Abstract
There is accumulating evidence that entity referred to as “essential tremor” (ET) is not a single disease. It may be a family of diseases better referred to as “the essential tremors”. This review will summarize the following evidence: (1) the presence of etiological heterogeneity, (2) the heterogeneity of findings in postmortem studies, thus suggesting several diseases, (3) recent discussion that age of onset may be an important marker of disease heterogeneity, (4) clinical expansion of the concept of “ET” in recent years to include a broader range of tremor phenomenology, other motor features (gait ataxia), other involuntary movements (dystonia), and non-motor features (cognitive problems, psychiatric problems), some of which could be primary, (5) heterogeneity of pharmacological response profile and clinical progression, (6) association of ET with Parkinson's disease, Alzheimer's disease and possibly progressive supranuclear palsy, with the possibility that some ET patients are more predisposed to develop one of these.
Keywords: essential tremor, disease, classification, pathology, genetics, clinical, heterogeneity, neurodegeneration
Introduction
Although tremor and, more specifically, essential tremor (ET) have long been known to humans [1,2,3], knowledge of ET has grown markedly over the past decade [4]. Indeed, the familiar neurological condition long ago labeled “essential tremor” (ET) [1] is becoming more difficult to fully encapsulate. Current thinking about the fundamental nature of this diathesis is evolving. Indeed, it is quite likely that “ET” is a family of related diseases, unified by a common symptom/sign, namely, action tremor (i.e., tremor during voluntary movement). In this sense, the label “the essential tremors” would be more appropriate. This review will summarize and discuss the relevant data.
Methods
The author used Pubmed (1966 to April 2013) to cross search the terms “tremor”, “essential tremor” AND “etiology”, “genes”, “familial”, “environmental”, “toxins”, “causes”, “epidemiology”, “clinical”, “phenotype”, “brain”, “cerebellum”, “Purkinje”, “Lewy body”, “degeneration”, “treatment”, and “medication” and reviewed all English language papers. The author supplemented this review with published peer-reviewed papers in his own files.
Results
Etiologic Heterogeneity in ET
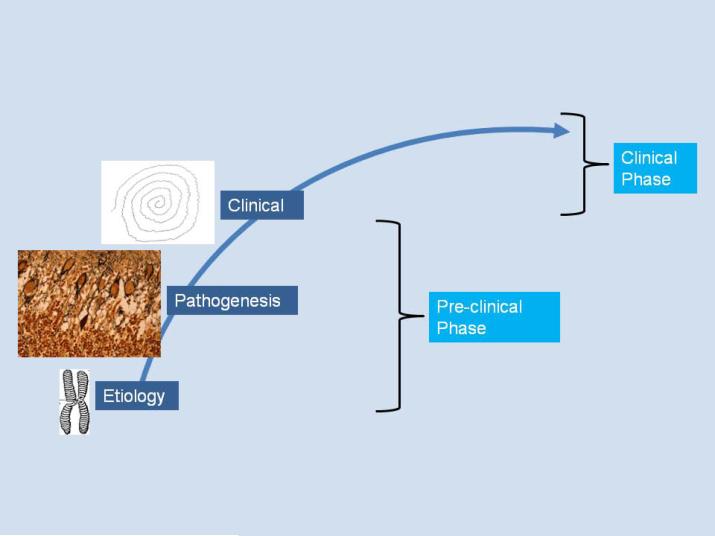
Disease etiology refers to the initial or primary cause of the disease, and it is comprised either of genetic or environmental factors; in some cases, both genetic and environmental factors may be operating alone or in combination (Figure 1) [5]. ET is a worldwide condition with a high prevalence [6,7,8,9,10,11,12]. The disorder also has a relatively restricted phenotype in the sense that the number of clinical features is relatively small. It is likely that there are many gene carriers, and that carriers of different genes express an identical or nearly identical set of clinical features. Linkage studies have demonstrated at least three loci (2p22, 3q13 and 6p23) that are of possible significance in ET [13,14,15]. More recently, genome wide association studies (GWAS) have identified a number of common variants that are associated with a modest elevation in risk of ET [16,17]. Thus, with respect to ET, the presence of multiple genes (genetic heterogeneity) is accepted [18,19]. Some of these genes are likely to be rare, as seems to be the case with the fused in sarcoma (FUS) gene [20,21,22], further suggesting that the eventual number of known identified genes might be sizable. If one takes the one gene-one disease approach [23], the presence of such genetic heterogeneity suggests that ET is not one disease; rather ET would be a family of diseases. Leaving aside this one source of heterogeneity (i.e., genetic heterogeneity), one must also consider other sources of etiological heterogeneity. It is generally agreed upon that there are both familial and sporadic forms of ET [24], thereby more widely indicating the presence of etiological heterogeneity beyond the domain of genes. The cause(s) of these sporadic forms of ET are fully unknown, yet in association studies, a wide array of neurotoxins has been investigated, and several of these, including harmane, lead, ethanol, could play a role in disease etiology [25]. Studies are suggesting that some of the toxins are of greater importance in the familial than sporadic form of ET [26], suggesting the presence of either gene-environment interaction or a two-hit (increased genetic susceptibility followed by exposure to environmental factor) model of disease. It is unclear how these various genes and environmental factors operate in a single population and across different populations. Regardless, the presence of etiological heterogeneity strongly suggests the possibility of a multiplicity of disease entities rather than only one.
Figure 1.
The disease process in ET as an etiological-biological-clinical continuum.
Heterogeneous Pathogenesis
Disease pathogenesis, which follows etiology, refers to the cascade of molecular, cellular, and then tissue/organ-based changes that occur after the disease is set in motion. It follows etiology (Figure 1).
In various neurodegenerative diseases of aging, the presence of pathological heterogeneity has led investigators to question the unitary nature of the diathesis. Thus, although we refer to Alzheimer's disease (AD) as a single disease entity with a common neuropathological hallmark, there are clearly distinct patterns/distributions of these features [27]. Some have even indicated that we must conceptualize the ADs “as a heterogeneous disorder or a family of Alzheimer diseases based on specific mechanism(s) rather than a single, neurodegenerative disorder dominated by beta-amyloid neuritic plaques and tau-based neuronal neurofibrillary change [28].” The absence of Lewy-bodies in some PD cases has also led investigators to raise the issue as to whether PD is one or more diseases [23]. As will be discussed below, in the setting of mounting evidence of pathological heterogeneity, a similar issue is now arising in ET as well.
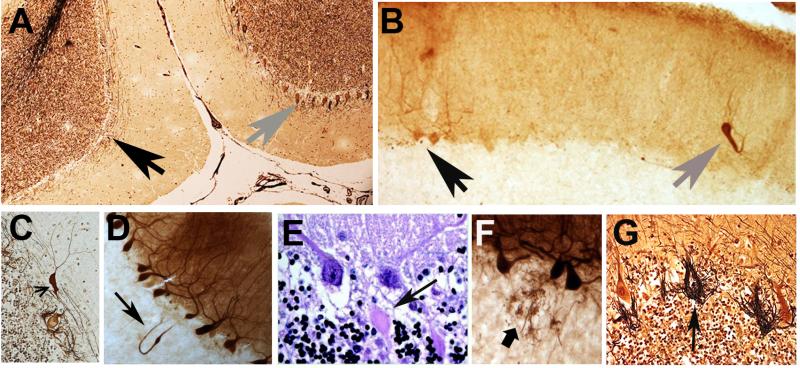
ET is increasingly being viewed as a disease of the cerebellum and its connections [29-34]. A variety of changes have been described in the ET cerebellum on postmortem examination, and these seem to be centered on the Purkinje cell and surrounding neuronal populations (i.e., Basket cells) (Figure 2) [29]. These changes resemble to some extent the types of changes seen in patients with disorders of cerebellar degeneration [35-37].
Figure 2.
A variety of structural microscopic changes have been described in the ET cerebellum on postmortem examination, and these seem to be centered on the Purkinje cell and surrounding neuronal populations (i.e., Basket cells). Cerebellar cortical sections in ET cases: A. Relatively normal Purkinje cell layer (gray arrow on right) near an area with segmental loss of Purkinje cells (black arrow on left). Bielschowsky stain. B. A heterotopic Purkinje cell (gray arrow) is adjacent to two normal Purkinje cells (black arrow). Calbindin-stained section. C. A swelling (arrow) of the Purkinje cell dendrite. Bielschowsky stain. D. A torpedo, thickened axon, and recurrent axonal collateral (arrow). Calbindin stain. E. Two torpedoes adjacent to their respective Purkinje cell soma. Luxol fast blue Hematoxylin and eosin stain. F. Sprouting of Purkinje cell axonal end processes in the upper granular layer. Calbindin stain. G. Hypertrophic Basket cell processes and an empty basket (arrow). Bielschowsky stain.
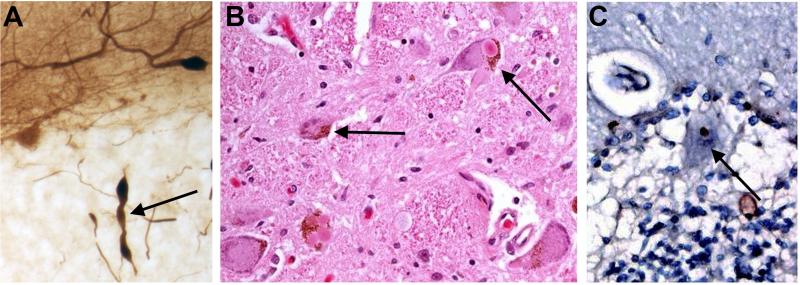
One feature that has been reported consistently across studies is the heterogeneity of pathological findings in ET [38-40]. Indeed, this had led earlier investigators to conclude that there was no consistent pattern of pathology [39], yet in reality, the heterogeneity does follow certain preliminary patterns (Figure 3). Thus, in those brain banks that have carefully quantified a full range of pathological changes in the cerebellum (New York), the majority of ET cases have a clearly identifiable set of postmortem structural changes and have been referred to as “cerebellar ET” (Figure 2) [38]. A smaller portion has brainstem Lewy bodies [41,42], and an even smaller portion has intranuclear inclusions in the cerebellum and elsewhere [43,44]. The number of ET brains that have come to postmortem is still small, and enlargement of the sample size is needed to better understand the basic patterns and make full sense of this emerging picture of pathological heterogeneity. It is even possible that “cerebellar ET” itself might be comprised of several disorders, with each characterized by a slightly different signature of postmortem changes in the cerebellum.
Figure 3.
Three different ET cases showing a heterogeneity of pathological findings in the ETs. A. Representing cerebellar pathology, a Purkinje cell axon is shown with three torpedoes. Calbindin stain. B. Multiple Lewy bodies in the locus ceruleus. Hematoxylin and eosin stain. C. Ubiquitin positive Purkinje cell intranuclear inclusion.
An additional consideration is that there are differences across brain banks with respect to some findings, with one bank unable to detect Purkinje cell loss in ET, for example [34,45]. Different findings across brain banks likely reflect differences in sampling, differences in case definition, and other important methodological issues like sample size [29,38,39,41,46]; it is also possible that these differences reflect true heterogeneity.
Age of Onset Tremor: A Marker of Heterogeneity
Although ET may arise at any age [47,48], from infancy through advanced age, this does not mean that the incidence and prevalence of disease are randomly distributed with respect to age. The incidence of ET follows a blueprint, with a rising incidence during advanced aging [49,50]. There has been some recent discussion that later onset ET (> age 65 years) should not be regarded as ET per se, but should be regarded as “senile tremor” [51]. Although the notion that age of onset may be a disease feature of mechanistic interest, this particular choice of this cut point does not have much biological support. Furthermore, when other disorders (e.g., AD, PD) occur at or after the age of 65, one does not refer to them as “senile dementia” or “senile parkinsonism”. The notion that ET must occur prior to the age of 65 is unfounded; indeed, it has been demonstrated in familial forms of the disorder that penetrance is not even complete by age 65 [52]. The term “senile tremor” is an arcane term that was used early in the last century, and it is not a particularly useful construct today. A more biologically meaningful age-related observation is that younger onset ET cases are more likely to be familial and, presumably, genetic [53]. Hence, certain distinct classes of etiologies (i.e., genes rather than environmental factors) may influence the timing of disease onset and possibly the expression of disease. Thus, age of onset heterogeneity likely reflects etiological and biological heterogeneity.
Clinical Features and Clinical Heterogeneity
Some degree of action tremor is nearly universal among adults - physiological tremor and enhanced physiological tremor [54]. In addition, a wide range of tremor disorders has been described [55]. There is a tendency for clinicians to be “lumpers”, over-applying the diagnosis “ET” to many of these conditions, and treating ET as if it were formless [56,48,57]. Yet far from being featureless and nondescript, the disease, ET, is characterized by specific and consistently described pattern(s) of tremor: (1) kinetic tremor > postural tremor [58,59], (2) involvement of specific joints in specific directions (e.g., wrist > metacarpal, wrist flexion-extension > wrist rotation) [60], (3) intention tremor of the arms in approximately 50% of cases [61,62], (4) rest tremor as a late feature in up to 20% of cases [63], (5) arm tremor preceding cranial tremor, of which there is a female preponderance [64,65], (6) prevalence of neck > jaw > tongue/cheek/forehead tremor [66], (7) a tendency for tremor severity to increase over time [67,68], and (8) the co-occurrence of cerebellar features aside from intention tremor (e.g., gait ataxia) in many patients [69,70]. Thus, in addition to these consistencies of pattern (i.e., a symptom complex), there is a general course/prognosis across patients.
With an increase in case-control studies over the past 5 – 10 years, which have supplemented older case series (i.e., cases without controls), there has been a growing appreciation that the clinical phenomenology that can accompany the base features of ET can be quite varied in nature and differentially expressed across patients (i.e., different phenotypes) [71]. First, there is an evolving awareness and acceptance of the presence of cerebellar features in some but not all patients [64,72,73,74,75,76,77,78]. In some unknown proportion of patients, these are more marked. Their pathophysiological significance is not fully clear. Second, it is becoming clear that other involuntary movements (e.g., dystonia) may co-occur with ET in some individuals, especially in familial forms of ET [79,80]. This raises the question as to whether mild torticollis and other features of dystonia may occur in longstanding/severe and/or familial forms of ET without the need to invoke a second or alternative diagnosis [79]. Third, non-motor features (cognitive problems ranging from mild to severe [81,82,83,84,85,86,87], and psychiatric problems [88,89,90,91,92], some of which could be primary [93,94]) are increasingly being seen as a feature of some patients (e.g., those with older age of onset) [95]. Heterogeneity of clinical progression has also been pointed out [96,97,98]. Together, these data point to a general yet ill-defined sense that clinical heterogeneity is likely to be an expression of disease state (e.g., disease duration and severity at the time point of the clinical observation) but that it may also reflect diverse disease trait(s) (phenotype, patho-mechanisms).
Heterogeneity of Pharmacological Response Phenotype
Nearly all of the treatments that have shown to be effective for ET, to date, involve the enhancement of a single and specific brain neurotransmitter system (i.e., the gamma amino butyric acid [GABA] -ergic system). This would include the barbiturates, primidone, benzodiazepines, gabapentin, and even various alcohols [99]. This indicates that there is a specific central target of underlying biological importance. Beta-blockers probably likely act at a very distal (i.e., peripheral) site [99].
For a variety of clinical trials in ET, treatment graphs (“before” and “after” treatment) clearly show that a sizable portion (30 – 60%) of patients has little if any response to medication; the remaining subgroup of patients do show evidence of a response [100,101,102,103]. Some of this heterogeneity could be accounted for by differences in disease duration along with the probability that the underlying biological substrate advances over time (i.e., likely a reflection of disease “state” rather than “trait”), leaving less room for clinical responsiveness [104], as occurs in PD [105] and AD [106,107]. Some of these differences, though, could be “trait” differences (i.e., separate diseases that are all lumped into and labeled as “ET”). Of interest is that there is less response heterogeneity with deep brain stimulation surgery, with the large majority of ET patients showing a sizable response to the intervention [108]. This is likely because the intervention is acting at the level of the thalamus, which is a more distal-final common nodal point for the receipt of diatheses-affected pathways that emerge from various more proximal points within the cerebellar system in ET.
Do Different “ET”s Lead to Different Outcomes?
In case-control and cohort studies, ET has been linked with several other neurodegenerative conditions [109]. Though considered controversial [110], there are no other epidemiological data to the contrary [111]. Thus there is a reported association of ET with PD [112,113,114,115], ET with AD [95,116,117] and ET with PSP [39,118]. It will be important to identify the subgroups of patients who are at increased risk for each of these entities. One would expect that these subgroups of prevalent ET cases might have basic pathophysiological features that distinguish them from those who are not at increased risk.
Discussion
Debates over nosology and disease classification can become quite intense, yet they often serve to bring to the fore useful discussion points, and they can facilitate clinical synthesis. Diverse etiological and neuropathological entities may produce clinical syndromes that are indistinguishable from one another; we know this is the case, for example, in PD [23]. This has led prior authorities to conclude that there is no one PD, and that the term “Parkinson's diseases” would be more appropriate [23]. A similar issue is now raised with respect to ET [79].
The presence of heterogeneity across multiple domains (etiologic, pathologic, age of onset, clinical features and clinical progression, pharmacological response phenotype, and possibly relationship with other diseases) strongly favors the notion that the etiological-pathological-clinical-therapeutic continuum that defines disease (Figure 1), and which occurs in ET, is not a single entity. Rather, there are likely to be several ETs. ET is thus likely to be a family of diseases for which the term “the essential tremors” is a more suitable term and a more useful construct for future basic and pharmacotherapeutic investigations.
While one may question whether ET is no more than a syndrome, there is little evidence to support this. A syndrome is a set of symptoms that occur together (i.e., a symptom complex). For example sore throat, coughing, sneezing, rhinorrhea, and malaise are a set of symptoms that often occur together, comprising an upper respiratory syndrome. In contrast with a syndrome, a disease is an entity characterized by a symptom complex as well as a specific course/prognosis (which may be variable), a specific etiology, and a set of organ-based changes in function or structure (whether known or unknown). For example, rhinoviral nasopharyngitis and influenza virus are diseases that are both characterized by an upper respiratory syndrome, yet the etiologies (i.e., infectious agents), clinical course and tissue-based changes are distinct. Action tremor of the arms, rather than ET, is perhaps syndromic. ET is an entity characterized by a symptom complex as well as a specific course/prognosis (which can be variable), a set of etiologies (many of which have not been specifically identified), and a set of organ-based changes in function or structure (still under active investigation and discussion).
An important issue is whether this question really matters? As in PD [23], the answer is “yes”, as the question has implications for future research and clinical care. First, studies of disease etiology are less likely to identify disease risk factors if the case group in actuality consists of several rather than one disease. Indeed, a given risk factor, whether genetic or environmental, is likely to be specific to a particular one of the ETs. Similarly, studies of basic disease mechanisms will not benefit from this form of diagnostic misclassification. Disease mechanisms could be very different across members of the ETs. In the clinical domain, it would be important from a prognostic and disease course vantage point to know if one were dealing with a single entity or several. Finally, clinical trials and, in the future, neuroprotective trials for the ETs are destined to do poorly if we are lumping multiple diseases together under the term “ET”.
“ET” is a old nosological entity [1]. Our understanding of the diathesis has evolved over the past 100 years, as has our concept of “disease” in general [23]. Heterogeneity is evident on multiple fronts, as reviewed above. Recognizing and organizing that heterogeneity will be an important scientific task, with the goal being to better understand the causes and processes that underlie the ETs.
Acknowledgments
Funding: This research was supported by National Institutes of Health Grant R01 NS042859, R01 NS39422, and R01 NS073872.
Footnotes
Disclosure: The author declares that there are no conflicts of interest and no competing financial interests.
References
- 1.Louis ED, Broussolle E, Goetz CG, Krack P, Kaufmann P, Mazzoni P. Historical underpinnings of the term essential tremor in the late 19th century. Neurology. 2008;71:856–859. doi: 10.1212/01.wnl.0000325564.38165.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koehler PJ, Keyser A. Tremor in Latin texts of Dutch physicians: 16th-18th centuries. Mov Disord. 1997;12:798–806. doi: 10.1002/mds.870120531. [DOI] [PubMed] [Google Scholar]
- 3.Louis ED. Essential tremor. Arch Neurol. 2000;57:1522–1524. doi: 10.1001/archneur.57.10.1522. [DOI] [PubMed] [Google Scholar]
- 4.Benito-Leon J. Essential tremor: from a monosymptomatic disorder to a more complex entity. Neuroepidemiology. 2008;31:191–2. doi: 10.1159/000154933. [DOI] [PubMed] [Google Scholar]
- 5.Ottman R. Gene-environment interaction: definitions and study designs. Prev Med. 1996;25:764–770. doi: 10.1006/pmed.1996.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–541. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 7.Bergareche A, De La Puente E, López De Munain A, Sarasqueta C, De Arce A, Poza JJ, Martí-Massó JF. Prevalence of essential tremor: a door-to-door survey in bidasoa, spain. Neuroepidemiology. 2001;20(2):125–128. doi: 10.1159/000054771. [DOI] [PubMed] [Google Scholar]
- 8.Liu HC, Wang SJ, Fuh JL, Liu CY, Lin KP, Lin CH, Wang PN, Lin KN, Wang HC, Chen HM, Chang R, Larson EB, Wu GS, Chou P, Teng EL. The Kinmen Neurological Disorders Survey (KINDS): a study of a Chinese population. Neuroepidemiology. 1997;16(2):60–68. doi: 10.1159/000109672. [DOI] [PubMed] [Google Scholar]
- 9.Louis ED, Thawani SP, Andrews HF. Prevalence of essential tremor in a multiethnic, community-based study in northern Manhattan, New York, N.Y. Neuroepidemiology. 2009;32(3):208–214. doi: 10.1159/000195691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benito-León J. How common is essential tremor? Neuroepidemiology. 2009;32(3):215–216. doi: 10.1159/000195692. [DOI] [PubMed] [Google Scholar]
- 11.Louis ED, Hafeman D, Parvez F, Alcalay RN, Islam T, Siddique AB, Patwary TI, Melkonian S, Argos M, Levy D, Ahsan H. Prevalence of essential tremor in Araihazar, Bangladesh: a population-based study. Neuroepidemiology. 2011;36(2):71–76. doi: 10.1159/000323389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benito-León J. Essential tremor: one of the most common neurodegenerative diseases? Neuroepidemiology. 2011;36(2):77–78. doi: 10.1159/000323572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JJ, Jankovic J, Lombardi RQ, Pucilowska J, Ashizawa T, Ruszczyk MU. Haplotype analysis of the ETM2 locus in familial essential tremor. Neurogenetics. 2003;4:185–189. doi: 10.1007/s10048-003-0151-2. [DOI] [PubMed] [Google Scholar]
- 14.Gulcher JR, Jónsson P, Kong A, Kristjánsson K, Frigge ML, Kárason A, Einarsdóttir IE, Stefánsson H, Einarsdóttir AS, Sigurthoardóttir S, Baldursson S, Björnsdóttir S, Hrafnkelsdóttir SM, Jakobsson F, Benedickz J, Stefánsson K. Mapping of a familial essential tremor gene, FET1, to chromosome 3q13. Nat Genet. 1997;17:84–87. doi: 10.1038/ng0997-84. [DOI] [PubMed] [Google Scholar]
- 15.Shatunov A, Sambuughin N, Jankovic J, Elble R, Lee HS, Singleton AB, Dagvadorj A, Ji J, Zhang Y, Kimonis VE, Hardy J, Hallett M, Goldfarb LG. Genomewide scans in North American families reveal genetic linkage of essential tremor to a region on chromosome 6p23. Brain. 2006;129:2318–2331. doi: 10.1093/brain/awl120. [DOI] [PubMed] [Google Scholar]
- 16.Thier S, Lorenz D, Nothnagel M, Poremba C, Papenqut F, Appenzeller S, Paschen S, Hofschulte F, Hussl AC, Hering S, Poewe W, Asmus F, Gasser T, Schöls L, Christensen K, Nebel A, Schreiber S, Klebe S, Deuschl G, Kuhlenbäumer G. Polymorphisms in the glial glutamate transporter SLC1A2 are associated with essential tremor. Neurology. 2012;79:243–248. doi: 10.1212/WNL.0b013e31825fdeed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stefansson H, Steinberg S, Petursson H, Gustafsson O, Gudjonsdottir IH, Jonsdottir GA, Palsson ST, Jonsson T, Saemundsdottir J, Bjornsdottir G, Böttcher Y, Thorlacius T, Haubenberger D, Zimprich A, Auff E, Hotzy C, Testa CM, Miyatake LA, Rosen AR, Kristleifsson K, Rye D, Asmus F, Schöls L, Dichgans M, Jakobsson F, Benedikz J, Thorsteinsdottir U, Gulcher J, Kong A, Stefansson K. Variant in the sequence of the LINGO1 gene confers risk of essential tremor. Nat Genet. 2009;41:277–279. doi: 10.1038/ng.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aridon P, Ragonese P, De Fusco M, Salemi G, Casari G, Savettieri G. Further evidence of genetic heterogeneity in familial essential tremor. Parkinsonism Relat Disord. 2008;14:15–18. doi: 10.1016/j.parkreldis.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Kovach MJ, Ruiz J, Kimonis K, Mueed S, Sinha S, Higgins C, Elble S, Elble R, Kimonis VE. Genetic heterogeneity in autosomal dominant essential tremor. Genet Med. 2001;3:197–199. doi: 10.1097/00125817-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Merner ND, Girard SL, Catoire H, Bourassa CV, Belzil VV, Riviere JB, Hince P, Levert A, Dionne-Laporte A, Spiegelman D, Noreau A, Diab S, Szuto A, Fournier H, Raelson J, Belouchi M, Panisset M, Cossette P, Dupré N, Bernard G, Chouinard S, Dion PA, Rouleau GA. Exome sequencing identifies FUS mutations as a cause of essential tremor. Am J Hum Genet. 2012;91:313–319. doi: 10.1016/j.ajhg.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parmalee N, Mirzozoda K, Kisselev S, Merner N, Dion P, Rouleau G, Clark L, Louis ED. Genetic analysis of the FUS/TLS gene in essential tremor. Eur J Neurol. 2013;20:534–539. doi: 10.1111/ene.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labbe C, Soto-Ortolaza AI, Rayaprolu S, Harriott AM, Strongosky AJ, Uitti RJ, Van Gerpen JA, Wszolek ZK, Ross OA. Investigating the role of FUS exonic variants in Essential Tremor. Parkinsonism Relat Disord. 2013;19(8):755–777. doi: 10.1016/j.parkreldis.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiner WJ. There is no Parkinson disease. Arch Neurol. 2008;65:705–708. doi: 10.1001/archneur.65.6.705. [DOI] [PubMed] [Google Scholar]
- 24.Louis ED, Ford B, Frucht S, Barnes LF, Tang M-X, Ottman R. Risk of tremor and impairment from tremor in relatives of patients with essential tremor: a community-based family study. Ann Neurol. 2001;49:761–769. doi: 10.1002/ana.1022. [DOI] [PubMed] [Google Scholar]
- 25.Louis ED. Environmental Epidemiology of Essential Tremor. Neuroepidemiology. 2008;31:139–149. doi: 10.1159/000151523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louis ED, Benito-Leon J, Moreno-Garcia S, Vega S, Romero JP, Bermejo-Pareja F, Gerbin M, Viner AS, Factor-Litvak P, Jiang WD, Zheng W. Blood harmane (1-methyl-9H-pyrido[3,4-b]indole) concentration in essential tremor cases in Spain. Neurotoxicology. 2013;34:264–268. doi: 10.1016/j.neuro.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitwell JL, Dickson DW, Murray ME, Weigand SD, Tosakulwong N, Senjem ML, Knopman DS, Boeve BF, Parisi JE, Petersen RC, Jack CR, Jr., Josephs KA. Neuroimaging correlates of pathologically defined subtypes of Alzheimer's disease: a case-control study. Lancet Neurol. 2012;11:868–877. doi: 10.1016/S1474-4422(12)70200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorelick PB. Role of inflammation in cognitive impairment: results of observational epidemiological studies and clinical trials. Ann N Y Acad Sci. 2010;1207:155–162. doi: 10.1111/j.1749-6632.2010.05726.x. [DOI] [PubMed] [Google Scholar]
- 29.Louis ED. Essential tremor: evolving clinicopathological concepts in an era of intensive post-mortem enquiry. Lancet Neurol. 2010;9:613–622. doi: 10.1016/S1474-4422(10)70090-9. [DOI] [PubMed] [Google Scholar]
- 30.Axelrad JE, Louis ED, Honig LS, Flores I, Ross GW, Pahwa R, Lyons FE, Faust PL, Vonsattel JPG. Reduced purkinje cell number in essential tremor: a postmortem study. Arch Neurol. 2008;65:101–107. doi: 10.1001/archneurol.2007.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo SH, Erickson-Davis C, Gillman A, Faust PL, Vonsattel JPG, Louis ED. Increased number of heterotopic Purkinje cells in essential tremor. J Neurol Neurosurg Psychiatry. 2011;82:1038–1040. doi: 10.1136/jnnp.2010.213330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erickson-Davis CR, Faust PL, Vonsattel JPG, Gupta S, Honig LS, Louis ED. “Hairy baskets” associated with degenerative Purkinje cell changes in essential tremor. J Neuropathol Exp Neurol. 2010;69:262–271. doi: 10.1097/NEN.0b013e3181d1ad04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuo SH, Tang G, Louis ED, MA K, Babij R, Balatbat M, Cortes E, Vonsattel JPG, Yamamoto A, Sulzer D, Faust PL. Lingo-1 expression is increased in essential tremor cerebellum and is present in the basket cell pinceau. Acta Neuropathol. 2013;125:879–889. doi: 10.1007/s00401-013-1108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Louis ED, Faust PL, Vonsattel JPG. Purkinje cell loss is a characteristic of essential tremor: Towards a more mature understanding of pathogenesis. Parkinsonism Relat Disord. 2012;18:1003–1004. doi: 10.1016/j.parkreldis.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Bebin EM, Bebin J, Currier RD, Smith EE, Perry TL. Morphometric studies in dominant olivopontocerebellar atrophy. Comparison of cell losses with amino acid decreases. Arch Neurol. 1990;47:188–192. doi: 10.1001/archneur.1990.00530020094021. [DOI] [PubMed] [Google Scholar]
- 36.Kume A, Takahashi A, Hashizume Y, Asai J. A histometrical and comparative study on Purkinje cell loss and olivary nucleus cell loss in multiple system atrophy. J Neurol Sci. 1991;101:178–186. doi: 10.1016/0022-510x(91)90043-7. [DOI] [PubMed] [Google Scholar]
- 37.Seidel K, Siswanto S, Brunt ER, den Dunnen W, Korf HW, Rub U. Brain pathology of spinocerebellar ataxias. Acta Neuropathol. 2012;124:1–21. doi: 10.1007/s00401-012-1000-x. [DOI] [PubMed] [Google Scholar]
- 38.Louis ED, Faust PL, Vonsattel JPG, Honig LS, Rajput A, Robinson CA, Rajput A, Pahwa R, Lyons KE, Ross GW, Borden S, Moskowitz CB, Lawton A, Hernandez N. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 39.Rajput A, Robinson CA, Rajput AH. Essential tremor course and disability: A clinicopathologic study of 20 cases. Neurology. 2004;62:932–936. doi: 10.1212/01.wnl.0000115145.18830.1a. [DOI] [PubMed] [Google Scholar]
- 40.Shill H, Adler CH, Sabbagh MN, Conoor DJ, Caviness JN, Hentz JG, Beach TG. Pathological changes in prospectively-ascertained essential tremor subjects. Neurology. 2008;70:1452–1455. doi: 10.1212/01.wnl.0000310425.76205.02. [DOI] [PubMed] [Google Scholar]
- 41.Ross GW, Dickson D, Cerosimo M. Pathological investigation of essential tremor. Neurology. 2004;62(7):A537–A538. [Google Scholar]
- 42.Louis ED, Honig LS, Vonsattel JPG, Maraganore DM, Borden S, Moskowitz CB. Essential tremor associated with focal nonnigral Lewy bodies: a clinicopathologic study. Arch Neurol. 2005;62:1004–1007. doi: 10.1001/archneur.62.6.1004. [DOI] [PubMed] [Google Scholar]
- 43.Louis ED, Erickson-Davis C, Pahwa R, Lyons KE, Garber A, Moskowitz CB, Lawton A, Faust PL, Vonsattel JPG. Essential tremor with ubiquitinated Purkinje cell intranuclear inclusions. Acta Neuropathol. 2010;119:375–377. doi: 10.1007/s00401-010-0641-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Louis ED, Mazzoni P, Ma KJ, Moskowitz CB, Lawton A, Garber A, Vonsattel JPG. Essential tremor with ubiquitinated intranuclear inclusions and cerebellar degeneration. Clin Neuropathol. 2012;31:119–126. doi: 10.5414/NP300414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rajput AH, Robinson CA, Rajput ML, Robinson SL, Rajput A. Essential tremor is not dependent upon cerebellar Purkinje cell loss. Parkinsonism Relat Disord. 2012;18:626–628. doi: 10.1016/j.parkreldis.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 46.Shill HA, Adler CH, Beach TG. Pathology in essential tremor. Parkinsonism Relat Disord. 2012;18(suppl 1):S135–S137. doi: 10.1016/S1353-8020(11)70042-6. [DOI] [PubMed] [Google Scholar]
- 47.Louis ED. Age of onset: can we rely on essential tremor patients to report this? Data from a prospective, longitudinal study. Neuroepidemiology. 2013;40(2):93–98. doi: 10.1159/000341903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Louis ED, Hernandez N, Rabinowitz D, Ottman R, Clark LN. Predicting age of onset in familial essential tremor: how much does age of onset run in families? Neuroepidemiology. 2013;40(4):269–273. doi: 10.1159/000345253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benito-Leon J, Bermejo-Pareja F, Louis ED. Incidence of essential tremor in three elderly populations of central Spain. Neurology. 2005;64:1721–1725. doi: 10.1212/01.WNL.0000161852.70374.01. [DOI] [PubMed] [Google Scholar]
- 50.Rajput AH, Offord KP, Beard CM, Kurland LT. Essential tremor in Rochester, Minnesota: a 45-year study. J Neurol Neurosurg Psychiatry. 1984;47:466–470. doi: 10.1136/jnnp.47.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deuschl G, Elble R. Essential tremor - Neurodegenerative or nondegenerative disease towards a working definition of ET. Mov Disord. 2009;24:2033–2041. doi: 10.1002/mds.22755. [DOI] [PubMed] [Google Scholar]
- 52.Louis ED, Ford B, Frucht S, Ottman R. Mild tremor in relatives of patients with essential tremor: what does this tell us about the penetrance of the disease? Arch Neurol. 2001;58:1584–1589. doi: 10.1001/archneur.58.10.1584. [DOI] [PubMed] [Google Scholar]
- 53.Louis ED, Dogu O. Does age of onset in essential tremor have a bimodal distribution? Data from a tertiary referral setting and a population-based study. Neuroepidemiology. 2007;29:208–212. doi: 10.1159/000111584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Louis ED, Ford B, Pullman S, Baron K. How normal is ‘normal’? Mild tremor in a multiethnic cohort of normal subjects. Arch Neurol. 1998;55:222–227. doi: 10.1001/archneur.55.2.222. [DOI] [PubMed] [Google Scholar]
- 55.Louis ED. Essential tremor. Clin Geriatr Med. 2006;22:843–857. doi: 10.1016/j.cger.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 56.Jain S, Lo SE, Louis ED. Common misdiagnosis of a common neurological disorder: how are we misdiagnosing essential tremor? Arch Neurol. 2006;63:1100–1104. doi: 10.1001/archneur.63.8.1100. [DOI] [PubMed] [Google Scholar]
- 57.Louis ED, Applegate LM, Rios E. ICD-9 CM code 333.1 as an identifier of patients with essential tremor: a study of the positive predictive value of this code. Neuroepidemiology. 2007;28(3):181–185. doi: 10.1159/000104096. [DOI] [PubMed] [Google Scholar]
- 58.Brennan KC, Jurewicz EC, Ford B, Pullman SL, Louis ED. Is essential tremor predominantly a kinetic or a postural tremor? A clinical and electrophysiological study. Mov Disord. 2002;17:313–316. doi: 10.1002/mds.10003. [DOI] [PubMed] [Google Scholar]
- 59.Louis ED. The primary type of tremor in essential tremor is kinetic rather than postural: cross-sectional observation of tremor phenomenology in 369 cases. Eur J Neurol. 2013;20:725–727. doi: 10.1111/j.1468-1331.2012.03855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thenganatt MA, Louis ED. Distinguishing essential tremor from Parkinson's disease: bedside tests and laboratory evaluations. Expert Rev Neurother. 2012;12:687–696. doi: 10.1586/ern.12.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deuschl G, Wenzelburger R, Loffler K, Raethjen J, Stolze H. Essential tremor and cerebellar dysfunction clinical and kinematic analysis of intention tremor. Brain. 2000;123(Pt 8):1568–1580. doi: 10.1093/brain/123.8.1568. [DOI] [PubMed] [Google Scholar]
- 62.Louis ED, Frucht SJ, Rios E. Intention tremor in essential tremor: Prevalence and association with disease duration. Mov Disord. 2009;24:626–627. doi: 10.1002/mds.22370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cohen O, Pullman S, Jurewicz E, Watner D, Louis ED. Rest tremor in patients with essential tremor: prevalence, clinical correlates, and electrophysiologic characteristics. Arch Neurol. 2003;60:405–410. doi: 10.1001/archneur.60.3.405. [DOI] [PubMed] [Google Scholar]
- 64.Hubble JP, Busenbark KL, Pahwa R, Lyons K, Koller WC. Clinical expression of essential tremor: effects of gender and age. Mov Disord. 1997;12:969–972. doi: 10.1002/mds.870120620. [DOI] [PubMed] [Google Scholar]
- 65.Hardesty DE, Maraganore DM, Matsumoto JY, Louis ED. Increased risk of head tremor in women with essential tremor: longitudinal data from the Rochester Epidemiology Project. Mov Disord. 2004;19:529–533. doi: 10.1002/mds.20096. [DOI] [PubMed] [Google Scholar]
- 66.Louis ED, Rios E, Applegate LM, Hernandez NC, Andrews HF. Jaw tremor: prevalence and clinical correlates in three essential tremor case samples. Mov Disord. 2006;21:1872–1878. doi: 10.1002/mds.21069. [DOI] [PubMed] [Google Scholar]
- 67.Putzke JD, Whaley NR, Baba Y, Wszolek ZK, Uitti RJ. Essential tremor: predictors of disease progression in a clinical cohort. J Neurol Neurosurg Psychiatry. 2006;77:1235–1237. doi: 10.1136/jnnp.2006.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Louis ED, Agnew A, Gillman A, Gerbin M, Viner AS. Estimating annual rate of decline: prospective, longitudinal data on arm tremor severity in two groups of essential tremor cases. J Neurol Neurosurg Psychiatry. 2011;82:761–765. doi: 10.1136/jnnp.2010.229740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bares M, Lungu OV, Husarova I, Gescheidt T. Predictive motor timing performance dissociates between early diseases of the cerebellum and Parkinson's disease. Cerebellum. 2010;9:124–135. doi: 10.1007/s12311-009-0133-5. [DOI] [PubMed] [Google Scholar]
- 70.Louis ED, Rios E, Rao AK. Tandem gait performance in essential tremor: clinical correlates and association with midline tremors. Mov Disord. 2010;25:1633–1638. doi: 10.1002/mds.23144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Louis ED. Factor analysis of motor and nonmotor signs in essential tremor: are these signs all part of the same underlying pathogenic process? Neuroepidemiology. 2009;33(1):41–46. doi: 10.1159/000211952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rao AK, Uddin J, Gillman A, Louis ED. Cognitive motor interference during dual-task gait in essential tremor. Gait Posture. 2013;38:403–409. doi: 10.1016/j.gaitpost.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoskovcová M, Ulmanová O, Sprdlík O, Sieger T, Nováková J, Jech R, Růžička E. Disorders of balance and gait in essential tremor are associated with midline tremor and age. Cerebellum. 2013;12:27–34. doi: 10.1007/s12311-012-0384-4. [DOI] [PubMed] [Google Scholar]
- 74.Klebe S, Stolze H, Grensing K, Volkmann J, Wenzelburger R, Deuschl G. Influence of alcohol on gait in patients with essential tremor. Neurology. 2005;65:96–101. doi: 10.1212/01.wnl.0000167550.97413.1f. [DOI] [PubMed] [Google Scholar]
- 75.Stolze H, Petersen G, Raethjen J, Wenzelburger R, Deuschl G. The gait disorder of advanced essential tremor. Brain. 2001;124:2278–2286. doi: 10.1093/brain/124.11.2278. [DOI] [PubMed] [Google Scholar]
- 76.Trillenberg P, Fuhrer J, Sprenger A, Hagenow A, Kömpf D, Wenzelburger R, Deuschl G, Heide W, Helmchen C. Eye-hand coordination in essential tremor. Mov Disord. 2006;21:373–379. doi: 10.1002/mds.20729. [DOI] [PubMed] [Google Scholar]
- 77.Helmchen C, Hagenow A, Miesner J, Sprenger A, Rambold H, Wenzelburger R, Heide W, Deuschl G. Eye movement abnormalities in essential tremor may indicate cerebellar dysfunction. Brain. 2003;126:1319–1332. doi: 10.1093/brain/awg132. [DOI] [PubMed] [Google Scholar]
- 78.Leegwater-Kim J, Louis ED, Pullman SL, Floyd AG, Borden S, Moskowitz CB, Honig LS. Intention tremor of the head in patients with essential tremor. Mov Disord. 2006;21:2001–2005. doi: 10.1002/mds.21079. [DOI] [PubMed] [Google Scholar]
- 79.Louis ED, Hernandez N, Alcalay RN, Tirri DJ, Ottman R, Clark LN. Prevalence and features of unreported dystonia in a family study of “pure” essential tremor. Parkinsonism Relat Disord. 2013;19:359–362. doi: 10.1016/j.parkreldis.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hedera P, Phibbs FT, Fang JY, Cooper MK, Charles PD, Davis TL. Clustering of dystonia in some pedigrees with autosomal dominant essential tremor suggests the existence of a distinct subtype of essential tremor. BMC Neurol. 2010;10:66. doi: 10.1186/1471-2377-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lombardi WJ, Woolston DJ, Roberts JW, Gross RE. Cognitive deficits in patients with essential tremor. Neurology. 2001;57:785–790. doi: 10.1212/wnl.57.5.785. [DOI] [PubMed] [Google Scholar]
- 82.Duane DD, Vermilion KJ. Cognitive deficits in patients with essential tremor. Neurology. 2002;58:1706. doi: 10.1212/wnl.58.11.1706. [DOI] [PubMed] [Google Scholar]
- 83.Bermejo-Pareja F. Essential tremor--a neurodegenerative disorder associated with cognitive defects? Nat Rev Neurol. 2011;7:273–282. doi: 10.1038/nrneurol.2011.44. [DOI] [PubMed] [Google Scholar]
- 84.Frisina PG, Tse W, Halbig TD, Libow LS. The pattern of cognitive-functional decline in elderly essential tremor patients: an exploratory-comparative study with Parkinson's and Alzheimer's disease patients. J Am Med Dir Assoc. 2009;10:238–242. doi: 10.1016/j.jamda.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 85.Gasparini M, Bonifati V, Fabrizio E, Fabbrini G, Brusa L, Lenzi GL, Meco G. Frontal lobe dysfunction in essential tremor: a preliminary study. J Neurol. 2001;248:399–402. doi: 10.1007/s004150170181. [DOI] [PubMed] [Google Scholar]
- 86.Kim JS, Song IU, Shim YS, Park JW, Yoo JY, Kim YI, Lee KS. Impact of tremor severity on cognition in elderly patients with essential tremor. Neurocase. 2010;16:50–58. doi: 10.1080/13554790903193216. [DOI] [PubMed] [Google Scholar]
- 87.Lacritz LH, Dewey R, Jr., Giller C, Cullum CM. Cognitive functioning in individuals with “benign” essential tremor. J Int Neuropsychol Soc. 2002;8:125–129. doi: 10.1017/s1355617702001121. [DOI] [PubMed] [Google Scholar]
- 88.Louis ED, Huey ED, Gerbin M, Viner AS. Apathy in essential tremor, dystonia, and Parkinson's disease: a comparison with normal controls. Mov Disord. 2012;27:432–434. doi: 10.1002/mds.24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Woods SP, Scott JC, Fields JA, Poquette A, Troster AI. Executive dysfunction and neuropsychiatric symptoms predict lower health status in essential tremor. Cogn Behav Neurol. 2008;21:28–33. doi: 10.1097/WNN.0b013e3181684414. [DOI] [PubMed] [Google Scholar]
- 90.Louis ED, Huey ED, Gerbin M, Viner AS. Depressive traits in essential tremor: impact on disability, quality of life, and medication adherence. Eur J Neurol. 2012;19:1349–1354. doi: 10.1111/j.1468-1331.2012.03774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Louis ED, Benito-Leon J, Bermejo-Pareja F. Self-reported depression and anti-depressant medication use in essential tremor: cross-sectional and prospective analyses in a population-based study. Eur J Neurol. 2007;14:1138–1146. doi: 10.1111/j.1468-1331.2007.01923.x. [DOI] [PubMed] [Google Scholar]
- 92.Miller KM, Okun MS, Fernandez HF, Jacobson CE, 4th, Rodriguez RL, Bowers D. Depression symptoms in movement disorders: comparing Parkinson's disease, dystonia, and essential tremor. Mov Disord. 2007;22:666–672. doi: 10.1002/mds.21376. [DOI] [PubMed] [Google Scholar]
- 93.Louis ED. Essential tremor as a neuropsychiatric disorder. J Neurol Sci. 2010;289:144–148. doi: 10.1016/j.jns.2009.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Louis ED, Okun MS. It is time to remove the ‘benign’ from the essential tremor label. Parkinsonism Relat Disord. 2011;17:516–520. doi: 10.1016/j.parkreldis.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Benito-Leon J, Louis ED, Bermejo-Pareja F. Elderly-onset essential tremor is associated with dementia. Neurology. 2006;66:1500–1505. doi: 10.1212/01.wnl.0000216134.88617.de. [DOI] [PubMed] [Google Scholar]
- 96.Louis ED, Ford B, Barnes LF. Clinical subtypes of essential tremor. Arch Neurol. 2000;57:1194–1198. doi: 10.1001/archneur.57.8.1194. [DOI] [PubMed] [Google Scholar]
- 97.Louis ED, Faust PL, Vonsattel JPG, Honig LS, Henchcliffe C, Pahwa R, Lyons KE, Rios E, Erickson-Davis C, Moskowitz CB, Lawton A. Older onset essential tremor: More rapid progression and more degenerative pathology. Mov Disord. 2009;24:1606–1612. doi: 10.1002/mds.22570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Louis ED, Hernandez N, Ionita-Laza I, Ottman R, Clark LN. Does rate of progression run in essential tremor families? Slower vs. faster progressors. Parkinsonism Relat Disord. 2013;19:363–366. doi: 10.1016/j.parkreldis.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rincon F, Louis ED. Benefits and risks of pharmacological and surgical treatments for essential tremor: disease mechanisms and current management. Expert Opin Drug Saf. 2005;4:899–913. doi: 10.1517/14740338.4.5.899. [DOI] [PubMed] [Google Scholar]
- 100.Zesiewicz TA, Ward CL, Hauser RA, Sanchez-Ramos J, Staffetti JF, Sullivan KL. A double-blind placebo-controlled trial of zonisamide (zonegran) in the treatment of essential tremor. Mov Disord. 2007;22:279–282. doi: 10.1002/mds.21282. [DOI] [PubMed] [Google Scholar]
- 101.Sasso E, Perucca E, Calzetti S. Double-blind comparison of primidone and phenobarbital in essential tremor. Neurology. 1988;38:808–810. doi: 10.1212/wnl.38.5.808. [DOI] [PubMed] [Google Scholar]
- 102.Findley LJ, Calzetti S. Double-blind controlled study of primidone in essential tremor: preliminary results. Br Med J (Clin Res Ed) 1982;285:608. doi: 10.1136/bmj.285.6342.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gironell A, Kulisevsky J, Barbanoj M, Lopez-Villegas D, Hernandez G, Pascual-Sedano B. A randomized placebo-controlled comparative trial of gabapentin and propranolol in essential tremor. Arch Neurol. 1999;56:475–480. doi: 10.1001/archneur.56.4.475. [DOI] [PubMed] [Google Scholar]
- 104.Louis ED. Treatment of Essential Tremor: Are there Issues We are Overlooking? Front Neurol. 2011;2:91. doi: 10.3389/fneur.2011.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Seibyl J, Russell D, Jennings D, Marek K. Neuroimaging over the course of Parkinson's disease: from early detection of the at-risk patient to improving pharmacotherapy of later-stage disease. Semin Nucl Med. 2012;42:406–414. doi: 10.1053/j.semnuclmed.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 106.Holland D, McEvoy LK, Desikan RS, Dale AM. Enrichment and stratification for predementia Alzheimer disease clinical trials. PLoS One. 2012;7:e47739. doi: 10.1371/journal.pone.0047739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sperling RA, Karlawish J, Johnson KA. Preclinical Alzheimer disease-the challenges ahead. Nat Rev Neurol. 2013;9:54–58. doi: 10.1038/nrneurol.2012.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schuurman PR, Bosch DA, Bossuyt PM, Bonsel GJ, van Someren EJ, de Bie RM, Merkus MP, Speelman JD. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med. 2000;342:461–468. doi: 10.1056/NEJM200002173420703. [DOI] [PubMed] [Google Scholar]
- 109.Bermejo-Pareja F. An old problem not yet resolved: the association of several neurodegenerative disorders. Neuroepidemiology. 2011;37(1):11–12. doi: 10.1159/000329662. [DOI] [PubMed] [Google Scholar]
- 110.Adler CH, Shill HA, Beach TG. Essential tremor and Parkinson's disease: lack of a link. Mov Disord. 2011;26:372–377. doi: 10.1002/mds.23509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.LaRoia H, Louis ED. Association between essential tremor and other neurodegenerative diseases: what is the epidemiological evidence? Neuroepidemiology. 2011;37:1–10. doi: 10.1159/000328866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Benito-Leon J, Louis ED, Bermejo-Pareja F. Risk of incident Parkinson's disease and parkinsonism in essential tremor: a population based study. J Neurol Neurosurg Psychiatry. 2009;80:423–425. doi: 10.1136/jnnp.2008.147223. [DOI] [PubMed] [Google Scholar]
- 113.Benito-Leon J, Louis ED, Bermejo-Pareja F. Risk of incident Parkinson's disease and Parkinsonism in essential tremor: A population-based study. Neurology. 2008;70:A191. doi: 10.1136/jnnp.2008.147223. [DOI] [PubMed] [Google Scholar]
- 114.Tan EK, Lee SS, Fook-Chong S, Lum SY. Evidence of increased odds of essential tremor in Parkinson's disease. Mov Disord. 2008;23:993–997. doi: 10.1002/mds.22005. [DOI] [PubMed] [Google Scholar]
- 115.Rocca WA, Bower JH, Ahlskog JE, Elbaz A, Grossardt BR, McDonnell SK, Schaid DJ, Maraganore DM. Increased risk of essential tremor in first-degree relatives of patients with Parkinson's disease. Mov Disord. 2007;22:1607–1614. doi: 10.1002/mds.21584. [DOI] [PubMed] [Google Scholar]
- 116.Louis ED, Benito-Leon J. Alzheimer's disease, Parkinson's disease and essential tremor: three common degenerative diseases with shared mechanisms? Eur J Neurol. 2010;17:765–766. doi: 10.1111/j.1468-1331.2010.02976.x. [DOI] [PubMed] [Google Scholar]
- 117.Bermejo-Pareja F, Louis ED, Benito-Leon J. Risk of incident dementia in essential tremor: A population-based study. Mov Disord. 2007;22:573–580. doi: 10.1002/mds.21553. [DOI] [PubMed] [Google Scholar]
- 118.Louis ED, Babij R, Ma K, Cortes E, Vonsattel JPG. Essential tremor followed by progressive supranuclear palsy: postmortem reports of 11 patients. J Neuropathol Exp Neurol. 2013;72:8–17. doi: 10.1097/NEN.0b013e31827ae56e. [DOI] [PMC free article] [PubMed] [Google Scholar]