a 7 T bilateral T1w contrast-enhanced examination are shown in Figure 4. Note the excellent FS and signal uniformity in both breasts, allowing differentiation of normal breast tissue. Subtracted images allow visualization of the internal enhancement pattern. In another subject, 0.6 mm isotropic images highlight the 7 T SNR advantage and allow visualization of small structures that are difficult to visualize in standard resolution images (Figure 5).
Figure 4.
Three-plane views of a 7 T bilateral dynamic contrast-enhanced examination: pre-contrast (top row), 5 min post-contrast (middle row), and the subtracted images (bottom row). The images demonstrate high signal uniformity, uniform fat suppression, and excellent coverage. of both breasts and pectoralis muscle. 5 min post-contrast images are shown because of lack of enhancement at earlier time-points in the asymptomatic subject.
Figure 5.
Three-plane views of T1w FS unilateral acquisitions at standard (left column, 1.1 × 1.1 × 1.6 mm3) and high (right column, 0.6 mm isotropic) resolution. Zoomed high resolution images show crisp fat/water contrast and delineation of structures which are difficult to visualize in the standard resolution image.
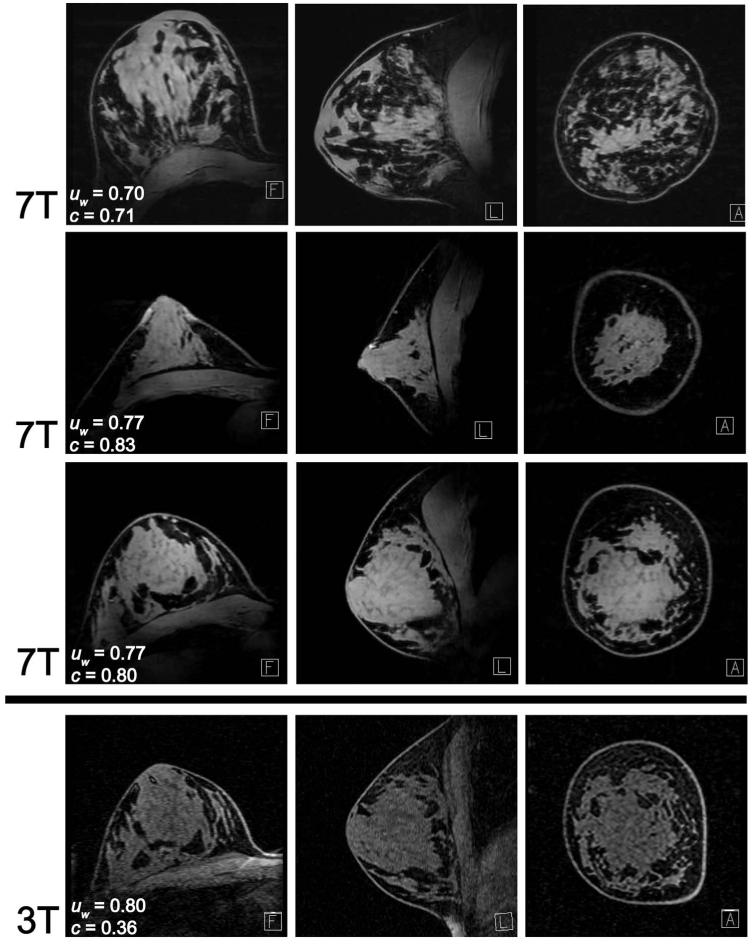
Excellent 7 T FS and image uniformity are demonstrated in T1w images from three subjects with various anatomic size and tissue composition (Figure 6), indicating insensitivity to typical high-field obstacles such as variable coil loading and patient-specific coil-tissue interaction. Image uniformity was remarkably similar at both field strengths; in unilateral images in 10 subjects, uw = 0.76 ± 0.08 at 7 T and 0.79 ± 0.04 at 3 T (P = 0.24). In bilateral images in 1 subject, uw = 0.81 at 7 T and 0.79 at 3 T.
Figure 6.
7 T T1w FS images from three subjects with various breast shape and density demonstrate consistent water signal uniformity (uw) and high fat/water contrast (c). Baseline 3 T images (bottom row) have similar signal uniformity as 7 T images from the same subject (third row) but inferior fat/water contrast.
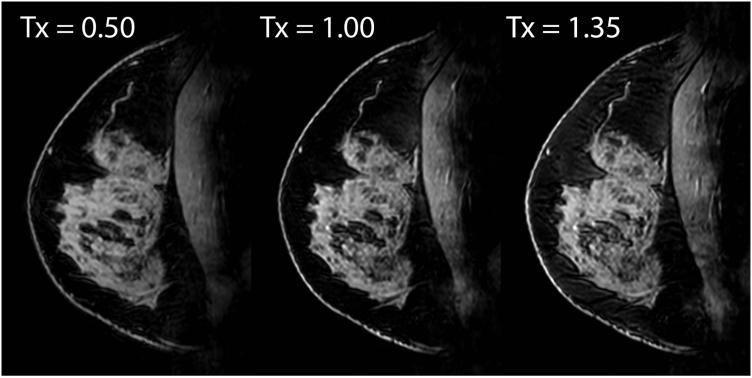
Adiabatic inversion-based FS was effective over a wide range of pulse amplitudes, indicating robustness against B1+ variation at 7 T (Figure 7). In unilateral T1w FS images in 10 subjects, fat/water contrast was c = 0.76 ± 0.04 at 7 T and 0.30 ± 0.10 at 3 T (P < 1×10−6). The small standard deviation in fat/water contrast at 7 T indicates consistent FS in all subjects. In bilateral T1w FS images in 1 subject, c = 0.77 at 7 T and 0.21 at 3 T.
Figure 7.
7 T T1w FS images with three different SPAIR pulse amplitudes demonstrate FS robustness over a wide range of B1+ variation. Unit transmit indicates the amplitude at which the FS pulse was nominally calibrated in the peripheral fat.
DISCUSSION
Breast MRI has emerged as an important tool in the evaluation of mammographically-occult breast lesions, in staging breast cancer, and evaluation of treatment response. The utility of 7 T MRI for breast cancer detection and consistent 7 T/3 T BI-RADS lesion assessment has been recently demonstrated (6,12-14), indicating that the technical developments presented here can be applied to augment the potential of lesion detection at 7 T. Specifically, the developed 7 T coil provided excellent image uniformity and its bilateral design provided a significant advancement in utility over unilateral coils. Bilateral exams, which had been difficult to perform at 7 T due to the lack of a satisfactory coil, are a key advantage of DCE-MRI of the breast in particular as the simultaneous assessment of the contrast agent uptake in both breasts is of utmost importance to rule out contralateral lesions. Further, the customized adiabatic inversion-based FS method provided effective and robust FS in a range of subjects by reducing the impact of B1+ inhomogeneity. In combination with effective FS, the high water signal strength inherent at 7 T and the high efficiency of the developed bilateral coil provided excellent fat/water contrast.
While an image uniformity penalty may be expected at 7 T due to the reduced RF wavelength, we found 3 T and 7 T image uniformity to be similar. This remarkable result can be partially attributed to the 7 T coil structure. Excluding tissue effects, the 7 T solenoid can be expected to provide improved uniformity compared to the 3 T receive-array which has the familiar properties of high sensitivity in the periphery and a drop in the center, which can be expected to reduce image uniformity. Although tissue loading can be expected to reduce uniformity in the 7 T solenoid, it should be noted that 3D T1w GRE sequences partially mitigate this factor due to their relative insensitivity to B1+ uniformity. Therefore, it is not surprising that 7 T image uniformity (0.76 ± 0.08 in unilateral acquisitions in 10 subjects) and B1+ uniformity (0.76 in a bilateral acquisition in 1 subject) were similar.
Quantitative image comparison at different magnetic field strengths is challenging due to the abundance of associated variables such as coil geometry and pulse sequence parameters. For example, due to the inversion recovery time required for the SPAIR FS technique, 7 T acquisition times were approximately 1.5 times longer than 3 T acquisitions with matched resolution (Table 1). Although this increase in 7 T scan time can be expected to improve SNR, this factor is expected to be negligible compared to the inherent gain in signal strength at 7 T. Nonetheless, to reduce bias due to minor differences in 7 T/3 T sequence parameters, we assessed image quality through image uniformity and fat/water contrast measurements. Image uniformity is a convenient metric because it does not depend on SNR and is therefore relatively insensitive to sequence parameters. Similarly, fat/water contrast depends on signal strength but not on SNR. Further, 7 T and 3 T SNR was measured in 2D GRE acquisitions with identical imaging parameters, rather than in 3D T1w FS acquisitions where identical parameters were not feasible.
One reason that the 7 T SNR advantage over 3 T (Figure 2, Figure 3) was substantially greater than the theoretical 7/3-fold advantage was the 7 T coil aperture was 1.7 times smaller than that of the 3 T coil. The 3 T receive-array used in this study is biopsy-compatible which reduces its SNR performance because of the increased distance between the coil and tissue and due to its “open” design in which the coil loops are large to accommodate the biopsy apparatus. 3 T biopsy-incompatible coils with reduced aperture and coil element size such as that described in (31) typically provide substantial SNR improvement, although the improvement can be primarily limited to the peripheral tissue. A 7 T versus 3 T SNR comparison using breast coils with identical geometries would have eliminated this influential variable. However, the main goal of this work was to demonstrate the feasibility of 7 T bilateral breast imaging and to provide 3 T benchmarks that facilitate rough comparisons. Design and implementation of identical coil geometries at both field strengths was not only beyond the scope of this work, but also not appropriate due to distinct criteria. For example, 3 T coil designs typically focus on signal reception optimization (31), while 7 T coil designs are more complex in that they must facilitate both transmit and receive capability.
We utilized the 7 T SNR advantage for unilateral 0.6 mm isotropic acquisitions, which can be expected to improve lesion contour visualization. While the scan was designed to demonstrate the feasibility of high-resolution imaging, it should be noted that an acceleration factor of approximately 6 is required to realize a bilateral acquisition with 0.6 mm isotropic resolution along with acceptable temporal resolution for dynamic imaging. Given the high baseline SNR at 7 T, the required acceleration could be readily achieved through parallel imaging, compressed sensing (32-33), or partial k-space acquisition without deleteriously impacting image quality. It should be recognized that the 7 T transmit/receive coil described here was limited to a parallel imaging acceleration rate of 2. To improve parallel imaging performance, a second-generation coil could include a dedicated multi-channel receive array, such as that described in Ref. (7,31). It is expected that a receive array would improve SNR in the peripheral tissue while maintaining the high SNR in the central tissue that was achieved with the volume coil described here. Of course, the majority of fibroglandular tissue is in the central breast, perhaps limiting the impact of peripheral SNR gain on baseline image quality. On the transmit side, a second-generation coil could include orthogonal transmit coils to enable quadrature excitation (for example, saddle or solenoid coils that generate field in the left/right direction could be added to the existing solenoids), which can be expected to improve transmit efficiency and reduce SAR by up to a factor of √2.
One drawback of the 7 T coil was low signal strength in the posterior breast and in the pectoralis muscle directly superior or directly inferior to the breast. This is because the magnetic field generated and detected by the solenoid is primarily in the head-foot direction in these regions. Regions of the pectoralis muscle posterior to the center of the breast and posterior to the left and right sides of each breast could be visualized because the magnetic field generated was primarily in the anterior-posterior direction. The axillary lymph nodes could generally be visualized, although the signal strength was weak. A future implementation of a 7 T solenoid breast coil could be tapered to a larger diameter near the chest wall to improve visibility of the posterior breast, pectoralis muscle, and axillary lymph nodes.
Fat suppression can be challenging at 7T due to technical hurdles stemming from inhomogeneous B1+. We utilized SPAIR FS because its adiabatic pulse provides inherent robustness against B1+ inhomogeneity. In combination with the inherently high fibroglandular signal strength at 7T, SPAIR FS provided a greater than two-fold gain in fibroglandular/fat contrast over 3T, which can be expected to improve delineation of fibroglandular tissue borders. While adiabatic pulses require a high level of power, SAR was not a limiting factor in the acquisitions performed in this study; the SAR limit was reached at a nominal imaging flip angle of roughly 15°, which is greater than the Ernst angle (assuming TR = 5 ms and T1 > 80 ms). We found that 1 pulse per 60 to 100 lines of acquired k-space (the standard value is 40) provided a good tradeoff between FS efficacy and SAR. Additionally, the local 7 T transmit coil partially mitigated the increased SAR penalty associated with greater magnetic field strength. The 7 T coil required 70% less voltage (91% less power) than the 3 T body coil to achieve similar excitation
Fat suppressed imaging was performed exclusively in this study due to its prevalence in United States clinical protocols. However, a discussion on non-FS imaging is warranted given its prevalence in many other parts of the world. The most apparent advantages of non-FS imaging are greater SNR in fibroglandular tissue, reduced acquisition time, and less sensitivity to B0 and B1+ inhomogeneity compared to FS imaging. Yet, sequence parameters must be carefully considered in order to realize these advantages at 7 T. Artifacts associated with fat/water chemical shift increase are much stronger at 7 T and must be minimized by selecting an echo time at which fat and water are “in-phase” or by increasing the receiver bandwidth, albeit with a SNR penalty. Further, artifacts due to patient motion may be more apparent in subtracted non-FS images due to the high level of background fat signal. Further study is clearly required to determine the utility of non-FS imaging at 7 T.
The Dixon chemical species separation method was advantageous for water and fat ROI selection for the following reasons: 1) variable tissue distribution among subjects would make manual ROI selection tedious and subject to observer variability, 2) pixels were included from all regions of the breast which improves measurement authenticity by removing bias that may exist if only central or peripheral pixels were included, 3) pixels that contained a mixture of fat and water were excluded, and 4) B0 field measurements were necessarily included and were useful for shim analysis.
In conclusion, we developed a custom bilateral breast coil and optimized a T1w FS pulse sequence to translate the raw 7 T SNR advantage into superior image quality. Excellent image uniformity and fat/water contrast was demonstrated in both unilateral and bilateral acquisitions without prolonged B0 or B1+ calibration procedures which can be burdensome in 7 T body imaging. 7 T breast images provided superb detail in fibroglandular tissue structures which may be beneficial for lesion identification since morphology is the main determinant in assessing the likelihood of malignancy (34). The 7 T SNR advantage was exploited for 0.6 mm isotropic acquisitions that improved fibroglandular tissue visualization beyond that typically available at 3 T. One drawback of the 0.6 mm isotropic scan was the long acquisition time (6.5 min).
Combined with a multi-coil receive array, increased 7 T SNR will allow flexible application of parallel imaging and compressed sensing, thereby enabling dynamic contrast-enhanced imaging with improved spatial/temporal resolution (33). We acknowledge that evaluation of pathology is required to assess the proposed coil and sequence with regards to its diagnostic potential and plan to explore this potential in a subset of patients who will undergo bilateral contrast-enhanced breast MRI at 7 T.
ACKNOWLEDGEMENTS
The authors thank Dr. Ray F. Lee of Princeton University for devising the dual-solenoid prototype, Dr. Cem Murat Deniz of NYU Langone Medical Center for instructive discussions on flip angle mapping, Cornel Stefanescu of NYU Langone Medical Center for construction of patient positioning devices, and Dr. Martijn Cloos of NYU Langone Medical Center for supplying the DREAM sequence.
Partial funding for this work was provided by NIH grant R01 EB002568.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Elsamaloty H, Salah Elzawai M, Mohamma S, Herial N. Increasing Accuracy of Detection of Breast Cancer with 3-T MRI. Am J Roentgenol. 2008;192:1142–1148. doi: 10.2214/AJR.08.1226. [DOI] [PubMed] [Google Scholar]
- 3.Katscher U, Djamshidi K, Voigt T, Ivancevic M, Abe H, Newstead G, Keupp J. Estimation of breast tumor conductivity using parabolic phase fitting. ISMRM; Melbourne, Australia: 2012. p. 3482. [Google Scholar]
- 4.Sodickson DK, Alon L, Deniz CM, Brown R, Zhang B, Wiggins GC, Cho GY, Ben Eliezer N, Novikov DS, Lattanzi R, Duan Q, Sodickson L, Zhu Y. Local Maxwell Tomography Using Transmit-Receive Coil Arrays for Contact-Free Mapping of Tissue Electrical Properties and Determination of Absolute RF Phase. Melbourne, Australia; ISMRM: 2012. p. 387. [Google Scholar]
- 5.Brown R, McGorty K, Moy L, DeGregorio S, Sodickson DK, Wiggins GC. Sub-Millimeter Breast Imaging and Relaxivity Characterization at 7T. ISMRM; Montreal, Canada: 2011. p. 3092. [Google Scholar]
- 6.Korteweg MA, Veldhuis WB, Visser F, Luijten PR, Mali WP, van Diest PJ, van den Bosch MA, Klomp DJ. Feasibility of 7 Tesla breast magnetic resonance imaging determination of intrinsic sensitivity and high-resolution magnetic resonance imaging, diffusion-weighted imaging, and (1)H-magnetic resonance spectroscopy of breast cancer patients receiving neoadjuvant therapy. Invest Radiol. 2011;46(6):370–376. doi: 10.1097/RLI.0b013e31820df706. [DOI] [PubMed] [Google Scholar]
- 7.Zheng T, Yang X, Finnerty M, Heilman J, Herczak J, Fujita H, Wiggins G, Brown R, Stoeckel B. A 7-Tesla High Density Tx/Rx Mammography Coil. ISMRM; . Montreal, Canada: 2011. p. 3819. [Google Scholar]
- 8.Vaughan JT, Snyder CJ, DelaBarre LJ, Bolan PJ, Tian J, Bolinger L, Adriany G, Andersen P, Strupp J, Ugurbil K. Whole-body imaging at 7T: preliminary results. Magn Reson Med. 2009;61(1):244–248. doi: 10.1002/mrm.21751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krautmacher C, Willinek WA, Tschampa HJ, Born M, Traber F, Gieseke J, Textor HJ, Schild HH, Kuhl CK. Brain tumors: full- and half-dose contrast-enhanced MR imaging at 3.0 T compared with 1.5 T--Initial Experience. Radiology. 2005;237(3):1014–1019. doi: 10.1148/radiol.2373041672. [DOI] [PubMed] [Google Scholar]
- 10.Elster AD. How much contrast is enough?. Dependence of enhancement on field strength and MR pulse sequence. Eur Radiol. 1997;7(Suppl 5):276–280. doi: 10.1007/pl00006908. [DOI] [PubMed] [Google Scholar]
- 11.Chang KJ, Kamel IR, Macura KJ, Bluemke DA. 3.0-T MR imaging of the abdomen: comparison with 1.5 T. Radiographics. 2008;28(7):1983–1998. doi: 10.1148/rg.287075154. [DOI] [PubMed] [Google Scholar]
- 12.Stehouwer BL, Klomp DWJ, Luijten PR, Houwert KAF, van Diest PJ, Mali WPTM, van den Bosch MAAJ, veldhuis WB. Feasibility of contrast-enhanced and high-resolution 7 Tesla MRI in patients with suspicious breast lesions. ISMRM; Melbourne, Australia: 2012. p. 1474. [Google Scholar]
- 13.Stehouwer BL, Klomp DWJ, Luijten PR, Mali WPTM, van den Bosch MAAJ, Veldhuis WB. Dynamic contrast-enhanced MRI of the breast at 7T and 3T; initial results of an intra-individual comparison of BI-RADS-MRI lesion assessment. ISMRM; Melbourne, Australia: 2012. p. 1485. [Google Scholar]
- 14.Umutlu L, Maderwald S, Kraff O, Theysohn JM, Kuemmel S, Hauth EA, Forsting M, Antoch G, Ladd ME, Quick HH, Lauenstein TC. Dynamic contrast-enhanced breast MRI at 7 Tesla utilizing a single-loop coil: a feasibility trial. Acad Radiol. 2010;17(8):1050–1056. doi: 10.1016/j.acra.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 15.Klomp D, Raaijmakers A, Korteweg M, van de Bank B, Possanzini C, Boer V, Visser F, van de Berg N, Luijten P. High resolution MR imaging and spectroscopy of the human breast at 7T using a focused field RF coil setup. ISMRM; Stockholm, Sweden: 2010. p. 2469. [Google Scholar]
- 16.Lee RF, Moy L, Brown R, McGorty K, Stefanescu C, Wang Y, Peck N. 7T high resolution breast MRI. ISMRM; Seattle, WA: 2006. p. 2900. [Google Scholar]
- 17.Brown R, Storey P, McGorty K, Raya J, Sodickson DK, Wiggins GC, Moy L. Toward improved T1-weighted breast imaging at 7T: preliminary results and comparison with 3T. ISMRM; Melbourne, Australia: 2012. p. 2981. [Google Scholar]
- 18.Lazebnik M, Popovic D, McCartney L, Watkins CB, Lindstrom MJ, Harter J, Sewall S, Ogilvie T, Magliocco A, Breslin TM, Temple W, Mew D, Booske JH, Okoniewski M, Hagness SC. A large-scale study of the ultrawideband microwave dielectric properties of normal, benign and malignant breast tissues obtained from cancer surgeries. Phys Med Biol. 2007;52(20):6093–6115. doi: 10.1088/0031-9155/52/20/002. [DOI] [PubMed] [Google Scholar]
- 19.Kuhl CK, Kooijman H, Gieseke J, Schild HH. Effect of B1 inhomogeneity on breast MR imaging at 3.0 T. Radiology. 2007;244(3):929–930. doi: 10.1148/radiol.2443070266. [DOI] [PubMed] [Google Scholar]
- 20.Tozaki M, Fukuma E. 1H MR spectroscopy and diffusion-weighted imaging of the breast: are they useful tools for characterizing breast lesions before biopsy? AJR Am J Roentgenol. 2009;193(3):840–849. doi: 10.2214/AJR.08.2128. [DOI] [PubMed] [Google Scholar]
- 21.Baltzer PA, Dietzel M, Burmeister HP, Zoubi R, Gajda M, Camara O, Kaiser WA. Application of MR mammography beyond local staging: is there a potential to accurately assess axillary lymph nodes? evaluation of an extended protocol in an initial prospective study. Am J Roentgenol. 2011;196(5):W641–647. doi: 10.2214/AJR.10.4889. [DOI] [PubMed] [Google Scholar]
- 22.Duck F. Physical Properties of Tissue: A Comprehensive Reference Work. Elsevier Science & Technology Books; New York: 1990. p. 28. [Google Scholar]
- 23.Gabriel S, Lau RW, Gabriel C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys Med Biol. 1996;41(11):2271–2293. doi: 10.1088/0031-9155/41/11/003. [DOI] [PubMed] [Google Scholar]
- 24.Kellman P, McVeigh ER. Image reconstruction in SNR units: a general method for SNR measurement. Magn Reson Med. 2005;54(6):1439–1447. doi: 10.1002/mrm.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42(5):952–962. [PubMed] [Google Scholar]
- 26.Nehrke K, Bornert P. DREAM - A Novel Approach for Robust, Ultra-Fast, Multi-Slice B1 Mapping. ISMRM; Melbourne, Australia: 2012. p. 605. [DOI] [PubMed] [Google Scholar]
- 27.Breton E, McGorty K, Wiggins GC, Axel L, Kim D. Image-guided radio-frequency gain calibration for high-field MRI. NMR Biomed. 2010;23(4):368–374. doi: 10.1002/nbm.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fautz HP, Vogel M, Gross P, Kerr A, Zhu Y. B1 mapping of coil arrays for parallel transmission. ISMRM; Toronto, Ontario: 2008. p. 1247. [Google Scholar]
- 29.Reeder SB, Wen Z, Yu H, Pineda AR, Gold GE, Markl M, Pelc NJ. Multicoil Dixon chemical species separation with an iterative least-squares estimation method. Magn Reson Med. 2004;51(1):35–45. doi: 10.1002/mrm.10675. [DOI] [PubMed] [Google Scholar]
- 30.Glover GH, Schneider E. Three-point Dixon technique for true water/fat decomposition with B0 inhomogeneity correction. Magn Reson Med. 1991;18(2):371–383. doi: 10.1002/mrm.1910180211. [DOI] [PubMed] [Google Scholar]
- 31.Nnewihe AN, Grafendorfer T, Daniel BL, Calderon P, Alley MT, Robb F, Hargreaves BA. Custom-fitted 16-channel bilateral breast coil for bidirectional parallel imaging. Magn Reson Med. 2011;66(1):281–289. doi: 10.1002/mrm.22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58(6):1182–1195. doi: 10.1002/mrm.21391. [DOI] [PubMed] [Google Scholar]
- 33.Kim S, Feng L, Moy L, Moccaldi M, Block KT, Sodickson DK, Otazo R. Highly-Accelerated Golden-Angle Radial Acquisition with Joint Compressed Sensing and Parallel Imaging Reconstruction for Breast DCE-MRI. ISMRM; Melbourne, Australia: 2012. p. 1468. [Google Scholar]
- 34.Nunes LW, Schnall MD, Orel SG. Update of breast MR imaging architectural interpretation model. Radiology. 2001;219(2):484–494. doi: 10.1148/radiology.219.2.r01ma44484. [DOI] [PubMed] [Google Scholar]