Abstract
The first record of millipedes (Diplopoda) being regularly used for food by humans (the Bobo people of Burkina Faso) is given, including information on how the millipedes are prepared. The species in question are Tymbodesmus falcatus (Karsch, 1881) and Sphenodesmus sheribongensis (Schiøtz, 1966) (Gomphodesmidae) and an unidentified species of Spirostreptidae. New information on the nutritional value of millipedes is provided; unsaturated fatty acids, calcium, and iron contents are particularly high. The millipedes' defensive secretions, hydrogen cyanide and benzoquinones, present a severe challenge for the spread of millipedes as an everyday food source. On the other hand, the possibility that benzoquinones may act as insect-repellents, as known from studies on nonhuman primates, and that sublethal cyanide ingestion may enhance human innate resistance to malaria, suggests promising ethnomedical perspectives to our findings.
1. Introduction
Small vertebrates and invertebrates, especially insects, the so called minilivestock, have been considered a promising resource for Earth's human population that will reach 9 billion humans in 2050 [1–3] and are potential candidates for reducing the higher and increasing impact on resources represented by larger livestock and inland fish production [4]. Information on traditional local use of these small animals is an important starting point for studying minilivestock as potential food resources for humans [5–8]. In addition, local use of invertebrates may have unexpected ethnomedical implications.
Millipedes (Diplopoda) have so far not been in focus as minilivestock. Indeed, most orders of millipedes (Glomerida, Polyzoniida, Siphonocryptida, Platydesmida, Siphonophorida, Callipodida, Julida, Spirobolida, Spirostreptida, and Polydesmida) are known for their chemical defenses and, unlike their relatives, the centipedes (Chilopoda), which in several cultures (China, Alto Orinoco in Venezuela, and Korea) have been used as medical remedies and/or food items [9, 10], no information on millipedes as human food has been available until now. A wide spectrum of chemicals has been identified from millipede defensive secretions [11], the most widespread ones being benzoquinones (in most cylindrical millipedes, superorder Juliformia) and hydrogen cyanide derived from mandelonitrile and related compounds (in most flatbacked millipedes, superorder Merocheta). These toxic, smelly chemicals make millipedes unattractive for most predators, although there are some animals, vertebrates as well as invertebrates, which eat millipedes [12, 13], and some are even specialized on a millipede diet, for example, assassin bugs (family Reduviidae) of the subfamily Ectrichodiinae [14] and beetle larvae of the family Phengodidae [15]. A few vertebrates are reported to eat millipedes as for instance banded mongoose (Mungos mungo) [16]. Some birds and nonhominid primates use toxic millipedes for “self-anointment,” presumably exploiting an insect-repellent effect of the millipedes' defensive chemicals, especially benzoquinones [17–20]. We have, however, not been able to trace any record of millipedes being used as food in any human society, but now we can report the consumption of millipedes by the Bobo population of Burkina Faso, a region where entomophagy has been extensively described [21].
The millipedes which are used as human food by the Bobo belong to two families: Gomphodesmidae and Spirostreptidae.
Gomphodesmidae (flatbacked millipedes of the order Polydesmida) (Figures 1 and 2) belong to the “cyanogenic” millipedes; the family is endemic to the African continent south of the Sahara, includes 146 named species [22], and was monographed by Hoffman (2005) [23]. The only gomphodesmid species previously recorded from Burkina Faso is Tymbodesmus falcatus (Karsch, 1881), collected in Ouagadougou [23]. One of the gomphodesmid species used for food at Kou village in Burkina Faso is indeed T. falcatus (D. Vandenspiegel det.), the other is Sphenodesmus sheribongensis (Schiøtz, 1966) (HE det.). T. falcatus is known from Mali, Burkina Faso, Nigeria, Sudan, and Central African Republic; S. sheribongensis was previously known from Ghana, Ivory Coast, and Nigeria [23]. Lewis [24] studied the life history and ecology of both species in Zaria, Northern Nigeria. Both have a two-year life cycle. Juvenile stadia of S. sheribongensis live entirely in the soil, whereas adults can be extremely abundant on the soil surface during the first part of the rainy season (from May to July). T. falcatus is similar, except that the last juvenile (subadult) stadium is seasonally surface active like the adults, albeit in smaller numbers.
Figure 1.

Live gomphodesmid millipede (Tymbodesmus falcatus) from Kou. S. Tchibozo phot., 2011.
Figure 2.

gomphodesmid millipede from Kou after boiling. S. Tchibozo phot., 2012.
Spirostreptidae (cylindrical millipedes of the order Spirostreptida) (Figure 3) belong to the “quinone” millipedes: the family is near-endemic to the Afrotropical and Neotropical regions, includes 275 named species [22], and was monographed by Krabbe [25]. No species of Spirostreptidae have been recorded from Burkina Faso, and the one occurring at Kou has not yet been identified.
Figure 3.

Spirostreptid millipede from Kou. S. Tchibozo phot., 2012.
We do not have any strong evidence that T. falcatus and S. sheribongensis actually produce hydrogen cyanide, nor that the spirostreptid in question produces quinones. The general occurrence of these substances in the respective higher taxa to which the species belong, however, is strong circumstantial evidence that this is actually so, although recent studies have demonstrated a larger diversity in millipede defensive chemicals than previously assumed [26–28].
Until this study, the nutritional value of millipedes has never been assessed. Considering that the muscle volume of millipede is small, they will constitute a poor source of protein. Their guts mainly contain soil and litter remains [24], but their calcified exoskeleton might constitute a considerable source of calcium, which may constitute 13–17% of the dry weight [29–31] or 9% of the fresh weight [32], In fact, millipedes are considered an essential source of calcium for egg production in certain birds [30, 33, 34]. In this work we present data based on Tymbodesmus falcatus, one of the species eaten by the Bobo people.
2. Material and Methods
2.1. Sampling and Ethnobiological Data Collection
Observations and interviews with the Bobo people were made by ST in 2011 and 2012 in Burkina Faso (Kou, 11°10.88′ N, 004°26.62′ W, altitude 351 m, near Bobo-Dioulasso). Exemplars of the edible millipedes were collected and are now preserved in Musée Royale de l'Afrique Centrale (Tervuren, Belgium) and the Natural History Museum of Denmark (Copenhagen). Five Bobo people, especially women, were interviewed for information on collection and preparation of the edible millipedes.
2.2. Nutritional Analysis
In order to assess the nutritional value of this unconventional food we determined various nutritional parameters of a whole Tymbodesmus falcatus male specimen. The raw specimen was preserved in 70% ethanol, subsequently dried and homogenized by cryomilling (SPEX Freezer/Mill 6770). The resulting powder was used for analysis of chitin, fatty acids, amino acids, and metal content. No appropriately preserved specimens of the other species in question were available. Dry weight of millipedes was measured on oven-dried specimens and kept in alcohol for 45 years (collected in Nigeria by J.G.E. Lewis).
2.2.1. Pyrolysis-GC/MS for Fatty Acid Analysis
100 μg of the sample was placed in a quartz tube, and 4.5 μL of a diluted, aqueous solution of tetramethylammonium hydroxide was added. The samples were subsequently pyrolyzed at 450°C for 10 s with a CDS 5250 pyrolysis autosampler attached to a Thermo Trace GC Ultra/MD 800 gas chromatography/mass spectrometry system. Volatile products were separated on a Supelco SP 2330 column (30 m, ID 0.32 mm, 0.2 μm film thickness) with helium 4.6 as carrier gas (2 mL·min−1) and identified by comparison to reference compounds as well as interpretation of their EI mass spectra and comparison to NIST 2002, Wiley, and NBS electronic libraries. The pyrolysis interface was kept at 300°C and the GC/MS interface at 280°C; the GC was programmed from 100°C (1 min) to 230°C (5 min) at a rate of 10°C min−1. The mass spectrometer was operated in EI mode (70 eV) at a source temperature of 200°C [35].
2.2.2. Solid State NMR for Chitin
Chitin was determined by Solid State NMR; all spectra were recorded on a narrow-bore 11.7 T instrument (500 MHz, 1 H Larmor frequency) at magic angle spinning rates of 10.0 kHz at 300 K. 13C chemical shifts are given in reference to tetramethylsilane TMS, using the sharp resonance of TMS as external calibration. A basic cross polarization experiment with total suppression of sidebands with a cross-polarization contact time of 2 ms was employed, with an effective acquisition time of 27.9 ms and a recycling delay of 5 s. The magic angle was adjusted using the 79Br resonance of KBr, and the actual sample temperature was determined using the 207Pb resonance of Pb(NO3)2 for calibration [36].
2.2.3. Amino Acid Analysis
The powder obtained after cryomilling was hydrolysed by refluxing with hydrochloric acid containing 5% phenol for 24 hours under exclusion of oxygen. The complete sample was then evaporated to dryness, redissolved in water, and analyzed with HPLC/MS using 0.5 mL·min−1 of a water acetonitrile gradient (100% water for 2 minutes and in 17 minutes to 30% acetonitrile which is held for a further 3 minutes) on a Waters AccQ Tag column (3.9 × 150 mm). Quantification was done using extracted ion chromatograms. With this procedure arginine, cysteine, and histidine could not be analyzed and leucine/isoleucine as well as glutamine/lysine could not be separated; therefore, the concentration of those amino acids is given as a sum.
2.2.4. Metal Analysis
Metal content was analysed with inductively coupled plasma optical emission spectrometry (ICP-OES) according to EN ISO 11885 from a commercial laboratory.
3. Results
3.1. Collection and Preparation of Millipedes at Kou Village
According to the interviewed villagers in Kou, millipedes are collected under bricks around houses made of straw and under decomposing wood. Once collected, the millipedes are placed in a pot with water filtered through firewood ashes, for 3–5 minutes until boiling. Then they are removed and left to dry on a roof for 3 days. Such preparation is specific for millipedes and different from those described for other arthropods in West Africa, especially for insect maggots and weevils, which are mainly roasted and fried [21]. The dried millipedes are placed in a tomato sauce to which is added the traditional African mustard known as soumbala (fermented seeds of the néré tree, Parkia biglobosa, very widely consumed in Burkina Faso and West Africa in general), shea butter oil, and tô (a paste made from maize or sorghum flour). For some meals, the millipedes replace meat.
3.2. Nutritional Values of Tymbodesmus falcatus
Proteins represent 25% of total dry weight (calculated as the sum of amino acids); the amino acid profile (Table 1) is similar to that of insects and crustaceans, for example, crickets and shrimps [37]. Unsaturated fatty acids constitute a relevant fraction (40%) of total fatty acids (Table 2, Figure 4), however lower than those described for widely consumed and appreciated edible insects [38]. Calcium levels (Table 3) are very high (17.4% of dry weight) which is higher than previously published values [29–32].
Table 1.
Tymbodesmus falcatus amino acid analysis (based on dry matter).
| Amino acid | mg/mg |
|---|---|
| Alanin | 0.0676 |
| Asparagin | 0.0137 |
| Asparaginic acid | 0.0164 |
| Glutamin + lysin | 0.0217 |
| Glutamic acid | 0.0000 |
| Glycin | 0.0000 |
| Isoleucin + leucin | 0.0283 |
| Methionin | 0.0056 |
| Phenylalanin | 0.0132 |
| Prolin | 0.0062 |
| Serin | 0.0142 |
| Threonin | 0.0251 |
| Tryptophan | 0.0053 |
| Tyrosin | 0.0151 |
| Valin | 0.0193 |
|
| |
| Total | 0.2518 |
Table 2.
Fatty acid distribution as determined by pyrolysis-GC/MS analysis. Unsaturated fatty acids constitute 40% of total fatty acids of Tymbodesmus falcatus.
| No. | ID | Name | t R | Percent |
|---|---|---|---|---|
| 1 | 10:0 | Caprylic acid | 3 | 0.3 |
| 2 | 12:0 | Lauric acid | 4.33 | 0.5 |
| 3 | 14:0 | Myristic acid | 5.82 | 3.1 |
| 4 | 15:0 | Pentadecanoic acid | 6.56 | 1.0 |
| 5 | 16:0 | Palmitic acid | 7.37 | 43.1 |
| 6 | 16:1 n9 | Sapienic acid | 7.61 | 0.2 |
| 7 | 16:1 n7 | Palmitoleic acid | 7.67 | 1.5 |
| 8 | 17:0 | Margaric acid | 8.01 | 0.8 |
| 9 | 18:0 | Stearic acid | 8.73 | 7.8 |
| 10 | 18:1 n9 | Oleic acid | 9.06 | 36.8 |
| 11 | 18:1 n7 | Vaccenic acid | 0.0 | |
| 12 | 18:2 n6 | Linoleic acid | 9.5 | 1.6 |
| 13 | 18:3 n6 | Gamma linolenic acid | 0.0 | |
| 14 | 20:0 | Arachidic acid | 10 | 1.1 |
| 15 | 18:3 n3 | Alpha linolenic acid | 10.08 | 0.1 |
| 16 | 18:2 x | Octadecenoic acid | 10.24 | 0.5 |
| 17 | 20:1 n9 | Gadoleic acid | 0.0 | |
| 18 | 18:2 x | Octadecenoic acid | 10.34 | 0.5 |
| 19 | 18:2 x | Octadecenoic acid | 10.55 | 0.3 |
| 20 | 20:2 | Eicosadienoic acid | 0.0 | |
| 21 | 20:3 | Eicosatrienoic acid | 0.0 | |
| 22 | 22:0 | Behenic acid | 11.2 | 0.1 |
| 23 | 20:4 n6 | Arachidonic acid | 0.0 | |
| 24 | 21:0 | Heneicosylic acid | 11.77 | 0.1 |
| 25 | 20:5 n3 | Timnodonic acid | 0.0 | |
| 26 | 24:0 | Lignoceric acid | 12.33 | 0.4 |
| 27 | 22:5 n6 | Docosapentaenoic acid | 0.0 | |
| 28 | 22:6 n3 | Docosahexaenoic acid | 0.0 | |
|
| ||||
| Total | 100.0 | |||
Figure 4.
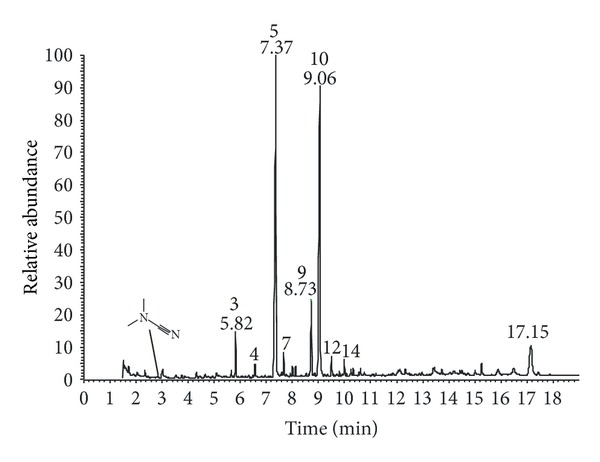
THM-GC/MS profile of Tymbodesmus falcatus; retention times (t R) refer to compounds listed in Table 2: palmitic acid 18 : 1 n9 (t R: 7.37) (5) and oleic acid 16 : 0 (t R: 9.06) (10) constitute 80% of total fatty acids.
Table 3.
Tymbodesmus falcatus metal contents (based on dry matter) and DRIs for a pregnant women of 19–30 yrs, by IOM 2004 [39].
| Metal | mg/kg | DRI (mg/day) |
|---|---|---|
| Pb | 6.2 | — |
| Cd | <0.5 | — |
| Ca | 174,000 | 1,000 |
| Fe | 10,600 | 27 |
| K | 2,610 | 4,700 |
| Cu | 789 | 1 |
| Mg | 4,990 | 350 |
| Na | 1,630 | 1,500 |
| Zn | 160 | 11 |
The dry weight of individual Tymbodesmus falcatus was 0.42–0.54 g (mean 0.46, n = 3) and of the smaller Sphenodesmus sheribongensis, 0.08–0.11 g (mean 0.09, n = 4). Spirostreptidae vary very much in size; live weights of up to 80 g have been measured (HE unpublished). Considering that the Ca content of a single Tymbosdesmus f. is about 80–90 mg (174 mg/g × 0.46 g), consumption of around 12-13 gomphodesmid individuals will provide 1000 mg/day, (the Dietary Reference Intake (DRI), by IOM 2004 [39]). Also iron content (100,600 mg/kg) is important considering that only 6 individuals provide the adequate amount for a women during pregnancy (DRI: 27 mg/day) and only 2-3 for men (DRI: 8 mg/day) [39].
Chitin constitutes around 5% of the total dry weight, a low amount compared to Ca, but it is only located in the exoskeleton (incl. legs).
A trace level of dimethylcyanamide revealed in the GC/MS (see Figure 4) is the only direct evidence of cyanogenic compounds in our sample.
4. Discussion
The gomphodesmid millipedes utilized by the Bobo people are quite small (max. 5 cm long), but gomphodesmids may occur in very large numbers at certain times of the year. Thus, Lewis [24] stated that 50 specimens of S. sheribongensis could be collected in 10–15 minutes at the beginning of the rainy season in Zaria, Nigeria and even (pers. comm.) that it was sometimes impossible to take a step without crushing several individuals. Barbetta et al. [40] used 1200 specimens of Haplogomphodesmus pavani (Demange, 1965) for their biochemical study, an indication that also this species can be quite abundant. Spirostreptids may occasionally form huge swarms, for example, in Ghana and Zimbabwe [41]. They are sometimes of considerable size, up to 30 cm long. Considering the regular and high levels of some essential nutrients (Ca, Fe, PUFA) and seasonal abundance of millipedes, they likely represent a significant type of minilivestock for the Bobo people. The ethnobiological/ethnopharmacological uses of many unconventional species, that is, earthworms and insects, are documented in several cultures, being part of a complex system of specific traditional knowledge adapted to local resource availability and to very variable hygienic standards [42, 43] that may not fit with the food-quality/food-safety standards imposed in industrialized countries [44].
As mentioned above, gomphodesmids belong to the “cyanogenic” millipedes [11]. Cyanogenesis in millipedes and other arthropods has not been studied with state-of-art methodology [41], but the presence of hydrogene cyanide and its precursors has been demonstrated in many species of the order Polydesmida [11, 26, 27, 45, 46], including two species of Gomphodesmidae; HCN has been identified in the secretion of Astrodesmus laxus (Gerstäcker 1873) from East Africa [47], and its precursor, mandelonitrile, in Haplogomphodesmus pavani [40]. Although there is no direct information on the secretions of T. falcatus and S. sheribongensis, there is no reason to believe that they have lost the cyanogenic function. They have the same complement of defense glands as the majority of gomphodesmids, including A. laxus and H. pavanii, that is, 11 pairs of glands opening along the sides of the body [23].
Benzoquinones have been detected in several spirostreptid species [11, 28, 48]. In Figure 3 the defensive glands can be seen as a series of darker spots, one on each diplosegment except the very first and last ones.
Some birds and mammals (see Table 4) have been described to use “quinone” millipedes for self-anointing in order to control ectoparasites and mosquito biting rate, and some mammals like opossum, coatis, skunk, mongoose, and lemurs are known to consume toxic millipedes—mainly Spirostreptidae—after different treatments such as prey-rolling, handling, and salivating [13, 49–52]. The complex behaviours that precede ingestion require a considerable investment of time and energy and are evidently necessary, maybe in order to reduce toxicity of the millipedes to be eaten [53, 54]. The use of benzoquinonigenic plants for self-anointing in orangutans [54] and owl monkeys [55] supports the hypothesis that benzoquinones are used by primates for their specific biochemical defensive properties. Contrarily, use and consumption of gomphodesmid cyanogenic millipedes seems to be rare even in most omnivorous mammals, except opossum [56].
Table 4.
Summary of the current knowledge about utilization of millipedes by mammals as anointing and/or food item.
| Mammals | Millipedes | Chemicals | Use | Reference |
|---|---|---|---|---|
| Marsupialia | ||||
| Opossum (Didelphis albiventris) |
Leptodesmus dentellus (Chelodesmidae) Gymnostreptus olivaceus (Spirostreptidae) |
Benzoquinones and Cyanogenics | Consumption and sniffing | [47] |
|
| ||||
| Carnivora | ||||
| White-nosed coatis (Nasua narica) | Orthoporus sp. (Spirostreptidae) | Benzoquinones | Consumption after prey-rolling treatment | [13] |
| Meerkat-mangoose (Suricata suricatta) | ? | ? | Consumption after treatment | [49, 51] |
| Striped skunk (Mephitis mephitis) | ? | ? | Consumption | [52] |
|
| ||||
| Primates | ||||
| Capuchin monkeys (Cebus sp.) | Orthoporus dorsovittatus (Spirostreptidae) | Benzoquinones | Self-anointing | [19] |
| Capuchin monkeys (C. olivaceus) | Orthoporus dorsovittatus (Spirostreptidae) | Benzoquinones | Self-anointing | [18] |
| Owl monkeys (Aotus sp.) | Anadenobolus monilicornis (Rhinocricidae) | Benzoquinones | Self-anointing | [55] |
| Black lemurs (Elemur macaco) | Charactopygus sp. (Spirostreptidae) | Benzoquinones ? | Self-anointing | [17] |
| Lemurs (Varecia rubra and Eulemur fulvus albifrons) | ? | ? | Self-anointing and consumption after handling and salivating | [50, 53, 72] |
| Humans, Bobo population |
Tymbodesmus falcatus and Sphenodesmus sheribongensis (Gomphodesmidae) unknown Spirostreptidae |
Benzoquinones and Cyanogenics | Consumption after boiling and drying | This study |
4.1. Cyanide in Traditional Foods
The use of millipedes as human food is absolutely exceptional, but the use of food items containing significant levels of cyanide is widespread [26]. Over 2000 plant species contain cyanide as a defense against insects and other herbivores [26] and the most important cyanogenic crop is cassava (Manihot esculenta, Crantz), a staple food of hundreds of millions of humans in the tropics [57, 58].
In the Amazons—the centre of domestication of cassava—the more toxic bitter varieties named yuca amarga are the most intensively cultivated because of their resistance to pest insects and rodents. However, cassava domestication largely preceded malaria ingression in the New World [59] and the indigenous preparation is aimed at avoiding the cyanogenic compounds, consisting of specific postharvest operations: grinding, squeezing, toasting, and fermentation [58, 60]. Boiling alone is not enough to avoid the toxicity of yuca amarga [58], and only the sweet varieties of yuca dulce, which are low in glycosides, can be consumed safely in soups or fried (MGP personal observation in Alto Orinoco, Venezuela).
The Bobo people subboil the millipedes, as part of the preparation for meals. This treatment may degrade the cyanogenic compounds by releasing the hydrogen cyanide gas, thereby detoxifying the gomphodesmids.
Nonetheless, the short (3–5 minutes) subboiling and natural drying appear to be a specific treatment for millipedes, maybe aimed at preserving part of their chemicals. Moreover, benzoquinones are not readily soluble in water and the most characteristic juliform benzoquinone, toluquinone, is insoluble in water [61]; thus substantial amounts of them may remain in the millipede bodies even after cooking.
4.2. Biocultural Perspective
African populations consume raw and subboiled bitter cassava [58, 60, 62, 63], as well as many other bitter food items that would not be tolerated by other populations. It has been proposed that the reduced sensitivity to bitterness is an ancient adaptation (70 kY BP) [64] of the human bitter-taste specific receptor to bitter antimalarial compounds (e.g., flavonoids), which are still abundant in the West African diet [62, 65]. Cassava contains the cyanogenic glucoside compounds linamarin and lotaustralin that, once ingested, are metabolized to thiocyanate and cyanate. Notably, these metabolites are biologically active although less toxic than cyanide and, at levels of expected dietary intake, cyanide-related compounds (e.g., cyanate) are able to modify essential proteins of Plasmodium falciparum and inhibit parasite survival [66].
Considering that malaria represents the main cause of mortality in the adult sub-Saharan population and that Burkina Faso is endemic for several Plasmodium species [67, 68], biocultural adaptations aimed at controlling this pathogen are strongly expected. Natural benzoquinones are known to exert an antiplasmodic activity in vitro [69], and their metabolites might act as systemic repellents against mosquitoes or as antimalarial prophylaxis in the Bobos. Moreover, studies on West African populations have demonstrated strong links between malaria, sub-lethal cyanide intake from bitter cassava, and sickle-cell anemia [60, 70], a genetic pathology affecting erythrocytes that confers protection against Plasmodium. Thus, after demonstrating that cyanide interacts with hemoglobins, partially compensating sickle-cell dysfunctionality [60, 71], some authors proposed that the abundant consumption of bitter foods could enhance biological fitness in West African populations exposed to malaria [62, 70]. Therefore, both ethological and bioanthropological evidences suggest that “toxic” millipedes consumed by the Bobo people take part in a complex biocultural mechanism for malaria control.
5. Concluding Remarks
Key topics of this paper are summarized in Figure 5. Whether millipedes will ever become a major actor in minilivestock husbandry may be dubious, but the Bobo people have shown that they constitute a helpful food source for an ever-growing human population, especially in rural Africa. In addition, the potential of millipede chemicals for deterring mosquitoes and for influencing Plasmodium and other parasites constitutes a promising field of research.
Figure 5.

Scheme of the exceptional use of toxic millipedes: as demonstrated for capuchin monkeys, that during the rainy season use millipedes' secretions against mosquitoes, Bobo people in Burkina Faso may consume millipedes for their antiparasite effect.
Acknowledgments
The authors are grateful to the Bobo people at Kou for sharing their knowledge with them, to The Fonds Francophone des Inforoutes (FFI) and the Coopération Belge au Développement for funding the 2011 expedition in Burkina Faso, to Bakary Sanou for help with field work, to Didier VandenSpiegel for identification of T. falcatus, and to Mika Zagrobelny for her attempt—against all odds—to isolate cyanogenic compounds from alcohol-preserved millipede specimens. The NMR spectrometers were acquired in collaboration with the University of South Bohemia (CZ) with financial support from the European Union through the EFRE INTERREG IV ETC-AT-CZ programme (Project M00146, “RERI-uasb"). Several people helped with discussion and suggestions, in particular, Natalie Vasey, Jean Zida, Robert O. Malley, William C. McGrew, Linda C. Jackson, Marco Pombi, Salvatore Musumeci, and Paul Weldon.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.FAO. Forest insects as food: humans bite back. In: Durst PB, Johnson DV, Leslie RN, Shono K, editors. Proceedings of a Workshop on Asia-Pacific Resources and their Potential for Development; February 2008; Thailand Chiang Mai; http://www.fao.org/docrep/012/i1380e/i1380e00.pdf. [Google Scholar]
- 2.Turner NJ, Luczaj LJ, Migliorini P, et al. Edible and tended wild plants, traditional ecological knowledge and agroecology. Critical Reviews in Plant Sciences. 2011;30(1-2):198–225. [Google Scholar]
- 3.Rumpold BA, Schlüter OK. Potential and challenges of insects as an innovative source for food and feed production. Innovative Food Science and Emerging Technolologies. 2013;17:1–11. [Google Scholar]
- 4.Paoletti MG, Gomiero T, Pimentel D. Introduction to the special issue: towards a more sustainable agriculture. Critical Reviews in Plant Sciences. 2011;30(1-2):2–5. [Google Scholar]
- 5.Paoletti MG, Bukkens S. Minilivestock: sustainable use of biodiversity for human food. Ecology of Food and Nutrition. 1997;36(2–4):95–346. [Google Scholar]
- 6.Paoletti MG, editor. Ecological Implications of Minilivestock: Potential of Insects, Rodents, Frogs and Sails. Enfield, NH, USA: Science; 2005. [Google Scholar]
- 7.van Huis A. Insects eaten in Africa (Coleoptera, Hymenoptera, Diptera, Heteroptera, Homoptera) In: Paoletti MG, editor. Ecological Implications of Minilivestock. Enfield, NH, USA: Science; 2005. pp. 231–244. [Google Scholar]
- 8.Oonincx DGAB, van Itterbeeck J, Heetkamp MJW, van den Brand H, van Loon JJA, van Huis A. An exploration on greenhouse gas and ammonia production by insect species suitable for animal or human consumption. PLoS ONE. 2010;5(12) doi: 10.1371/journal.pone.0014445.e14445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pemberton RW. Contemporary use of insects and other arthropods in traditional Korean medicine (Hanbang) in South Korea and elsewhere. In: Paoletti MG, editor. Ecological Implications of Minilivestock. Enfield, NH, USA: Science; 2005. pp. 459–474. [Google Scholar]
- 10.Paoletti MG, Dufour DL. Edible invertebrates among Amazonian Indians: a critical review of disappearing knowledge. In: Paoletti MG, editor. Ecological Implications of Minilivestock. Enfield, NH, USA: Science; 2005. pp. 293–342. [Google Scholar]
- 11.Eisner D T, Alsop, Hicks K, Meinwald J. Defensive secretions of millipedes. In: Bettini S, editor. Arthropod Venoms. Vol. 48. Berlin, Germany: Springer; 1978. pp. 41–72. (Handbuch der Experimentellen Pharmakologie). [Google Scholar]
- 12.Hopkin SP, Read HJ. The Biology of Millipedes. Oxford Science; 1992. [Google Scholar]
- 13.Weldon PJ, Cranmore CF, Chatfield JA. Prey-rolling behavior of coatis (Nasua spp.) is elicited by benzoquinones from millipedes. Naturwissenschaften. 2006;93(1):14–16. doi: 10.1007/s00114-005-0064-z. [DOI] [PubMed] [Google Scholar]
- 14.Forthman M, Weirauch C. Toxic associations: a review of the predatory behaviors of millipede assassin bugs (Hemiptera: Reduviidae: Ectrichodiinae) European Journal of Entomology. 2012;109:147–153. [Google Scholar]
- 15.Eisner T, Eisner M, Attygalle AB, Deyrup M, Meinwald J. Rendering the inedible edible: circumvention of a millipede’s chemical defense by a predaceous beetle larva (Phengodidae) Proceedings of the National Academy of Sciences of the United States of America. 1998;95(3):1108–1113. doi: 10.1073/pnas.95.3.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rood JP. Population dynamics and food habits of the banded mongoose. East Africa Wildlife Journal. 1975;13(2):89–111. [Google Scholar]
- 17.Birkinshaw CR. Use of millipedes by black lemurs to anoint their bodies. Folia Primatologica. 1999;70(3):170–171. doi: 10.1159/000021691. [DOI] [PubMed] [Google Scholar]
- 18.Valderrama X, Robinson JG, Attygalle AB, Eisner T. Seasonal anointment with millipedes in a wild primate: a chemical defense against insects? Journal of Chemical Ecology. 2000;26(12):2781–2790. [Google Scholar]
- 19.Weldon PJ, Aldrich JR, Klun JA, Oliver JE, Debboun M. Benzoquinones from millipedes deter mosquitoes and elicit self-anointing in capuchin monkeys (Cebus spp.) Naturwissenschaften. 2003;90(7):301–304. doi: 10.1007/s00114-003-0427-2. [DOI] [PubMed] [Google Scholar]
- 20.Sazima I. Anting behaviour with millipedes by the dendrocolaptid bird Xiphocolaptes albicollis in Southeastern Brazil. Biota Neotropica. 2009;9(1):249–252. [Google Scholar]
- 21.Malaisse F. Human consumption of Lepidoptera, termites, orthoptera, and ants in Africa. In: Paoletti MG, editor. Ecological Implications of Minilivestock. Enfield, NH, USA: Science; 2005. pp. 175–230. [Google Scholar]
- 22.Shear WA. Class diplopoda de blainville in gervais 1844. In: Zhang -Q Z, editor. Animal Biodiversity: An Outline of Higher-Level Classification and Survey of Taxonomic Richness. Vol. 3148. 2011. pp. 159–164. (Zootaxa). [DOI] [PubMed] [Google Scholar]
- 23.Hoffman RL. Monograph of the Gomphodesmidae, A family of African Polydesmoid Millipedes. Vienna, Austria: Verlag des Naturhistorischen Museums Wien; 2005. [Google Scholar]
- 24.Lewis JGE. The life history and ecology of the millipede Tymbodesmus falcatus (Polydesmida: Gomphodesmida) in Northern Nigeria with notes on Sphenodesmus sheribongensis . Journal of Zoology. 1971;164:551–563. [Google Scholar]
- 25.Krabbe E. (Abhandlungen des Naturwissenschaftlichen Vereins in Hamburg (NF)).Systematik der Spirostreptidae. 1982;24 [Google Scholar]
- 26.Zagrobelny M, Bak S, Møller BL. Cyanogenesis in plants and arthropods. Phytochemistry. 2008;69(7):1457–1468. doi: 10.1016/j.phytochem.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 27.Shear WA, Jones TH, Miras HM. A possible phylogenetic signal in milliped chemical defenses: the polydesmidan milliped Leonardesmus injucundus Shelley & Shear secretes p-cresol and lacks a cyanogenic defense (Diplopoda, Polydesmida, Nearctodesmidae) Biochemical Systematics and Ecology. 2007;35(12):838–842. [Google Scholar]
- 28.Smolanoff J, Demange JM, Meinwald J, Eisner T. 1,4-Benzoquinones in African millipeds. Psyche. 1975;82(1):78–80. [Google Scholar]
- 29.Reichle DE, Shaks MH, Crossley DA. Calcium, potassium, and sodium content of forest floor arthropods. Annals of the Entomological Society of America. 1969;62(1):57–62. [Google Scholar]
- 30.Gist CS, Crossley DA., Jr. The litter arthropod community in a Southern Appalachian Hardwood Forest: numbers, biomass and mineral element content. The American Midland Naturalist Journal. 1975;93(1):107–122. [Google Scholar]
- 31.Graveland J, van Gijzen T. Arthropods and seeds are not sufficient as calcium sources for shell formation and skeletal growth in passerines. Ardea. 1994;82(2):299–314. [Google Scholar]
- 32.Nakamura K, Taira J. Distribution of elements in the millipede, Oxidus gracilis C. L. Koch (Polydesmida: Paradoxosomatidae) and the relation to environmental habitats. Biometals. 2005;18(6):651–658. doi: 10.1007/s10534-005-4575-z. [DOI] [PubMed] [Google Scholar]
- 33.Bureš S, Weidinger K. Sources and timing of calcium intake during reproduction in flycatchers. Oecologia. 2003;137(4):634–641. doi: 10.1007/s00442-003-1380-7. [DOI] [PubMed] [Google Scholar]
- 34.Hames RS, Lowe JD, Swarthout SB, Rosenberg KV. Understanding the risk to neotropical migrant bird species of multiple human-caused stressors: elucidating processes behind the patterns. Ecology and Society. 2006;11(1, article 24) [Google Scholar]
- 35.Hood-Nowotny R, Schwarzinger B, Schwarzinger C, et al. An analysis of diet quality, how it controls fatty acid profiles, isotope signatures and stoichiometry in the malaria mosquito Anopheles arabiensis . PLoS ONE. 2012;7(10) doi: 10.1371/journal.pone.0045222.e45222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kramer KJ, Hopkins TL, Schaefer J. Applications of solids NMR to the analysis of insect sclerotized structures. Insect Biochemistry and Molecular Biology. 1995;25(10):1067–1080. [Google Scholar]
- 37.Collavo A, Glew RH, Yunk-Sheng H, et al. House cricket small scale farming. In: Paoletti MG, editor. Ecological Implications of Minilivestock. Enfield: Science; 2005. pp. 519–544. [Google Scholar]
- 38.Fontaneto D, Tommaseo-Ponzetta M, Galli C, Risé P, Glew RH, Paoletti MG. Differences in fatty acid composition between aquatic and terrestrial insects used as food in human nutrition. Ecology of Food and Nutrition. 2011;50(4):351–367. doi: 10.1080/03670244.2011.586316. [DOI] [PubMed] [Google Scholar]
- 39.IOM. Recommended Intakes for Individuals, Elements. USA: Food and Nutrition Board. Institute of Medicine, National Academies; 2004. http://www.iom.edu/Global/News%20Announcements/~/media/Files/Activity%20Files/Nutrition/DRIs/DRI_Summary_Listing.pdf. [Google Scholar]
- 40.Barbetta M, Casnati G, Pavan M. Sulla presenza di D-(+)mandelonitrile nella secrezione difensiva del miriapode Gomphodesmus pavani Dem. Memorie Della Società Entomologica Italiana. 1966;45:169–176. [Google Scholar]
- 41.Dangerfield JM, Telford SR. Aggregation in the tropical millipede Alloporus uncinatus (Diplopoda: Spirostreptidae) Journal of Zoology. 1993;230(3):503–511. [Google Scholar]
- 42.DeFoliart GR, Dunkel FV, Gracer D. The food insects newsletter: chronicle of changing culture. Aardvark Global. 2009 [Google Scholar]
- 43.Cooper EL, Balamurugan M, Chih-Yang Huang, et al. Earthworms dilong: ancient, inexpensive, noncontroversial models may help clarify approaches to integrated medicine emphasizing neuroimmune systems. Evidence-Based Complementary and Alternative Medicine. 2012;2012:11 pages. doi: 10.1155/2012/164152.164152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belluco S, Losasso C, Maggioletti M, Alonzi CC, Paoletti A Ricci MG. Edible insects in a food safety and nutritional perspective: a critical review. Comprehensive Reviews in Food Science and Food Safety. 2013;12(3):296–313. [Google Scholar]
- 45.Makarov SE, Ćurčić BPM, Tešević VV, et al. Defensive secretions in three species of polydesmids (Diplopoda, Polydesmida, Polydesmidae) Journal of Chemical Ecology. 2010;36(9):978–982. doi: 10.1007/s10886-010-9847-6. [DOI] [PubMed] [Google Scholar]
- 46.Kuwahara Y, Shimizu N, Tanabe T. Release of hydrogen cyanide via a post-secretion Schotten-Baumann reaction in defensive fluids of polydesmoid millipedes. Journal of Chemical Ecology. 2011;37(3):232–238. doi: 10.1007/s10886-011-9920-9. [DOI] [PubMed] [Google Scholar]
- 47.Eisner HE, Wood WF, Eisner T. Hydrogen cyanide production in North American and African polydesmoid millipedes. Psyche. 1975;82(1):20–23. [Google Scholar]
- 48.Deml R, Huth A. Benzoquinones and hydroquinones in defensive secretions of tropical millipedes. Naturwissenschaften. 2000;87(2):80–82. doi: 10.1007/s001140050014. [DOI] [PubMed] [Google Scholar]
- 49.Doolan SP, Macdonald DW. Diet and foraging behaviour of group-living meerkats, Suricata suricatta, in the Southern Kalahari. Journal of Zoology. 1996;239(4):697–716. [Google Scholar]
- 50.Vasey N. Circadian rhythms in diet and habitat use in red ruffed lemurs (Varecia rubra) and white-fronted brown lemurs (Eulemur fulvus albifrons) American Journal of Physical Anthropology. 2004;124(4):353–363. doi: 10.1002/ajpa.10357. [DOI] [PubMed] [Google Scholar]
- 51.Eisner T, Davis JA. Mongoose throwing and smashing millipedes. Science. 1967;155(3762):577–579. doi: 10.1126/science.155.3762.577. [DOI] [PubMed] [Google Scholar]
- 52.Slobodchikoff CN. Experimental studies of tenebrionid beetle predation by skunks. Behaviour. 1978;66:313–322. [Google Scholar]
- 53.Vasey N. Niche separation in Varecia variegata rubra and Eulemur fulvus albifrons: II. Intraspecific patterns. American Journal of Physical Anthropology. 2002;118(2):169–183. doi: 10.1002/ajpa.10054. [DOI] [PubMed] [Google Scholar]
- 54.Morrogh-Bernard HC. Fur-rubbing as a form of self-medication in Pongo pygmaeus . International Journal of Primatology. 2008;29(4):1059–1064. [Google Scholar]
- 55.Zito M, Evans S, Weldon PJ. Owl monkeys (Aotus spp.) self-anoint with plants and millipedes. Folia Primatologica. 2003;74(3):159–161. doi: 10.1159/000070649. [DOI] [PubMed] [Google Scholar]
- 56.Santori RT. Discrimination of millipedes by the opossum Didelphis albiventris (Marsupiala, Didelphidae) Journal of Advanced Zoology. 1998;19(2):118–119. [Google Scholar]
- 57.FAO. Roots, Tubers, Plantains and Bananas in Human Nutrition. Rome, Italy: FAO; 1990. http://www.fao.org/docrep/t0207e/T0207E00.htm#Contents/ [Google Scholar]
- 58.Wilson WM, Dufour DL. Why “bitter” cassava? Productivity of “bitter” and “sweet” cassava in a Tukanoan Indian settlement in the Northwest Amazon. Economic Botany. 2002;56(1):49–57. [Google Scholar]
- 59.Carter R, Mendis KN. Evolutionary and historical aspects of the burden of malaria. Clinical Microbiology Reviews. 2002;15(4):564–594. doi: 10.1128/CMR.15.4.564-594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jackson LC, Oseguera M, Medrano S, Kim YL. Carbamylation of hemoglobin in vivo with chronic sublethal dietary cyanide: implications for hemoglobin S. Biochemical Medicine and Metabolic Biology. 1988;39(1):64–68. doi: 10.1016/0885-4505(88)90059-x. [DOI] [PubMed] [Google Scholar]
- 61.Nithianandam VS, Kaleem K, Chertok F, Erhan S. Quinone-amine polymers. V. Syntheses and solubilities of several diamine-p-benzoquinone oligomers (PAQ) Journal of Applied Polymer Science. 1991;42(11):2893–2897. [Google Scholar]
- 62.Jackson LC. Ecological modelling of human-plant-parasite coevolutionary triads: heoretical perspectives on the interrelationships of human HbPS, GGPD, Manihot esculenta, Vicia faba, and Plasmodium fakiparum . In: Greene L, Danubio M, editors. Adaptation to Malaria: The Interaction of Biology and Culture. New York, NY, USA: Academic; 1996. [Google Scholar]
- 63.Jackson LC. The coevolutionary relationship of humans and domesticated plants. American Journal of Physical Anthropology. 1996;101(23):161–176. [Google Scholar]
- 64.Soranzo N, Bufe B, Sabeti PC, et al. Positive selection on a high-sensitivity allele of the human bitter-taste receptor TAS2R16 by the N172 allele may have driven the signal 8 of selection at an early stage of human evolution. Current Biology. 2005;15(14):1257–1265. doi: 10.1016/j.cub.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 65.Maranz SS. An alternative paradigm for the role of antimalarial plants in Africa. The Scientific World Journal. 2012;2012:9 pages. doi: 10.1100/2012/978913.978913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagel RL, Raventos C, Tanowitz HB, Wittner M. Effect of sodium cynanate on Plasmodium falciparum in vitro. Journal of Parasitology. 1980;66(3):483–487. [PubMed] [Google Scholar]
- 67.Rihet P, Abel L, Traoré Y, Traoré-Leroux T, Aucan C, Fumoux F. Human malaria: segregation analysis of blood infection levels in a suburban area and a rural area in Burkina Faso. Genetic Epidemiology. 1998;15(5):435–450. doi: 10.1002/(SICI)1098-2272(1998)15:5<435::AID-GEPI1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 68.Wang S-J, Lengeler C, Smith TA, et al. Rapid urban malaria appraisal (RUMA) I: epidemiology of urban malaria in Ouagadougou. Malaria Journal. 2005;4, article 43 doi: 10.1186/1475-2875-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grellier P, Maroziene A, Nivinskas H, Šarlauskas J, Aliverti A, Čenas N. Antiplasmodial activity of quinones: roles of aziridinyl substituents and the inhibition of Plasmodium falciparum glutathione reductase. Archives of Biochemistry and Biophysics. 2010;494(1):32–39. doi: 10.1016/j.abb.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 70.Jackson LC. Two evolutionary models for the interactions of dietary organic cyanogens, hemoglobins, and falciparum malaria. American Journal of Human Biology. 1990;2(5):521–532. doi: 10.1002/ajhb.1310020508. [DOI] [PubMed] [Google Scholar]
- 71.Gillette PN, Manning JM, Cerami A. Increased survival of sickle-cell erythrocytes after treatment in vitro with sodium cyanate. Proceedings of the National Academy of Sciences of the United States of America. 1971;68(11):2791–2793. doi: 10.1073/pnas.68.11.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vasey N. Niche separation in Varecia variegata rubra and Eulemur fulvus albifrons: I. Interspecific patterns. American Journal of Physical Anthropology. 2000;112:411–431. doi: 10.1002/1096-8644(200007)112:3<411::AID-AJPA10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]


