Abstract
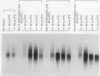
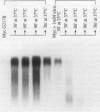
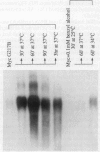
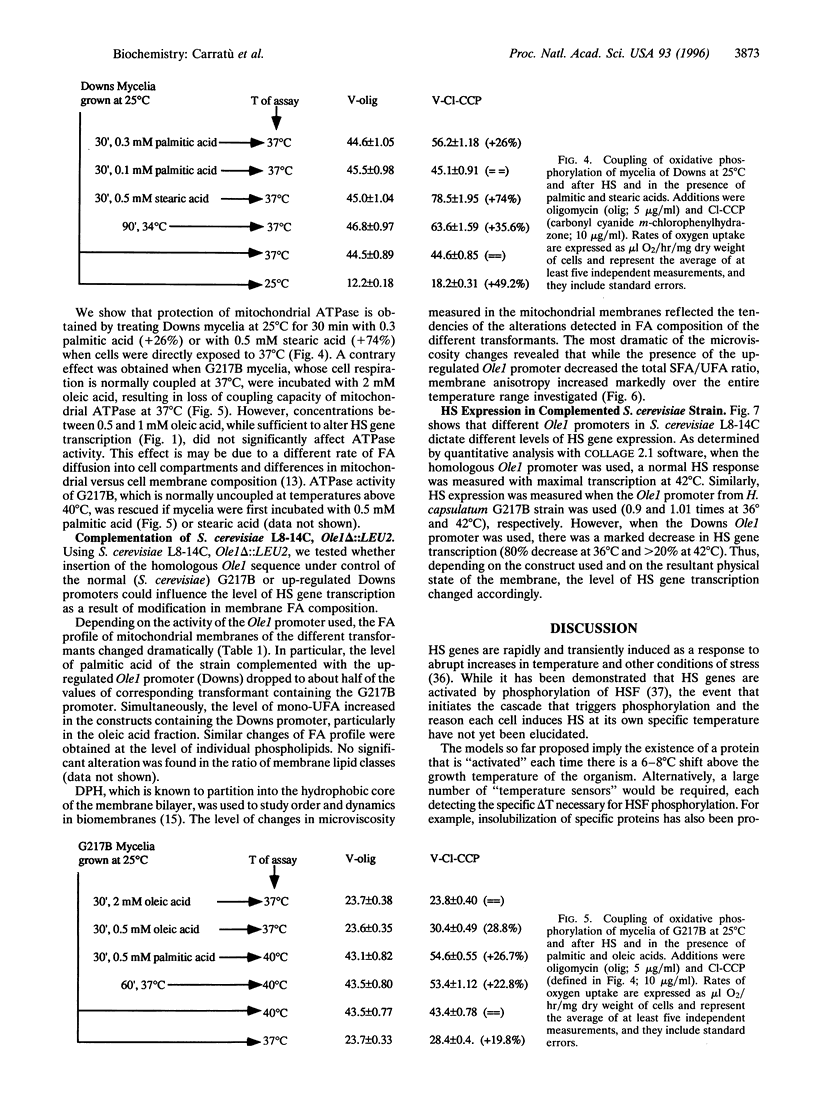
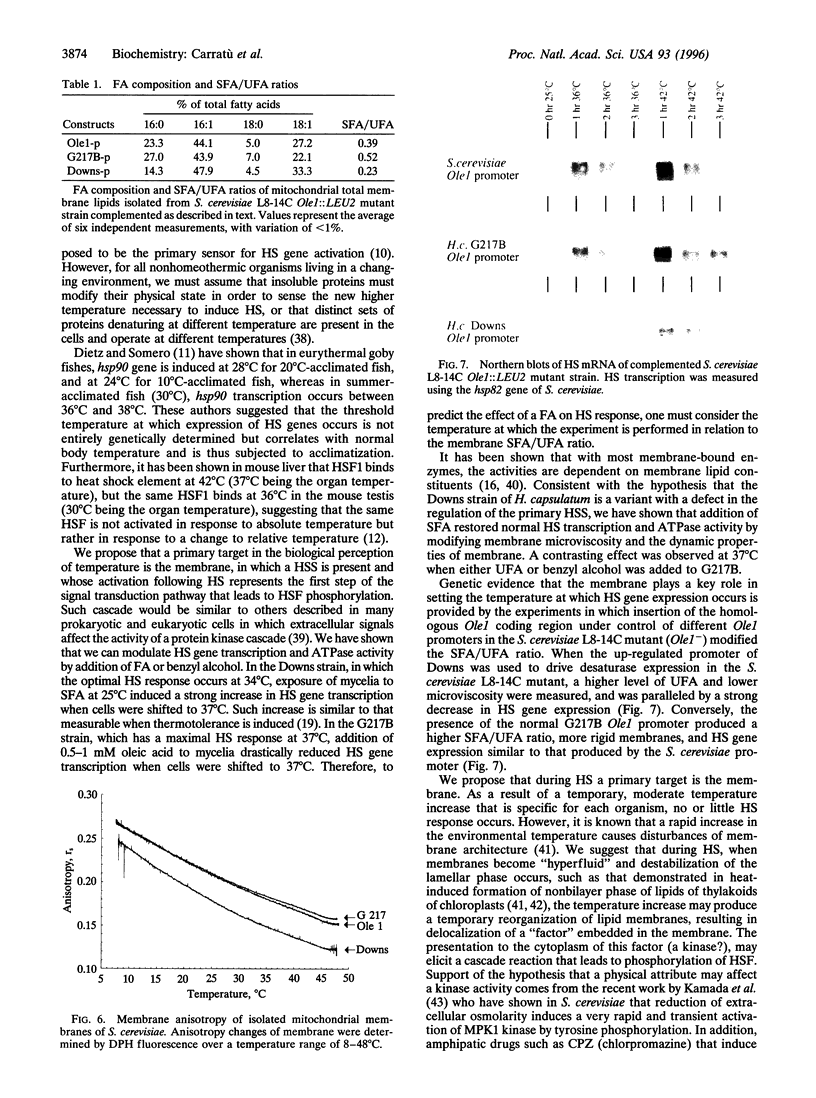
Addition of a saturated fatty acid (SFA) induced a strong increase in heat shock (HS) mRNA transcription when cells were heat-shocked at 37 degrees C, whereas treatment with an unsaturated fatty acid (UFA) reduced or eliminated the level of HS gene transcription at 37 degrees C. Transcription of the delta 9-desaturase gene (Ole1) of Histoplasma capsulatum, whose gene product is responsible for the synthesis of UFA, is up-regulated in a temperature-sensitive strain. We show that when the L8-14C mutant of Saccharomyces cerevisiae, which has a disrupted Ole1 gene, is complemented with its own Ole1 coding region under control of its own promoter or Ole1 promoters of H. capsulatum, the level of HS gene transcription depends on the activity of the promoters. Fluorescence anisotropy of mitochondrial membranes of completed strains corresponded to the different activity of the Ole1 promoter used. We propose that the SFA/UFA ratio and perturbation of membrane lipoprotein complexes are involved in the perception of rapid temperature changes and under HS conditions disturbance of the preexisting membrane physical state causes transduction of a signal that induces transcription of HS genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Berbee M. L., Taylor J. W. Convergence in ascospore discharge mechanism among pyrenomycete fungi based on 18S ribosomal RNA gene sequence. Mol Phylogenet Evol. 1992 Mar;1(1):59–71. doi: 10.1016/1055-7903(92)90036-g. [DOI] [PubMed] [Google Scholar]
- Caruso M., Sacco M., Medoff G., Maresca B. Heat shock 70 gene is differentially expressed in Histoplasma capsulatum strains with different levels of thermotolerance and pathogenicity. Mol Microbiol. 1987 Sep;1(2):151–158. doi: 10.1111/j.1365-2958.1987.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Clos J., Rabindran S., Wisniewski J., Wu C. Induction temperature of human heat shock factor is reprogrammed in a Drosophila cell environment. Nature. 1993 Jul 15;364(6434):252–255. doi: 10.1038/364252a0. [DOI] [PubMed] [Google Scholar]
- Craig E. A., Gross C. A. Is hsp70 the cellular thermometer? Trends Biochem Sci. 1991 Apr;16(4):135–140. doi: 10.1016/0968-0004(91)90055-z. [DOI] [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Dietz T. J., Somero G. N. The threshold induction temperature of the 90-kDa heat shock protein is subject to acclimatization in eurythermal goby fishes (genus Gillichthys). Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3389–3393. doi: 10.1073/pnas.89.8.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M. F., Hovanessian A. G., Bensaude O. Heat-shock-induced denaturation of proteins. Characterization of the insolubilization of the interferon-induced p68 kinase. J Biol Chem. 1991 May 25;266(15):9707–9711. [PubMed] [Google Scholar]
- Gargano S., Di Lallo G., Kobayashi G. S., Maresca B. A temperature-sensitive strain of Histoplasma capsulatum has an altered delta 9-fatty acid desaturase gene. Lipids. 1995 Oct;30(10):899–906. doi: 10.1007/BF02537480. [DOI] [PubMed] [Google Scholar]
- Guidon P. T., Jr, Hightower L. E. Purification and initial characterization of the 71-kilodalton rat heat-shock protein and its cognate as fatty acid binding proteins. Biochemistry. 1986 Jun 3;25(11):3231–3239. doi: 10.1021/bi00359a023. [DOI] [PubMed] [Google Scholar]
- Hazel J. R. Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu Rev Physiol. 1995;57:19–42. doi: 10.1146/annurev.ph.57.030195.000315. [DOI] [PubMed] [Google Scholar]
- Kamada Y., Jung U. S., Piotrowski J., Levin D. E. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 1995 Jul 1;9(13):1559–1571. doi: 10.1101/gad.9.13.1559. [DOI] [PubMed] [Google Scholar]
- Karin M. Signal transduction and gene control. Curr Opin Cell Biol. 1991 Jun;3(3):467–473. doi: 10.1016/0955-0674(91)90075-a. [DOI] [PubMed] [Google Scholar]
- Kingston R. E., Schuetz T. J., Larin Z. Heat-inducible human factor that binds to a human hsp70 promoter. Mol Cell Biol. 1987 Apr;7(4):1530–1534. doi: 10.1128/mcb.7.4.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Quirk P. G., Guffanti A. A. Uncoupler-resistant mutants of bacteria. Microbiol Rev. 1990 Mar;54(1):52–65. doi: 10.1128/mr.54.1.52-65.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambowitz A. M., Kobayashi G. S., Painter A., Medoff G. Possible relationship of morphogenesis in pathogenic fungus, Histoplasma capsulatum, to heat shock response. Nature. 1983 Jun 30;303(5920):806–808. doi: 10.1038/303806a0. [DOI] [PubMed] [Google Scholar]
- Lee J. A., Cossins A. R. Temperature adaptation of biological membranes: differential homoeoviscous responses in brush-border and basolateral membranes of carp intestinal mucosa. Biochim Biophys Acta. 1990 Jul 24;1026(2):195–203. doi: 10.1016/0005-2736(90)90064-u. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Los D., Horvath I., Vigh L., Murata N. The temperature-dependent expression of the desaturase gene desA in Synechocystis PCC6803. FEBS Lett. 1993 Feb 22;318(1):57–60. doi: 10.1016/0014-5793(93)81327-v. [DOI] [PubMed] [Google Scholar]
- Maresca B., Cossins A. R. Cell physiology. Fatty feedback and fluidity. Nature. 1993 Oct 14;365(6447):606–607. doi: 10.1038/365606a0. [DOI] [PubMed] [Google Scholar]
- Medoff G., Maresca B., Lambowitz A. M., Kobayashi G., Painter A., Sacco M., Carratu L. Correlation between pathogenicity and temperature sensitivity in different strains of Histoplasma capsulatum. J Clin Invest. 1986 Dec;78(6):1638–1647. doi: 10.1172/JCI112757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara K. Structure and regulation of rat liver microsomal stearoyl-CoA desaturase gene. J Biochem. 1990 Dec;108(6):1022–1029. doi: 10.1093/oxfordjournals.jbchem.a123301. [DOI] [PubMed] [Google Scholar]
- Minchiotti G., Gargano S., Maresca B. Molecular cloning and expression of hsp82 gene of the dimorphic pathogenic fungus Histoplasma capsulatum. Biochim Biophys Acta. 1992 May 7;1131(1):103–107. doi: 10.1016/0167-4781(92)90106-a. [DOI] [PubMed] [Google Scholar]
- Minchiotti G., Gargano S., Maresca B. The intron-containing hsp82 gene of the dimorphic pathogenic fungus Histoplasma capsulatum is properly spliced in severe heat shock conditions. Mol Cell Biol. 1991 Nov;11(11):5624–5630. doi: 10.1128/mcb.11.11.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczko M., Schönfisch B., Voos W., Pfanner N., Rassow J. The mitochondrial ClpB homolog Hsp78 cooperates with matrix Hsp70 in maintenance of mitochondrial function. J Mol Biol. 1995 Dec 8;254(4):538–543. doi: 10.1006/jmbi.1995.0636. [DOI] [PubMed] [Google Scholar]
- Morimoto R. I. Cells in stress: transcriptional activation of heat shock genes. Science. 1993 Mar 5;259(5100):1409–1410. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- Patriarca E. J., Kobayashi G. S., Maresca B. Mitochondrial activity and heat-shock response during morphogenesis in the pathogenic fungus Histoplasma capsulatum. Biochem Cell Biol. 1992 Mar-Apr;70(3-4):207–214. doi: 10.1139/o92-031. [DOI] [PubMed] [Google Scholar]
- Patriarca E. J., Maresca B. Acquired thermotolerance following heat shock protein synthesis prevents impairment of mitochondrial ATPase activity at elevated temperatures in Saccharomyces cerevisiae. Exp Cell Res. 1990 Sep;190(1):57–64. doi: 10.1016/0014-4827(90)90143-x. [DOI] [PubMed] [Google Scholar]
- Quinn P. J., Joo F., Vigh L. The role of unsaturated lipids in membrane structure and stability. Prog Biophys Mol Biol. 1989;53(2):71–103. doi: 10.1016/0079-6107(89)90015-1. [DOI] [PubMed] [Google Scholar]
- Rabindran S. K., Haroun R. I., Clos J., Wisniewski J., Wu C. Regulation of heat shock factor trimer formation: role of a conserved leucine zipper. Science. 1993 Jan 8;259(5092):230–234. doi: 10.1126/science.8421783. [DOI] [PubMed] [Google Scholar]
- Rodgers W., Glaser M. Distributions of proteins and lipids in the erythrocyte membrane. Biochemistry. 1993 Nov 30;32(47):12591–12598. doi: 10.1021/bi00210a007. [DOI] [PubMed] [Google Scholar]
- Rouser G., Kritchevsky G., Simon G., Nelson G. J. Quantitative analysis of brain and spinach leaf lipids employing silicic acid column chromatography and acetone for elution of glycolipids. Lipids. 1967 Jan;2(1):37–40. doi: 10.1007/BF02531998. [DOI] [PubMed] [Google Scholar]
- Sarge K. D., Bray A. E., Goodson M. L. Altered stress response in testis. Nature. 1995 Mar 9;374(6518):126–126. doi: 10.1038/374126a0. [DOI] [PubMed] [Google Scholar]
- Shearer G., Jr, Birge C. H., Yuckenberg P. D., Kobayashi G. S., Medoff G. Heat-shock proteins induced during the mycelial-to-yeast transitions of strains of Histoplasma capsulatum. J Gen Microbiol. 1987 Dec;133(12):3375–3382. doi: 10.1099/00221287-133-12-3375. [DOI] [PubMed] [Google Scholar]
- Sloots J. A., Aitchison J. D., Rachubinski R. A. Glucose-responsive and oleic acid-responsive elements in the gene encoding the peroxisomal trifunctional enzyme of Candida tropicalis. Gene. 1991 Aug 30;105(1):129–134. doi: 10.1016/0378-1119(91)90524-f. [DOI] [PubMed] [Google Scholar]
- Somero G. N. Proteins and temperature. Annu Rev Physiol. 1995;57:43–68. doi: 10.1146/annurev.ph.57.030195.000355. [DOI] [PubMed] [Google Scholar]
- Sorger P. K. Heat shock factor and the heat shock response. Cell. 1991 May 3;65(3):363–366. doi: 10.1016/0092-8674(91)90452-5. [DOI] [PubMed] [Google Scholar]
- Sorger P. K., Pelham H. R. Purification and characterization of a heat-shock element binding protein from yeast. EMBO J. 1987 Oct;6(10):3035–3041. doi: 10.1002/j.1460-2075.1987.tb02609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter P., Spatz L., Corcoran D., Rogers M. J., Setlow B., Redline R. Purification and properties of rat liver microsomal stearyl coenzyme A desaturase. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4565–4569. doi: 10.1073/pnas.71.11.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stukey J. E., McDonough V. M., Martin C. E. Isolation and characterization of OLE1, a gene affecting fatty acid desaturation from Saccharomyces cerevisiae. J Biol Chem. 1989 Oct 5;264(28):16537–16544. [PubMed] [Google Scholar]
- Vigh L., Los D. A., Horváth I., Murata N. The primary signal in the biological perception of temperature: Pd-catalyzed hydrogenation of membrane lipids stimulated the expression of the desA gene in Synechocystis PCC6803. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9090–9094. doi: 10.1073/pnas.90.19.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]