Abstract
Background
The disproportionately higher incidence of, and mortality from colorectal cancer (CRC) among African Americans (AA) led the American College of Gastroenterology to recommend screening starting at age 45 in 2005.
Aim
To determine the prevalence of colorectal neoplasia among 40–49 years old inner city African Americans (AA) and Hispanic Americans (HA).
Methods
We reviewed the medical records of 2435 inner city AA and HA who underwent colonoscopy regardless of indication and compared the prevalence of colorectal neoplasia between AA and HA patients. We used logistic regression models to calculate odds ratios (OR) and 95% confidence intervals (CI).
Results
There were 2,163 AA and 272 HA. There were 57% women in both groups. A total of 158 (7%) AA and 9 (3%) HA (P = 0.014) underwent the procedures for CRC screening. When compared to HA, AA had higher prevalence of any polyp (35% versus 18%, OR = 2.53; 95% CI: 1.82–3.52). Overall, AA had higher prevalence of colorectal neoplasia (adenoma and cancer) when compared to HA (16% versus 10%; OR = 1.68; 95% CI: 1.10–2.56).
Conclusion
We observed a higher frequency of colorectal neoplasia among 40–49 year-old AA as compared to HA suggesting an increased susceptibility to CRC risk in this population.
Keywords: Colorectal Adenoma, African Americans, Hispanic Americans, colorectal cancer
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths among men and third for women of age group 40–59 years. Multiple oarameters including age, sex, socioeconomic status, health care access, high quality screening, timely diagnosis and treatment as well as the colonic subsite of neoplasia are factors suggested to affect the risk and course of the disease [1–3]. CRC is thought to be a largely preventable and curable disease with screening and early detection.
African Americans (AA) have 20% higher incidence of CRC and 45% higher mortality from CRC than Caucasian Americans (CA) [4]. Several studies have shown that AAs are more likely to be diagnosed with advanced stage CRC and to have poorer survival [1, 5, 6]. Furthermore, AA have less favorable prognosis than Caucasians after CRC diagnosis [1, 7]. Therefore, in 2005, the American College of Gastroenterology recommends screening for AAs starting at age 45 [8] Colonic adenoma prevalence has been found to be higher among AA and Hispanic Americans (HA) and both groups are at greater risk of having proximal adenomas [9].
Hispanic Americans (HA) are the fastest growing minority in the United States [10]. It is predicted that there will be around 129 million Hispanics by year 2060, representing 31% of the nation’s population by that time [11]. HAs have the lowest burden of CRC and lowest screening rates among all ethnic groups in the United States [10].
However, up to 10% of CRCs are diagnosed among individuals younger than 50 years of age [5, 6, 12]. There is limited information on the frequency of adenoma, the common precursor for CRC and main target for screening among persons under the age of 50 years [13]. In this study, we compared the prevalence of colorectal neoplasia among inner city African American and Hispanic patients who were between the ages of 40–49.
Materials and Methods
Patients
We reviewed the medical records of 21,201 patients who underwent colonoscopy from January 2000 through December 2010 at Howard University Hospital, an inner city tertiary institution in Washington DC. We identified AA and HA who were 40–49 years old. Race classification was based on self-identification. Subjects with Familial Adenomatous Polyposis (FAP), Hereditary Non-polyposis Colorectal Cancer (HNPCC) and family history of colon cancer were excluded.
Ascertainment of primary outcome
We included all colonoscopies regardless of indications. Patients’ selection was accomplished using data from the medical coding and billing department. Electronic medical records of all patients including their colonoscopy reports were reviewed and abstracted manually. We defined advanced adenoma as adenomas ≥ 1 cm in diameter, villous or tubulovillous histology, or high grade dysplasia [14–16]. Polyps located from cecum to the splenic flexure were considered proximal and from descending colon to the rectum were classified as distal. The Institutional Review Board of Howard University approved the study.
Statistical analysis
Distribution of variables was explored by table of frequency or median (IQR). Categorical variables including sex, indication for colonoscopy, and histopathological diagnosis and polyp location were explored and compared using chi square tests. We used logistic regression models to evaluate factors that were associated with adenoma/cancer (or neoplasia) detection. We used a biologic clinical approach to select the primary list of variables to be entered into the model, i.e. variables which were clinically relevant to risk of neoplasia were entered and the final model was developed based on a backward stepwise approach. P values less than 0.05 were considered significant. Data analysis was performed using STATA 12.0 (StataCorp, College Station, TX)
Results
African Americans undergo screening more than Hispanics
A total of 2,435 individuals age 40–49 (Table 1) underwent colonoscopy during the study period. Of the eligible 2435 subjects, 2163 (89%) were AA and 272 (11%) were HA. The AA were older than the HA (median age 46 years vs. 45 years, P< 0.001). There were 57% female in each group. Most of the colonoscopies in both groups were diagnostic. Gastrointestinal bleeding was the most common indication for colonoscopy (40% among AA and 37% among HA). HA were more likely to undergo colonoscopy as part of an investigation for abdominal pain than AA (36% versus. 14%; P < 0.001) However, AA were more likely to undergo CRC screening procedures than HA (7% vs. 3%; p= 0.014) (Table 1).
Table 1.
Demographic and clinical characteristics of colonoscopies by ethnicity.
| AA n=2163 | HAs n= 272 | P value | |
|---|---|---|---|
|
| |||
| Age, median (IQR) | 46 (44–48) | 45 (42–47) | <0.001 |
|
| |||
| Female sex, no (%) | 1232 (57%) | 154 (57%) | 0.9 |
|
| |||
| Screening colonoscopy, no (%) | 0.014 | ||
| Yes | 158(7%) | 9(3%) | |
| No | 2005(93%) | 263(97%) | |
|
| |||
| Non-screening indication of colonoscopy, no (%) | <0.001 | ||
| Abdominal pain | 278 (14%) | 96 (36%) | |
| GI bleeding | 800 (40%) | 96 (37%) | |
| Anemia | 121 (6%) | 11 (4%) | |
| Other/Nonspecific | 806 (40%) | 60 (23%) | |
|
| |||
| Polyp, no (%) | 753 (35%) | 50 (18%) | <0.001 |
|
| |||
| Polyp location, polyp no (%) | 0.6 | ||
| Proximal colon | 642 (85%) | 44 (88%) | |
| Distal colon | 111 (15%) | 6 (12%) | |
Histopathology of polyps
The frequency of polyp was higher in AA (35% vs. 18%, P<0.001). The location of polyps in both population higher in proximal compared to distal (Table 1, P=0.6). Although in AA the frequency of tubular adenoma was lower (37% vs. 60%) and that of mixed and hyperplastic polyps was almost as twice than in HAs (41% vs. 24% and 9% vs. 5% respectively) the difference was not statistically significant (P = 0.06). Polyp preponderance was found in proximal colon in both the groups with more common in AAs than HAs (64% vs. 50% and 15% vs. 12%, P = 0.021).
Advanced Adenomatous Polyp and Hyperplastic Pattern
Advanced adenoma in AA was significantly more frequent in males than females (18% vs. 11%, P = 0.004). In both ethnic groups, the frequency of hyperplastic polyp was not associated with age. In multiple logistic regression, the AA group was associated with OR of 2.23 (95% CI = 1.06–4.66, P = 0.034) for diagnosing hyperplastic polyp during colonoscopy than the HA group.
Neoplasia by Age and Ethnicity
The frequency of finding neoplasia at colonoscopy increased from 9% at 40 years to 20% at 49 years old (P = 0.005) in AA, however, this frequency distribution remained the same in HA (P = 0.3; Figure 1).
Figure 1.
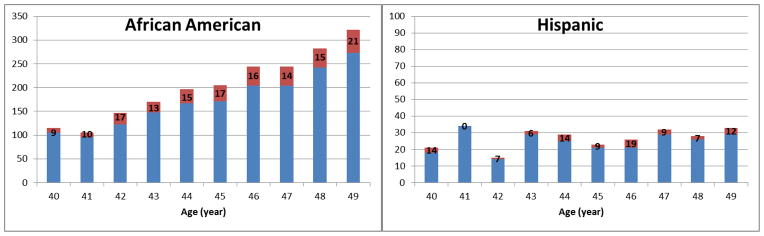
Percentage of normal (blue) and neoplasia (adenoma and CRC, red) findings by age and race (number in each bar shows the percentage of neoplasia finding in corresponding age and race group).
Colorectal neoplasia by race
The frequency of any neoplasia (including adenoma and cancer) among AA was 16% vs. 10% among HA (P<0.001). In a logistic regression analysis, age (OR: 1.07; 95% CI: 1.02–1.11; P = 0.002), AA race (OR: 1.68; 95% CI: 1.10–2.56; P = 0.017) and male gender (OR: 1.50; 95% CI: 1.20–1.88; P<0.001) were associated with higher risk of neoplasia. The frequency of advanced adenoma among AA was 5% vs. 1% among HA (P=0.004). In a logistic regression analysis, age (OR: 1.09; 95% CI: 1.02–1.18; P = 0.017), AA race (OR: 4.41; 95% CI: 1.39–14.01; P = 0.012) and male gender (OR: 1.79; 95% CI: 1.21–2.63; P=0.003) were associated with higher risk of advanced adenoma. Neither calendar year nor colonoscopy indication were significant predictors of neoplasia prevalence (Table 2).
Table 2.
Prevalence of adenoma and advanced adenoma at colonoscopy
| Race-ethnicity | (%) with neoplasia | OR (95% CI) | (%) with advanced adenoma | OR (95% CI) |
|---|---|---|---|---|
| Hispanic (n = 272) | 10% | Reference | 1% | Reference |
| African Americans (n = 2,163) | 16% | 1.68 (1.10–2.56)* | 5% | 4.41 (1.39–14.01)* |
Adjusted for age and gender.
Discussion
CRC is the third most common cancer in African Americans living in the United States and second leading cause of cancer related deaths in 2013 [1, 17]. Colonic adenoma prevalence has been found to be higher in African Americans and Hispanic Americans and both groups are at greater risk of having proximal adenomas in absence of distal pathology [9].
Epidemiological, genetic studies, molecular studies should allow us to improve risk qualification of patients with CRC and allow high risk patients use preventive modalities, early treatment, and efficient surveillance for the early detection of disease recurrence [18]. Adenoma is a precancerous lesion and its size, number and histologic features determine follow up colonoscopy surveillance pattern. We found that AA had increased odds of adenoma detection than HA during colonoscopy performed among those who were 40–49 years. Increasing age and male gender were also associated with increased odds of neoplasia.
There was 7% vs. 3% colonoscopies for screening and the rest was for symptom assessment in AAs and HAs respectively. Although the AAs were twice more likely to undergo screening colonoscopy than HAs, which might be attributed to a combination of increased awareness and access to healthcare delivery and also the reduction of screening age to 45 for AAs but not HAs, the pattern of increasing frequency of colonic lesions by age during this period raises concerns. We also found that most common symptoms in HAs was abdominal pain as opposed to blood in stool and non-specific symptoms in AAs. However, Pain is a subjective phenomenon and HAs might have a lower pain threshold than AAs.
In our logistic regression analysis, AA race and male sex and age were significantly associated with higher risk of neoplasia and advanced adenoma.
We are not aware of any previous study that has compared AA to HA among persons who were 40–49 years in terms of prevalence of colorectal neoplasia for a direct comparison to our study.
Our finding of higher frequency of polyps in both populations is comparable to the results of Lebwohl et al [9]. The rate of adenoma among blacks who were 50–59 years of age was reported to be 19% and increased with the age. The prevalence of adenoma among African American patients 40–49 year was 16% in our analysis.
Our study many limitations. First, this is a single center study; with a heterogeneity of the patients’ place of birth that was not accounted for. Higher frequency of neoplasia in AAs could be due to several unmeasured confounding factors including difference in lifestyle or access to clinical services and may not be solely attributed to genetic predisposition. Second, sessile serrated adenoma was not included in the study since it was not well known or catalogued at the beginning of our inclusive dates in this study. So, we did not have any robust information on the prevalence of these lesions. Third, in the current CRC screening guidelines, the age for screening is 50. Therefore, we are aware and it is expected that most colonoscopies performed among younger patients are more likely to be for diagnostic purposes. Further research with a larger sample size including screening younger urban minorities from multiple health care centers can illustrate the hidden pattern of colonic adenoma in relation to age and geography. Addition of Caucasians as a control group might help to clearly define the disparity in terms of disease process in young African Americans and Hispanic Americans.
In conclusion, we found that AA are more likely to have prevalent colorectal neoplasia at younger ages when compared to HA. Earlier CRC screening as suggested by the American College of Gastroenterology (ACG) may represent an important component of the interventions to reduce CRC disparities.
Acknowledgments
This project has been funded in whole or in part with Federal funds National Institutes of Health, through RCMI.
References
- 1.Jemal A, et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, Featuring the Burden and Trends in Human Papillomavirus (HPV)-Associated Cancers and HPV Vaccination Coverage Levels. J Natl Cancer Inst. 2013;105(3):175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laiyemo AO, et al. Short- and long-term risk of colorectal adenoma recurrence among whites and blacks. Gastrointest Endosc. 2013;77(3):447–54. doi: 10.1016/j.gie.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laiyemo AO, et al. Race and colorectal cancer disparities: health-care utilization vs different cancer susceptibilities. J Natl Cancer Inst. 2010;102(8):538–46. doi: 10.1093/jnci/djq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–41. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 5.Alexander DD, et al. African-American and Caucasian disparities in colorectal cancer mortality and survival by data source: an epidemiologic review. Cancer Biomark. 2007;3(6):301–13. doi: 10.3233/cbm-2007-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wudel LJ, Jr, et al. Disparate outcomes in patients with colorectal cancer: effect of race on long-term survival. Arch Surg. 2002;137(5):550–4. doi: 10.1001/archsurg.137.5.550. discussion 554–6. [DOI] [PubMed] [Google Scholar]
- 7.Hashiguchi Y, et al. Impact of race/ethnicity on prognosis in patients who underwent surgery for colon cancer: analysis for white, African, and East Asian Americans. Ann Surg Oncol. 2012;19(5):1517–28. doi: 10.1245/s10434-011-2113-5. [DOI] [PubMed] [Google Scholar]
- 8.Rex DK, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104(3):739–50. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 9.Lebwohl B, et al. Risk of colorectal adenomas and advanced neoplasia in Hispanic, black and white patients undergoing screening colonoscopy. Aliment Pharmacol Ther. 2012;35(12):1467–73. doi: 10.1111/j.1365-2036.2012.05119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jafri NS, et al. Incidence and survival of colorectal cancer among Hispanics in the United States: a population-based study. Dig Dis Sci. 2013;58(7):2052–60. doi: 10.1007/s10620-012-2454-3. [DOI] [PubMed] [Google Scholar]
- 11.US Census Bureau Projections Show a Slower Growing, Older, More Diverse Nation a Half Century from Now. 2012 https://www.census.gov/newsroom/releases/archives/population/cb12-243.html.
- 12.Jemal A, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen SP, et al. Gender as a risk factor for advanced neoplasia and colorectal cancer: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7(6):676–81. e1–3. doi: 10.1016/j.cgh.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Lieberman DA, et al. Prevalence of polyps greater than 9 mm in a consortium of diverse clinical practice settings in the United States. Clin Gastroenterol Hepatol. 2005;3(8):798–805. doi: 10.1016/s1542-3565(05)00405-2. [DOI] [PubMed] [Google Scholar]
- 15.Lieberman DA, et al. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343(3):162–8. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman D. Progress and challenges in colorectal cancer screening and surveillance. Gastroenterology. 2010;138(6):2115–26. doi: 10.1053/j.gastro.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 18.Avital I, et al. Evidence-based Guidelines for Precision Risk Stratification-Based Screening (PRSBS) for Colorectal Cancer: Lessons learned from the US Armed Forces: Consensus and Future Directions. J Cancer. 2013;4(3):172–92. doi: 10.7150/jca.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]


